Abstract
Background
Risk for foetal Down syndrome (DS) increases as maternal age increases. Non‐invasive screening (maternal serum triple test) for DS is routinely offered to pregnant women to provide risk estimates and suggest invasive amniocentesis for definitive pre‐natal diagnosis to high‐risk women.
Objective
We examined women's decision process with regard to pre‐natal screening, and specifically, the degree to which they take into account triple serum screening results when considering whether or not to undergo amniocentesis.
Design
Semi‐structured phone interviews were conducted to assess recall of DS screening results, understanding of risk estimates and their effect on women's decision whether to undergo amniocentesis. The study included 60 pregnant Israeli women (half younger than 35 and half advanced maternal age – AMA), with normal DS screening results and no known ultrasound abnormalities.
Results
Age appeared to determine the decision process. The vast majority of AMA women had amniocentesis, many of them before receiving their DS screening results. Most AMA participants knew that their risk estimate was ‘normal’, but still considered themselves at high risk due to their age. Procedure‐related risk (miscarriage) and other factors only had a minor effect on their decision. A minority of younger women had amniocentesis. Younger women mentioned procedure‐related risk and having normal screening results as the main factors affecting their decision not to have amniocentesis.
Conclusion
Age 35 is an anchor for the pre‐determination regarding performing or avoiding amniocentesis. AMA women mention ‘age’ as their main reason to have amniocentesis and considered it an independent risk factor.
Keywords: advanced maternal age, amniocentesis, decision making, Down syndrome, screening, statistical risk estimates
Introduction
Statistical risk estimates are important in various life situations. They also comprise a major component of the genetic counselling process which integrates the interpretation of family and medical histories to assess the chance of disease occurrence or recurrence.1
As genetic counsellors strive to provide precise information and assist in the decision‐making process of the counselees, an effort is made to provide the most accurate numerical risk estimates, under the premise that this information is a significant factor in the final decision. Yet, several studies have shown that risk information is interpreted by counselees in a personal manner and that their decisions are related to their subjective perceptions of risks rather than the ‘objective’ risk.2, 3, 4 People have a difficulty interpreting risk estimates, especially when the magnitude of the risk is small.5 Probabilities, by their very nature, involve an element of uncertainty, yet people tend to interpret them in a binary or in a more absolute manner as if something will either happen or not happen.6 Further, work on patients' recall of their own risk estimates has demonstrated that patients are often inaccurate and that individuals coming from families with a history of a disease tend to believe they are in greater risk than was assigned to them by the doctor, based on their genetics.7
The risk for foetal Down syndrome (DS) increases with the increase in maternal age, from 1 : 1600 at age 20 to 1 : 30 at age 45. At age 35, the risk is approximately 1 : 350.8 Non‐invasive screening (maternal serum triple test) for DS is routinely offered to pregnant women with the purpose of providing risk estimates and offering invasive pre‐natal diagnosis (usually amniocentesis) to appropriate subgroups of women considered to be at higher risk. Screening for DS risk, performed by analysing markers in maternal serum, is recommended to every pregnant woman in the US, Israel and many European countries. The main goal of the test is to assess the risk of foetal DS,9 which should inform the decision whether or not to undergo amniocentesis that provides a definitive diagnosis for DS. Risk assessment is based on measuring the amount of several chemicals in the mother's blood and a statistical model calculating the risk given her age at the end of pregnancy. Risk figures are usually presented in a probabilistic 1 : XXX format with a range of 1 : 1–1 : 20 000. A cut‐off is used to distinguish ‘normal’ (low‐risk) from ‘abnormal’ (high‐risk) results. In Israel, where the present study took place, the Ministry of Health sets the cut‐off at 1 : 380 so that if the result indicates a higher risk, amniocentesis is recommended and funded.10, 11 Additionally, when the woman is advanced maternal age (AMA) or if various ultrasound abnormalities are detected, amniocentesis is government funded regardless of the screening test results.12 In Israel, all women who undergo the screening for DS receive the test result that states the basic risk of DS due to age alone and the adjusted risk according to the test. It is widely believed by geneticists and genetic counsellors that this information facilitates the decision of whether amniocentesis should be performed.13, 14 However, Lawson found that negative perceptions of having a child with DS had a more significant influence on decisions made than the numeric probability for the outcome.15
Compared with other western countries, Israeli women tend to perform many tests during pregnancy16 including genetic carrier screening, ultrasound scans and biochemical screening for DS. Based on the latest survey of the Israeli Ministry of Health, 64% of pregnant Jewish AMA women had triple test screening and 47% had amniocentesis.17 Of Jewish women younger than 35, 61% had the triple test screening and 10% had amniocentesis. Israeli Jewish population is very diverse with regard to religiosity. One of the main factors affecting the decision regarding amniocentesis is religiosity probably as religious women do not accept the option of pregnancy termination for a foetus with DS. Among secular Jewish women of all ages, 89% had triple test screening and 32% had amniocentesis. Secular women over 35 were 4.8 times more likely to have amniocentesis than ultraorthodox women.
Amniocentesis is associated with a risk of miscarriage. The exact magnitude of this risk is cited differently in different places, ranging from 1 : 100 to 1 : 1600,18, 19, 20 although in the official Israeli consent form that is used in all medical facilities, the risk is stated as 1 : 200. Thus, the decision to perform the procedure without a medical indication can lead to significant negative consequences.
A question that arises in this context is what factors affect the decision regarding the screening test and amniocentesis. A study performed in Israel found that the use of amniocentesis by low‐risk women was associated with older age, having more information and being under more social pressure.21 In a study carried out before screening for DS was available, French et al.22 found that in a sample of highly educated women, there was no relationship between knowledge and the decision to obtain amniocentesis. It was previously noted that decision by AMA patients regarding amniocentesis may not always correlate clinically with DS screening results.23 Researchers have found that screening for DS decreased amniocentesis uptake among AMA women.24, 25 Johnson et al.26 found that AMA women were more likely to undergo amniocentesis when their screening‐based risk was higher than their age‐related risk, while Vergani et al.27 indicated that the key determinant of the choice regarding amniocentesis in AMA women was the a priori opinion of the woman towards the procedure.
The main objective of this study was to examine both comprehension and use of numeric risk information in medical decision making. We investigated the understanding and recall of the DS screening results and the way this information affected the decision to undergo amniocentesis with a special emphasis on age as a factor in the decision. Specifically, we examined whether women understand the statistical risk estimates of DS screening tests as well as the risk associated with amniocentesis and whether they use this information in conjunction with other factors, when making their decision about amniocentesis.
Methods
Approval for this research was obtained from the Sheba Medical Center medical ethics committee. Names and phone numbers of women who had normal (low‐risk) results of second trimester triple test screening performed at the Genetic Institute of the Sheba Medical Center were obtained. A written explanation about the research and a possible future phone interview was sent by mail to all prospective participants.
Women who had medical indications for amniocentesis (ultrasound findings, family history of DS or high statistical risk estimate on first trimester screening) were excluded from the sample. We also excluded those who did not speak fluent Hebrew. Approximately, 2 months after they received their screening results, women were contacted by phone and asked for their consent to participate in the semi‐structured interview. The interview was conducted after the decision regarding amniocentesis was reached, so as not to influence the woman's decision. There were a total of 311 eligible participants during the research period.
To reach the pre‐determined number of 60 participants, women were randomly selected, and at least one attempt to call each one of them was made, until the desired sample size of 60 was reached. Six women among the 74 that were reached by phone on the first attempt refused to participate. The main reason was time constrains due to the uninterrupted time slot required to complete the interview.
Eight additional women were later excluded due to medical indications for amniocentesis, which were revealed in the interview. This places the response rate to our study at 81%. Sample size was calculated as to compare the proportion of women in the below‐ and above‐AMA groups, respectively, on proportion that undergo amniocentesis (yes/no). A sample of 30 in each group would be enough to detect a large effect at power of 0.80 (P < 0.05, 2‐tailed).28
After verbal consent was obtained, a phone interview that lasted 11.81 min on average (with a standard deviation of 4.14 min) was conducted. All women were interviewed by a single interviewer (JGC). Overall, 60 women were interviewed: 30 were under the age of 35 and 30 were over 35, thus considered AMA for government funding purposes. Of the 60 interviews, 52 were recorded and transcribed. The remaining eight were analysed based on the questionnaire filled during the phone conversation. All 60 participants were Jewish. Forty‐four (73.3%) described themselves as secular, 9 (15%) as traditional and 4 (6.6%) as religious. Three women did not answer the question regarding religious beliefs. With regard to education, 15 (25%) had high school education, 25 (41.6%) had an academic degree, and 18 (30%) had a masters or doctoral (MD or PhD) degree. Two women did not answer the question regarding education level.
Questions included assessment of:
Knowledge and recall of screening test (triple test) purpose, amniocentesis risk, threshold for abnormal results and own risk estimate.
Understanding of screening results.
Whether or not amniocentesis was performed.
The reasons for performing or declining amniocentesis, especially the effect of maternal age, family history, and available public funding.
The questionnaire is presented as Data S1.
Statistical analysis was performed using spss v19.
Results
The decision of the patients regarding amniocentesis is presented in Table 1. While the vast majority of AMA women (86.7%) had amniocentesis, only a small minority of younger women (6.6%) chose to undergo the procedure. The Pearson's correlation between age (as a continuous variable) and performing amniocentesis is 0.768 (P < 0.001).
Table 1.
Age, risk and amniocentesis performance: participants' characteristics with regards to age, risk estimate and amniocentesis performance are presented
| Sample | ‘Young’ | ‘AMA’ | All |
|---|---|---|---|
| Sample size | 30 | 30 | 60 |
| Average age | 29.1 (23–35) | 37.6 (35–42) | 33.4 |
| Average risk | 1 : 10 866 (1 : 590–1 : 20 000) | 1 : 5050 (1 : 410–1 : 20 000) | 1 : 7958 |
| Had amniocentesis (rate) (%) | 2 (6.6) | 26 (86.7) | 28 (46.7) |
AMA, advanced maternal age.
The average risk of the 28 women who had amniocentesis was 1 : 5753, vs. 1 : 9888 for the 32 women who did not have the test. Even though this is a statistically significant difference (P < 0.05), both risks are in the normal range and are much lower than the 1 : 380 cut‐off for ‘high‐risk’ results (P < 0.01). The Pearson's correlation between risk at the DS screening test (as a continuous variable) and performing amniocentesis is 0.278 (P < 0.05).
When controlling for risk, the correlation between age and amniocentesis (r = 0.747) is still highly significant (P < 0.01). However, when controlling for age, there is no significant correlation (r = 0.074) between the risk estimates and amniocentesis. The above correlations were calculated for the entire sample, regardless of whether or not the woman waited for the triple serum screening results before deciding on an amniocentesis. About a third of the women (17) did not wait to receive the screening test result before deciding on an amniocentesis (see Fig. 1). When only the 43 women who had waited for screening result (see below) were included, the significance of the above correlations did not change (correlation for age and amniocentesis controlled for risk of 0.642, P < 0.01).
Figure 1.
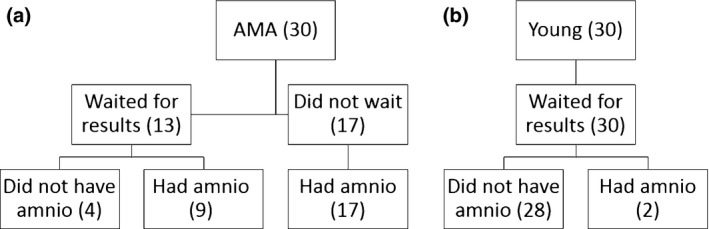
Flow chart representing whether women waited for screening results before deciding on amniocentesis performance among advanced maternal age (a) and Young (b) women.
The Pearson's correlation between education level (a scale of 1 = high school to 4 = PhD or MD) and amniocentesis performance is 0.362 (P value 0.05). However, when controlled for age, the very small remaining correlation (−0.026) is negative and not statistically significant.
Within the AMA group, the correlation between education and amniocentesis uptake (0.118) is not significant and is lost when controlled for age.
The correlation between education level and recall of screening results was −0.059 (P value 0.05). Thus, education hardly affected recall.
The correlation between religiosity (on a scale of 1 = secular to 4 = orthodox) and amniocentesis performance is −0.265 (P value 0.05). When controlling for age, this correlation remains similar (−0.236), but is no longer statistically significant. In any case, of our participants, only four defined themselves as religious (score of 3).
Among the 43 women who had the statistical results at the time of the decision regarding amniocentesis, only 17 (40%) said that they received an explanation about the triple test by a medical practitioner (doctor or nurse) before having it. Thus, the majority of women either did not have the test results at their disposal while deciding on amniocentesis or did not have an explanation regarding the purpose of triple serum screening and its role in determining whether to have amniocentesis.
Is the decision affected by accurate recall of risk estimates?
At the time of interview, participants were asked to recall the risk estimates for DS and for amniocentesis‐related miscarriage. By that time, all women had received their screening results (thus had the necessary information to answer) and had reached their final decision regarding amniocentesis. The results presented in Table 2 show that while a relatively small fraction of women could accurately recall the risk estimates for DS and amniocentesis‐related miscarriage, more women who had amniocentesis accurately recalled the procedure‐related risk, while more women who did not have amniocentesis accurately recalled their screening results.
Table 2.
Recall of risk estimates and amniocentesis performance: patients (who did, or did not, have amniocentesis) recall of both Down syndrome (DS) screening risk based on their screening results and amniocentesis‐related miscarriage risk (1 : 200)
| Had amniocentesis (28) | Did not have amniocentesis (32) | All (60) | |
|---|---|---|---|
| Can accuratelya quote screening result (%) | 3 (11) | 13 (41) | 16 (27) |
| Recalls result as ‘normal’ (%) | 28 (100) | 32 (100) | 60 (100) |
| Can accuratelya quote amniocentesis‐related risk (%) | 12 (43) | 6 (19) | 18 (30) |
| Can accuratelya quote both the screening result and amniocentesis risk (%) | 3 (11) | 6 (19) | 9 (15) |
| Cannot accurately quote either risk (%) | 10 (36) | 18 (56) | 28 (47) |
Among those that could quote any number at all, a response within a 10% range from the actual risk estimate was accepted as accurate.
For almost a third of the participants (17), the issue of whether the screening results affected their amniocentesis decision was irrelevant as they had amniocentesis before receiving their screening results. This means that the screening results could not have been taken into account when deciding on amniocentesis.
As shown in Table 2, five of the 28 women who had amniocentesis (18%) quoted procedure‐related risk as 1 : 1000. This risk estimate is mentioned during counselling at the Sheba Medical Center and appears in the information brochure, but is not written in the consent forms that state the risk as 1 : 200.
Is the decision to undergo amniocentesis affected by level of risks?
When asked whether the risk estimates (risk for DS and procedure‐related risk) were important for their decision making, ten of the ‘Young’ (33%) and eight of the AMA (27%) women replied that they were helpful. Eleven of these 18 (9 ‘Young’ and 2 AMA) did not have amniocentesis (61%).
Eleven of our 60 participants had risk estimates in the highest range, above 1 : 1000 (1 : 410–800). However, only six of them were aware of the result at the time of the decision. Seven of these 11 women, all of whom were above age 35, had amniocentesis. Two of the six women who waited for their screening results had amniocentesis. On the other end of the spectrum, 14 of our 60 participants had the lowest possible risk estimate of 1 : 20 000. Among them, three had amniocentesis (two were AMA and one was 34 years old). One of these AMA women did not wait for the result before undergoing amniocentesis.
Factors that influence the decision regarding amniocentesis in AMA and Young women
The 52 transcribed interviews were searched for factors that the participants mentioned as important for their decision (and were not presented as multiple‐choice answers). Each participant could mention several different reasons. These results are shown in Table 3.
Table 3.
The reasons for having/not having amniocentesis: presented in descending order of popularity (from top to bottom). The reasons were inferred during the interview not as a multiple‐choice or menu‐itemed question
| Age group | AMA | AMA | Young | Young | Total |
|---|---|---|---|---|---|
| Amniocentesis (number of interviewsa) | Had amniocentesis (24) | Did not have amniocentesis (4) | Had amniocentesis (2) | Did not have amniocentesis (22) | |
| Ageb | 21c | 6 | 27 | ||
| Procedure risk | 3 | 3 | 14 | 20 | |
| Doctor's recommendationb | 7 | 1 | 9 | 17 | |
| Normal statistical risk estimate | 4 | 11 | 15 | ||
| Certainty that foetus is normal | 8 | 2 | 10 | ||
| Previous amnio | 8 | 8 | |||
| Government funding | 4 | 4 | |||
| Previous children/healthy family | 4 | 4 | |||
| Abnormal child is worse than miscarriage | 3 | 3 | |||
| Family/friends | 2 | 1 | 3 | ||
| Amnio detects other abnormalities except DS | 1 | 1 | 1 | 3 | |
| Having control | 2 | 2 | |||
| Had exposure to DSd | 2 | 2 | |||
| May not terminate a DS pregnancy | 1 | 1 | 2 | ||
| Fear of needles | 1 | 1 |
AMA, advanced maternal age; DS, Down syndrome.
Based on the 52 fully transcribed interviews only.
‘Age’ and ‘doctor's recommendation’ were mentioned by AMA women as a reason to have amniocentesis and by Young women as a reason not to have the procedure.
Among the 21 AMA women who mentioned age as a reason for amniocentesis, 14 were asked what that risk was. Only five of them (36%) could accurately quote that risk.
Had exposure to information about families with Down syndrome.
Age is the most significant factor cited by AMA women as a reason to have amniocentesis. Next factors are: wanting certainty (as opposed to statistical estimates), having performed amniocentesis in a previous pregnancy and the recommendation of the doctor. The Young women mentioned procedure‐related risk as the most common reason for not having amniocentesis, and others are having a normal statistical estimate, doctor's recommendation and age. Age and doctor's recommendation were mentioned by both groups, but as opposite influences.
Table 4 brings citations from the women as pertaining to the most popular reasons they mentioned for either performing or not performing amniocentesis. The citations allow hearing the women's voices and their unique interpretations of the reasons that led them to test or to refrain from testing.
Table 4.
Representative quotes for the most commonly stated reasons
| Age group | AMA | AMA | Young | Young |
|---|---|---|---|---|
| Amniocentesis | Yes | No | Yes | No |
| Agea |
‘My age was what led me to have the test’ ‘Because of my age we made a decision to have the test regardless of the results’ |
‘I did not have an amnio because of my age (29)’ | ||
| Procedure risk | ‘Because there is a risk to both mother and fetus we debated whether to have it’ | ‘I was afraid of the risk’ |
‘I think that the test is dangerous and it's best to avoid it if it's unnecessary’ ‘Once I understood that the procedure has a risk, even a small one, I decided not to do it’ |
|
| Doctor's recommendationa |
‘My doctor referred me for amnio anyway because of my age’ ‘My doctor said that since I am 38 it is better to have the test than take unnecessary risks’ |
‘My doctor said that I don't need to have the test because the (screening) result was normal’ a‘My doctor didn't want to look at my results and said that since I am 35 I must have an amnio’ |
‘I asked my doctor, he said that I don't need it (amniocentesis)’ ‘I discussed it with my doctor and he did not recommend (amniocentesis)’ |
|
| Normal statistics |
‘My result was very good so I did not have an amnio’ ‘I had a risk estimate of 1 : 9400 which is good’ |
‘It was a low risk, that's why I did not have the amnio’ | ||
| Certainty |
‘I feel that it is important for me to make sure that there is no problem with the baby, as much as currently possible’ ‘It was Important for me to have certainty regarding birth defects’ |
‘The statistics wasn't enough for me’ | ||
| Previous Amniocentesis | ‘I had an amnio in all my previous pregnancies’ |
AMA, advanced maternal age.
Two women claimed that they were reprimanded by their doctor for not having amniocentesis in their age.
Hypothetical risk scenarios
To evaluate the role of risk estimates in women's decisions, we asked two hypothetical questions regarding predicted behaviour in two risk‐level scenarios.
Among the 32 women who did not have amniocentesis, 19 (59%) women (16 of them ‘Young’) said they would consider having amniocentesis at 1 : 100 risk for DS. An additional 3 (9%) women (two of them ‘Young’) said they would ‘maybe’ have amniocentesis at that risk level. This result shows that the majority of these women would consider a high‐risk estimate as important.
Among the 27 women who had amniocentesis, only one (36 years old, 1 : 740 risk for DS) said that she ‘might have considered not having amniocentesis’ at a 1 : 10 000 risk for DS. This shows that the risk level was not a meaningful factor in the decision for most women who decided to have amniocentesis in this study.
Discussion
This study aimed to examine pregnant women's decision process with regard to pre‐natal screening, and specifically, the degree to which they take into account triple serum screening results when considering whether or not to undergo amniocentesis.
A recent systematic review29 that attempted to identify the factors influencing the uptake of invasive pre‐natal testing by AMA women has found that it is difficult to draw firm conclusions as to the influential factors. The researchers looked at ‘external’ and ‘psychological’ factors, but did not examine women's subjective risk assessments or their main reasons for having amniocentesis. Our current study fills this gap as we have directly asked our participants about the factors that affected their decision and explored their subjective risk perception.
Our results show that being above age 35 had a strong influence on women's use of the results to decide whether they would perform amniocentesis. Thus, low statistical risk for DS did not affect the decision to have amniocentesis for women over 35, who were likely to undergo amniocentesis regardless of the results. Notably, the majority of AMA participants did not plan to have triple serum screening or to wait for its results before undergoing amniocentesis. AMA women stated that age was the most significant factor for their decision to have amniocentesis although most of them did not know the exact risk associated with their age. Young women stated the risk of amniocentesis as the most significant reason for avoiding the procedure. DS risk was also important for the decision about having the procedure for young women.
For our participants, age 35, which is associated with both physician recommendations and government funding for amniocentesis, served as an anchor in the decision to perform amniocentesis. While most women could not state their age‐related or screening‐based risks, they clearly considered being over 35 years of age as a determining factor. So much so that most of the AMA women did not take statistical risk estimates into account when deciding about amniocentesis. Most of our AMA participants knew that their risk estimate was ‘normal’, but still considered themselves at high risk due to their age. Other factors, such as procedure‐related risk, were secondary and had only a minor effect on the decision.
Younger women, on the other hand, did state the procedure‐related risk and having a normal screening result as the two main factors affecting their decision not to have amniocentesis. Even though the majority of the young women could not recall their screening result, they were four times more likely to accurately recall it than AMA women. It thus seems that the decision process of the young women is more informed than that of the AMA women. Several of the AMA women said that in previous pregnancies, they did not have amniocentesis because their screening results were normal, but their point of view was different now that they were ‘old’.
A small minority of AMA women mentioned procedure risk, normal risk estimates and the fact that amniocentesis detects other abnormalities besides DS, as their reasons for action. Thus, women choose to undergo amniocentesis for the certain detection of DS disregarding the actual risk and other factors. It is possible that if a proper explanation about the screening test was given, women would still have chosen to have the procedure, but would have stated other relevant reasons and have arrived to the decision in a more informed way, based on better knowledge and understanding. Previous studies that explored this issue did not specifically compare AMA and younger women; thus, our results provide a unique perspective on the subject. While it was previously found that subjective factors, and not knowledge, are important for the decision, the dual interpretation of age as a high‐ or low‐risk factor is very important for the understanding of the decision process of women with low screening risk for DS.
Several contextual factors should be considered when evaluating Israeli women's decision process regarding amniocentesis. The first is the effect of differential government funding. While young women only receive funding for amniocentesis if they have abnormal screening results or ultrasound findings, AMA women have free amniocentesis without any medical indication. Our participants appeared to interpret funding as a medical recommendation and as a social norm. It is interesting to explore whether AMA women would still have amniocentesis if it was not funded. We did not specifically ask this question; however, the women did not mention the test being free as a reason to have it, and young women did not mention cost as a significant reason not to have the test. It is also worth mentioning that funding for amniocentesis for AMA women is available in many countries.
The second contextual factor is the attitude of the medical community towards pre‐natal testing for AMA women. It is important to note that the majority of our AMA participants had the blood drawn for the triple test immediately before having the amniocentesis. This practice is based on the recommendation of some doctors and geneticists to have the triple serum test regardless of amniocentesis, as it can detect risk for Smith–Lemli–Opitz syndrome, X–linked Ichthyosis and other conditions (all unrelated to maternal age) by detecting extremely low levels of estriol (UE3) in maternal blood.30 Five AMA women mentioned this as their main reason to have the triple test. These women emphasized that the estimated risk for DS was not significant for them. This timing of the triple serum screening suggests that the test is seen as part of routine pre‐natal care and is not considered as a decision factor for amniocentesis by either the women or their medical caretakers. Further, most women reported that they did not receive an explanation about the triple test from a medical practitioner, and some found the information on the internet and in the media. It is possible that lack of discussion and limited understanding of the purpose and importance of the test contributed to the disregard of its results during decision making. The disregard of a screening test when deciding about a diagnostic test may be relevant to other medical situations. It is in agreement with the previously mentioned findings of Linnenbringer et al.7 regarding the overestimate of individual Alzheimer risk despite the results of genetic screening.
The effect of physicians’ attitudes on the actions and choices of their patients was previously explored by Gurmankin et al.31 who found that physicians’ recommendations can lead people to make decisions that go ‘against what is best and against what they would otherwise prefer’. Heckerling et al.32 showed that, according to patients, the choice of pre‐natal test was made entirely or mostly by the physician in 14% of cases and was shared equally between patient and physician in 37% of cases. According to a recent survey, over 60% of Israeli obstetricians would recommend amniocentesis to AMA women with normal screening results.33 Our results suggest that the common attitudes of the medical community (referred to by our participants as ‘doctor's recommendation’) have a significant effect on the decisions of individual women. The current study design could not parse the women's attitudes and actions from the attitudes and recommendations of their physicians. Furthermore, two of our AMA participants mentioned being reprimanded by their physician for not having amniocentesis, and others mentioned feeling considerable pressure from their environment to have the procedure.
It seems plausible that the combination of funding that is based on age alone and the recommendations of many physicians are perceived by women as an independent risk factor. If the policy makers and the medical community insinuate that amniocentesis is indicated after age 35, it is hard to expect the non‐expert women to make an independent decision.
In western countries, a woman's informed choice is considered a basic principle in the carrying out of pre‐natal screening and diagnosis that are presented as offering new reproductive choices for women and couples.34 A definition of informed choice as adapted from O'Connor and O'Brien‐Pallas35 is one that is based on relevant knowledge, consistent with the decision‐maker's values and behaviourally implemented. We show a significant lack of knowledge among our participants, thus casting doubt whether the decision to undergo amniocentesis is indeed informed.
This study has several limitations. The sample size was relatively small and not completely representative of the general Israeli population, but it does represent a large population of secular, educated, Israeli women who utilize many pre‐natal tests.
There is a significant correlation, of medium magnitude, between education and amniocentesis uptake in our sample of women. However, as education is highly correlated with age, age is confounding this correlation. There is a very weak negative correlation between education and recall of screening results; thus, this sample being highly educated should not alter our conclusions as for the significance of age for the decision.
A certain limitation in this study is that the women were interviewed 1–2 months after they have received their screening results and reached their decision regarding amniocentesis. It is possible that the time lag contributed to the relatively low recall rates of the risk estimates and that at the time of decision, the women had better knowledge of their risks. However, the majority of AMA women chose not to wait for their screening results before having amniocentesis, and others claimed that the risk estimates had no effect on their decision. The small number of the remaining women did somewhat limit the statistical power of the comparisons between AMA and young women.
We chose to explore the effect of the second trimester triple marker screen rather than the first trimester screening or the integrated screening that may have higher specificity and sensitivity. The triple test is the only test that was uniformly recommended and funded for all pregnant women at the time of the study. As most women did not recall their results or incorporate them into the decision, we believe that the effect of other statistical tests would not differ.
Future research could further explore the decision process of AMA women undergoing amniocentesis. One potential direction for doing so is in the context of physician attitudes and recommendations as well as the effect of funding policies.
It was previously shown that the use of decision aids can improve informed choice36, 37 which opens a possibility for further exploration of the process among pregnant women in Israel and other places. New technologies, such as non‐invasive testing for foetal trisomy and microarray‐based techniques, are being recently introduced into the pre‐natal testing field. Many are offered directly to consumers. Understanding of decision processes can help in the integration of these technologies into existing practices by both patients and medical community.
Funding sources
This research was supported by The Israel Notional Institute of Health Policy Research.
Conflict of interest
No conflict of interest has been declared.
Supporting information
Data S1. Questionnaire.
References
- 1. Resta R, Biesecker BB, Bennett RL et al A new definition of genetic counseling: National Society of Genetic Counselors’ task force report. Journal of Genetic Counseling, 2006; 15: 77–83. [DOI] [PubMed] [Google Scholar]
- 2. Lippman‐Hand A, Fraser FC. Genetic counseling: provision and reception of information. American Journal of Medical Genetics, 1979; 3: 113–127. [DOI] [PubMed] [Google Scholar]
- 3. Wertz DC, Sorenson JR, Heeren TC. Clients' interpretation of risks provided in genetic counseling. American Journal of Human Genetics, 1989; 39: 253–264. [PMC free article] [PubMed] [Google Scholar]
- 4. Shiloh S, Saxe L. Perception of risk in genetic counselling. Psychology and Health, 1989; 3: 45–46. [Google Scholar]
- 5. Miron‐Shatz T, Hanoch Y, Graef D, Sagi M. Presentation format, numeracy, and emotional reactions: the case of prenatal screening tests. Journal of Health Communication, 2009; 14: 439–450. [DOI] [PubMed] [Google Scholar]
- 6. Hallowell N, Green JM, Statham H, Murton F, Richards MPM. Recall of numerical risk estimates and counsellees' perceptions of the importance of risk information following genetic counselling for breast and ovarian cancer. Psychology Health and Medicine, 1997; 2: 149–169. [Google Scholar]
- 7. Linnenbringer E, Roberts J, Hiraki S, Cupples L, Green R. “I know what you told me, but this is what I think:” perceived risk of Alzheimer disease among individuals who accurately recall their genetics‐based risk estimate. Genetics in Medicine, 2010; 12: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hook EB. Rates of chromosomal abnormalities at different maternal ages. Obstetrics and Gynecology, 1981; 58: 282–285. [PubMed] [Google Scholar]
- 9. Cheng EY, Luthy DA, Zebelman AM, Williams MA, Lieppman RE, Hickok DE. A prospective evaluation of a second‐trimester screening test for fetal Down syndrome using maternal serum alpha‐fetoprotein, hCG, and unconjugated estriol. Obstetrics and Gynecology, 1993; 81: 72–77. [PubMed] [Google Scholar]
- 10. Israeli Ministry of Health policy statement, 2007; 15/2007.
- 11. Israeli Ministry of Health policy statement, 2013; 6/2013.
- 12. Israeli Ministry of Health policy statement, 1992; 36/92.
- 13. Beaman JM, Goldie DJ. Second trimester screening for Down's syndrome: 7 year experience. Journal of Medical Screening, 2001; 8: 128–131. [DOI] [PubMed] [Google Scholar]
- 14. Zikmund‐Fisher BJ, Fagerlin A, Keeton K, Ubel PA. Does labeling prenatal screening test results as negative or positive affect a woman's responses? American Journal of Obstetrics and Gynecology, 2007; 197: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawson KL. Contemplating selective reproduction: the subjective appraisal of parenting a child with a disability. Journal of Reproductive and Infant Psychology, 2001; 19: 73–82. [Google Scholar]
- 16. Remennick L. The quest for the perfect baby: why do Israeli women seek prenatal genetic testing? Sociology of Health and Illness, 2006; 28: 21–53. [DOI] [PubMed] [Google Scholar]
- 17. Romano‐Zelicha O, Shohat T. Israeli Ministry of Health publication number 343, 2011.
- 18. Tabor A, Madsen M, Obel EB, Philip J, Bang J, Gaard‐Pedersen B. Randomized controlled trial of genetic amniocentesis in 4606 low‐risk women. The Lancet, 1986; 327: 1287–1293. [DOI] [PubMed] [Google Scholar]
- 19. Eddleman KA, Malone FD, Sullivan L et al Pregnancy loss rates after midtrimester amniocentesis. Obstetrics and Gynecology, 2006; 108: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 20. Odibo AO, Gray DL, Dicke JM, Stamilio DM, Macones GA, Crane JP. Revisiting the fetal loss rate after second‐trimester genetic amniocentesis: a single center's 16‐year experience. Obstetrics and Gynecology, 2008; 111: 589–595. [DOI] [PubMed] [Google Scholar]
- 21. Lesser Y, Rabinowitz J. Elective amniocentesis in low‐risk pregnancies: decision making in the era of information and uncertainty. American Journal of Public Health, 2001; 91: 639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. French BN, Kurczynski TW, Weaver MT, Pituch MJ. Evaluation of the health belief model and decision making regarding amniocentesis in women of advanced maternal age. Health Education Quarterly, 1992; 19: 177–186. [DOI] [PubMed] [Google Scholar]
- 23. Marini T, Sullivan J, Naeem R. Decisions about amniocentesis by advanced maternal age patients following maternal serum screening may not always correlate clinically with screening results: need for improvement in informed consent process. American Journal of Medical Genetics, 2002; 109: 171–175. [DOI] [PubMed] [Google Scholar]
- 24. Wray AM, Ghidini A, Alvis C, Hodor J, Landy HJ, Poggi SH. The impact of first‐trimester screening on AMA patients' uptake of invasive testing. Prenatal Diagnosis, 2005; 25: 350–353. [DOI] [PubMed] [Google Scholar]
- 25. Nakata N, Wang Y, Bhatt S. Trends in prenatal screening and diagnostic testing among women referred for advanced maternal age. Prenatal Diagnosis, 2010; 30: 198–206. [DOI] [PubMed] [Google Scholar]
- 26. Johnson JP, Streets K, Fitzgerald J et al Influence of triple‐marker screen risk versus a priori risk in decision for amniocentesis in women of advanced maternal age. Prenatal Diagnosis, 1998; 18: 979–986. [DOI] [PubMed] [Google Scholar]
- 27. Vergani P, Locatelli A, Biffi A et al Factors affecting the decision regarding amniocentesis in women at genetic risk because of age 35 years or older. Prenatal Diagnosis, 2002; 22: 769–774. [DOI] [PubMed] [Google Scholar]
- 28. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd edn New York: Academic Press, 1988. [Google Scholar]
- 29. Godino L, Turchetti D, Skirton H. A systematic review of factors influencing uptake of invasive fetal genetic testing by pregnant women of advanced maternal age. Midwifery, 2013; 29: 1235–1243. [DOI] [PubMed] [Google Scholar]
- 30. Palomaki GE, Bradley LA, Knight GJ, Craig WY, Haddow JE. Assigning risk for Smith‐Lemli‐Opitz syndrome as part of 2nd trimester screening for Down's syndrome. Journal of Medical Screening, 2002; 9: 43–44. [DOI] [PubMed] [Google Scholar]
- 31. Gurmankin AD, Baron J, Hershey JC, Ubel PA. The role of physicians' recommendations in medical treatment decisions. Medical Decision Making, 2002; 22: 262–271. [DOI] [PubMed] [Google Scholar]
- 32. Heckerling PS, Verp MS, Albert N. The role of physician preferences in the choice of amniocentesis or chorionic villus sampling for prenatal genetic testing. Genetic Testing, 1998; 2: 61–66. [DOI] [PubMed] [Google Scholar]
- 33. Srebnik N, Miron‐Shatz T, Rolison JJ, Hanoch Y, Tsafrir A. Physician recommendation for invasive prenatal testing: the case of the “precious baby”. Human Reproduction, 2013; 28: 3007–3011. [DOI] [PubMed] [Google Scholar]
- 34. Santalahti P, Hemminki E, Latikka A, Ryynae M. Women's decision‐making in prenatal screening. Social Science and Medicine, 1998; 46: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 35. O'Connor A, O'Brien‐Pallas LL. Decisional conflict In: Mcfarlane GK, Mcfarlane EA. (eds) Nursing Diagnosis and Intervention. Toronto: Mosby, 1989: 486–496. [Google Scholar]
- 36. Drake ER, Engler‐Todd L, O'Connor AM, Surth LC, Hunter A. Development and evaluation of a decision aid about prenatal testing for women of advanced maternal age. Journal of Genetic Counseling, 1999; 8: 217–233. [DOI] [PubMed] [Google Scholar]
- 37. Bekker HL, Hewson J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomized controlled trial. Prenatal Diagnosis, 2004; 4: 265–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Questionnaire.


