Abstract
Background
According to surveys, many patients with advanced cancer wish to receive survival information.
Objective
This study investigated information preferences by offering patients a decision aid (DA) with information on expected survival for two treatment options: supportive care with or without second‐line palliative chemotherapy. Predictors of accepting survival information were explored.
Design
Eligible patients in this multicentre prospective study were offered second‐line chemotherapy for advanced breast or colorectal cancer. A nurse presented a DA on second‐line treatment and asked patients whether they desired information on (i) adverse events, (ii) tumour response and (iii) survival. Data on 50 clinical and psychosocial patient characteristics were collected from inclusion forms and patient questionnaires.
Results
Seventy‐seven patients received a DA; median age 62 years (range 32–80), 61% female, 77% colorectal cancer. Fifty‐seven patients (74%; 95% CI 64–84) desired survival information. Four psychosocial characteristics (e.g. deliberative decision style) independently predicted information desire. However, the use of these characteristics to predict information desire hardly outperformed a simple prediction rule.
Conclusions
Many patients desired information on expected survival when deciding about second‐line treatment. However, our exploratory analysis indicated that patients desiring this information could not be identified based on their clinical or psychosocial characteristics. These findings can help encourage candid discussions about expected survival. Health professionals should be careful not to make implicit assumptions of information desire based on patient characteristics, but to explicitly ask patients if survival information is desired, and act accordingly.
Keywords: decision aids, information desire, palliative chemotherapy, predictors, survival information, treatment decision
Introduction
A central component of the communication with patients with advanced cancer is the discussion of prognosis, including expected survival. Health professionals may be concerned that providing survival information could be contrary to patients' wishes or best interests.1 While patients with advanced cancer indeed fear bad news, many wish to receive survival information to make treatment decisions and plan the future.2 Candid conversations about prognosis can establish an open atmosphere, improve patients' sense of control and facilitate more realistic expectations.3 Surveys found that many patients with advanced cancer (44, 59, 80 and 88%, respectively) stated a desire for survival information.4, 5, 6, 7 It remains unclear, however, how many patients will accept survival information when it is actually offered by a health professional.8
Previous studies have tried to characterize patients desiring survival information. In these studies, patients were asked whether they desired to discuss expected survival6, 7, 9 or whether they desired these discussions had taken place.4, 10 Among patients with advanced cancer, a higher information desire was observed for men4, 10 and for patients with higher education,7 more pain,7 or more symptoms of depression.6 In addition, among patients with cancer across all disease stages, stating a desire for survival information was associated with lower age, lower death avoidance and worse prognosis.9
This study will assess preferences of patients with advanced breast or colorectal cancer for receiving survival information when deciding whether or not to start second‐line palliative chemotherapy. Patients' information desire will be assessed by actually offering information on the expected benefits and risks of chemotherapy, using a decision aid (DA). Clinical and psychosocial patient characteristics associated with desiring survival information will be explored.
Methods
Design
This study was part of a randomized trial (Netherlands Trial Register; NTR1113) conducted in 17 hospitals in the Netherlands which was described in detail elsewhere.11 In short, the target population consisted of patients with advanced breast or colorectal cancer facing the decision whether or not to start second‐line palliative chemotherapy. To identify these patients, patients who were in remission after first‐line chemotherapy or were receiving first‐line chemotherapy were preselected. Exclusion criteria were labile personality structure (as assessed by the physician), a Karnofsky performance score lower than 60, and insufficient Dutch language proficiency. The study was approved by the regional ethics review committee and the research ethics committees of all participating centres.
Procedure
The medical oncologist or nurse assessed the potential eligibility of consecutive patients. Professionals were instructed not to mention that explicit survival information could be provided, to avoid losing patients not desiring such information. Professionals asked patients for permission to be approached by the researcher, who obtained written informed consent.
At inclusion, patients were sent a baseline questionnaire with sociodemographic and psychosocial variables hypothesized to be associated with information desire. These patients were monitored for disease progression and the ensuing treatment decision whether or not to start second‐line palliative chemotherapy. Patients who were offered second‐line treatment were randomly assigned to receive (i) the usual treatment‐related information from the oncologist (control group) or (ii) the usual treatment‐related information from the oncologist followed by a DA from a nurse (intervention group) (1 : 2 ratio).
This study focused on the patients in the intervention group, who received the DA in a subsequent consultation with a nurse, typically within a week after the consultation with the oncologist who mentioned disease progression and treatment options. DAs were developed for 11 chemotherapeutic regimens commonly used as second‐line treatment for advanced breast or colorectal cancer, based on systematic reviews of the literature for the two tumour types.12, 13 In the systematic review on benefits and risks of second‐line irinotecan for advanced colorectal cancer, 25 phase II and 5 phase III studies were identified. Median survival was established using the single direct randomized comparison between patients receiving BSC plus second‐line irinotecan and patients receiving BSC alone.12 In the vast body of literature on second‐line chemotherapy for breast cancer, no randomized studies comparing any of the second‐line chemotherapeutic regimens to BSC alone were found. A meta‐analysis was performed to establish the median survival for each of the selected chemotherapeutic regimens; no differences in effectiveness were found between the regimens.13 In the DAs, the median expected survival of 12 months with chemotherapy was presented together with a question mark for expected survival without chemotherapy, and an explanation that it is not known whether, or in what way, the survival of patients with advanced breast cancer is influenced by second‐line chemotherapy.
Figure 1 shows a summary of the information provided in a DA for colorectal cancer, and a full DA is available in the online supplement.
Figure 1.

Example of the summary page of a decision aid for colorectal cancer.
The consultation started with an introduction in which the DA, the two treatment options (supportive care with or without second‐line palliative chemotherapy), and an example of risk information were presented. Then, the nurse proceeded to offer information on risks and benefits in three separate items: (i) adverse events; (ii) tumour response; and (iii) survival. For each item, the nurse first elaborated on the type of information that could be expected (e.g. implications of a serious adverse event, the temporary nature of tumour response, the concept of median survival) and then asked the patient whether the information was desired or not. If desired, the nurse provided the information. The instructions for the nurse, including the explanation of the concept of median survival, are included in the online supplement.
Measures
Table 1 gives an overview of the measurements of information desire and potential predictors.
Table 1.
Overview of measurements of information desire and predictors
| Variable | Operationalization | Timing of measurements | |
|---|---|---|---|
| Inclusion | Interview with DA | ||
| Information desire | P | ||
| Predictors of information desire | |||
| Sociodemographics (n = 8) | |||
| Age | P | ||
| Living situation | P | ||
| Working status | P | ||
| Having children | P | ||
| Having grandchildren | P | ||
| Education | P | ||
| Religion | P | ||
| Gender | O | ||
| Tumour and treatment characteristics (n = 7) | |||
| Date of initial diagnosis of disease | O | ||
| Date of diagnosis metastatic disease | O | ||
| Tumour location | O | ||
| Tumour status | O | ||
| Previous palliative chemotherapy | O | ||
| First‐line chemotherapy in study setting | O | ||
| Estimate of patient survival | 3–6, 6–9, 9–12, >12 months | Oa | |
| Decision‐related measures (n = 2) | |||
| Treatment preference | Chemotherapy+BSC/BSC alone/do not know | P | |
| Strength of treatment preference | 1–4 (not strong–very strong)b | P | |
| Well‐being (n = 13) | |||
| General health | 0–10 (worst‐best imaginable) | P | |
| Anxiety and depression | HADS Anxiety and Depression Scale | P | |
| Cancer Worries | Adapted Lerman's Cancer Worry Scale | P | |
| Health‐related quality of life (n = 9)c | EORTC QLQ‐C15‐PAL | P | |
| Coping (n = 9) | |||
| Coping with cancer (n = 3)d | Mental Adjustment to Cancer Scale | P | |
| Decision style (n = 4)e | Michigan Assessment of Decision Style | P | |
| Participation preference | Problem‐Solving Decision‐Making Scale | P | |
| Death avoidance | Death Avoidance Scale | P | |
| Information‐related measures (n = 5) | |||
| Information preference | 0–10 (I want to know nothing‐everything there is to know) | P | |
| Amount of information received | 1–7 (way too little–way too much) | P | |
| Subjective numeracy (n = 3)f | Subjective Numeracy Scale | P | |
| Knowledge‐related measures (n = 1) | |||
| Subjective knowledge | 1–10 (very bad–excellent) | P | |
| Treatment attitudes (n = 5) | |||
| Striving for length or quality of life (n = 2)g | QQ‐Questionnaire | P | |
| Perceived benefits and harms of first‐line chemotherapy (n = 2)h | 1–4 (much–none) | P | |
| Time since last chemotherapy | 1–5 (currently under treatment–more than a year ago) | P | |
P, patient‐reported; O, oncologist‐reported; BSC, best supportive care; DA, decision aid.
Reported on the progression form when disease progression occurred.
For patients who were undecided, strength of the treatment preference was scored as ‘0’.
Including physical and emotional functioning, fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation.
Including helplessness/hopelessness, cognitive avoidance, fighting spirit.
Including decision avoidance, deferring responsibility, information seeking, deliberation.
Including the total scale and the ability and preference subscales.
Including striving for length of life and striving for quality of life.
Including benefits and harms of first‐line palliative chemotherapy.
Information desire
During the interview with the DA, the nurse registered the main outcome measure: whether or not the patient wanted to see the information on survival (see above in ‘Procedure’).
Potential predictors of information desire
Sociodemographic variables were collected through the baseline questionnaire and the inclusion form (Table 1). Oncologists were instructed to record tumour and treatment characteristics on the inclusion form and to estimate patient survival on the progression form. At the start of the interview, the nurse asked for the patient's treatment preference and if applicable, the strength of this preference. Measures on well‐being included general health, anxiety and depression,14 cancer worries15 and health‐related quality of life.16 Questions on coping included coping with cancer,17 decision style,18 participation preference19 and death avoidance.9, 20 Information‐related measures included the amount of information preferred,21 the amount of information received and numeracy22, 23 (i.e. the ability to handle basic probability concepts). Patients rated their own baseline knowledge (subjective knowledge) on cancer and on benefits and risks of treatment options. Patients' attitudes were measured with questions on striving for length (quantity) and quality of life,24 questions on the patients' perceived amount of benefits and risks experienced during first‐line chemotherapy and a question on the time since last chemotherapeutic treatment.
Statistical analysis
To examine selective attrition, the characteristics of patients receiving the DA were compared with patients experiencing progressive disease who were not randomized, using an independent‐samples t‐test or chi‐square test. As the main outcome of this study, we calculated the percentage of patients, including the 95% confidence interval of that percentage, who accepted the survival information. Next, an extensive exploratory analysis of potential predictors of accepting survival information was performed. Patients desiring survival information were compared with patients not desiring that information, using chi‐square tests. In case of missing data, scale values were calculated only if at least half of the items were available, by imputing the mean of the available items. Data were dichotomized by a median split, except for HADS anxiety and depression scales which were dichotomized using a clinical cut‐off point of 8.25 Patient characteristics associated with desiring survival information at a level of P < 0.2 in a bivariate analysis were entered stepwise in a multivariable logistic regression model, adding additional variables with a P value of <0.05. The use of a higher value of P for the selection of variables in the bivariate analysis is generally recommended because the use of the traditional value of P < 0.05 can result in missing important predictors, concealed by confounding.
Results
Participants
Of 441 patients assessed for potential eligibility, 86 (20%) did not meet the inclusion criteria, and 34 patients (8%) were not approached by the oncologist and therefore the inclusion criteria could not be verified (see Fig. 2). Of the 321 patients asked for the study, 263 (82%) gave informed consent. Of them, 92 patients (35%) were not faced with the decision on second‐line palliative chemotherapy and therefore did not belong to the target population of this study. Another 43 patients (16%) faced the treatment decision but were not randomized and dropped out of the study. Of the 128 patients who experienced disease progression and were randomized, 83 were assigned to the intervention group, of which 77 (93%) received the DA.
Figure 2.
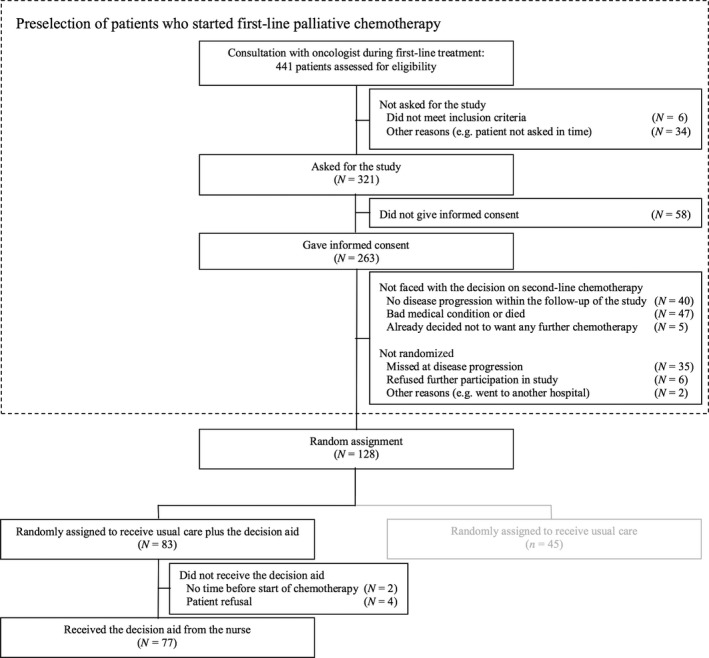
Patient flow chart.
No significant differences were found between the 43 patients with progressive disease who were not randomized and the 77 patients who received the DA on the variables of gender (60 vs. 61% female; P = 0.872), age (mean 59.6 vs. 61.0; P = 0.470), education (college education 25 vs. 30%; P = 0.592), employment (45 vs. 41%; P = 0.751), information preference at baseline (mean scores 8.3 vs. 8.5; P = 0.458) and general health (mean scores 6.7 vs. 6.4; P = 0.422). However, non‐randomized patients more often had a tumour of the breast (vs. colon or rectum) than patients receiving the DA (43 vs. 23%; P = 0.027).
Information desire
Of 77 patients receiving the DA, 74 (96%) desired the information on adverse events, 70 (91%) desired the information on tumour response, and 57 (74; 95% CI 64–84) desired the information on survival.
Potential predictors of information desire
Of 50 patient characteristics considered, 15 (30%) were associated with information desire at the level P < 0.2 (see Table 2). Patients who desired survival information were more likely to be unemployed or male, more recently received the initial diagnosis of disease, and more often had a tumour of the colon or rectum (vs. breast). In addition, desiring survival information was associated with more symptoms of nausea and vomiting, pain and dyspnoea. Patients desiring survival information employed less cognitive avoidant and fighting spirit cancer coping styles, deferred less responsibility and deliberated more regarding decision making. Desire for survival information was higher in patients with a lower perceived numerical ability, lower subjective knowledge and perception of greater benefit and lesser harm from first‐line chemotherapy. The multivariable model was fitted twice; once with all 15 predictors and once with 14 predictors, as the variable ‘employment status’ was only available for a subgroup of patients under age 65 (retirement age in the Netherlands at the time of the study) (n = 53). Both models showed that greater perceived benefit of first‐line chemotherapy (OR 7.4; 95% CI 1.8–30.8), lower cognitive avoidance (OR 0.1; 95% CI 0.0–0.7), lower fighting spirit (OR 0.2; 95% CI 0.0–0.8) and higher deliberation decision style (OR 4.9; 95% CI 1.1–21.1) were four independent predictors of desiring survival information. The odds ratios presented are derived from the model fitted with 14 predictors.
Table 2.
Patient characteristics associated with information desire at P < 0.2.
| Acceptance of survival information | Bivariate analysis | ||||
|---|---|---|---|---|---|
| Yes n = 57 (74%) | No n = 20 (26%) | χ2 | OR (95% CI) | P | |
| Sociodemographics | |||||
| Working statusa | |||||
| Unemployed | 24 (80%) | 6 (20%) | 4.4 | 0.3 (0.1–0.9) | 0.037 |
| Employed | 11 (52%) | 10 (48%) | |||
| Gender | |||||
| Male | 26 (87%) | 4 (13%) | 4.4 | 0.3 (0.1–1.0) | 0.037 |
| Female | 31 (66%) | 16 (34%) | |||
| Tumour and treatment characteristics | |||||
| Time since initial diagnosis of disease | |||||
| Short (<35 months) | 33 (87%) | 5 (13%) | 5.9 | 0.3 (0.1–0.8) | 0.015 |
| Long (≥35 months) | 24 (63%) | 14 (37%) | |||
| Tumour location | |||||
| Breast | 11 (61%) | 7 (39%) | 1.9 | 2.3 (0.7–7.0) | 0.165 |
| Colon or rectum | 46 (78%) | 13 (22%) | |||
| Well‐being: HRQoL | |||||
| HRQoL: nausea and vomiting | |||||
| Low (0) | 32 (68%) | 15 (32%) | 3.3 | 2.9 (0.9–9.9) | 0.068 |
| High (>0) | 25 (86%) | 4 (14%) | |||
| HRQoL: pain | |||||
| Low (<16.7) | 32 (70%) | 14 (30%) | 1.9 | 2.2 (0.7–6.9) | 0.167 |
| High (≥16.7) | 25 (83%) | 5 (17%) | |||
| HRQoL: dyspnoea | |||||
| Low (0) | 34 (69%) | 15 (31%) | 2.5 | 2.5 (0.7–8.6) | 0.117 |
| High (>0) | 23 (85%) | 4 (15%) | |||
| Coping | |||||
| Coping with cancer: cognitive avoidance | |||||
| Low (<2.5) | 30 (88%) | 4 (12%) | 6.1 | 0.2 (0.1–0.8) | 0.014 |
| High (≥2.5) | 27 (64%) | 15 (36%) | |||
| Coping with cancer: fighting spirit | |||||
| Low (<3) | 31 (86%) | 5 (14%) | 4.7 | 0.3 (0.1–0.9) | 0.031 |
| High (≥3) | 26 (65%) | 14 (35%) | |||
| Decision style: deferring responsibility | |||||
| Low (<4.7) | 40 (80%) | 10 (20%) | 1.9 | 0.5 (0.2–1.4) | 0.169 |
| High (≥4.7) | 17 (65%) | 9 (35%) | |||
| Decision style: deliberation | |||||
| Low (<4.4) | 23 (62%) | 14 (38%) | 6.5 | 4.1 (1.3–13.1) | 0.011 |
| High (≥4.4) | 34 (87%) | 5 (13%) | |||
| Information‐related measures | |||||
| Subjective Numeracy: ability subscale | |||||
| Low (<5) | 30 (83%) | 6 (17%) | 2.6 | 0.4 (0.1–1.2) | 0.108 |
| High (≥5) | 27 (68%) | 13 (33%) | |||
| Knowledge‐related measures | |||||
| Subjective knowledge | |||||
| Low (<6.3) | 31 (86%) | 5 (14%) | 4.0 | 0.3 (0.1–1.0) | 0.045 |
| High (≥6.3) | 26 (67%) | 13 (33%) | |||
| Treatment attitudes | |||||
| Perceived benefits first‐line chemotherapy | |||||
| Low (<2) | 13 (54%) | 11 (46%) | 8.3 | 5.1 (1.6–15.8) | 0.004 |
| High (≥2) | 42 (86%) | 7 (14%) | |||
| Perceived harms first‐line chemotherapy | |||||
| Low (<2) | 10 (91%) | 1 (9%) | 1.9 | 0.3 (0.0–2.3) | 0.172 |
| High (≥2) | 47 (73%) | 17 (27%) | |||
Selection of 51 patients below the age of 65 (retirement age in the Netherlands at the time of the study).
Applying these four characteristics, desiring survival information was correctly predicted in 60 of 72 patients with complete data (83% correct). Incorrect predictions included overestimations for eight patient (11%) and underestimations for four patients (6%). By way of comparison, simply assuming that all 72 patients would accept survival information already correctly identified 55 patients desiring information (76%).
Discussion
This study showed that the large majority of patients with advanced breast or colorectal cancer (74%) desired to be informed about survival when facing a decision on second‐line palliative treatment. An extensive exploration of patient characteristics associated with actual acceptance of survival information yielded four characteristics, related to past experience and coping with cancer and decision‐making styles, associated with desiring survival information. However, these characteristics were not very helpful in correctly identifying patients desiring survival information. Simply assuming that all patients desire survival information would already identify most of these patients correctly.
Previous studies reported that a large proportion of patients with advanced cancer (44, 59, 80 and 88%, respectively) stated a desire for survival information.4, 5, 6, 7 In the present study, we went a step further by offering treatment‐related information to patients who faced a palliative treatment decision. This information was offered by a nurse using a DA. We found that a high percentage of patients (74%) wished to receive the information when it was actually offered to them.
Some of the patient characteristics associated with acceptance of survival information have previously been reported to be associated with a stated desire for survival information. This includes the finding that men4, 10 and patients with more pain7 were more likely to desire survival information. Likewise, our data mildly suggested that patients experiencing more nausea, vomiting and dyspnoea desired more information. We will not elaborate on these findings because of the limited practical value for predicting information desire. Reported predictors of preferences for survival information not confirmed in this study include higher education,7, 9 higher depression scores6 and lower death avoidance.9 Furthermore, the previously reported relation between higher desire for survival information and worse patient‐reported prognosis9 was not confirmed when oncologists' estimate of survival was used. Age was not found to be related to desiring survival information; previous studies showed mixed results for age.7, 9 Hypothesized predictors of desiring survival information that were not confirmed in this study included (strength of) patients' treatment preference, cancer worries, information and participation preferences, and striving for length vs. quality of life.
Despite extensive modelling, patients desiring survival information could not be identified using psychosocial and clinical characteristics. We do not recommend that any of the characteristics are used to decide whether or not to offer survival information to a patient. Apart from the fact that the identified characteristics are not easily assessable, (at least) 11% of patients would receive undesired survival information, while another 6% would be denied desired information.
The main strength of this study is that patients' desire for survival information was investigated by offering information to patients who actually faced a treatment decision. To minimize selection bias, we preselected patients who would potentially face the treatment decision, applying few exclusion criteria, and instructed professionals not to tell patients beforehand that detailed survival information would be offered in the DA. A satisfactory informed consent rate of 82% was achieved.
A limitation to the generalizability of the results is that we selected only patients with advanced breast or colorectal cancer, deciding about second‐line palliative chemotherapy. For both tumour types, a substantial majority of patients (78% of colorectal and 61% of patients with breast cancer, respectively) desired survival information. The generalizability of our findings to patients with other tumour types or patients facing other palliative treatment decisions needs further study.
The exploration of predictors of accepting survival information was extensive, but has several limitations. Patients filled out the baseline questionnaire several months (median 3; IQR 0–9) before the survival information was offered. It is possible that certain patient characteristics (e.g. well‐being) changed over these months. Thus, the predictive performance of the model might have been better had more recent patient data been used. However, a lengthy questionnaire at the time of disease progression was judged to be infeasible. The generalizability of the identified patient characteristics to other populations is questionable. First, there is the issue of multiple testing. In the bivariate analysis, 15 of 50 patient characteristics (30%) were found to be associated with information desire, while 10 characteristics (20%; P < 0.2) were expected to be found due to chance alone. Second, the multivariable regression model is likely overfitted, for instance due to the high number of patient characteristics in relation to the number of patients.26 Larger studies are needed to confirm the findings on patients' information desire and potential predictors.
The finding that many patients wanted to receive information on expected survival regarding second‐line chemotherapy can help encourage candid discussions between health professionals and patients. Our recommendation to health professionals is not to make implicit assumptions of information desire based on patient characteristics, but to explicitly ask patients if survival information is desired, and then act accordingly. The use of open‐ended questions can help to elicit a patient's most important questions and concerns as well as the preferred level of candidness, to guide the provision of information by the clinician.27 For example, a physician might ask ‘How much would you like to know?’ or more specifically ‘Do you want me to tell you how long patients can live with this kind of cancer with or without chemotherapy? What kind of information do you want me to cover?’.3, 28
DAs can support professionals and patients in conversations about treatment options by providing numerical estimates of expected survival, including visual aids, for each treatment option. DAs are proven to be effective at improving knowledge and realistic perceptions of outcomes and increasing patients' involvement.29 In the current study, DAs were offered to patients by nurses, because nurses usually spend more time with patients than physicians, and some nurses are already highly involved in supporting treatment decisions.30, 31 A possible amendment to the DAs used in this study, as recommended in a recent study exploring preferences of people with a cancer experience, is the presentation of three survival scenarios (best case, worst case and typical survival) instead of median survival.32 These scenarios would better convey the variation in survival duration and help patients to hope for the best while planning for the worst.
Conclusion
Our findings indicate that many patients with advanced breast or colorectal cancer want to receive survival information when deciding about second‐line palliative chemotherapy. It is, however, difficult to identify those patients who desire the information. Candid conversations about expected survival are particularly relevant in the context of treatment decisions, when the potential benefits of treatment have to be weighed up against the risks. Decision aids can be valuable tools to support professionals and patients in these conversations.
Conflict of interest
No conflicts of interest have been declared.
Source of funding
This work was supported by the Dutch Cancer Society, Amsterdam, the Netherlands (grant number KUN 2006‐3465).
Acknowledgements
The authors are grateful to all patients and health professionals for investing their precious time in this study. Patients were accrued by the Medical Centre Alkmaar, the Jeroen Bosch Hospital in Den Bosch; the Slingeland Hospital in Doetinchem; the Catharina Hospital in Eindhoven; the Medical Spectrum Twente in Enschede, the St. Anna Hospital in Geldrop, the University Medical Centre Groningen in Groningen; the St Jansdal Hospital in Harderwijk; the Elkerliek Hospital in Helmond; the Medical Centre Leeuwarden; the Radboud University Medical Centre in Nijmegen; the Bernhoven Hospital in Oss; the St. Antonius Hospital in Nieuwegein and Utrecht; the Bernhoven Hospital in Veghel; the Máxima Medical Centre in Veldhoven; VieCuri Medical Centre in Venlo/Venray; and the Isala Clinics in Zwolle. Eva Volmeijer, Mirte Sprengers and Sanne Hobbelink are thanked for their assistance with the data collection.
References
- 1. Hagerty RG, Butow PN, Ellis PM, Dimitry S, Tattersall MH. Communicating prognosis in cancer care: a systematic review of the literature. Annals of Oncology, 2005; 16: 1005–1053. [DOI] [PubMed] [Google Scholar]
- 2. Butow PN, Dowsett S, Hagerty R, Tattersall MH. Communicating prognosis to patients with metastatic disease: what do they really want to know? Supportive Care in Cancer, 2002; 10: 161–168. [DOI] [PubMed] [Google Scholar]
- 3. Clayton JM, Hancock KM, Butow PN et al Clinical practice guidelines for communicating prognosis and end‐of‐life issues with adults in the advanced stages of a life‐limiting illness, and their caregivers. Medical Journal of Australia, 2007; 186: S77, S9, S83–S108. [DOI] [PubMed] [Google Scholar]
- 4. Elkin EB, Kim SH, Casper ES, Kissane DW, Schrag D. Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. Journal of Clinical Oncology, 2007; 25: 5275–5280. [DOI] [PubMed] [Google Scholar]
- 5. Kutner JS, Steiner JF, Corbett KK, Jahnigen DW, Barton PL. Information needs in terminal illness. Social Science and Medicine, 1999; 48: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 6. Hagerty RG, Butow PN, Ellis PA et al Cancer patient preferences for communication of prognosis in the metastatic setting. Journal of Clinical Oncology, 2004; 22: 1721–1730. [DOI] [PubMed] [Google Scholar]
- 7. Pardon K, Deschepper R, Stichele RV, Bernheim J, Mortier F, Deliens L. Preferences of advanced lung cancer patients for patient‐centred information and decision‐making: a prospective multicentre study in 13 hospitals in Belgium. Patient Education and Counseling, 2009; 77: 421–429. [DOI] [PubMed] [Google Scholar]
- 8. Manson NC. Why do patients want information if not to take part in decision making? Journal of Medical Ethics, 2010; 36: 834–837. [DOI] [PubMed] [Google Scholar]
- 9. Kaplowitz SA, Campo S, Chiu WT. Cancer patients' desires for communication of prognosis information. Health Communication, 2002; 14: 221–241. [DOI] [PubMed] [Google Scholar]
- 10. Fletcher KM, Prigerson HG, Maciejewski PK. Women know, and men wish they knew, prognostic information in advanced cancer. Journal of Clinical Oncology, 2012; 30: (suppl; abstr 9037). [Google Scholar]
- 11. Oostendorp LJ, Ottevanger PB, van der Graaf WT, Stalmeier PF. Assessing the information desire of patients with advanced cancer by providing information with a decision aid, which is evaluated in a randomized trial: a study protocol. BMC Medical Informatics and Decision Making, 2011; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oostendorp LJ, Stalmeier PF, Pasker‐de Jong PC, Van der Graaf WT, Ottevanger PB. Systematic review of benefits and risks of second‐line irinotecan monotherapy for advanced colorectal cancer. Anti‐Cancer Drugs, 2010; 21: 749–758. [DOI] [PubMed] [Google Scholar]
- 13. Oostendorp LJ, Stalmeier PF, Donders AR, van der Graaf WT, Ottevanger PB. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: a systematic review. The Lancet. Oncology, 2011; 12: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 14. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scand, 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 15. Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychology, 1991; 10: 259–267. [DOI] [PubMed] [Google Scholar]
- 16. Groenvold M, Petersen MA, Aaronson NK et al The development of the EORTC QLQ‐C15‐PAL: a shortened questionnaire for cancer patients in palliative care. European Journal of Cancer, 2006; 42: 55–64. [DOI] [PubMed] [Google Scholar]
- 17. Watson M, Greer S, Young J, Inayat Q, Burgess C, Robertson B. Development of a questionnaire measure of adjustment to cancer: the MAC scale. Psychological Medicine, 1988; 18: 203–209. [DOI] [PubMed] [Google Scholar]
- 18. Pierce P. Michigan Assessment of Decision Style (MADS). Ann Arbor, MI: University of Michigan, 1995. [Google Scholar]
- 19. Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Archives of Internal Medicine, 1996; 156: 1414–1420. [PubMed] [Google Scholar]
- 20. Klug L, Sinha A. Death acceptance: a two component formulation and a scale. OMEGA–Journal of Death and Dying, 1987; 18: 229–235. [Google Scholar]
- 21. Blanchard CG, Labrecque MS, Ruckdeschel JC, Blanchard EB. Information and decision‐making preferences of hospitalized adult cancer patients. Social Science and Medicine, 1988; 27: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 22. Fagerlin A, Zikmund‐Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Medical Decision Making, 2007; 27: 672–680. [DOI] [PubMed] [Google Scholar]
- 23. Zikmund‐Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Medical Decision Making, 2007; 27: 663–671. [DOI] [PubMed] [Google Scholar]
- 24. Stiggelbout AM, de Haes JC, Kiebert GM, Kievit J, Leer JW. Tradeoffs between quality and quantity of life: development of the QQ Questionnaire for Cancer Patient Attitudes. Medical Decision Making, 1996; 16: 184–192. [DOI] [PubMed] [Google Scholar]
- 25. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 26. Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression‐type models. Psychosomatic Medicine, 2004; 66: 411–421. [DOI] [PubMed] [Google Scholar]
- 27. Ngo‐Metzger Q, August KJ, Srinivasan M, Liao S, Meyskens FL Jr. End‐of‐Life care: guidelines for patient‐centered communication. American Family Physician, 2008; 77: 167–174. [PubMed] [Google Scholar]
- 28. Back AL, Arnold RM. Discussing prognosis: “how much do you want to know?” talking to patients who are prepared for explicit information. Journal of Clinical Oncology, 2006; 24: 4209–4213. [DOI] [PubMed] [Google Scholar]
- 29. Stacey D, Bennett CL, Barry MJ et al Decision aids for people facing health treatment or screening decisions. Cochrane Database Systematic Review, 2011: CD001431. [DOI] [PubMed] [Google Scholar]
- 30. Barthow C, Moss C, McKinlay E, McCullough L, Wise D. To be involved or not: factors that influence nurses' involvement in providing treatment decisional support in advanced cancer. European Journal of Oncology Nursing, 2009; 13: 22–28. [DOI] [PubMed] [Google Scholar]
- 31. Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp KG. Physician, patient, and contextual factors affecting treatment decisions in older adults with cancer and models of decision making: a literature review. Oncology Nursing Forum, 2012; 39: E70–E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiely BE, McCaughan G, Christodoulou S et al Using scenarios to explain life expectancy in advanced cancer: attitudes of people with a cancer experience. Supportive Care in Cancer, 2013; 21: 369–376. [DOI] [PubMed] [Google Scholar]


