Abstract
Background
Self‐management is recommended for patients with chronic conditions, but its use with cancer survivors is underexplored. Optimal strategies for achieving lifestyle changes in cancer survivors are not known.
Objective
We aimed to determine feasibility, acceptability and preliminary efficacy of self‐management‐based nutrition and physical activity interventions for cancer survivors.
Design, setting and participants
Adult survivors (n = 25) during (Group 1 , n = 11) or post (Group 2, n = 14)‐curative chemotherapy for solid tumours, most (n = 20, 80%) with breast cancer, were recruited prospectively from a single clinical centre.
Intervention
The Flinders Living Well Self‐Management Program, a generic self‐management care planning programme, was utilized to establish patient‐led nutrition and exercise goals within a tailored 12‐week intervention. Fortnightly progress reviews occurred with assessments at baseline, 6 and 12 weeks.
Results
Most participants (84%) found the intervention acceptable/very acceptable. Both groups showed a trend towards significant improvement in the self‐management capability ‘knowledge about changing risk factors’ (P = 0.047); Group 2 showed a trend towards significantly improved ‘psychological impacts’ (P = 0.007). Goal ratings improved for both groups (P = 0.001). Quality of life improved for both groups for emotional functioning (P = 0.03). Physical functioning improved for Group 2 (P = 0.05); however, most symptom domains worsened for Group 1, as expected given their treatment stage.
Discussion and conclusions
Self‐management interventions are feasible for this population. In particular, building self‐management capacity during the active phase of patients' cancer treatment provides health and psychosocial benefits. Larger randomized controlled trials are required to further determine efficacy. Further translational research is also needed to determine acceptability,feasibility, enablers and barriers for clinicians embedding this approach into routine cancer survivorship care.
Keywords: cancer, exercise, nutrition, prevention (risk factors), self‐management (self‐care), survivor
Introduction
With improved cancer survival, more people are living with long‐term consequences of cancer and its treatment. In Australia, there are approximately 700 000 cancer survivors and this figure is increasing by 2.5% each year.1 Cancer is associated with increased risk of chronic diseases.2 Lifestyle factors associated with diet and exercise are important contributors to this risk3 and to cancer recurrence and reduced cancer‐specific survival.4, 5 The cause of this health burden is multifactorial, including effects of cancer itself and long‐term adverse impacts (late effects) of cancer and its treatment including musculoskeletal pain, fatigue, depression and cognitive impairment, which can have significant impacts on the person's capacity to self‐manage their health.2, 6, 7, 8
Current approaches to cancer care do not adequately engage cancer survivors to self‐manage their long‐term needs and non‐cancer issues such as health lifestyle management or management of comorbidity.9, 10, 11 A US survey of cancer survivors12 showed that up to 29% had unmet physical needs and up to 45% had unmet emotional needs, both needs which are important to successful self‐management of health.13 Various approaches to provision of treatment summaries and survivorship care plans have been explored among cancer survivors.14, 15 Notably, the focus of these survivorship care plans has been on cancer‐specific management, rather than patient‐led identification of self‐management needs, strengths and barriers which may influence their lifestyle behaviour and engagement in care plans.16 Service user involvement in cancer care has been found to benefit their capacity to live well with cancer, refocusing their lives, ‘in a positive, purposeful and productive way’.17
Little is known about how cancer survivors manage lifestyle risk factors for ill health once primary cancer treatment is complete, and how they can be supported to do this. The assumption held by the community is that life will somehow get back to normal.11, 18 During the active phase of treatment, many patients with cancer lose physical strength and condition. Many attempt to adopt more healthy lifestyles by paying particular attention to their diet, exercise levels, alcohol use, smoking and stress management; however, many put such considerations on hold.19, 20
Promoting each person's capacity to self‐manage their health within a more collaborative framework of provision of care and self‐management support is a priority focus of health‐care systems in Australia and internationally. This is particularly important as health‐care burden continues to grow.13, 21, 22 Self‐management support provided through a partnership between the patient and support providers reverses the focus on telling patients what they ‘should do’ to one where the patient is supported in addressing their own agenda.23, 24 It is integral in delivering more person‐centred care which promotes greater patient autonomy and control, and patient/health professional collaboration, and re‐establishing patients' personal ownership of health. People with high self‐efficacy are also more likely to engage in self‐management behaviours.25, 26 This may be especially important for people who have experienced cancer and survived, particularly because many patients with cancer report heightened feelings of fear and powerlessness in the face of a cancer diagnosis and the threat of its recurrence.27, 28
Self‐management approaches have been shown to be effective for many chronic conditions and risk factors,29, 30 and preliminary research suggests superior outcomes when incorporating self‐management models into health‐care delivery for cancer.15, 18, 31 The aim of this paper was to report the feasibility, acceptability and preliminary efficacy of the Flinders Program, a well‐established approach to self‐management of a range of non‐cancer conditions, in different contexts and populations.32, 33, 34 Our hypothesis was that this patient‐led self‐management approach would be feasible and acceptable to cancer survivors and would improve their nutrition and exercise behaviours and quality of life regardless of stage of treatment. Effectiveness measures of the study, including anthropometric, strength, body composition and functional exercise capacity, are reported elsewhere.35
Methods
Sample
Participants were men and women diagnosed with solid tumours, treated with curative intent, assigned to two patient groups: Group 1) those currently receiving chemotherapy (within 4 weeks of commencing treatment) and Group 2) patients following active treatment (within 8 weeks of treatment completion which included chemotherapy +/− radiotherapy or surgery). All participants were aged 18 years and over, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1.36 Patients were excluded if they were pregnant or planned to become pregnant during the study period, and if their treating clinical oncologist assessed them as possessing a level of cognitive impairment, mental illness and/or physical disability that would impair their capacity to provide consent and participate in the intervention.
Recruitment procedure
The study received ethics approval from the Southern Adelaide Clinical Human Research Ethics Committee. Eligible patients were identified between August 2012 and March 2013 and invited to participate by medical oncologists and cancer care coordinators, as well as using flyers and by mailed letters of invitation containing opt‐in forms and reply paid envelopes. Once eligibility was confirmed, signed consent was obtained and a mutually acceptable time for baseline assessments and intervention was arranged.
Intervention
The Flinders Living Well Self‐Management Program (FLW Program) was utilized. It is an evidence‐based, structured interview process, using cognitive behavioural and motivational processes that allow for assessment of self‐management behaviours, enablers and barriers to change, and collaborative identification of problems and goals, leading to the development of an individualized person‐centred self‐management care plan.37, 38, 39 This is the first time that these tools have been tested in a cancer population. They include the following steps:
The Living Well Scale (LWS): A patient Likert‐rated questionnaire adapted (for prevention and risk factors) from the Partners in Health Scale.40 It is a validated tool within the Flinders Program which is based on the WHO and Australian National Chronic Disease Strategy principles of self‐management.41 It enables measurement of perceived change over time where 0 = more favourable and 8 = less favourable self‐management capacity. Self‐management‐rated capacities include knowledge of risk factors, actions taken, access to services and ability to discuss health concerns, general health, and social and psychological impacts of managing risk factors.
The Cue and Response Interview (C&R): An adjunct to the LWS using open‐ended questions or cues to explore the patient's responses to the LWS in more depth, with the patient and worker comparing their Likert ratings to identify agreed good self‐management and agreed issues that need to be addressed as part of a self‐management care plan. It enables the strengths and barriers to self‐management to be explored and checks assumptions that either the worker or patient may have, as part of a motivational process.
The Problems and Goals (P&G) Assessment: Defines a problem statement from the patient's perspective (the problem, its impact and how it makes them feel) and identifies specific, measurable, achievable, realistic and timely (SMART) goals that they can work towards. It is Likert‐rated, allowing measurement of progress over time where 0 = not a problem and 8 = a significant problem; and goal statements: 0 = no progress towards achievement and 8 = achieved.
Living Well Care Plan: Self‐management issues, aims, steps to achieve them, who is responsible and date for review.
The research officer (SZ‐a qualified dietician) received training in the use of the tools from a Flinders Program accredited trainer (SL). Fidelity during the intervention was assured by the research officer delivering all Flinders Program interventions and by meeting with the accredited trainer following their first three care planning interventions and being assessed as demonstrating competence in use of the tools. The research officer worked with participants to develop tailored nutrition and physical activity goals, with interventions of their choice to support goal attainment, delivered over a 12‐week period. All participants could choose from a range of nutrition and physical activity supports in addition to personalized actions outlined on their care plan. Nutrition and physical activity services included home exercise programmes, supervised exercise classes, supermarket tours and 1‐on‐1 dietary counselling. These were delivered by the various health‐care providers; settings, formats and number of sessions varied. Reviews of progress with actions on their care plans and progress towards these goals occurred fortnightly at 2,4,8 and 10 weeks via telephone (by the project's dietetics honours student), with Flinders Program assessments at baseline, 6 and 12 weeks (performed by SZ). Participants were also asked to complete a brief survey at the completion of the study, asking about their experience of using the FLW Program and other interventions. This survey asked about their perceptions of the intervention, including its content, format and delivery, location, the acceptability of review periods and mechanisms of assessment. Several objective and exploratory measures of nutrition and physical activity status were used in addition to the FLW Program. The results of these are reported in detail elsewhere.35 Quality of life was assessed using the EORTC‐QLQ‐c30, chosen because of its rigour and applicability to cancer survivors to measure global quality of life, physical, social and emotional role functions and individual cancer‐specific symptom domains.42 See Appendix S1 for further details of all assessment and evaluation measures and their delivery.
Data analysis
Mixed‐effects models were used to assess the changes in nutrition, physical activity and other goal scores over the 12 weeks of the intervention. We also used mixed‐effects models to assess the changes in each of the 10 LWS domain scores over the 12 weeks. In each model, we included time, group and a time × group interaction term. Subject was included as a random intercept and time was entered as a categorical variable. A global P‐value was obtained for time to assess the overall change over time, and a global P‐value for the time × group interaction term was used to assess whether there was any overall difference in scores between the two groups across time. Within‐group differences across time were also compared using the mixed‐model estimates. All analyses were carried out in Stata (version 13, StataCorp, Texas, USA). To adjust for the multiple comparisons performed within the same instrument, we used P < 0.005 (0.05/10) and P < 0.0033 (0.05/15) as our two‐sided Type 1 error rates for the LWS score domains and the quality of life scores, respectively. Data in figures are displayed as mean and 95% confidence interval. Data in tables are displayed as mean ± SD unless otherwise stated.
Results
Sample, recruitment and retention
Twenty‐five cancer survivors participated in the study (Group 1 = 11; Group 2 = 14). Most were women with breast cancer (80%). Other cancer types were ovarian, colorectal, lung and brain cancer. Average age of the total cohort was 49.9 (±9.9) years, with Group 1 participants being generally older than Group 2 participants [53.1 (± 8.7) and 47.4 (±10.4), respectively]. Group 2 participants were more likely to be in couple relationships than Group 1 participants. Educational attainment was mixed across both groups, with Group 2 having more participants with higher educational attainment. Only one participant in Group 1 had a comorbid chronic condition, whereas more Group 2 participants had comorbid chronic conditions. Body mass index was similar across the groups with the average being 25.8 (±6.6) for the total sample (see Table 1).
Table 1.
Characteristics of the study sample (n = 25)
| Group 1 (n = 11) | Group 2 (n = 14) | Total survivors (n = 25) | |
|---|---|---|---|
| Gender | |||
| Female | 10 (91) | 13 (93) | 23 (92) |
| Age (years) | 53.1 ± 8.7 | 47.4 ± 10.4 | 49.9 ± 9.9 |
| <65 | 9 (82) | 12 (86) | 21 (84) |
| ≥65 | 2 (18) | 2 (14) | 4 (16) |
| Body Mass Index (BMI)a | 25.9 ± 3.3 | 25.7 ± 8.5 | 25.8 ± 6.6 |
| Marital status | |||
| Married/De facto | 7 (64) | 13 (93) | 20 (80) |
| Divorced | 1 (9) | 1 (7) | 2 (8) |
| Single | 3 (27) | 0 (0) | 3 (12) |
| Ethnicity | |||
| Caucasian | 10 (91) | 13 (93) | 23 (92) |
| Asian | 0 (0) | 1 (7) | 1 (4) |
| Other | 1 (9) | 0 (0) | 1 (4) |
| Education | |||
| Did not complete secondary schoolb | 4 (36) | 1 (7) | 5 (20) |
| Completed secondary school | 4 (36) | 4 (29) | 8 (32) |
| TAFEb | 1 (9) | 3 (21) | 4 (16) |
| Tertiary education | 2 (18) | 6 (43) | 8 (32) |
| Cancer | |||
| Breast | 10 (91) | 10 (71) | 20 (80) |
| Ovarian | 0 (0) | 2 (14) | 2 (8) |
| Colorectal | 0 (0) | 1 (7) | 1 (4) |
| Lung | 1 (9) | 0 (0) | 1 (4) |
| Brain | 0 (0) | 1 (7) | 1 (4) |
| Chemotherapy | 11 (100) | 14 (100) | 25 (100) |
| Surgery | 11 (100) | 14 (100) | 25 (100) |
| Radiotherapy | 6 (55) | 9 (64) | 15 (60) |
| Most common comorbid conditions | |||
| Overweight or obese (as above) | 5 (45) | 10 (71) | 15 (60) |
| Chronic obstructive pulmonary disease | 1 (9) | 4 (29) | 5 (20) |
| Hypertension | 0 (0) | 3 (21) | 3 (12) |
| Impaired glucose tolerance | 0 (0) | 3 (21) | 3 (12) |
Data presented as n (%) or mean ± SD.
World Health Organisation BMI definitions: acceptable weight (18.5–24.9 kg/m2) overweight (25–29.9 kg/m2) obese (30–39.9 kg/m2).
In Australia, students usually turn 18 years of age in their final year of secondary schooling. TAFE students are those who have finished secondary schooling/or left prior to completion who undertake vocational courses.
Seventy‐two patients were identified as being potentially eligible and were approached to participate between August and November 2012. Of these, 25 were confirmed eligible and agreed to participate (35% recruitment rate). No major demographic differences were observed between those who consented vs. declined. The main reason for declining was lifestyle changes not being considered a priority. Twenty‐two of the initial 25 participants (91%) completed the FLW Program and other assessments at 6 weeks and 20 participants (80%) completed them (Group 1 = 9; Group 2 = 11) at 12 weeks. All missing assessments are accounted for by withdrawals (n = 4). These withdrawals were due to cancer recurrence (n = 1), coping issues (n = 2) and chemotherapy‐related complications (n = 1). For the EORTC‐QLQ‐c30, one (4%) participant's forms were incomplete and unable to be utilized.
Living well self‐management capabilities
There were no significant changes across time for any of the domains (see Table 2 and Fig. 1a–j). However, there was a trend towards a significant increase over time for ‘knowledge about changing risk factors’ scores (P = 0.047) and a trend towards differences between groups in changes in scores for ‘psychological impact’ (P = 0.05) and ‘ability to discuss health with health professionals’ (P = 0.06):
Table 2.
The Living Well Scale scores by domain at baseline, 6 and 12 weeks
| Baseline | 6 weeks | 12 weeks | P‐value for timea | P‐value for time × groupa | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (±SD) | n | Mean (±SD) | n | Mean (±SD) | |||
| Knowledge about risk factors | ||||||||
| Total survivors | 25 | 6.3 ± 1.6 | 21 | 7.0 ± 1.4 | 22 | 7.4 ± 0.7 | 0.37 | |
| Group 1 | 11 | 6.5 ± 1.4 | 9 | 7.1 ± 1.4 | 9 | 7.2 ± 0.8 | ||
| Group 2 | 14 | 6.1 ± 1.7 | 12 | 6.8 ± 1.5 | 13 | 7.5 ± 0.7 | 0.54 | |
| Knowledge about changing risk factors | ||||||||
| Total survivors | 25 | 25 ± 5.6 | 21 | 7.0 ± 1.2 | 22 | 7.3 ± 0.6 | 0.047 | |
| Group 1 | 11 | 5.8 ± 2.2 | 9 | 7.0 ± 1.7 | 9 | 7.2 ± 0.7 | ||
| Group 2 | 14 | 5.4 ± 1.9 | 12 | 6.9 ± 0.8 | 13 | 7.3 ± 0.6 | 0.83 | |
| Taking action | ||||||||
| Total survivors | 25 | 5.2 ± 1.5 | 21 | 6.0 ± 1.3 | 22 | 6.3 ± 1.2 | 0.24 | |
| Group 1 | 11 | 5.4 ± 1.5 | 9 | 5.8 ± 6.2 | 9 | 5.8 ± 6.6 | ||
| Group 2 | 14 | 5.1 ± 1.6 | 12 | 6.2 ± 0.9 | 13 | 6.6 ± 0.9 | 0.26 | |
| General health impact | ||||||||
| Total survivors | 25 | 5.2 ± 2.2 | 21 | 5.8 ± 2.0 | 22 | 5.9 ± 1.7 | 0.88 | |
| Group 1 | 11 | 5.2 ± 2.5 | 9 | 5.3 ± 2.1 | 9 | 5.0 ± 2.2 | ||
| Group 2 | 14 | 5.3 ± 2.0 | 12 | 6.2 ± 1.9 | 13 | 6.5 ± 0.9 | 0.21 | |
| Social impact | ||||||||
| Total survivors | 25 | 6.2 ± 2.2 | 21 | 6.1 ± 1.3 | 22 | 6.7 ± 1.3 | 0.98 | |
| Group 1 | 11 | 6.4 ± 2.2 | 9 | 6.2 ± 1.1 | 9 | 6.2 ± 1.4 | ||
| Group 2 | 14 | 6.1 ± 2.3 | 12 | 6.1 ± 1.5 | 13 | 7.0 ± 1.2 | 0.45 | |
| Living situation impact | ||||||||
| Total survivors | 25 | 6.0 ± 2.0 | 21 | 5.9 ± 1.8 | 22 | 6.0 ± 2.2 | 0.93 | |
| Group 1 | 11 | 6.2 ± 1.7 | 9 | 6 ± 1.9 | 9 | 5.9 ± 2.0 | ||
| Group 2 | 14 | 5.9 ± 2.2 | 12 | 5.8 ± 1.8 | 13 | 6.2 ± 2.4 | 0.84 | |
| Psychological impact | ||||||||
| Total survivors | 25 | 5.1 ± 2.0 | 21 | 5.1 ± 2.2 | 22 | 5.6 ± 1.9 | 0.56 | |
| Group 1 | 11 | 4.9 ± 1.9 | 9 | 4.2 ± 2.5 | 9 | 4.4 ± 2.2 | ||
| Group 2 | 14 | 5.2 ± 2.2 | 12 | 5.8 ± 1.7 | 13 | 6.5 ± 1.1 | 0.05 | |
| Accessibility to services | ||||||||
| Total survivors | 25 | 5.8 ± 1.8 | 21 | 5.2 ± 2.2 | 22 | 6.0 ± 1.9 | 0.43 | |
| Group 1 | 11 | 5.9 ± 1.9 | 9 | 5.1 ± 2.6 | 9 | 5.2 ± 2.4 | ||
| Group 2 | 14 | 5.7 ± 1.8 | 12 | 5.3 ± 1.9 | 13 | 6.5 ± 1.2 | 0.31 | |
| Ability to discuss health | ||||||||
| Total survivors | 25 | 5.7 ± 2.1 | 21 | 5.0 ± 2.1 | 22 | 5.8 ± 2.5 | 0.42 | |
| Group 1 | 11 | 5.8 ± 1.7 | 9 | 5.1 ± 2.0 | 9 | 4.7 ± 2.9 | ||
| Group 2 | 14 | 5.6 ± 2.4 | 12 | 5.0 ± 2.3 | 13 | 6.6 ± 1.9 | 0.06 | |
| Staying healthy | ||||||||
| Total survivors | 25 | 4.9 ± 1.9 | 21 | 4.6 ± 2.1 | 22 | 5.7 ± 1.9 | 0.43 | |
| Group 1 | 11 | 5.7 ± 1.7 | 9 | 5.1 ± 2.4 | 9 | 5.9 ± 1.9 | ||
| Group 2 | 14 | 4.2 ± 1.8 | 12 | 4.3 ± 2.0 | 13 | 5.5 ± 1.9 | 0.38 | |
The Living Well Scale is a self‐rated Likert scale which measures a person's skill and ability to self‐manage.
Using a mixed‐effects model with time and time × group as fixed effects and the subject as a random effect.
Figure 1.
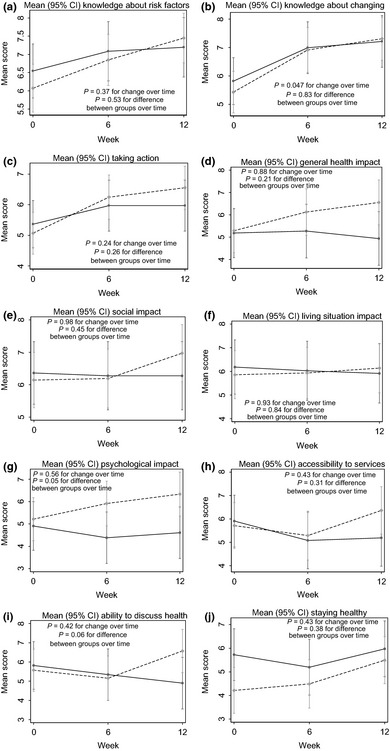
(a–j) Changes in Living Well Scale scores over the 12‐week intervention. Mean (95% CI) changes in scores over the 12‐week intervention for each of the 10 LWS domains. ( ) Group 1; (
) Group 1; ( ) Group 2.
) Group 2.
For ‘psychological impact’, scores for Group 1 did not change across time (P = 0.29 and P = 0.54 at 6 and 12 weeks, respectively), but there was a trend for scores in Group 2 to be improved at week 12 compared to baseline (P = 0.007) (Fig. 1).
For ‘ability to discuss health’, scores for Group 1 did not change across time (P = 0.49 and P = 0.19 compared to baseline at 6 and 12 weeks, respectively). Scores for Group 2 increased non‐significantly (P = 0.49 and P = 0.09 compared to baseline at weeks 6 and 12, respectively).
Problems and goals
Sixty‐eight percentage of participants (n = 17) set problem statements (Group 1 n = 7; Group 2 n = 10). For participants who did not set problem statements, their predominant reported reason for this was that they did not want to dwell on the negatives present in their lives as a result of the presence of cancer. These participants expressed a strong desire to set goals. Group 1 and Group 2 participants were just as likely/unlikely to set a problem statement. There were trends in decreasing problem ratings with mean (±SD) baseline rating at 5.76 (±1.75) and mean 12‐week rating at 3.17 (±44), where 0 = no problem and 8 = a significant problem. Although problem ratings improved, statistical significance was not achieved (0.12) due to the small sample. Of the participants who identified problem statements, 36% (n = 9) identified weight (either gain or loss) as their biggest problem, followed by body image 8% (n = 2), cancer (for example, diagnosis and fear of recurrence) 8% (n = 2), weight and fitness 4% (n = 1), smoking 4% (n = 1), motivation 4% (n = 1), work‐life commitments 4% (n = 1) and physical ability 4% (n = 1). Thirty‐two percentage of participants with problem statements (n = 8) had complete fortnightly problem ratings data across the intervention period. Missing data on problem ratings were due to this aspect being overlooked during follow‐up with the research assistant or receiving less priority by participants as part of interactions at these follow‐up sessions. Examples of problem statements and their ratings over the study period are displayed below:
| Examples of Problem Statements | |||||||
| Group 1 Problem Statement: I see my main problem as gaining weight, which might lead me to be less active which makes me sad, disappointed and angry. (50 female, breast cancer) | |||||||
| Ratings | Baseline | 2 weeks | 4 weeks | 6 weeks | 8 weeks | 10 weeks | 12 weeks |
| 6 | 5 | 5 | 4 | 2 | 0 | 0 | |
| Group 2 Problem Statement: My biggest problem is getting going (motivation)/started. I have become lazy, unmotivated and I sleep in and just lose days. I feel frustrated. (67 female, breast cancer) | |||||||
Thirty‐seven physical activity goals were set by the 25 participants over the 12‐week intervention period. Continuous data (i.e. each goal rated at baseline, 2, 4, 6, 8, 10 and 12 weeks) are available on 17 goals (46%). This percentage does not reflect dropouts or participants not achieving their goals but was because we allowed participants flexibility with their goals and making changes over the intervention, as part of the FLW Program process. For example, if they set a goal to perform supervised exercise three times per week and found it too difficult to attend on‐site exercise at week 6, they may have changed to home exercise and hence set a new goal. Therefore, that participant had two incomplete rating of goals sets due to the revaluation they did at week 6. Another example was if their goal was achieved by week 6 and the participant wished to add another goal. There was a significant increase in physical activity goal scores across the 12 weeks (P < 0.001). There was no significant difference between Group 1 and Group 2 (P = 0.50).
Seventy‐four nutrition goals were set by the 25 participants (on average setting three goals each). Complete data (as per above) were available on 53 goals (72%). The three most commonly set nutrition goals were increasing vegetable consumption, decreasing extras (includes foods that provide excess calories, salt and were of low nutrient value) and increasing consumption of reduced fat dairy. Participants were more likely to re‐evaluate their physical activity goals changing one for another, whereas nutrition goals remained more constant with additional ones added. There was a significant increase in nutrition goal scores across the 12 weeks (P < 0.001). There was no significant difference between Group 1 and Group 2 (P = 0.36).
Sixteen other goals were set by 16 participants at baseline. Complete data were available on 11 goals (69%). Fifteen (94%) of the other goals set were related to weight management. This included weight maintenance, loss and, in one circumstance, gain. One person set a goal around attending social activities. There was a significant increase in other goal scores across the 12 weeks (P < 0.001). There was no significant difference between Group 1 and Group 2 (P = 0.24).
Goal achievement significantly improved (P = 0.001) across the total sample, from 0.9 (±2.1) at baseline to 6.9 (±1.9) at 12 weeks for physical activity goals, and 2.4 (±2.2) at baseline and 7.1 (±1.2) at 12 weeks for nutrition goals. There was no significant difference between Group 1 and Group 2 for goal achievement (see Table 3 and Fig. 2a–c).
Table 3.
Self‐rated physical activity, nutrition and other goals scores at baseline, 6 and 12 weeks
| Baseline | Week 6 | Week 12 | P‐value for timea | P‐value for time × groupa | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (±SD) | n | Mean (±SD) | n | Mean (±SD) | |||
| Physical activity goals | ||||||||
| Total survivor goals | 32 | 0.9 ± 2.1 | 29 | 5.8 ± 3.0 | 25 | 6.9 ± 1.9 | <0.001 | |
| Group 1 goals | 14 | 0.8 ± 1.8 | 11 | 5.5 ± 3.3 | 9 | 6.4 ± 2.2 | 0.50 | |
| Group 2 goals | 18 | 0.9 ± 2.3 | 18 | 6 ± 2.9 | 16 | 7.2 ± 1.8 | ||
| Nutrition goals | ||||||||
| Total survivor goals | 73 | 2.4 ± 2.2 | 68 | 6.6 ± 1.7 | 61 | 7.1 ± 1.2 | <0.001 | |
| Group 1 goals | 33 | 2.5 ± 2.4 | 28 | 6.8 ± 1.5 | 25 | 6.9 ± 1.3 | 0.37 | |
| Group 2 goals | 40 | 2.4 ± 2.2 | 40 | 6.5 ± 1.9 | 36 | 7.3 ± 1.0 | ||
| Other goals (e.g. weight management, improving social activities) | ||||||||
| Total survivor goals | 16 | 0.8 ± 2.2 | 15 | 4.2 ± 2.8 | 13 | 5.2 ± 3.2 | <0.001 | |
| Group 1 goals | 7 | 1.9 ± 3.2 | 6 | 4.2 ± 3.4 | 5 | 5.2 ± 3.6 | 0.24 | |
| Group 2 goals | 9 | 0.0 ± 0.0 | 9 | 4.2 ± 2.4 | 8 | 5.1 ± 3.2 | ||
Self‐rated goals are measured on a Likert scale from 0 to 8, where 0 is no success and 8 is complete success.
Using a mixed‐effects model with time and time × group as fixed effects and the subject as a random effect.
Figure 2.
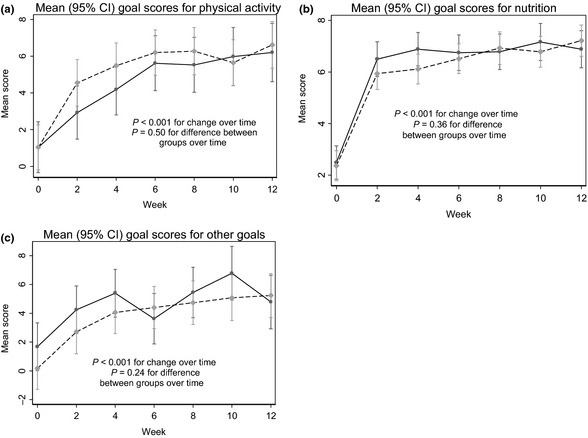
(a–c) Changes in self‐rated physical activity, nutrition and other goal scores over the 12‐week Intervention. Mean (95% CI) changes in scores over the 12‐week intervention for physical activity, nutrition and other goals. ( ) Group 1; (
) Group 1; ( ) Group 2.
) Group 2.
Quality of life
For functioning domains of the EORTC QLQ c30 scale, where higher scores equal better functioning, changes in QOL measures of emotional functioning showed a non‐statistically significant improvement (P = 0.031) for the total sample, and global health, physical functioning and social functioning also showed non‐statistically significant improvements for Group 2. Group 2s global health status increased clinically from a mean (±SD) of 71.53 (±17.57) at baseline to 83.33 (±11.24) at 12 weeks (P = 0.023). Group 1 showed clinically significant physical functioning decline between baseline and 6 weeks, and baseline and 12 weeks (87.41, 77.04 and 75.56 respectively). For symptom domains, where higher scores equal worse symptoms, Group 1 had clinically significant increases for dyspnoea, with more variable patterns in global, functional and other symptom scores observed. Unfavourable trends in physical and social functioning and fatigue, insomnia and diarrhoea symptoms were witnessed, although not significant. Within these symptom domains, Group 1 showed clinically significant worsening of insomnia between week 6 and week 12 (40.74 and 55.56, respectively) and worsening of fatigue between baseline and 12 weeks (39.51 and 51.85, respectively) (see Table 4).
Table 4.
Descriptive statistics for quality of life scores at baseline, 6 and 12 weeks
| Baseline | 6 Weeks | 12 Weeks | P‐value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Global health status | ||||||||||
| Total survivors | 21 | 66.67 | 21.89 | 21 | 65.87 | 25.8 | 21 | 72.62 | 21.43 | 0.059 |
| Group 1 | 9 | 60.19a | 26.28 | 9 | 50a | 229.17 | 9 | 58.33 | 23.94 | 0.374 |
| Group 2 | 12 | 71.53a | 17.57 | 12 | 77.78 | 15.21 | 12 | 83.33a | 11.24 | 0.023 |
| Physical functioning | ||||||||||
| Total survivors | 21 | 88.89 | 10.61 | 21 | 86.98 | 15.27 | 21 | 86.67 | 17.51 | 0.778 |
| Group 1 | 9 | 87.41ab | 9.69 | 9 | 77.04aא | 17.67 | 9 | 75.56b≠ | 21.34 | 0.164 |
| Group 2 | 12 | 90ǂ | 11.54 | 12 | 94.44א | 7.43 | 12 | 95ǂ≠ | 7.03 | 0.050 |
| Role functioning | ||||||||||
| Total survivors | 21 | 74.6 | 28.68 | 21 | 74.6 | 31.01 | 21 | 77.78 | 22.57 | 0.827 |
| Group 1 | 9 | 62.96 | 29.79 | 9 | 53.7€ | 35.14 | 9 | 59.26£ | 16.9 | 0.626 |
| Group 2 | 12 | 83.33 | 25.62 | 12 | 90.28€ | 15.01 | 12 | 91.67£ | 15.08 | 0.531 |
| Emotional functioning | ||||||||||
| Total survivors | 21 | 79.31 | 21.81 | 21 | 71.11aǂ | 25.97 | 21 | 82.62aǂ | 13.73 | 0.031 |
| Group 1 | 9 | 76.55a | 16.79 | 9 | 62.04ab | 31.21 | 9 | 76.85b | 13.68 | 0.241 |
| Group 2 | 12 | 81.39 | 25.47 | 12 | 77.92 | 20 | 12 | 86.94 | 12.61 | 0.149 |
| Cognitive functioning | ||||||||||
| Total survivors | 21 | 79.37 | 18.93 | 21 | 76.19 | 23.9 | 21 | 79.37 | 20.35 | 0.617 |
| Group 1 | 9 | 70.37a | 20.03 | 9 | 59.26aß | 25.15 | 9 | 66.67ð | 22.05 | 0.508 |
| Group 2 | 12 | 86.11 | 15.62 | 12 | 88.89ß | 12.97 | 12 | 88.89ð | 12.97 | 0.751 |
| Social functioning | ||||||||||
| Total survivors | 21 | 70.64 | 23.51 | 21 | 74.6 | 29.16 | 21 | 76.98 | 28.61 | 0.602 |
| Group 1 | 9 | 62.96 | 23.24 | 9 | 53.7Þ | 27.36 | 9 | 55.56ø | 27.64 | 0.802 |
| Group 2 | 12 | 76.39abǂ | 22.98 | 12 | 90.28aǂÞ | 19.41 | 12 | 93.06bø | 16.6 | 0.037 |
| Fatigue | ||||||||||
| Total survivors | 21 | 32.27 | 20.46 | 21 | 30.68 | 21.35 | 21 | 33.32 | 24.35 | 0.282 |
| Group 1 | 9 | 39.51a | 15.82 | 9 | 44.44Ɣ | 15.71 | 9 | 51.85aƕ | 22.22 | 0.237 |
| Group 2 | 12 | 26.84 | 22.46 | 12 | 20.36Ɣ | 19.44 | 12 | 19.43ƕ | 15.07 | 0.222 |
| Nausea and vomiting | ||||||||||
| Total survivors | 21 | 6.35 | 11.15 | 21 | 7.14 | 9.96 | 21 | 3.97 | 7.27 | 0.386 |
| Group 1 | 9 | 12.96ƛ | 13.89 | 9 | 12.96Ʊ | 11.11 | 9 | 7.4 | 8.78 | 0.571 |
| Group 2 | 12 | 1.39ƛ | 4.81 | 12 | 2.78Ʊ | 6.49 | 12 | 1.39 | 4.81 | 0.647 |
| Pain | ||||||||||
| Total survivors | 21 | 21.43 | 24.23 | 21 | 18.25 | 16.59 | 21 | 15.08 | 18.18 | 0.272 |
| Group 1 | 9 | 31.48 | 28.19 | 9 | 27.78Ƹ | 14.43 | 9 | 29.63ϕ | 18.22 | 0.927 |
| Group 2 | 12 | 13.89 | 18.58 | 12 | 11.11Ƹ | 14.79 | 12 | 4.17ϕ | 7.54 | 0.353 |
| Dyspnoea | ||||||||||
| Total survivors | 21 | 12.7a | 19.65 | 21 | 22.22 | 32.2 | 21 | 25.4a | 25.61 | 0.131 |
| Group 1 | 9 | 14.81ab¥ɫ | 17.57 | 9 | 44.44a¥ϡ | 33.33 | 9 | 44.44bɫɄ | 23.57 | 0.026 |
| Group 2 | 12 | 11.11 | 21.71 | 12 | 5.56ϡ | 19.25 | 12 | 11.11Ʉ | 16.41 | 0.348 |
| Insomnia | ||||||||||
| Total survivors | 21 | 33.33 | 36.51 | 21 | 28.57 | 28.45 | 21 | 36.51 | 33.17 | 0.259 |
| Group 1 | 9 | 48.15 | 44.44 | 9 | 40.74a | 32.39 | 9 | 55.56aɅ | 33.33 | 0.407 |
| Group 2 | 12 | 22.22 | 25.95 | 12 | 19.44 | 22.29 | 12 | 22.22Ʌ | 25.94 | 0.606 |
| Appetite loss | ||||||||||
| Total survivors | 21 | 14.29 | 16.9 | 21 | 14.29 | 19.92 | 21 | 11.11 | 19.24 | 0.609 |
| Group 1 | 9 | 22.22 | 16.67 | 9 | 18.52 | 24.22 | 9 | 18.52 | 24.22 | 0.853 |
| Group 2 | 12 | 8.33 | 15.08 | 12 | 11.11 | 16.41 | 12 | 5.56 | 12.97 | 0.237 |
| Constipation | ||||||||||
| Total survivors | 21 | 17.46 | 20.05 | 21 | 12.7 | 24.67 | 21 | 15.87 | 29.1 | 0.650 |
| Group 1 | 9 | 25.93 | 22.22 | 9 | 22.22 | 33.33 | 9 | 22.22 | 37.27 | 0.962 |
| Group 2 | 12 | 11.11 | 16.41 | 12 | 5.56 | 12.97 | 12 | 11.11 | 21.71 | 0.402 |
| Diarrhoea | ||||||||||
| Total survivors | 21 | 6.35 | 13.41 | 21 | 9.52 | 18.69 | 21 | 7.94 | 17.97 | 0.736 |
| Group 1 | 9 | 14.81Ɏ | 17.57 | 9 | 22.22ʡ | 23.57 | 9 | 18.52ξ | 24.22 | 0.764 |
| Group 2 | 0 | 0Ɏ | 0 | 0 | 0ʡ | 0 | 0 | 0ξ | 0 | – |
| Financial difficulties | ||||||||||
| Total survivors | 21 | 28.57 | 33.81 | 21 | 25.4 | 36.37 | 21 | 25.4 | 34.81 | 0.629 |
| Group 1 | 9 | 37.04 | 38.89 | 9 | 37.04 | 42.31 | 9 | 37.04 | 42.31 | 1.000 |
| Group 2 | 12 | 22.22 | 29.59 | 12 | 16.67 | 30.15 | 12 | 16.67 | 26.6 | 0.402 |
Matched symbols (e.g. ǂǂ ) indicate statistically significant difference between pairs and matched letters (e.g. aa) indicate a clinically significance difference as defined as a 10‐point difference in functioning scores (Osaba et al 1998).
Acceptability and feasibility
On average, 21 (84%) participants who completed the final survey found the FLW Program acceptable or totally acceptable. Twenty‐one participants (84%) found the P&G acceptable or very acceptable, stating that they could tailor it to their needs. Of those that did not find these tools acceptable, three participants (12%) had withdrawn from the trial and one participant's data (4%) were incomplete. Participants' levels of engagement with each of the FLW Program tools suggest that goal setting was particularly feasible for cancer survivors, particularly nutrition goals. Their full participation in completing the FLW scale (which assessed their self‐management capabilities) across all intervention time points also suggests that this tool is convenient and feasible for use with this population. Further survey outcomes are not reported here.
Discussion
Our study demonstrated that cancer survivors, in the active phase of treatment or later in their cancer treatment trajectory, found the FLW Program acceptable as a means of helping them to develop and achieve their nutrition and physical activity goals. High levels of acceptability of the Flinders Program tools have been found in other studies.32, 33, 34
Many participants rated their baseline risk factor self‐management capacity as relatively good across the LWS domains. This differs from baseline ratings of self‐management capacity commonly observed in studies of populations with existing chronic diseases.32, 33, 34 In those studies, physical and psychosocial impacts are particularly challenging and often rated poorly. This is likely because of the long‐standing nature of chronic disease and its insidious capacity to impact negatively on the individual's resources and supports over time, and also because multiple comorbidities can make self‐management challenging.43 Cancer, on the other hand, may affect those with no other chronic conditions prior to the cancer diagnosis. The support networks of cancer sufferers may even improve as a result of the cancer diagnosis, and they have been shown as important for survivorship.44
Many participants did not want to set problems, and our findings showed that, for participants with complete data on their problem rating, their problem did not change over time. Likely reasons for this are the short 12‐week time period, the nature of the population and the context of their cancer treatment stage in which we would not expect problems related to nutrition and physical activity to improve. However, they appeared more motivated to talk about goals. This may be related to the journey many cancer survivors take as part of discovering they have cancer and fortifying themselves to fight it45 Unlike chronic conditions, which require accommodations46 and ‘living with’ and addressing problems as they arise as part of long‐term self‐management,31, 47 cancer is something we are socialized to see as ‘fighting’ and ‘surviving’. This is an important distinction, with significant implications for how cancer as a chronic disease is perceived.48
As confirmed in the results, participants found goal setting acceptable because it remained flexible to their real‐world experience and needs, and was not rigid or with artificially imposed timeframes for achievement (i.e. participants were able to achieve their goals at their own pace). Participants were also more inclined to set nutrition goals than physical activity goals, suggesting that they felt more able or willing to work towards nutrition goals at these stages of their cancer treatment. In addition, more nutrition goals were worked on continuously over the total intervention period than physical activity goals (72 and 46%, respectively). Reasons for this are unclear and require further investigation.
When considering differences between groups, the post‐chemotherapy group (Group 2) did better generally and reported better quality of life. Worsening physical functioning related to problems with dyspnoea, insomnia and fatigue in Group 1 would be expected, given participants in this group were in the active phase of chemotherapy treatment. Despite this, those currently receiving chemotherapy appeared to benefit also from early intervention to promote healthy lifestyle self‐management. The results indicate that there is value in starting self‐management support during the active phase of treatment with curative intent. Davies and Batehup49 argue that conversations with patients about self‐management need to begin at point of diagnosis, as part of a collaborative, empowering and interactive relationship between patient and health‐care provider. They also argue that there needs to be a shift in perception by clinicians from expert to enabler and by patients from passive recipients to active participants in their care. This is similarly argued in the chronic disease area.47 However, self‐management is more than just knowledge acquisition. Supporting patients' self‐efficacy or confidence to self‐manage is one of the most important roles that clinicians can play because it is one of the most significant personal attributes affected after cancer diagnosis, treatment and survivorship.26, 49 Self‐management support tools such as the FLW Program appear to be acceptable and feasible for use with this population.
This study involved a small sample. Most participants were women with breast cancer. We cannot be certain whether this was due to recruitment bias, their greater motivation or willingness to participate compared to people with other cancer types, or some other reason. Oncologists and cancer care coordinators at the centre from which participants were recruited serve patients will a wide range of cancer diagnoses. Further research is needed to investigate potential gender differences and acceptability and feasibility for patients with different cancer types. People with more comorbidity might have chosen not to participate, due to issues related to health literacy, but also potential burden of tasks that the patient with cancer is facing where these issues may take a lower priority. Also, the study involved an intervention tailored to the needs of the individual and thus was delivered in diverse settings by a diverse range of providers. This approach introduces the variability of the delivery of interventions which may impact on efficacy, but at the same time facilitates adaptation to different settings and is fundamental to the tailored approach. Participants were specifically encouraged to set physical activity and nutrition goals, whereas the spirit of self‐management within chronic condition management is for the patient to freely determine their goals. Further research using an RCT design, with a larger sample, with intervention occurring over a longer time period and more rigorous protocols for data collection is needed. Further translational research is also needed to determine acceptability, feasibility, enablers and barriers for clinicians embedding this approach into routine cancer survivorship care.
Conflict of interest
The authors can confirm that they have no financial relationship with the organization that sponsored the research. They have full control of all primary data and they agree to allow the journal to review their data if requested.
Source of funding
This research was supported by the Flinders Centre for Innovation in Cancer, Flinders Medical Centre, Adelaide, South Australia, Australia. The intervention was made possible through the recruitment support of the Department of Oncology at Flinders Medical Centre, Adelaide, South Australia, Australia.
Supporting information
Appendix S1. Study interventions and measures.
Acknowledgements
We wish to thank the study participants, the oncology staff who assisted with recruitment and Flinders EFM gym for their contributions to the physical activity arm of the intervention.
References
- 1. AIHW (Australian Institute of Health and Welfare), AACR (Australasian Association of Cancer Registries) . Cancer in Australia: An Overview, 2008. Cancer Series no. 46. Canberra, ACT: Australian Institute of Health and Welfare; Available at: http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442454588, accessed 16 January 2014. [Google Scholar]
- 2. Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population‐based national sample. Journal of the National Cancer Institute, 2004; 96: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 3. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Research, 2011; 13: R64 Available at: http://www.biomedcentral.com/content/pdf/bcr2901.pdf, accessed 16 January 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. Journal of Clinical Oncology, 2005; 23: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 5. Demark‐Wahnefried W, Platz EA, Ligibel JA et al The role of obesity in cancer survival and recurrence. Cancer Epidemiology Biomarkers & Prevention, 2012; 21: 1244–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. Journal of Clinical Oncology, 2008; 26: 759–767. [DOI] [PubMed] [Google Scholar]
- 7. Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It's not over when it's over: long‐term symptoms in cancer survivors – a systematic review. International Journal of Psychiatry in Medicine, 2010; 40: 163–181. [DOI] [PubMed] [Google Scholar]
- 8. de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer Survivors and unemployment: A meta‐analysis and meta regression. JAMA: The Journal of the American Medical Association, 2009; 301: 753–762. [DOI] [PubMed] [Google Scholar]
- 9. Earle CC, Ganz PA. Cancer survivorship care: don't let the perfect be the enemy of the good. Journal of Clinical Oncology, 2012; 30: 3764–3768. [DOI] [PubMed] [Google Scholar]
- 10. Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press, 2005. [Google Scholar]
- 11. Del Guidice ME, Grunfeld E, Harvey BJ, Piliotis E, Verma S. Primary care physicians' views of routine follow‐up care of cancer patients. Journal of Clinical Oncology, 2009; 27: 3338–3345. [DOI] [PubMed] [Google Scholar]
- 12. Lance Armstrong Foundation . “I Learned To Live With It” Is Not Good Enough: Challenges Reported by Post‐Treatment Cancer Survivors In The Livestrong Surveys. A Livestrong Report. Austin, TX: Lance Armstrong Foundation, 2010. Available at: https://assets-livestrong-org.s3.amazonaws.com/media/site_proxy/data/a54320d560396f8742fa490257c442829f5be4b2.pdf, accessed 16 January 2014. [Google Scholar]
- 13. World Health Organization (WHO) . Care for Chronic Conditions: Building Blocks for Action. Geneva: World Health Organization, 2002. Available at: http://www.who.int/diabetesactiononline/about/icccreport/en/, accessed 16 January 2014. [Google Scholar]
- 14. Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. Journal of Clinical Oncology, 2011; 29: 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grunfeld E, Julian JA, Pond G et al Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. Journal of Clinical Oncology, 2011; 29: 4755–4762. [DOI] [PubMed] [Google Scholar]
- 16. Smith TJ, Snyder C. Is it time for (survivorship care) plan B? Journal of Clinical Oncology, 2011; 29: 4740–4742. [DOI] [PubMed] [Google Scholar]
- 17. Cotterell P, Harlow G, Morris C et al Service user involvement in cancer care: the impact on service users. Health Expectations, 2011; 14: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCorkle R, Ercolano E, Lazenby M et al Self‐management: Enabling and empowering patients living with cancer as a chronic illness. CA: A Cancer Journal for Clinicians, 2011; 61: 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. Journal of Clinical Oncology, 2006; 24: 3527–3534. [DOI] [PubMed] [Google Scholar]
- 20. Knols RH, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology, 2005; 23: 3830–3842. [DOI] [PubMed] [Google Scholar]
- 21. National Health Priority Action Council (NHPAC) . National Chronic Disease Strategy. Canberra, ACT: Australian Government Department of Health and Aging, 2005. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/pq-ncds-strat, accessed 16 January 2014. [Google Scholar]
- 22. National Health and Hospitals Reform Commission . A Healthier Future for all Australians: Final Report of the National Health and Hospitals Reform Commission. Canberra, ACT: National Health and Hospitals Reform Commission, 2009. Available at: http://www.health.gov.au/internet/nhhrc/publishing.nsf/content/nhhrc-report, accessed 16 January 2014. [DOI] [PubMed] [Google Scholar]
- 23. Thille PH, Russell GM. Giving patients responsibility or fostering mutual response‐ability: family physicians' constructions of effective chronic illness management. Qualitative Health Research, 2010; 20: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 24. Pulvirenti M, McMillan J, Lawn S. Empowerment, patient centred care and self‐management. Health Expectations 2012; 17: 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Regan‐Smith M, Hirschmann K, Lobst W, Battersby M. Teaching residents chronic disease management using the Flinders Model. Journal of Cancer Education, 2006; 21: 60–62. [DOI] [PubMed] [Google Scholar]
- 26. Kidd LA. Consequences, control and appraisal: cues and barriers to engaging in self‐management among people affected by colorectal cancer – a secondary analysis of qualitative data. Health Expectations 2012; 17: 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aujoulat I, Luminet O, Deccache A. The perspective of patients on their experience of powerlessness. Qualitative Health Research, 2007; 17: 772–785. [DOI] [PubMed] [Google Scholar]
- 28. Foster C, Fenlon D. Recovery and self‐management support following primary cancer treatment. British Journal of Cancer, 2011; 105: S21–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weingarten SR, Henning JM, Badamgarav E et al Interventions used in disease management programmes for patients with chronic illness—which ones work? Meta‐analysis of published reports British Medical Journal, 2002; 325: 925–932. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC130055/pdf/925.pdf, accessed 11 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chodosh J, Morton SC, Mojica W. Meta‐analysis: Chronic disease self‐management programs for older adults. Annals of Internal Medicine, 2005; 143: 427–438. [DOI] [PubMed] [Google Scholar]
- 31. Fenlon D, Foster C. Self‐Management Support: A Review of the Evidence, Working document to support the National Cancer Survivorship Self‐Management Work Stream. South Hampton: University of South Hampton, Macmillan Research Unit, 2009. Available at: http://www.ncsi.org.uk/wp-content/uploads/Self-Management-Support-A-Review-of-the-Evidence.pdf, accessed 16 January 2014. [Google Scholar]
- 32. Lawn S, Battersby MW, Pols RG, Lawrence L, Parry T, Urukalo M. The mental health expert patient: Findings from a pilot study of a generic chronic condition self‐management programme for people with mental illness. International Journal of Social Psychiatry, 2007; 53: 63–74. [DOI] [PubMed] [Google Scholar]
- 33. Battersby MW, Beattie J, Pols R, Smith D, Condon J, Blunden S. A randomised controlled trial of the Flinders Program™ . The Australian and New Zealand Journal of Psychiatry, 2013; 47: 451–462. [DOI] [PubMed] [Google Scholar]
- 34. Battersby MW, Kit JA, Pridcaux C, Harvey PW, Coffins JP, Mills PD. Research implementing the flinders model of self‐management support with Aboriginal people who have diabetes: findings from a pilot study. Australian Journal of Primary Health, 2008; 14: 66–74. [Google Scholar]
- 35. Zrim S, Miller M, Lawn S et al An innovative combined nutrition and physical activity (NAPA) intervention for cancer survivors. Poster presentation. Clinical Oncology Society of Australia (COSA) Annual Conference, 13–15 Nov 2012, Brisbane, Australia. [Google Scholar]
- 36. Oken MM, Creech RH, Tormey DC et al Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology, 1982; 5: 649–655. [PubMed] [Google Scholar]
- 37. Battersby M, Harvey P, Mills PD et al SA HealthPlus: a controlled trial of a state‐wide application of a generic model of chronic illness care. Milbank Quarterly, 2007; 85: 37–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flinders Human Behaviour & Health Research Unit (FHBHRU) . The Flinders Program™. Adelaide, SA: FHBHRU, 2013. Available at: http://www.flinders.edu.au/medicine/sites/fhbhru/self-management.cfm, accessed 16 January 2014. [Google Scholar]
- 39. Health SA. Do it for Life & Healthy Weight Storybook: A Passion for Prevention. Adelaide, SA: Government of South Australia, 2011. [Google Scholar]
- 40. Battersby M, Ask A, Reece M, Markwick M, Collins J. The partners in health scale: the development and psychometric properties of a generic assessment scale for chronic condition self‐management. Australian Journal of Primary Health, 2003; 9: 41–52. [Google Scholar]
- 41. Harley C, Pini S, Bartlett YK, Velikova G. Defining chronic cancer: patient experiences and self‐management needs. BMJ Supportive & Palliative Care, 2012; 2: 248–255. [DOI] [PubMed] [Google Scholar]
- 42. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. Journal of Clinical Oncology, 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 43. Vogeli C, Shields AE, Lee TA et al Multiple Chronic Conditions: Prevalence, Health Consequences, and Implications for Quality, Care Management, and Costs. Journal of General Internal Medicine, 2007; 22(Suppl. 3): 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beasley JM, Newcomb PA, Trentham‐Dietz A et al Social networks and survival after breast cancer diagnosis. Journal of Cancer Survivorship, 2010; 4: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hou WK, Law CC, Yin J, Fu YT. Resource loss, resource gain, and psychological resilience and dysfunction following cancer diagnosis: A growth mixture modeling approach. Health Psychology, 2010; 29: 484–495. [DOI] [PubMed] [Google Scholar]
- 46. Corbin JM, Strauss A. Unending Work and Care: Managing Chronic Illness at Home. San Francisco, CA: Jossey‐Bass Publishing Co, 1988. [Google Scholar]
- 47. Lorig K, Holman H. Self‐management education: History, definition, outcomes and mechanisms. Annals of Behavioural Medicine, 2003; 26: 1–7. Available at: http://link.springer.com/article/10.1207%2FS15324796ABM2601_01#page-1, accessed 16 January 2014. [DOI] [PubMed] [Google Scholar]
- 48. Tritter JQ, Calnan M. Cancer as a chronic illness? Reconsidering categorization and exploring experience. European Journal of Cancer Care, 2002; 11: 161–165. [DOI] [PubMed] [Google Scholar]
- 49. Davies NJ, Batehup L. Self‐management support for cancer survivors: guidance for developing interventions Self‐Management Support for Cancer Survivors: Guidance for Developing Interventions. London: National Cancer Survivorship Initiative Supported Self‐Management Work stream. Macmillan Cancer Support, Department of Health, 2010. Available at: http://www.ncsi.org.uk/wp-content/uploads/Guidance-for-Developing-Cancer-Specific-Self-Management-Programmes.pdf, accessed 16 January 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Study interventions and measures.


