Abstract
Background
Training health professionals in shared decision making (SDM) may influence their patients' intention to engage in SDM.
Objective
To assess the impact of DECISION+2, a SDM training programme for family physicians about the use of antibiotics to treat acute respiratory infections (ARIs), on their patients' intention to engage in SDM in future consultations.
Design
Secondary analysis of a multicentre clustered randomized trial.
Setting and participants
Three hundred and fifty‐nine patients consulting family physicians about an ARI in nine family practice teaching units (FPTUs).
Intervention
DECISION+2 (two‐hour online tutorial, two‐hour workshop, and decision support tools) was offered in the experimental group (five FPTUs, 162 physicians, 181 patients). Usual care was provided in the control group (four FPTUs, 108 physicians, 178 patients).
Outcome measure
Change in patients' intention scores (range −3 to +3) between pre‐ and post‐consultation.
Results
The mean ± SD [median] scores of intention to engage in SDM were high in both study groups before consultation (DECISION+2 group: 1.4 ± 1.0 [1.7]; control group: 1.5 ± 1.1 [1.7]) and increased in both groups after consultation (DECISION+2 group: 2.1 ± 1.1 [2.7]; control group: 1.9 ± 1.2 [2.3]). Change of intention, classified as either increased, stable or decreased, was not statistically associated with the exposure to the DECISION+2 programme after adjusting for the cluster design (proportional odds ratio = 1.5; 95% confidence interval = 0.8–3.0).
Conclusion
DECISION+2 had no significant impact on patients' intention to engage in SDM for choosing to use antibiotics or not to treat an ARI in future consultations. Patient‐targeted interventions may be necessary to achieve this purpose.
Keywords: clinician–patient communication, implementation, patient involvement in decision making, shared decision making, theory of planned behaviour
Introduction
In contrast with the paternalistic ‘doctor knows best’ approach to clinical decision making, shared decision making (SDM) involves active collaboration between patient and clinician.1 The importance of SDM emerges when health problems have multiple possible treatments, and neither the patient nor the clinician alone holds all the relevant information. In such situations, clinicians may know more about the consequences of each course of action (risks, benefits, costs), but patients know more about how much each of these consequences matters to them given their own values and preferences.1 When engaging in SDM, the parties pool their knowledge and identify together the patient's preferred course of action.1 The benefits of this approach have been observed in a wide range of clinical contexts.2, 3, 4 Compared with those receiving usual care, patients who use decision aids (SDM tools) tend to have better knowledge of the medical problem at hand, more comfort with the decision made and more realistic expectations about its potential consequences.2 Not surprisingly, then, an increasing proportion of patients and clinicians report a favourable intention to engage in SDM,5, 6 governments are providing more support for SDM7 and coverage in the biomedical literature has increased.8
According to a recent study involving pregnant women deciding whether or not to do a screening test for Down syndrome, physicians' attitudes towards SDM may be associated with their patients' intention to engage in SDM.9 While the study was not about the decision to use antibiotics for acute respiratory infections (ARIs), it laid the grounds for our rationale that training physicians in SDM has the potential to foster a positive attitude towards SDM, which in turn may influence patients' intention to engage in SDM. However, this remained to be tested. Consequently, we conducted a secondary analysis of the multicentre clustered randomized trial of DECISION+2,10 a SDM training programme for family physicians regarding the use of antibiotics for treating ARIs, and assessed its impact on patients' intention to engage in SDM for choosing to use antibiotics or not to treat an ARI in future consultations.
Methods
Study design
A detailed protocol and the main results of the multicentre parallel clustered randomized trial of DECISION+2 have been published elsewhere.10, 11 Briefly, all 12 family practice teaching units (FPTUs) affiliated with Laval University, Quebec City, Canada, were simultaneously randomized to the DECISION+2 group (experimental group) or the control group after stratifying for FPTU location (rural vs. urban). Physicians in participating FPTUs encountered one cohort of patients before the SDM training programme was offered to the DECISION+2 group and a second cohort of patients afterwards. Before the SDM training programme took place, patients in both the experimental and control groups received usual care. Our secondary analysis focused solely on the cohort of patients encountered after the training programme was offered to the FTPUs assigned to the experimental group (Fig. 1). This encounter with the second cohort of patients became our index consultation (baseline data collection) and was followed two weeks later by an interview (follow‐up data collection). Therefore, in contrast with patients in the control group, patients in the DECISION+2 group had experienced a consultation with a family physician from a FTPU in which SDM training had taken place.
Figure 1.
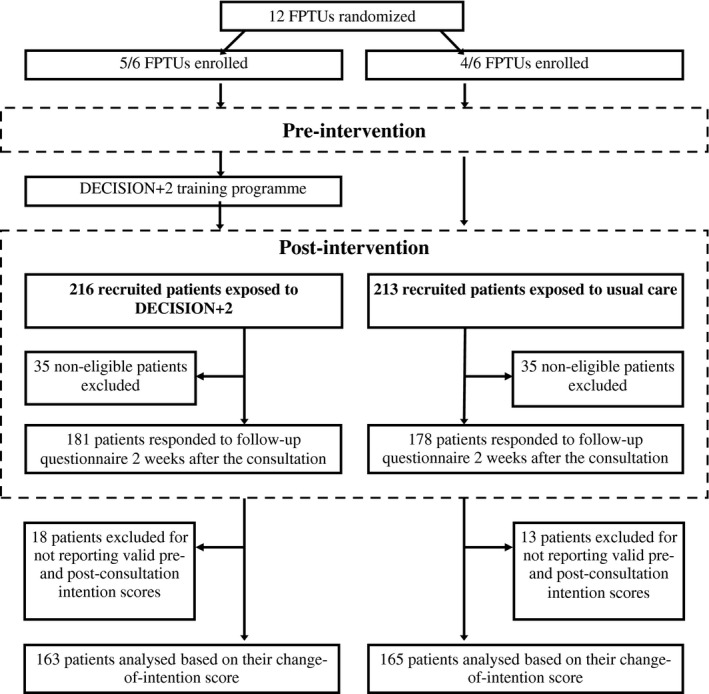
Flow of participants.
Participants and recruitment procedure
From July 2010 to April 2011, research assistants approached adults and children accompanied by an adult or legal guardian whose symptoms suggested an ARI prior to index consultations in the waiting rooms of participating FPTUs. The pre‐intervention cohort of patients (excluded from this analysis) was recruited between July and October 2010, while the post‐intervention cohort of patients (the one analysed here) was recruited between November 2010 and April 2011. Participating patients were eligible if (i) an ARI was diagnosed (acute bronchitis, otitis media, pharyngitis or rhinosinusitis) and (ii) use of antibiotics was discussed as a potential treatment by either the patient or the physician, based on a questionnaire completed immediately after the consultation. For this secondary analysis, we defined the participant as the person who engaged in the decision‐making process with the physician – the parent or legal guardian in the case of a child consulting for an ARI. Neither patients nor clinicians were aware that the impact of DECISION+2 on patients' intention to engage in SDM would be specifically assessed, and patients did not know whether their physician had been exposed to DECISION+2.
Interventions
DECISION+2 consists of a two‐hour online tutorial followed by a two‐hour on‐site workshop. The online tutorial addresses key components of the clinical decision‐making process about antibiotic treatment for common ARIs in primary care (bronchitis, otitis media, pharyngitis and rhinosinusitis). The online tutorial and workshop include videos, exercises and decision support tools to help clinicians communicate the probability of a bacterial ARI and the benefits and harms associated with the use of antibiotics. They also provide scripts to help clinicians clarify the values and preferences of patients. During index consultations, decision support tools were available in all walk‐in consultation rooms of FPTUs assigned to the DECISION+2 study group. In the control group, physicians were not offered DECISION+2 and did not receive the decision support tools. Therefore, patients in the control group were exposed to usual care. The DECISION+2 intervention took place between October and November 2010.
Data collection
Participating patients completed self‐administered questionnaires just before and immediately after the index consultation and responded to a follow‐up questionnaire two weeks later by telephone interview. Pre‐consultation, patients reported sociodemographic data, their preferred role in the decision‐making process based on the Control Preference Scale,12, 13 and whether they had prior knowledge of SDM by answering the simple question ‘Did you know about SDM before participating in this study?’ (response scale: yes or no).
Outcome measure
Our outcome measure was the patients' change of score between baseline (pre‐consultation) and follow‐up (two weeks after the consultation) regarding their intention to engage in SDM for choosing to use antibiotics or not to treat an ARI in future consultations. Before consultation and two weeks later, patients reported their intention14 (three items, Cronbach's alpha = 0.8). The three items measured on a 7‐point Likert scale were: (i) their likelihood of engaging in SDM, from very unlikely (−3) to very likely (+3), (ii) their odds of engaging in SDM, from very weak (−3) to very strong (+3) and (iii) their agreement with the statement ‘I intend to engage in SDM', from total disagreement (−3) to total agreement (+3). These items were developed for the pilot trial of DECISION+15 and were based on the theory of planned behaviour.14 The theory of planned behaviour provides a clear method to assess behavioural intention.14 It proposes three items each assessed on a 7‐point Likert scale. The sum of the three items divided by three is computed to derive the total score of intention (Cronbach alpha = 0.8). Our team has extensive experience with the measurement of behavioural intention using the theory of planned behaviour.9, 10, 15, 16, 17, 18, 19, 20
Statistical analysis
We computed descriptive statistics for all variables. Patients' scores (pre‐ and post‐consultation) regarding their intention to engage in SDM were computed as the mean of all items for which they reported valid responses, provided that they had done so for at least two out of three items.
Although patients' change‐of‐intention scores seemed normally distributed, they did not meet the normality assumptions according to the Shapiro–Wilk and Kolmogorov–Smirnov tests and displayed a very high kurtosis index. We therefore used ordered logistic regression instead of multiple linear regression to compare groups. We split our distribution of change‐of‐intention scores into terciles and labelled changes as ‘increased’ if they were ≥1, ‘stable’ if they were <1 and ≥0 and ‘decreased’ if they were <0. The resulting model respected the proportional odds assumption, that is, the odds ratios (OR) are assumed to be the same between change‐of‐intention scores that were ‘increased’ vs. ‘stable’/‘decreased’ and between scores that were ‘increased’/‘stable’ vs. ‘decreased’.
The potentially confounding and/or modifying effects of patients' gender, age, academic degrees, annual revenue, prior knowledge of SDM and preferred role in decision making were assessed by adding each relevant variable and interaction term to the model (adjusted for the cluster design). A variable was considered as a confounder if its inclusion had a significant impact on the predictions of the statistical model at P < 0.1 and if it changed the OR estimating the impact of DECISION+2 by 10% or more. A variable was considered as a modifier if the inclusion of an interaction term was significant at P < 0.05. Main analyses were conducted on the assumption that all patients in the DECISION+2 group received the same exposure (as if on an ‘intention‐to‐treat’ basis). We also explored the impact of each component of DECISION+2 completed by the physician separately (online tutorial, workshop, both or none). We performed statistical analysis using the Statistical Analysis System (SAS Institute Inc. 2010. SAS OnlineDoc® 9.3; SAS Institute Inc., Cary, NC, USA).
Results
Of 12 eligible FPTUs, nine participated in the study: four in the control group and five in the DECISION+2 group (Fig. 1). All 429 potentially eligible patients for our secondary analysis responded to the questionnaires. Of the 359 eligible patients, we collected valid intention scores both pre‐ and post‐consultation among 163 of 181 (90%) patients in the DECISION+2 group and 165 of 178 (93%) in the control group. Characteristics of eligible patients were balanced between groups (Table 1) and were similar to those of excluded patients (data not shown).
Table 1.
Characteristics of eligible patients
| Characteristica | DECISION+2 group (n = 181) | Control group (n = 178) |
|---|---|---|
| Age, mean ± SD | 39 ± 13 | 42 ± 14 |
| Women, n/N (%) | 131/170 (77) | 127/176 (72) |
| College degree or more, n/N (%) | 65/112 (58) | 94/149 (63) |
| Prior knowledge of SDM, n/N (%) | 66/180 (37) | 74/177 (42) |
| Annual revenue: | ||
| Lower than 30 000 CAD$, n/N (%) | 18/104 (17) | 28/141 (20) |
| Between 30 000 and 60 000 CAD$, n/N (%) | 36/104 (35) | 55/141 (39) |
| Higher than 60 000 CAD$, n/N (%) | 50/104 (48) | 58/141 (41) |
| Preferred role in decision makingb: | ||
| Passive, n/N (%) | 58/163 (36) | 65/165 (39) |
| Collaborative, n/N (%) | 53/163 (33) | 43/165 (26) |
| Active, n/N (%) | 52/163 (32) | 57/165 (35) |
For some characteristics, denominators may be smaller than sample size due to missing values.
We labelled a patient's preferred role as ‘passive’ if he/she stated preferring that ‘the physician decides alone’ or that ‘the physician decides after considering the patient's opinion’; as ‘collaborative’ if he/she stated preferring that ‘parties decide together’; and as ‘active’ if he/she stated preferring that ‘the patient decides alone’ or that ‘the patient decides after considering the physician's opinion’.12, 13
The mean ± SD [median] scores of intention to engage in SDM were high and skewed towards higher values in both study groups before consultation (DECISION+2 group: 1.4 ± 1.0 [1.7]; control group: 1.5 ± 1.1 [1.7]). After consultation, these scores increased in both groups and remained skewed towards higher values (DECISION+2 group: 2.1 ± 1.1 [2.7]; control group: 1.9 ± 1.2 [2.3]).
Figure 2 shows the distribution and descriptive statistics of patients' change‐of‐intention scores, and Table 2 the distribution of patients according to terciles of these scores (increased, stable, decreased) by study group. There was a higher proportion of patients with increased (or not decreased) intention to engage in SDM in the DECISION+2 group, but we found no statistically significant difference between groups after adjusting for the clustered design (intracluster correlation coefficient of change of intention within FPTUs = 0.05). Patients' gender, age, academic degrees, annual revenue, prior knowledge of SDM and preferred role in decision making did not have significant confounding or modifying effects on the association between change‐of‐intention scores (increased, stable, decreased) and exposure of FPTUs to DECISION+2.
Figure 2.
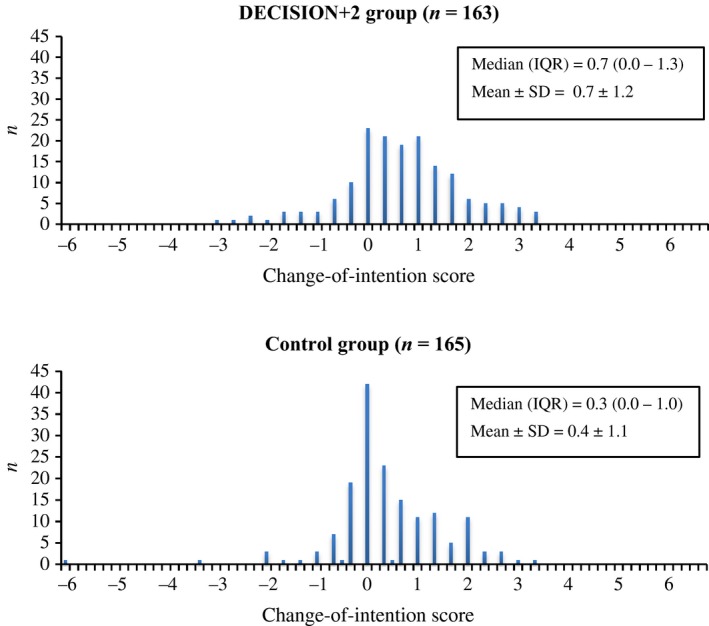
Distributions of patients’ change‐of‐intention scores in regard to their intention to engage in SDM.
Table 2.
Distribution of patients' change‐of‐intention scores to engage in SDM categorized in terciles by study groups
| Change‐of‐intention score | DECISION+2 group (N = 163), n (%) | Control group (N = 165), n (%) | Crude OR (95% CI)a | Adjusted OR (95% CI)a , b |
|---|---|---|---|---|
| Increased intention (change ≥1) | 70 (43) | 47 (28) | 1.6 (1.1–2.5) | 1.5 (0.8–3.0) |
| Stable intention (1> change ≥0) | 63 (39) | 81 (49) | ||
| Decreased intention (change <0) | 30 (18) | 37 (22) |
Reported odds ratio (OR)s were computed on cumulative odds under the proportional odds assumption: that is, ORs are assumed to be the same between intention scores that were ‘increased’ vs. ‘stable’/‘decreased’ and scores that were ‘increased’/'stable’ vs. ‘decreased’.
Adjustments were made to account for the clustered design.
Change‐of‐intention scores were more likely to increase (or not decrease) in patients exposed to physicians who completed both the DECISION+2 online tutorial and the live workshop or the live workshop only, but the differences were not statistically significant. Compared with patients in the control group, for DECISION+2 patients, the ordered logistic regression OR (95% CI) adjusted for clustered design were 1.8 (0.9–3.6) (physician completed whole course), 1.9 (0.7–5.1) (workshop only), 0.8 (0.2–2.7) (online tutorial only) and 1.0 (0.4–2.8) (physician did not complete any of the course components).
Discussion
In this secondary analysis of a clustered randomized trial, we found that after adjusting for the clustered design of the trial, DECISION+2 did not significantly affect patients’ intention to engage in SDM for choosing to use antibiotics or not to treat an ARI in future consultations. To the best of our knowledge, other than the earlier pilot trial of DECISION+,15 this is the first study to assess the impact of training health professionals in SDM on the intention of patients to engage in SDM. We also observed high scores of patient intention to engage in SDM in future consultations. These results lead us to discuss three main issues.
First, patient intention scores collected in the current study were noticeably higher (ceiling effect) than in the pilot study.15 This may be explained by the fact that the current study was conducted in FPTUs where patient engagement is integral to the curriculum and residents are continually reminded of its importance, while the pilot trial was conducted in private practices. This is congruent with results from another study in which pregnant women consulting physicians in FPTUs were surveyed regarding their intention to engage in SDM for prenatal screening.9 These women also reported very high intention to engage in SDM. This difference between FPTU clinical settings and private practices suggests that fostering a continuous learning approach among physicians in private medical practice may have the potential to influence their patients’ willingness to engage in SDM because their intention to start with is lower. Lastly, the literature indicates that not only do most patients want to engage in SDM, but there is an historical trend: in more recently published studies, more and more patients want to engage in SDM.5, 6 This could also partly explain our results.
Second, as these study results did not indicate that DECISION+2 had a statistically significant impact, it would be important to add a patient‐mediated intervention to future SDM implementation studies. At least three systematic reviews reporting on patients’ engagement in SDM indicate that both clinician‐ and patient‐targeted interventions are needed for SDM to take place during clinical encounters.2, 3, 4 Although DECISION+2 included a decision support tool that clinicians were invited to use with patients during consultation, we could not assess this with an objective measure. Therefore, in this study, we cannot assume that patients were activated with a patient‐mediated intervention such as the decision support tool. Future trials will need to test a combination of DECISION+2 and a patient‐mediated intervention such as a patient decision aid.
Third, although we did not observe that DECISION+2 had a statistically significant impact on the intention of patients to engage in SDM in future consultations, we cannot completely exclude this possibility, especially in patients with very low intention to start with. Indeed as this was a secondary analysis of a multicentre clustered randomized trial, it was not powered to detect a difference on our outcome of interest, namely a change in patient intention to engage in SDM in future consultations. However, based on these study results, if there is a difference, we expect this difference to be small and probably not clinically significant.
This study has limitations. First, as acknowledged above, the main trial was not designed per se for this specific study objective. Second, even though collecting follow‐up data through telephone interviews minimized loss of patients at follow‐up, both sets of intention scores should ideally have been collected using the same medium (paper or telephone) to prevent potential information bias. Regardless of the group, patients’ scores of intention to engage in SDM were significantly higher post‐consultation than at baseline, suggesting that factors unrelated to DECISION+2 may have increased average levels of post‐consultation scores. A ‘mode effect’21, 22, 23, 24, 25 may be involved, that is, for reasons of ‘social desirability’,23 some people may have felt less willing to reveal negative or neutral outlooks on SDM when they were interviewed by a real person than when they completed paper‐based questionnaires. Another possible explanation is the ‘mere measurement effect’, whereby the mere reporting of one's intention to perform a desirable behaviour increases one's likelihood (and therefore intention) of actually performing the behaviour. In our case, merely filling in the pre‐consultation questionnaire on intention to engage in SDM might have increased the likelihood of a positive response to the post‐consultation telephone interview.26, 27, 28, 29, 30, 31, 32 Whatever the reason, many patients’ intention to engage in SDM was so high to begin with that increased intention after consultation could not be detected. This ceiling effect could partially explain our inability to detect significant differences between groups. The lack of significant results may also reflect a lack of sensitivity of the measure of intention, although in previous studies focusing on other types of behavioural intentions, the measure appeared to be adequate.33, 34, 35 Last, we approached our research question with a proportional odds model to account for violations of normality assumptions. This methodological choice might have led to significant loss of information.
Conclusion
DECISION+2 had no significant impact on patients’ intention to engage in SDM for choosing to use antibiotics or not to treat an ARI in future consultations. Further studies are needed to document the impact of training health professionals in SDM on patients’ intention to engage in SDM in various clinical decision‐making contexts. Patient‐targeted interventions may be necessary to achieve this purpose.
Source of funding
During most of the process, Nicolas Couët held a Strategic Training in Health Research (STIHR) scholarship from Knowledge Translation Canada. France Légaré is Canada Research Chair in Implementation of Shared Decision Making in Primary Care.
Competing interests
The authors declare that they have no competing interests.
Acknowledgement
We thank Louisa Blair, who edited our written English.
References
- 1. Charles C, Gafni A, Whelan T. Decision‐making in the physician‐patient encounter: revisiting the shared treatment decision‐making model. Social Science & Medicine, 1999; 49: 651–661. [DOI] [PubMed] [Google Scholar]
- 2. Stacey D, Bennett CL, Barry MJ et al Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 2011; 10: CD001431. [DOI] [PubMed] [Google Scholar]
- 3. Légaré F, Turcotte S, Stacey D, Ratté S, Kryworuchko J, Graham ID. Patients’ perceptions of sharing in decisions. The Patient: Patient‐Centered Outcomes Research, 2012; 5: 1–19. [DOI] [PubMed] [Google Scholar]
- 4. Légaré F, Ratte S, Stacey D et al Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews, 2010; 5: CD006732. [DOI] [PubMed] [Google Scholar]
- 5. Williams N, Fleming C. Consumer and Provider Perspectives on Shared Decision Making: A Systematic Review of the Peer‐Reviewed Literature. Washington, DC: Center on Health Care Effectiveness: Mathematica Policy Research, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Education and Counseling, 2012; 86: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Härter M, van der Weijden T, Elwyn G. Policy and practice developments in the implementation of shared decision making: an international perspective. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen, 2011; 105: 229–233. [DOI] [PubMed] [Google Scholar]
- 8. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Affairs, 2013; 32: 276–284. [DOI] [PubMed] [Google Scholar]
- 9. Légaré F, St‐Jacques S, Gagnon S et al Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision‐making. Prenatal Diagnosis, 2011; 31: 319–326. [DOI] [PubMed] [Google Scholar]
- 10. Légaré F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision‐making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ: Canadian Medical Association Journal, 2012; 184: E726–E734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Légaré F, Labrecque M, Godin G et al Training family physicians and residents in family medicine in shared decision making to improve clinical decisions regarding the use of antibiotics for acute respiratory infections: protocol for a clustered randomized controlled trial. BMC Family Practice, 2011; 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strull WM, Lo B, Charles G. Do patients want to participate in medical decisions? JAMA: The Journal of the American Medical Association, 1984; 252: 2990–2994. [PubMed] [Google Scholar]
- 13. Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. The Canadian Journal of Nursing Research/Revue Canadienne de Recherche en Sciences Infirmieres, 1997; 29: 21. [PubMed] [Google Scholar]
- 14. Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes, 1991; 50: 179–211. [Google Scholar]
- 15. Légaré F, Labrecque M, LeBlanc A et al Training family physicians in shared decision making for the use of antibiotics for acute respiratory infections: a pilot clustered randomized controlled trial. Health Expectations, 2011; 14(S1): 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson‐Leduc P, Clayman ML, Turcotte S, Légaré F. Shared decision‐making behaviours in health professionals: a systematic review of studies based on the Theory of Planned Behaviour. Health Expectations, 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Légaré F, Stacey D, Briere N et al Healthcare providers’ intentions to engage in an interprofessional approach to shared decision‐making in home care programs: a mixed methods study. Journal of Interprofessional Care, 2013; 27: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guerrier M, Légaré F, Turcotte S, Labrecque M, Rivest L‐P. Shared decision making does not influence physicians against clinical practice guidelines. PLoS One, 2013; 8: e62537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allaire A‐S, Labrecque M, Giguere A, Gagnon M‐P, Legare F. What motivates family physicians to participate in training programs in shared decision making? Journal of Continuing Education in the Health Professions, 2012; 32: 98–107. [DOI] [PubMed] [Google Scholar]
- 20. Légaré F, Stacey D, Pouliot S et al Interprofessionalism and shared decision‐making in primary care: a stepwise approach towards a new model. Journal of Interprofessional Care, 2011; 25: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erhart M, Wetzel RM, Krügel A, Ravens‐Sieberer U. Effects of phone versus mail survey methods on the measurement of health‐related quality of life and emotional and behavioural problems in adolescents. BMC Public Health, 2009; 9: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravens‐Sieberer U, Erhart M, Wetzel R, Krügel A, Brambosch A. Phone respondents reported less mental health problems whereas mail interviewee gave higher physical health ratings. Journal of Clinical Epidemiology, 2008; 61: 1056–1060. [DOI] [PubMed] [Google Scholar]
- 23. De Leeuw ED. To mix or not to mix data collection modes in surveys. Journal of Official Statistics, 2005; 21: 233–255. [Google Scholar]
- 24. Rodriguez HP, von Glahn T, Rogers WH, Chang H, Fanjiang G, Safran DG. Evaluating patients’ experiences with individual physicians: a randomized trial of mail, internet, and interactive voice response telephone administration of surveys. Medical Care, 2006; 44: 167–174. [DOI] [PubMed] [Google Scholar]
- 25. Cheung YB, Goh C, Thumboo J, Khoo K‐S, Wee J. Quality of life scores differed according to mode of administration in a review of three major oncology questionnaires. Journal of Clinical Epidemiology, 2006; 59: 185–191. [DOI] [PubMed] [Google Scholar]
- 26. Godin G, Sheeran P, Conner M et al Which survey questions change behavior? Randomized controlled trial of mere measurement interventions. Health Psychology, 2010; 29: 636. [DOI] [PubMed] [Google Scholar]
- 27. Sandberg T, Conner M. A mere measurement effect for anticipated regret: impacts on cervical screening attendance. British Journal of Social Psychology, 2009; 48: 221–236. [DOI] [PubMed] [Google Scholar]
- 28. Godin G, Sheeran P, Conner M, Germain M. Asking questions changes behavior: mere measurement effects on frequency of blood donation. Health Psychology, 2008; 27: 179. [DOI] [PubMed] [Google Scholar]
- 29. Morwitz VG. The effect of survey measurement on respondent behaviour. Applied Stochastic Models in Business and Industry, 2005; 21: 451–455. [Google Scholar]
- 30. Morwitz VG, Fitzsimons GJ. The mere‐measurement effect: why does measuring intentions change actual behavior? Journal of Consumer Psychology, 2004; 14: 64–74. [Google Scholar]
- 31. Dholakia UM, Morwitz VG. The scope and persistence of mere‐measurement effects: evidence from a field study of customer satisfaction measurement. Journal of Consumer Research, 2002; 29: 159–167. [Google Scholar]
- 32. Chapman KJ. Measuring intent: there's nothing ‘mere’ about mere measurement effects. Psychology & Marketing, 2001; 18: 811–841. [Google Scholar]
- 33. Armitage CJ, Conner M. Efficacy of the theory of planned behaviour: a meta‐analytic review. British Journal of Social Psychology, 2001; 40: 471–499. [DOI] [PubMed] [Google Scholar]
- 34. Hardeman W, Johnston M, Johnston D, Bonetti D, Wareham N, Kinmonth AL. Application of the theory of planned behaviour in behaviour change interventions: a systematic review. Psychology and Health, 2002; 17: 123–158. [Google Scholar]
- 35. Godin G, Bélanger‐Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Science, 2008; 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


