Abstract
Context
In recent years, the continuous improvements in molecular biology techniques have made it possible to detect carriers for several genetic conditions, including cystic fibrosis (CF). In some countries, CF carrier screening is offered to increasing subset of the general population.
Offering of carrier screening at a population level should not be decided by local health authorities only, without consulting citizens' preferences. One way to involve citizens in the decision process might be to a Citizens' jury, a method of deliberative democracy.
Objective
The object of the study is to produce a recommendation statement about CF carrier screening using a Citizens' jury. As this is a new method in the field, the study also provided the opportunity to evaluate its effectiveness.
Design
The project is designed and managed by an executive committee. The whole process is superintended by a multidisciplinary scientific committee. The 16 members of the jury attend a 1 day meeting, assisted by a non‐medical and unbiased facilitator. Informative material was prepared and distributed 15 days before the jury meeting; during the meeting, experts and witnesses interact directly with all the jurors through questions and answers.
Results
All except one member of the jury felt positively about the Health Service actively providing population carrier screening for CF. The final statement was available to public, clinicians, researchers and decision‐makers.
Discussion
In general, a Citizens' jury is a feasible method for involving citizens in public health decision‐making process and in particular for obtaining a community view about CF carrier screening.
Keywords: citizens' jury, cystic fibrosis, cystic fibrosis carrier screening
In the last few decades, the continuous improvements in molecular biology techniques have made it possible to detect carriers for several genetic conditions, including cystic fibrosis (CF). CF is the most frequent life‐limiting autosomal recessive disease in people of Caucasian descent; it affects one in 3500 newborns in Europe1 and is caused by mutations in the cystic fibrosis trans membrane (CFTR) gene.2
Various learned societies have produced statements about CF carrier screening. The National Institutes of Health3 recommended that carrier testing for CF should be offered not only to adults with an a priori reproductive risk – that is those with a family history of CF – but also to couples from the general population planning a pregnancy. The NIH were followed by the American College of Obstetricians and Gynaecologists,4, 5 and the American College of Medical Genetics,6 which recommended widespread genetic testing for CF. The European CF Society produced a consensus document on standards for effective, safe and ethical CF carrier screening and suggested that the decision whether or not to do it should be left to individual countries or regions, in accordance with local legislation.7 Consequently, there has been a steady increase in CF carrier tests in the United States,8 Australia9, 10 and, to a lesser extent, in Europe.11
Attitudes towards carrier screening for CF have been recently reviewed.12 In general, healthcare providers take a positive attitude, stressing that important matters such as information and education, time constraints during the consultations and counselling are essential before organising a CF population screening. Preliminary results suggest that CF carrier screening may be cost‐effective5 – although the economic evaluation of CF screening showed wide heterogeneity in study design, models and costs13 – and can lessen the number of FC cases.9, 10 The decrease in births of children with CF following the implementation of widespread carrier testing indicates the drastic impact on the reproductive attitudes of couples found to be heterozygous.9
From a different and more general point of view, the systematic individuation of CF carriers could be considered as a relevant topic of a larger debate of increasing medicalization of the society. The size and the extent of this process can be described and measured in various ways,14 and his effects can be evaluated in positive or negative terms. On the one hand, the growing ability to identify pre‐clinical conditions, the gradual extension of the definition of illness and the lowering of the threshold of normality, as well as the proliferation of activities for early detection in all fields of medicine.15, 16 On the other hand it originates from the probabilistic nature of knowledge regarding the efficacy and safety of any kind of medical interventions, so it is necessary to treat a number ‘N’, getting bigger, of people to get a positive result in one individual (without knowing which one), while all the others do not benefit from the treatment to which they are subject (and do not know it). From these two trends comes out an increasing risk of mass iatrogenesis, so the prospective benefits of medicalization could be overcome by the disadvantages, not only in economic terms or social, but also of public health. In addition, the increasing consumption of resources produced by the medicalization undermines the fairness in access to care and is already damaging the sustainability of health systems themselves.17
For the reasons above mentioned, it may be therefore inappropriate to leave the decision about health – such as offer CF carrier screening to health authorities only – without consulting the general population for its preferences and values. Deliberative democracy approach is a reliable and reproducible way of involving potential recipients of the test, and citizens at large, in the decision.18, 19 Citizen's jury is one of the methods of deliberative democracy, it is based on decision‐making by a group of lay people20, 21 who have no vested interests, and who apply their common sense and experience, having been informed with the best possible evidence by expert witnesses.22
This study constitutes the first experience of Citizen's jury on CF carrier screening, and it is aimed to produce a recommendation statement about CF carrier screening using a deliberative democracy process. Moreover, as the Citizen's jury is a new method in the field, this was the opportunity to confirm the feasibility of the method and examine his value in eliciting public values on CF screening.
A group of lay citizens was selected, adequately informed and asked to answer the question: ‘Should the Health Service organize screening of the population with the aim of identifying healthy people who may have children with CF?’
Methods
We adopted the Citizen's jury methods as is a well tested method of deliberative democracy,23, 24 and it has been efficiently utilized in the context of screening programs.25, 26 This study was promoted by a multidisciplinary group including researchers, involved in partnership projects with citizens and patients, science communication experts and clinicians. The whole process was superintended by a multidisciplinary scientific committee where different skills were represented: genetics, general practice, reproductive medicine, counselling, organized screening, communication, laboratory medicine and healthcare organization. Advocate groups of patients and families were also involved. As the project was prompted by the experience published by the Verona group, the project was organized in the neighbouring Veneto region.11
The main question
At a first meeting, the promoters, together with the scientific committee, discussed the key question for the jury, as follows: ‘Should the Health Service organize screening of the population with the aim of identifying healthy people who may have children with CF?’ The main question was accompanied by five subquestions regarding information to be highlighted on CF and the genetic test, responsibility for the information and recommendations for future research. These were as follows:
What information should the health service give about tests and CF to people of child‐bearing age, so they can plan children consciously and responsibly?
What aspects of the test on the carrier should be highlighted?
Which aspects of CF should be highlighted?
Who should do the informing, in what context and how?
Are there unknown issues that should be researched scientifically regarding the test for healthy carriers?
The information booklet for the jury
An ad hoc informative material was presented as a booklet, based on a review of the literature, of consumer websites and websites of family‐patients organizations. The different drafts were examined step‐by‐step and discussed with the scientific committee and with the GRAL, a group of patients and consumers' representatives trained by PartecipaSalute, a research project to involve lay people, patients' associations and scientific‐medical representatives in the health debate.27 The language and layout of the booklet was carefully designed to be easily accessible and readable; it included some knowledge self‐tests, a list of pros and cons and a glossary.
The 26‐page booklet for the jury was been organized in six sections: what is the jury project, information on CF and its impact, what is the carrier screening test, information on the carrier test in relatives and the general population, information on carrier screening around the world and glossary. There are two do‐it‐yourself tests to review the information, one after the information on CF and the other after the carrier screening section. References to four selected websites are provided. Three extracts of the information booklet are displayed in Appendix 1.
The jury
The jury was composed of people with no personal or family history of CF.24, 28 To select the members, all the voluntary associations registered in the Verona area were contacted, and 878 associations were invited (418 cultural, 81 assistance, 164 sport, 26 religious and 189 social) by e‐mail, explaining the rationale of the project, objectives, rules, time required, date and place of the meeting. Thirty‐two associations answered, and we list 23 people interested in participating. On the basis of their representativeness – such as sex, age and education – we selected 16 people. Jurors selected belonged to blood donor associations (3), cultural associations (5), consumers associations (5) and patients associations not CF (3).
Two weeks before the jury meeting, all members were sent a copy of the information booklet, so they could discuss the key question and related subquestions. Each member received a fee for participation in the project (50 Euros).
The composition of the jury is summarized in Table 1.
Table 1.
Main characteristics of member of the jury
| N° (%) | |
|---|---|
| Male | 9 (56.2) |
| Female | 7 (43.8) |
| Age mean (range) – years | 52.5 (38–64) |
| Education | |
| Elementary | 2 (12.6) |
| Middle and high school | 7 (43.7) |
| University | 7 (43.7) |
One‐day jury meeting
Information to the jury was provided during the morning section where different experts presented the evidence available in the international literature on CF screening, the impact of CF on quality and quantity of life, and the roles and responsibilities of individuals and society relating to health decisions (four different invited speakers). In one session, the issues related to CF carrier screening were explored using a video of interviews to three carriers detected through screening (two men and one woman). A discussion for the jury members was scheduled at the end of each session. At the end of the morning section, there was a pros and cons debate between a clinician and a health policymaker. During all the morning section, the jurors actively participate with specific questions (such as impact of different drugs, more details on quality of life, patients' autonomy) and general comments (such as responsibility in conveying information, role of general practitioners/gynaecologists).
The 4 h behind close doors, afternoon section was dedicated to discussion among the jury members. The whole process was assisted by a non‐medical expert and unbiased facilitator with the role of:
Create a favourable climate for the exchange of points of view allowing everyone to express themselves freely and being the guarantor of full respect for all the opinions;
Ensure that the decision was not taken on the basis of incorrect or partial or emotional information, driving the jurors to consider all the elements that were provided by experts in the previous days and considering the benefits in the short and long term;
Maintain a high level of awareness of the jurors regarding the role and responsibilities arising from the decision;
Ensure that the decision was taken with the objective good of the entire community.
The argumentative style used for conducting the jury discussion meeting had been discussed in advance with the promoters and scientific committee, taking also into account pertinent literature. At the beginning of the jury discussion meeting the facilitator reiterated that neither the facilitator nor the promoters had any interest in one particular decision.
The final decision was reached by consensus as far as possible. The jurors identified one spokesperson responsible for organizing the first draft of the decision, presenting the results at the end of the discussion to the promoters and drafting the final document. The conclusion and recommendations were included in the final report, circulated, shared, amended and, finally, approved by all the jurors.
Rating
Before the 1‐day jury meeting and after its conclusion, all the members of the jury were asked to complete two questionnaires. The first questionnaire was filled in at the beginning of the jury meeting day before the start of the meeting. It included 10 closed questions: six testing the level of knowledge on FC, two regarding the Citizen's jury method, one regarding the information booklet and one regarding additional information searched the days before the meeting. The second questionnaire was filled at the end of the jury meeting. This questionnaire included seven closed questions: one regarding the information booklet, one about the speakers, one regarding the time dedicated to the jury discussion meeting, one regarding the facilitator and three regarding the Citizen's jury method.
Role of the funding source
The Italian Cystic Fibrosis Research Foundation sponsor of the project was not involved in the study design, preparation of information booklet for the jury, composition of the jury, meeting of the jury, writing the report or in the decision to submit for publication. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results: the jury statement
During the morning, the jury1 had a lively discussion with the experts. The jury discussion meeting started with jurors introducing themselves and being asked to express one reason in favour and one reason against with regard to the main question Should the Health Service organize screening of the population with the aim of identifying healthy people who may have children with CF? Different points of view were discussed among jurors and pros and cons written on a whiteboard. Then the jury was asked to try to re‐examine the arguments put forward in favour or against in order to assess their strengths and weaknesses.
At the end of the discussion, all except one of the jury members stated they felt positive about the Health Service providing population carrier screening for CF. Several issues were discussed during the jury discussion meeting: the screening is considered a way to allow/promote much equity among citizens (no barriers related to different information and awareness or to the cost of the test); the cost of the illness in terms of cure, care and quality of life of patients and families; the survival rate and the impact in term of depression; the future of research and the hope to achieve better conditions for patients. Although some jurors have addressed the issue of eugenics, the majority considered CF carrier screening a valuable way for conveying accurate information to couples and let them decide whether to take the test or not. The final statement approved by the jury is to be considered as a summary of the discussion among jurors; in fact, it is articulated in four different sections: human reasons, scientific reasons, economic reasons and social fairness. These reasons are reported here, while the answers to the subquestions are reported in Appendix 2.
Human reasons
The information provided by experts clearly shows how serious the illness is and its impact on the quality of life of patients and their families. The illness requires a commitment to constant treatment on a daily basis, which, however, is still not sufficient to prevent it progressing. Even though there has been a steady increase over the years, the average life expectancy of those who have CF still does not exceed 40 years. This means, among other things, constant concern among the parents of those who are sick, which in 35% of cases leads to depression. CF carrier screening is thus a potential tool available to the public to reduce the incidence of the disease and the burden of suffering for patients and their families.
Finally, while not dismissing the emotional impact of a positive test on a large number of people, it nevertheless seems a lesser evil than the possibility, always unforeseeable, of having a child with CF and, in perspective, is a positive element of awareness.
Scientific reasons
Unlike multifactorial diseases, the origin of CF is exclusively genetic. The tests currently on the market can identify with considerable accuracy most carriers of the genetic mutations that cause the disease. Although there is a residual risk that, despite a negative result, the person is in any case a carrier of the mutated gene, the fact that the test identifies 85% of carriers, on average, is considered enough to make it reliable.
The survey in the eastern part of Veneto,11 while not fulfilling the criteria for a scientific study, can be considered a valid experiment which involved gynaecologists and was not sponsored by any pharmaceutical company or organization with vested interests. The significant decrease in the incidence of Mediterranean thalassaemia as a result of screening offers a precedent with regard to CF screening and its outcome in terms of lowering its incidence. Screening for thalassaemia is also considered more similar to that for CF than other types of screening aimed at identifying existing diseases, such as those in oncology.
Economic reasons
Experts confirm that the cost of the test is expected to fall, while the cost of care will rise. A projection on a national level of the results in the eastern part of Veneto would lead to a reduction of the number of patients, to the point that the test costs could be easily covered in the medium to long term. This is even more likely considering that, with advances in research and improving health care, the average life expectancy of CF patients is destined to increase over the years but along with it the costs of care. In the worst case, if the incidence of CF were to be reduced by only one case per million inhabitants, this would led to complete coverage of screening costs, while reduction of the incidence by two to four cases per million would lead to a considerable saving of resources which could be used to finance research on new cures for this and other diseases.
Social fairness
At the moment, the test for CF carrier is available and can be requested by those who have the means to do so, even if they have no cases of CF in the family. This creates inequality among citizens which goes against the principle of equity, regardless of the fact that each citizen decides whether or not to undergo screening. Screening would place people who participate in the condition to make a better‐informed reproductive decision without affecting their freedom of choice.
While admitting that this is not a major problem for the NHS, the jury nevertheless felt that the choice taken is valid because it would not deprive the system of resources that could be allocated for other initiatives or improvement. It would actually free up some resources. The jury also acknowledged the high level of complexity involved in this kind of screening, but it believes that the Italian NHS is capable of taking on this task in ways that can be verified and gradually improved. Doubts from an organizational point of view can not constitute sufficient reasons for not implementing a screening scheme which, apart from having its own funding coverage, has high social value in terms of reducing suffering.
Finally, regarding the evaluation of the process, all the members of the jury completed the questionnaires. In the pre‐jury assessment, the majority of jurors rated easy or very easy to answer the question, and 10/16 jurors considered 1 day enough to reply the question. Most of the jurors received correct information by reading the booklet: more than 88% of right answer for three questions on FC, and carrier screening. Forty‐four percentage of the jurors collected also additional information, most through Internet.
At the end of the day, the jurors considered positive the experience, particularly the information received (71% good), the discussion organized during the 1‐day meeting (93% good) and the experience on the whole (64% very good and 36% good).
Discussion
Principal findings
The jury felt positively about the NHS providing the population carrier screening for CF. This result provides a comparable prospective respect to the expert deliberations.3, 5, 6, 7 The final statement was independently written by the jurors, and neither the promoters nor the scientific committee reviewed the text before publication. The final statement considers different aspects of carrier screening: human, scientific, economic and social fairness. None of these aspects was considered more important than the others, meaning that the complexity of the theme implied the utility of approaching it through a multimodal path where scientific, medical and technical aspects are matched with human and social ones. However, the statement was greeted favourably, although some members had criticisms, for example asking for more attention to the counselling aspects, noting inconsistencies in the psychological implications related to the test results or too much attention to the economic aspects. The question for the jury involves issues which many may consider bordering on eugenics and producing strong divergences in society. However, as was clear from the report by the facilitator on the jury's discussion, the eugenics question was hardly an issue for the jury, who was more concerned about the health of future generations of CF patients than by any other questions.
One can agree or disagree about the specific decision [that the NHS should organize screening for CF carriers], but what matters is that the members of the jury were able to freely discuss all aspects of the question and converged on a shared position. This process of consensus is the core of deliberative democracy. As shown by the answers to the questionnaires, the jury members assessed the experience as positive. The effectiveness of the method can also be judged from the fact that the jury produced a nearly unanimous statement, on the common ground of the best interests of society.
Strengths and weaknesses of the study
Although on the whole scientifically sound, the jury declarations include some imprecise statements, which outline the challenges of explaining the multifaceted aspects of a complex intervention like CF carrier screening. Such imperfections do not undermine our confidence in the general understanding of the issues by the jury, but seem anyway to suggest the necessity of longer pre‐deliberative explanatory sessions. This study received limited resources allowing only a 1‐day meeting. This may have meant that not all the members of the jury understood in the same way all the aspects of the CF carrier screening question. In particular, we are aware that the information and the discussion were mainly about the meaning and the limits of the genetic test and less about other important issues such as, for example, the pre‐ and post‐test counselling or the organizational aspects and financial challenge of a population screening.
The promoters were disappointed by the poor response to the invitation sent to the voluntary associations. The method of Citizens' jury is relatively new in Italy, which might partially explain the difficulty in recruitment. It was also clear, considering some of the responses obtained during the recruitment phase that these organizations prefer to be involved in initiatives closely related to their usual activities without venturing into unfamiliar situations.
It is quite possible that participants selected from voluntary associations are not really similar to people from society at large, but probably the main difference is their willingness to be involved in public debate. Our jury was composed only of people from the Verona area, so it cannot represent the social and cultural composition of other areas of the country. However, unlike representative democracy, representation is not an issue for direct deliberative democracy. In this case, the focus is not to ensure the proportional expression of the stakeholders for all possible points of view, but on involving lay people – with no direct and potentially conflicting interests on the topic – to judge only for the good of the society at large. The close analogy is with popular juries, where the members are random lay persons, but is widely accepted that they can take hard decisions ‘in the name of the people’. It would now be interesting to assess the method in different contexts, and we do plan to do other similar initiatives in other cities in Italy, with different historical and cultural inheritances.
Dissemination and usefulness of this study
The final statement of the jury has been widely disseminated through websites, ad hoc mail to representatives of patients' associations, health authorities, short reports on local bulletins, on national newspaper and magazines, and through the network of researchers and supporters of the Italian Cystic Fibrosis Research Foundation. As in Italy, several patient and family groups or patients' associations are closely involved in the debate on research and assistance for CF, it is possible that the jury statement might prove useful during the discussion of future investments. As a first result of the project, we can report increased attention to the question of CF carrier screening, not only between patients' and family groups but also within the medical and scientific community, which is discussing new initiatives in the field (CC, personnel communication).
On a more general level, this experience shows that the method of the Citizens' jury may be used to meet the challenge of the growing process of medicalization of society, which implies the need to inform and involve in the choices all the people that may be affected by positive and negative effects of interventions.22 To date, the only response to this need had been the individual informed consent, but the collective nature of most medical interventions should impose a preliminary information and sharing with the citizen target of any new medical initiatives, when this will have repercussions on a collective level, as well as on individual. All this in the wake of the patient‐centred‐care debate.29
The need for an information and a collective consensus before and in addition to the individual one is not yet part of the common heritage of awareness of citizens, their representatives and health professionals.30 Also, at the political and institutional level, it is not yet clear what is the best way to share the healthcare choices with citizens.31 In general, the classical forms of representative democracy do not seem appropriate for this purpose, because they in fact delegate the decisions to expert groups or restricted and self‐referential policy networks.
This Citizen's jury experience suggests that the development of innovative forms of deliberative democracy may be an appropriate response to the challenge of social sharing of health choices that have impact on the community.
Contributions
All the authors contributed the investigation. PM and SR had the idea for the study and designed the study. PM and WV coordinated all the phases of the project, reviewed the literature, collected data and developed the informative material for the jury. RS and CC collaborated to some phases of the project, contributed with data interpretation and reviewed the informative materials for the jury. All the authors critically reviewed the manuscript and approved the final version.
Source of funding
This project was funded by the Italian Cystic Fibrosis Research Foundation, grant FFC #9/2011 with the contribution of Euro 35 000. The Italian Cystic Fibrosis Research Foundation was not directly involved in any phase of the project.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We thank Dr. Nadia Oprandi for facilitating the jury process, the members of the jury [Bagnulo August Associazione Consumatori Europei, Battiato Mariagrazia Associazione Consumatori Europei, Bazzani Fausto Associazione FIDAS, Butti Marta Giaele ALIVE Accademia Musicale, Campedelli M. Teresa Associazione Fossa Bova, Corbioli Simone Associazione Interzona, Felis Giovanna Associazione Interzona, Fusari Giuseppe Associazione Consumatori Europei, Gamba Silvio Associazione FIDAS, Imperato Rolando Associazione FIDAS, Lollis Gianni Società Belle Arti, Solinas Carmen Movimento Consumatori, Tonello Wilma Donne Europee Federcasalinghe, Zoccatelli Franco Unione Parkinsoniani] and the members of CTS [Franco Berti, Lega Italiana Fibrosi Cistica, Firenze; Renata Bortolus, Azienda Ospedaliera Universitaria Integrata, Verona; Maria Cristina Rosatelli, Università di Cagliari, Cagliari; Paola Emilia Cicerone, Giornalista, Milano; Bruno Dallapiccola, Ospedale Bambin Gesù, Roma; Luciano Flor, ASL Trento, Trento; Sandra Perobelli, Azienda Ospedaliera Verona, Verona; Franco Raimo, Pediatra Libera Scelta, Verona; Manuela Seia, Ospedale Policlinico, Milano] for their participation.
Appendix 1. From the original information booklet distributed to the jury
The booklet was organized in different chapters, each characterized by a colour.
Blue: four pages regarding the jury method: what is a Citizens' jury, the individual and collective interests, results and impact, and Citizens' jury in the FC field.
Red: four pages regarding FC: what is the FC, the impact of FC on patient and family.

Green: four pages regarding the carrier text: what is the carrier screening, psychological aspects and counselling in the decision to test the carrier of the FC.

Yellow: six pages regarding the carrier screening in the general population: what is, the results from the literature, simulation of results in a sample of 10 000 people called to do FC carrier screening.
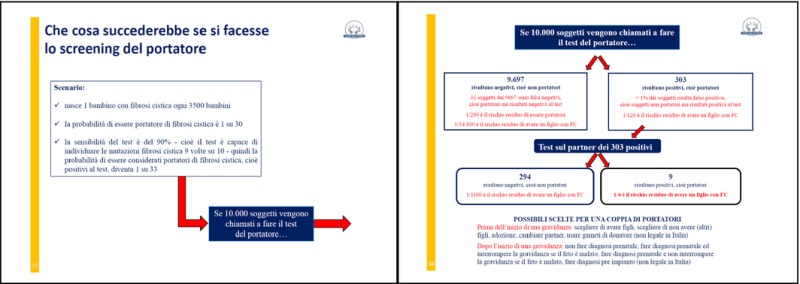
Purple: three pages regarding the test: what happens in other countries, what happens in Italy and the cost of the test.
Moreover, one page of introduction with additional references, two pages with text of knowledge ‘Check your knowledge’, one page with the glossary and one page with the list of people participant in the different committee.
Appendix 2.
What information should the health service give about tests and CF to people of child‐bearing age, so they can plan children consciously and responsibly?
It must be made clear that carriers are healthy people who will never develop the disease; two carriers have a 75% probability of having a healthy child and 25% of having a sick child. It should be specified that a negative test considerably lowers the probability of having a sick child, though this cannot be excluded completely and that, in the case of a positive test, carriers will need consultation to discuss the possible implications and alternative action.
What aspects of the test on the carrier should be highlighted?
It should first of all be clarified that the test organized by the NHS is free, but it must be taken voluntarily. The test method should be explained. It should be specified that it is a simple blood test and that a positive result does not mean that carrier has the disease. Carriers are healthy and will not develop CF, but they can transmit it. It should be explained that the test result is 85% reliable and that there is a residual risk of 0.8 (1/125) that a person whose test was negative is actually positive. It must be made clear that the test result must be viewed by a geneticist/expert and that test‐takers must not interpret the result on their own, particularly if positive. Clear assurance regarding the confidentiality of the test results must be given. It is not necessary to state that the test does not cover all the possible mutations; this is already implicit because the test is not 100% reliable, and explanations could cause confusion. It is also unnecessary to specify the test's possible psychological implications so as not to create alarm.
Which aspects of cystic fibrosis should be highlighted?
It is important to specify that CF is a genetic disease that is transmitted from both parents to their child and that it is a chronic, evolving, pervasive and debilitating disease. It should be explained in detail what it consists of, what organs are effected and how it is manifested. Information should be given on the average life expectancy and on the quality of life, including the psychological aspects of both the patient and the family (the sense of diversity, daily healthcare requirements, etc.). It should also be stressed that each case is unique.
Who should do the informing, in what context and how?
The most appropriate ways of sending screening invitations should be established by experts. However, the usual way of sending a simple letter/brochure to those eligible for screening (people of childbearing age) can be adopted in this case. It is believed that primary care doctors and, to a large extent, gynaecologists should encourage their patients to be screened. However, it is also clear that the test result must be discussed with a geneticist. It is recommended that tests be conducted in a limited number of laboratories, using a standardized analysis procedure.
Are there unknown issues that should be researched scientifically regarding the test for healthy carriers?
Research should concentrate on reducing the percentage of false‐negative tests and identifying the mutations that cause lung problems.
Note
Two hours after the beginning of the afternoon discussion, two participants, for personal reasons not related to the project, decided to leave the jury.
References
- 1. Southern KW, Munck A, Pollitt R et al A survey of newborn screening for cystic fibrosis in Europe. Journal of Cystic Fibrosis, 2007; 6: 57–65. [DOI] [PubMed] [Google Scholar]
- 2. Riordan JR, Rommens JM, Kerem B et al Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science, 1989; 245: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 3. Genetic testing for cystic fibrosis. NIH Consensus Statement, 1997; 15: 1–37. [PubMed] [Google Scholar]
- 4. Gregg AR, Simpson JL. Genetic screening for cystic fibrosis. Obstetrics and Gynecology Clinics of North America, 2002; 29: 329–340. [DOI] [PubMed] [Google Scholar]
- 5. Update on carrier screening for cystic fibrosis. ACOG Committee Opinion No. 486. Obstetrics and Gynecology, 2011; 117: 1028–1031. [DOI] [PubMed] [Google Scholar]
- 6. Grody WW, Cutting G, Klinger K et al Laboratory standards and guidelines for population‐based cystic fibrosis carrier screening. Genetics in Medicine, 2001; 3: 149–154. [DOI] [PubMed] [Google Scholar]
- 7. Castellani C, Macek M, Cassiman JJ et al Benchmark for cystic fibrosis carrier screening: a European consensus document. Journal of Cystic Fibrosis, 2010; 9: 165–178. [DOI] [PubMed] [Google Scholar]
- 8. Grody WW, Cutting GR, Watson MS. The cystic fibrosis mutation “arms race”: when less is more. Genetics in Medicine, 2007; 9: 739–744. [DOI] [PubMed] [Google Scholar]
- 9. McClaren BJ, Metcalfe SA, Amor DJ, Aitken M, Massie J. A case for cystic fibrosis carrier testing in the general population. Medical Journal of Australia, 2011; 194: 208–209. [DOI] [PubMed] [Google Scholar]
- 10. Norman R, van Gool K, Hall J, Delatycki M, Massie J. Cost‐effectiveness of carrier screening for cystic fibrosis in Australia. Journal of Cystic Fibrosis, 2012; 11: 281–287. [DOI] [PubMed] [Google Scholar]
- 11. Castellani C, Picci L, Tamanini A, Girardi P, Rizzotti P, Assael BM. Association between carrier screening and incidence of cystic fibrosis. JAMA, 2009; 302: 2573–2579. [DOI] [PubMed] [Google Scholar]
- 12. Janssens S, De Paepe A, Borry P. Attitudes of health care professionals toward carrier screening for cystic fibrosis. A review of the literature. Journal of Community Genetics, 2014; 5: 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radhakrishnan M, van Gool K, Hall J, Delatycki M, Massie J. Economic evaluation of cystic fibrosis screening: a review of the literature. Health Policy, 2008; 85: 133–147. [DOI] [PubMed] [Google Scholar]
- 14. Glasziou P, Moynihan R, Richards T, Godlee F. Too much medicine; too little care. BMJ, 2013; 347: f4247. [DOI] [PubMed] [Google Scholar]
- 15. Moynihan R, Doran E, Henry D. Disease mongering is now part of the global health debate. PLoS Medicine, 2008; 5: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tikkinen KAO, Leinonen JS, Guyatt GH, Ebrahim S, Järvinen TLN. What is a disease? Perspectives of the public, health professionals, and legislators. BMJ Open, 2012; 2: e001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Domenighetti G, Vineis P, De Pietro C, Tomada A. Ability to pay and equity in access to Italian and British National Health Services. European Journal of Public Health, 2010; 20: 500–503. [DOI] [PubMed] [Google Scholar]
- 18. Goodin RE, Dryzek JS. Deliberative impacts: the macro‐political uptake of mini‐publics. Politics & Society, 2006; 34: 219–244. [Google Scholar]
- 19. Lukensmeyer CJ, Hasselblad Torres L. Public deliberation: a manager's guide to citizen engagement, IBM Center for the Business of Government, 2006. Available at: http://www.whitehouse.gov/files/documents/ostp/opengov_inbox/ibmpubdelib.pdf, accessed 30 May 2014.
- 20. Department of Health . Choosing Health: Making Healthier Choices Easier. London: Department of Health, 2004. (UK Government White Paper). [Google Scholar]
- 21. King's Fund . Public Attitudes to Public Health Policy. London: King's Fund Publications, 2004. [Google Scholar]
- 22. Elwood P, Longley M. My health: whose responsibility? A jury decides. Journal of Epidemiology and Community Health, 2010; 64: 761–764. [DOI] [PubMed] [Google Scholar]
- 23. Lenaghan J. Involving the public in rationing decisions. The experience of citizens juries. Health Policy, 1999; 49: 45–61. [DOI] [PubMed] [Google Scholar]
- 24. The Jefferson Center . Citizens jury handbook, 2004. Available at: http://www.epfound.ge/files/citizens_jury_handbook.pdf, accessed 26 May 2014.
- 25. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens' deliberation. Health Policy, 2008; 85: 314–320. [DOI] [PubMed] [Google Scholar]
- 26. Rychetnik L, Doust J, Thomas R, Gardiner R, Mackenzie G, Glasziou PA. Community Jury on PSA screening: what do well‐informed men want the government to do about prostate cancer screening‐a qualitative analysis. BMJ Open, 2014; 4: e004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosconi P, Colombo C, Satolli R, Liberati A. PartecipaSalute, an Italian project to involve lay people, patients' associations and scientific‐medical representatives on the health debate. Health Expectations, 2007; 10: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuper R. Citizens Juries: The Hertfordshire Experience, Working Paper, University of Hertfordshire Business School, UK, 1996.
- 29. Tinetti ME, Basch E. Patients' responsibility to participate in decision making and research. JAMA, 2013; 309: 2331–2332. [DOI] [PubMed] [Google Scholar]
- 30. Richards T, Montori VM, Godlee F, Lapsley P. Let the patient revolution begin. BMJ, 2013; 346: f2614. [DOI] [PubMed] [Google Scholar]
- 31. Moynihan R. The future of medicine lies in truly shared decision making. BMJ, 2013; 346: f2789. [DOI] [PubMed] [Google Scholar]


