ABSTRACT
Gastric cancer is the fourth most common cancer and the second leading cause of cancer deaths worldwide. Additionally, it is well-known that metastatic cancer disease is a major cause of morbidity and mortality in cancer patients. Several investigations reported that HER-2 (ErbB-2 receptor) and Epstein-Barr virus (EBV) are important etiological factors in human gastric cancer, where either oncogene/oncovirus alone can derive a major event of cancer progression and metastasis via epithelial–mesenchymal transition (EMT). Herein, we discuss, for the first time, the possibility of HER-2/EBV-oncoproteins interaction in human gastric cancer initiation and/or progression.
KEYWORDS: cancer initiation and progression, EBV, gastric cancer, HER-2, oncogene/oncovirus cooperation
Gastric cancer is a major health problem worldwide, causing approximately one million deaths per year and ranking as the second leading cause of cancer-related deaths due to metastasis. Although the role of HER-2 (ErbB-2) in this tumor has already been extensively explored, and found to be frequently associated with invasion, high grade, and unfavorable prognosis, its overall prognostic role remains uncertain and appears to be stage-dependent.1 In this regard, the seminar paper by Van Cutsem et al.2 on gastric cancer elegantly discussed important parameters related to this malignancy, especially new and promising agents targeting the HER-2 receptor (as ∼20% of these cancers are HER-2 positive); Additionally, the authors highlighted the potential role of Epstein-Barr virus (EBV) in human gastric cancer especially gastroesophageal junction adenocarcinomas since 81% of these malignancies are positive for this virus in men.
HER-2 (ErbB-2, c-erbB2 or Her-2/neu), is a member of the HER-family that also includes HER-1 (Epidermal Growth Factor Receptor-EGFR, or ErbB1), HER-3 (ErbB-3) and HER-4 (ErbB-4); HER-2 is a proto-oncogene that encodes for a 185-kDa plasma membrane-bound receptor.3,4 The ErbB family belongs to transmembrane growth factor receptors defined by a glycosylated extracellular domain to which peptide growth factor ligands bind, a single transmembrane region, and a cytoplasmic domain with tyrosine kinase activity, which has a decisive role in the regulation of fundamental signaling transduction pathways in both normal and cancer cells.4,5 These receptors can be stimulated by extracellular signals and/or independent homodimerization or heterodimerization leading to the activation of downstream pathways such as mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K), phospholipase C and protein kinase C, inducing signal transduction and transcription (Fig. 1).5 As such, HER-2 gene amplification and protein overexpression are involved in the pathogenesis and progression of several human carcinomas, thus they are often considered as a poor prognostic factor.4,5 Since HER-2 is overexpressed in many epithelial malignancies including breast and gastric cancer, it has been regarded as an important potential therapeutic target for several human carcinomas especially breast.3 Accordingly, antibodies and tyrosine kinase inhibitors were developed to treat human carcinomas expressing HER-1 and HER-2 and/or HER-2 alone.5 To date, although numerous targeted agents have been tested in randomized trials, however, only trastuzumab (in HER2-positive patients) and ramucirumab (in an unselected population) are currently approved in gastric cancer management.6
Figure 1.
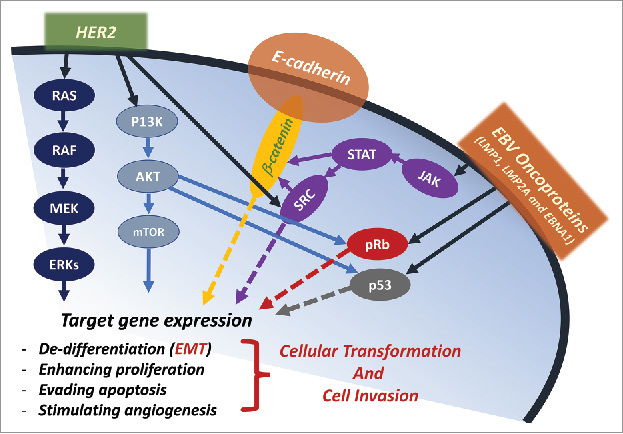
Schematic outline showing potential cooperation between HER-2 and EBV oncoproteins in human gastric cancer initiation and/or progression. We note that HER-2 and EBV oncoproteins share various downstream-signaling pathways, including SRC/β-catenin, PI3k/Akt/mTOR and RAS/MEK/ERK; thus, HER-2/EBV activation pathways could initiate cancer development and/or enhance malignancy progression via the deregulation of cell differentiation (EMT), proliferation, apoptosis and angiogenesis.
The EBV was discovered as the etiologic agent responsible of Burkitt's lymphoma, an unusual African pediatric lymphoma, in 1964, making it the first identified human tumor-causing virus.7,8 EBV is an ubiquitous human herpesvirus 4 (HHV4) that establishes latent infections in >90% of the adult population worldwide.7 This virus is composed of a linear, double-stranded DNA, protected by a 100nm in diameter icosahedral nucleocapsid composed by 162 capsomers, which is usually found in the episomal form inside cells.8 EBV genome has 172,000 bp and although it can potentially codify more than 85 proteins, only a few are well known: there are 6 nuclear antigens (EBNA 1, 2, 3A, 3B, and 3C, and EBNA-LP); 3 latent membrane proteins (LMPs 1, 2A, 2B) also known as latent genes; small non-polyadenylated RNAs, EBER 1 and 2; microRNAs (miR-BHRF1 and miR-BART); and several early lytic genes.9,10 EBV is considered an etiological factor in multiple malignancies of either lymphoid or epithelial origin, including Burkitt lymphoma, Hodgkin's lymphoma, gastric and nasopharyngeal carcinomas, suggesting its primary tropism for these tissue cells.8,10 To date, most studies have focused on EBV-encoded proteins such as the latent nuclear antigen EBNA1 and the latent membrane proteins “oncoproteins” LMP1, LMP2A and LMP2B, which have the ability to transform cells and cause gene alteration and consequently enhance cell proliferation, survival and invasion of transformed cells.11,12 However, EBV-associated tumors generally express a restricted repertoire of EBV-oncoproteins in a histological specific fashion,12 suggesting that the pathogenesis and progression of these tumors are not only regulated by these oncoproteins but also by other important factors such as EBV-encoded microRNAs, as demonstrated recently by Cai et al.13 In addition, it was revealed that EBV oncoproteins (LMP1 and EBNA1) can enhance gastric cancer progression via the initiation of the epithelial-mesenchymal transition (EMT) event by modulating PI3/Akt/GSK-β and Shc-MAPK/Erk1/Erk2 signaling pathways which can lead to nuclear accumulation of β-catenin and enhancing cancer metastasis (Fig. 1).13
As we mentioned above, it is known that activated HER-2 (hetero- and homo-dimer) proteins initiate phosphorylation events that lead to the activation of several signaling pathways, all of which are implicated in breast cancer progression.3 These pathways include STAT3, Erk1/ErK2, and PI3/Akt which can initiate EMT and consequently cancer progression and metastasis (Fig. 1).4,14 Based on the fact that oncovirus infection alone is not sufficient to induce neoplastic transformation of normal epithelial cells; the infected cells must undergo additional genetic changes and/or co-infection with another oncovirus to reach full transformation and consequently tumor formation. Therefore, we have demonstrated that HER-2 can cooperate with E6/E7 oncoproteins of HPV type 16 to induce cellular transformation of human normal oral epithelial (NOE) cells; this was accompanied by a de-localization of β-catenin from the undercoat membrane to the nucleus.15 Furthermore, we reported that cyclin D1 is the downstream target of HER-2/E6/E7 cooperation via the conversion of β-catenin's role from a cell-cell adhesion molecule to a nuclear transcriptional regulator.16 Finally, we were able to show that the cooperation effect of HER-2 with E6/E7 oncoproteins, in human oral epithelial cells, occurs via β-catenin tyrosine phosphorylation through pp60 (c-Src) kinase activation.4 On the other hand, we demonstrated that HER-2 can cooperate with E6/E7 of HPV type 16 to enhance breast tumorigenesis in vivo using double transgenic mice.4
Therefore, and based on Van Cutsem's paper and other investigations related to HER-2 and EBV in gastric cancer; in addition to our own study on HER-2/E6/E7 cooperation in human oral cells, we are proposing that HER-2 could cooperate with EBV oncoproteins in human gastric cancer development and/or progression. This initiation and enhancement of the EMT event can be concomitant with crosstalk between HER-2 and EBV signaling pathways eliciting a spectrum of genes deregulation linked with gastric carcinogenesis (Fig. 1). Meanwhile, it is important to mention that a previous study conducted by Zang et al.17 revealed that HER-2 expression in EBV-associated gastric carcinoma cases is less than EBV-negative gastric carcinomas, and none of LMP2A oncoprotein-positive cases showed a high expression of HER-2. Additionally, the same group pointed-out that overexpressing LMP2A of EBV in EBV-negative gastric cells could decrease the expression of HER-2. We believe that the data of this study need confirmation in addition to mechanistic investigation regarding the functional domain of LMP2A on the deregulation of HER2. However, and based on the large number of investigations regarding the overexpression of HER-2 and the presence of EBV in human gastric cancer especially gastroesophageal junction adenocarcinomas, we consider that HER-2 and EBV crosstalk can play an important role in human gastric carcinogenesis.
In conclusion, it is evident that HER-2 and EBV can be co-present in human gastric carcinomas, as illustrated by Van Cutsem et al.2 and others,18 which could provoke/enhance the initiation and progression of these cancers. Thus, the role of EBV and particularly the cooperation effect between its oncoporteins and HER-2 in gastric carcinogenesis needs thorough investigation. Therefore, we believe that more molecular and cellular studies, in addition to developing in vivo models, are necessary to elucidate the cooperation outcome of HER-2/EBV oncoproteins especially LMP1 and EBNA1 in this prevalent cancer, which may lead to the identification of new gene therapy targets. Finally, we think that future EBV vaccines could have a considerable impact on EBV-associated cancers and their metastatic forms,19 which are responsible for the majority of cancer-related deaths.
Funding Statement
Our research is supported by the College of Medicine and Qatar University.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Mrs. A. Kassab for her critical reading of the manuscript. We also would like to thank Dr. Asmaa Althani for her support of our work at the BRC.
References
- [1].Qi X, Liu Y, Wang W, Cai D, Li W, Hui J, Liu C, Zhao Y, Li G. Management of advanced gastric cancer: An overview of major findings from meta-analysis. Oncotarget 2016; 7(47):78180-205; https://doi.org/ 10.18632/oncotarget.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet 2016; 388(10060):2654-64; https://doi.org/ 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- [3].Yasmeen A, Bismar TA, Al Moustafa AE. ErbB receptors and epithelial-cadherin-catenin complex in human carcinomas. Future Oncol 2006; 2(6):765-81; PMID:17155902; https://doi.org/ 10.2217/14796694.2.6.765 [DOI] [PubMed] [Google Scholar]
- [4].Al Moustafa AE, Kassab A, Darnel A, Yasmeen A. High-risk HPV/ErbB-2 interaction on E-cadherin/catenin regulation in human carcinogenesis. Curr Pharm Des 2008; 14(22):2159-72; PMID:18781969; https://doi.org/ 10.2174/138161208785740216 [DOI] [PubMed] [Google Scholar]
- [5].Dokala A, Thakur SS. Extracellular region of epidermal growth factor receptor: a potential target for anti-EGFR drug discovery. Oncogene 2016; 36(17):2337-44 [DOI] [PubMed] [Google Scholar]
- [6].Fanotto V, Ongaro E, Rihawi K, Avallone A, Silvestris N, Fornaro L, Vasile E, Antonuzzo L, Leone F, Rosati G, et al.. HER-2 inhibition in gastric and colorectal cancers: tangible achievements, novel acquisitions and future perspectives. Oncotarget 2016; 7(42):69060-74; PMID:27542243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from burkitt's lymphoma. The Lancet 1964; 283(7335):702-3; https://doi.org/ 10.1016/S0140-6736(64)91524-7 [DOI] [PubMed] [Google Scholar]
- [8].Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res 2004; 10(3):803-21; PMID:14871955; https://doi.org/ 10.1158/1078-0432.CCR-0670-3 [DOI] [PubMed] [Google Scholar]
- [9].Kalla M, Göbel C, Hammerschmidt W. The lytic phase of epstein-barr virus requires a viral genome with 5-methylcytosine residues in CpG sites. J Virol 2012; 86(1):447-58; PMID:22031942; https://doi.org/ 10.1128/JVI.06314-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Neves M, Marinho-Dias J, Ribeiro J, Sousa H. Epstein-Barr virus strains and variations: Geographic or disease-specific variants? J Med Virol 2017; 89(3):373-87; PMID:27430663; https://doi.org/ 10.1002/jmv.24633 [DOI] [PubMed] [Google Scholar]
- [11].Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004; 4:757-68; PMID:15510157; https://doi.org/ 10.1038/nrc1452 [DOI] [PubMed] [Google Scholar]
- [12].Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore) 2015; 94:e792; PMID:25997049; https://doi.org/ 10.1097/MD.0000000000000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cai LM, Lyu XM, Luo WR, Cui XF, Ye YF, Yuan CC, Peng QX, Wu DH, Liu TF, Wang E, et al.. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene 2015; 34:2156-66; PMID:25347742; https://doi.org/ 10.1038/onc.2014.341 [DOI] [PubMed] [Google Scholar]
- [14].Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci (Schol Ed) 2012; 4:671-84; PMID:22202084; https://doi.org/ 10.2741/s292 [DOI] [PubMed] [Google Scholar]
- [15].Al Moustafa AE, Foulkes WD, Benlimame N, Wong A, Yen L, Bergeron J, Batist G, Alpert L, Alaoui-Jamali MA. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene 2004; 23:350-8; PMID:14724563; https://doi.org/ 10.1038/sj.onc.1207148 [DOI] [PubMed] [Google Scholar]
- [16].Al Moustafa AE, Foulkes WD, Wong A, Jallal H, Batist G, Yu Q, Herlyn M, Sicinski P, Alaoui-Jamali MA. Cyclin D1 is essential for neoplastic transformation induced by both E6/E7 and E6/E7/ErbB-2 cooperation in normal cells. Oncogene 2004; 23:5252-6; PMID:15229656; https://doi.org/ 10.1038/sj.onc.1207679 [DOI] [PubMed] [Google Scholar]
- [17].Zhang YW, Zhao XX, Tan C, Zhang ZG, Jiang Y, Chen JN, Wei HB, Xue L, Li HG, Du H, Shao CK. Epstein-Barr virus latent membrane protein 2A suppresses the expression of HER2 via a pathway involving TWIST and YB-1 in Epstein-Barr virus-associated gastric carcinomas. Oncotarget 2015; 6(1):207-20; PMID:25402957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang W, Morgan DR, Meyers MO, Dominguez RL, Martinez E, Kakudo K, Kuan PF, Banet N, Muallem H, Woodward K, et al.. Epstein-barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect Agent Cancer 2012; 7:21; PMID:22929309; https://doi.org/ 10.1186/1750-9378-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cohen JI. Epstein-barr virus vaccines. Clin Transl Immunology 2015; 4:e32; PMID:25671130; https://doi.org/ 10.1038/cti.2014.27 [DOI] [PMC free article] [PubMed] [Google Scholar]


