ABSTRACT
Contactin-2/transiently expressed axonal surface glycoprotein-1 (TAG-1) is a cell adhesion molecule belonging to the immunoglobulin superfamily (IgSF). It has six immunoglobulin-like extracellular domains and four fibronectin III-like ones, with anchoring to the cell membrane through glycosylphosphatidyl inositol. Contactin-2/TAG-1 is expressed in specific neurons transiently on the axonal surface during the fetal period. In postnatal stages, Contactin-2/TAG-1 is expressed in cerebellar granule cells, hippocampal pyramidal cells, and the juxtaparanodal regions of myelinated nerve fibers. In the embryonic nervous system, Contactin-2/TAG-1 plays important roles in axonal elongation, axonal guidance, and cellular migration. In the postnatal nervous system, it also plays an essential role in the formation of myelinated nerve fibers. Moreover, Contactin-2/TAG-1 has been linked to autoimmune diseases of the human nervous system. Taken together, Contactin-2/TAG-1 plays a central role in a variety of functions from development to disease.
KEYWORDS: axonin-1/SC2, cerebral cortex, commissural neuron, dorsal root ganglion, floor plate, juxtaparanode, L1, NrCAM, TAX-1
Introduction
Contactin-2/transiently expressed axonal surface glycoprotein-1 (TAG-1) is a cell-surface molecule belonging to the immunoglobulin superfamily (IgSF). This molecule was originally found to be recognized by the monoclonal antibody 4D7, which was raised against an antigen of the fetal rat brain; therefore, it was termed stage-specific neurite-associated protein (SNAP).1 Subsequently, this antigenic molecule was identified in the fetal rat brain/spinal cord and renamed TAG-1.2 Later, the cDNA for rat TAG-1 was generated and sequenced.3
Independent of the above reports, axonin-1/SC2 was identified in the chick.4 It was subsequently revealed that axonin-1/SC2 was a chick homolog of rat TAG-1 and human TAG-1/axonin-1 (TAX-1).5 Although the name “TAG-1” refers to the molecule found in rodents, in actuality, it is widely used for its human and chick homologues. Recently, while the unified name “Contactin-2” has come into use, familiarity with this name is weak, and this review will not use it on its own. Instead, the term Contactin-2/TAG-1 will be used when referring to the molecule found in all animal types, TAG-1 when referring to the rodent homolog, and TAX-1 and axonin-1/SC2 when referring to human and chick homologues, respectively. This distinction has been applied so that the animal type that applies to the research results can be discerned at a glance. As described later (see Fig. 2), chicken axonin-1/SC2 and rodent TAG-1 have different expression patterns in commissural axons and show some different functions. There is a possibility that they are not closely related to each other evolutionarily. Currently, the gene for all homologues has been universally named CNTN2 or Cntn2.
Figure 2.
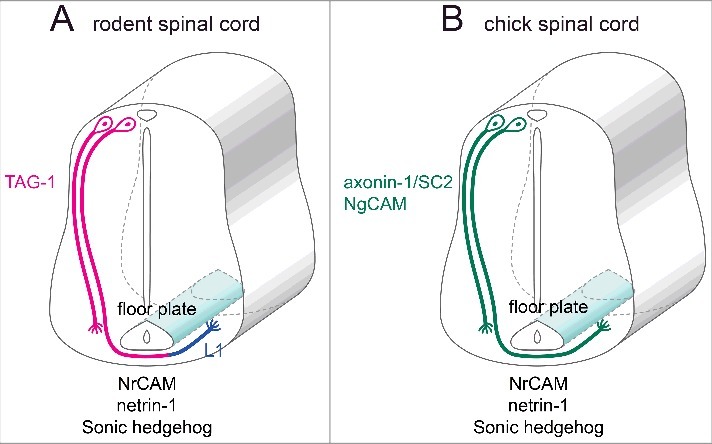
Molecules expressed in the spinal floor plate and commissural interneurons during the fetal period. (A) Molecules expressed in commissural axons and the floor plate of fetal rodents. (B) Molecules expressed in commissural axons and the floor plate of chick embryos.
Molecular structure
Domain structure and subfamily
Contactin-2/TAG-1 is a glycoprotein with a molecular weight of 135 kDa, and it is 1040 aa long in humans, rats, and mice, and 1035 aa long in the chick. Contactin-2/TAG-1 belongs to group II of the IgSF. Molecules of this group comprise multiple extracellular immunoglobulin-like domains and multiple fibronectin III-like domains and are expressed in the nervous system. Group II is further divided into subgroups, with Contactin-2/TAG-1 belonging to the Contactin subgroup. The Contactin subgroup comprises six different molecules named Contactin-1–6.
All six molecules of the Contactin subgroup, which includes Contactin-2/TAG-1, have six extracellular immunoglobulin-like domains and four fibronectin III-like domains, are anchored to the lipid bilayer of the cell membrane via glycosylphosphatidyl inositol, and are found on the membrane surface (Fig. 1A).
Figure 1.
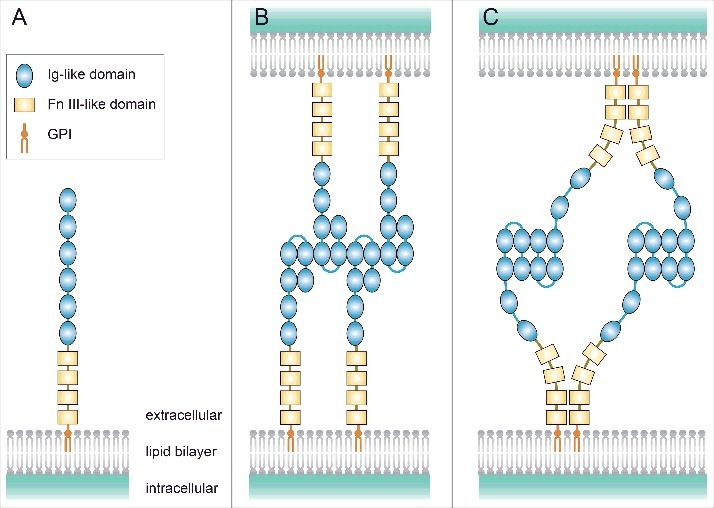
The domain structure and structural arrangements of Contactin-2/TAG-1. (A) Contactin-2/TAG-1 consists of six extracellular immunoglobulin (Ig)-like domains, four fibronectin (Fn) III-like domains, and glycosylphosphatidyl inositol (GPI) and is anchored to the lipid bilayer via GPI. (B) The zipper mode model proposed for axonin-1/SC2 in cell-to-cell interactions. (C) The four molecule mode model proposed for TAX-1 in cell-to-cell interactions.
Contactin-2/TAG-1 also includes a secreted form, and large amounts of soluble TAG-1 have been detected in the rat fetal brain.3 The β-site amyloid β precursor protein (APP)-cleaving enzyme 1 (BACE1) cleaves TAG-1 near its GPI anchor site and generates soluble TAG-1.6,7 The inhibition of BACE1 activity increases the levels of TAG-1 on the cell surface in vitro.8 As described later, TAG-1 is present on the cell surface and plays an important role in axonal growth and guidance of a variety of neurons and formation and maintenance of myelinated fibers. The surface level of TAG-1 is reduced by BACE1, which results in defects in these functions, while soluble TAG-1 may bind to cell surface molecules on other axons and in turn contribute to another axonal event. Hence, the cleavage of TAG-1 is thought to play a role in modifying the function of TAG-1 in vivo.
Structural arrangement
The crystalline structures of the N-terminus of axonin-1/SC2 and TAX-1 have been investigated (http://pdbj.org/mine/summary/1cs6, and http://pdbj.org/mine/summary/2om5, respectively).9,10 As for the mode of Contactin-2/TAG-1 in cell-to-cell interactions, the following two models have been proposed.9 The model proposed for axonin-1/SC2 is called “zipper mode,” which shows trans interactions between the second and third immunoglobulin-like domains (Fig. 1B). In contrast, the model proposed for TAX-1 is called “four molecule mode,” which shows not only trans interactions between the immunoglobulin-like domains, but also cis interactions of the fibronectin III-like domains (Fig. 1C). Additionally, Tzimourakas et al. assumed the possibility of trans interactions between fibronectin III-like domains; however, it has not been validated.11 Various binding patterns may be considered depending on the species (TAX-1, TAG-1, and axonin-1/SC2), sites (axon-axon and axon-glia), and periods.
Expression patterns in the nervous system
Expression patterns obtained by in situ hybridization staining for TAG-1 in the mouse brain (sagittal section) during the fetal periods (E11.5, E13.5, E15.5, E18.5) and postnatal periods (P4, P14, P28, P56) have been published in Allen Brain Atlas (http://www.brain-map.org/).
Contactin-2/TAG-1 is expressed in specific neurons. In particular, it has been reported to be transiently expressed on the surface of elongating axons and the nerve fibers of spinal commissural interneurons and embryonic spinal motor neurons.1 In the fetal rodent nervous system, TAG-1 expression has been observed in such locations as the cell bodies and nerve fibers of dorsal root ganglion cells (DRG), the lateral olfactory tract, the anterior commissure, the corpus callosum, and the molecular layer of the cerebellar cortex.1,12 Moreover, during the postnatal period, transient TAG-1 expression has been observed within the premigratory cells of the external granular layer in the cerebellum.3 Additionally, axonin-1/SC2 is expressed in the retinotectal projection system of chick embryos.13,14
In the nervous system of adult rodents, TAG-1 is expressed in cerebellar granule cells, olfactory bulb mitral cells, and the pyramidal cells of hippocampal Cornu Ammonis areas 1 and 3.15,16 Its expression has also been observed in the juxtaparanode of myelinated nerve fibers.17
Physiological functions
Binding proteins
TAG-1 and axonin-1/SC2 undergo homophilic binding.18-20 Moreover, studies have shown that TAX-1 binds to the IgSF member L121 and that axonin-1/SC2 binds to the neuron-glia cell adhesion molecule (NgCAM, an L1 chicken homolog).20,22,23 Furthermore, TAX-1 binds to the IgSF member Contactin-1/F3 and to the NgCAM-related cell-adhesion molecule (NrCAM);21 whereas axonin-1/SC2 also binds to NrCAM.24,25 Additionally, axonin-1/SC2 binds to the IgSF member neural cell adhesion molecule (NCAM), to neurocan and phosphacan known as the members of chondroitin sulfate proteoglycans, and to tenascin-C, a constituent of the extracellular matrix (ECM).26 One study also has shown that TAG-1/TAX-1 binds to the APP.27
The adhesion to the ECM is essential for neurons to extend their axons, which is called anchorage dependence. TAG-1 on the cell surface mediates the adhesion of axons to the ECM by binding to ECM molecules as described above. Thus, it is considered that the binding of TAG-1 and ECM molecules plays a central role in axonal growth and guidance.
Signaling cascade
Although Contactin-2/TAG-1 does not possess a transmembrane domain, it is associated with intracellular signaling cascades. Analyses of neurons from the cerebral cortex of mouse embryos have shown that TAG-1 controls neuron polarization via Lyn, a Src family tyrosine kinase.28 Similarly, in cerebellar granule cells of rat embryos, Lyn mediates the transmission of TAG-1 signals into cells.29 Furthermore, it is reported that Fyn, another Src family tyrosine kinase, mediates the transmission of axonin-1/SC2 signals into cells in the chick embryo.30 A recent report has shown that the receptor for activated C kinase 1 (RACK1) interacts with TAG-1.31 This study indicates that TAG-1 may mediate the effect of RACK1 on the growth and differentiation of glioma cells. Future research on signal cascades of TAG-1 is required.
Function in the fetal nervous system
The floor plate of the spinal cord secretes axonal guidance factors such as netrin-1 and sonic hedgehog to attract the axonal projections of spinal commissural interneurons. Their axons approach the ventral midline of the spinal floor plate, then pass through it to project toward the contralateral side.32 In fetal rodents, the axons of commissural neurons strongly express TAG-1 on their surface when approaching the floor plate. Once the commissural axons pass through the floor plate, however, TAG-1 expression fades out and L1 is expressed instead (Fig. 2A).2 In contrast, the axons of spinal commissural neurons in chick embryos express axonin-1/SC2 and NgCAM even after passing through the floor plate, exhibiting a slightly different expression pattern from that of rodents (Fig. 2B). Yet, although axonin-1/SC2 is involved in the fasciculation and guidance of commissural axons, it is not involved in axonal elongation.25,33 Moreover, to allow commissural axons to pass through the floor plate, axonin-1/SC2 on the surface of these axons is required to interact with NrCAM on the surface of the floor plate cells (Fig. 2B).25,34,35
Contactin-2/TAG-1, which is expressed on DRG axonal surfaces during the fetal period, is known to be involved in the elongation,3,36 fasciculation,36,37 and guidance37-41 of DRG axons. Additionally, the elongation of DRG axons is promoted by the homophilic binding of TAG-1 on the axonal surface not only to TAG-1 in the ECM but also to L1/NgCAM and β1 integrin.18,19,22 Contact-mediated interactions between axonin-1/SC2 expressed on the surface of DRG axons (afferent fibers) and other members of the IgSF molecules expressed in the spinal cord (NgCAM, NrCAM, and F11) are essential for guiding DRG axons to innervate specific target layers in the spinal cord.38 Furthermore, Contactin-2/TAG-1 on the DRG axonal surface is involved in the reception of chemorepellents secreted by the notochord or ventral spinal cord.37,39-41 Moreover, TAG-1 regulates the responsiveness of DRG axons to the axon repellent semaphorin 3A through endocytosis of its receptor complex (L1/Neuropilin-1).41,42
In the cerebral cortex of mouse embryos, TAG-1 mediates interactions between mature axons (pioneer axons) and immature neurons, and plays an important role in the axonal formation of the latter.28
TAG-1 is also important for the migration of various nerve cells. In the cerebral cortex of mouse embryos, neural stem cells are long and narrow; however, in TAG-1-deficient mouse embryos, these cells are shorter and their nuclei become immobile.43 Accordingly, the formation of the cerebral cortex layer is disorganized.43 Moreover, analysis of TAG-1-deficient fetal mice has shown that TAG-1 deficiency causes apoptosis of cells migrating to the surface layer and shrinkage of the lateral reticular nuclei in the caudal medulla.44 However, it has been suggested that TAG-1 is not involved in the migration of GABAergic interneurons from ganglionic eminences to the cerebral cortex.44
In the cerebellum of mouse embryos, TAG-1 is expressed on the surface of cerebellar granulocyte precursor cells, and prevents their premature differentiation.45 In addition, axonin-1/SC2 is essential for guiding the parallel fibers of cerebellar granule cells in chicks, but is not necessary for their elongation.46
Function in the adult nervous system
In the adult rodent nervous system, TAG-1 is expressed in both axons and the myelin sheath. TAG-1 is known to interact with both the contactin-associated protein-like 2 (Caspr2) and K+ channel via its immunoglobulin-like domains (Fig. 3).11 In the juxtaparanode region, TAG-1 forms cis-bonds with Caspr2 to create heterodimers on the axonal side, which in turn complex with TAG-1 on the myelin side (Fig. 3).17,47-49 These complexes are necessary for K+ channel clustering at the juxtaparanode.48,49 TAG-1 thus plays an essential role in the formation and maintenance of myelinated nerve fibers. Besides its role at the juxtaparanode, TAG-1 also co-localizes with Caspr2 at synapses.50
Figure 3.
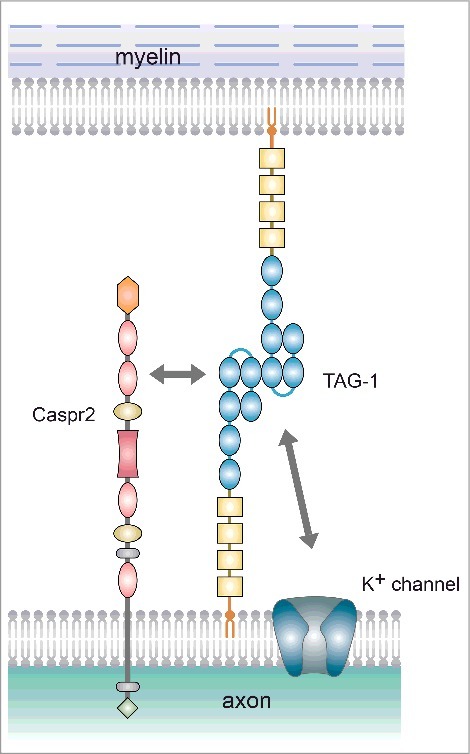
Molecular interactions at the juxtaparanode. At the juxtaparanode, TAG-1 is present both on the axonal and myelin sides, and forms a complex by binding to Caspr2 (Trans binding between TAG-1 molecules has been shown with the zipper mode model.).
Association with pathologies
Meinl's group has searched for targets of the autoimmune response in patients with multiple sclerosis (MS), an autoimmune disease of the central nervous system (CNS), and identified that TAX-1 is a target protein of autoantibodies in patients with MS.51
As described above, TAG-1/TAX-1 is a ligand for APP. The binding of TAG-1 and APP triggers the γ-secretase-dependent release of APP intracellular domain (AICD), resulting in the suppression of mouse neurogenesis.27 More recently, in vitro experiments have revealed that TAG-1 inhibits TGFβ2-induced cell death of neurons by reducing the binding between TGFβ2 and APP.52 Based on the common understanding that AICD leads to amyloid β formation and that the neuronal cell death could result in progression of Alzheimer's disease (AD), it may be inferred that TAG-1/TAX-1 affects the pathogenesis of AD.
TAX-1 is reported to be overexpressed in malignant gliomas.53 More recently, comprehensive bioinformatics analyses have shown that TAX-1 is linked to the oligodendroglioma to the highest degree.54 As described above, in vitro experiments have further shown that TAX-1 is involved in the growth and differentiation of glioma cells.31 Thus, the determination of Contactin-2/TAG-1 signal cascades may be essential for therapeutic approaches that target these diseases.
Genetic mutations
Genetic mutations in humans
TAX-1 has been mapped to chromosome 1 (1q32).55 SNP analysis of genes linked to chronic inflammatory demyelinating polyneuropathy (CIDP), an autoimmune disease of the peripheral nervous system, has shown that the TAX-1 gene affects CIDP's refractoriness and resistance to treatment.56,57 Furthermore, The TAX-1 gene has also been reported to be a gene responsible for cortical tremors and epilepsy.58
TAG-1-deficient mouse established in 2001 (C57/BL/6)
A Japanese research group has engineered a TAG-1-deficient mouse by deleting exons 2 through 5 of the TAG-1 gene.59 Compared to wild-type mice, these mice show no major abnormalities in appearance or brain tissue structure. However, an increased number of adenosine receptors in the hippocampus, susceptibility to events such as convulsive seizures, and other functional abnormalities of the nervous system are observed.59
Moreover, K+ channels and Caspr2 expression levels are reduced in the sciatic nerve fibers, and behavioral experiments revealed impairments in learning and memory, of these deficient mice.60 Furthermore, incomplete myelin formation is observed in the retinal ganglion cell axons of these deficient mice.61 Additionally, in these deficient mice the number of olfactory bulb mitral cells is reduced and abnormalities in olfactory behavior are clearly observed.62
TAG-1-deficient mouse established in 2003 (C57/BL/6J)
A British research group has engineered a TAG-1-deficient mouse by deleting exons 2 through 6 of the TAG-1 gene.48 These deficient mice are born at the Mendelian rate and show no obvious differences from wild-type mice. Morphological abnormalities similar to those in the TAG-1-deficient mice established by the Japanese group have been observed upon analysis of CNS tissues.48 Moreover, these mice have lower metabolic function than wild-type mice.63
Conclusions
Contactin-2/TAG-1 has been one of the best-known cell adhesion molecules for three decades in our field. Despite its importance, the comprehensive review that covers a wide range of its role in the nervous system has not yet been written. In this review, I first presented an overview of the role of Contactin-2/TAG-1 in the nervous system. In comparison to Contactin-1/F3, the signal cascade of Contactin-2/TAG-1 has not yet been elucidated in detail. I hope it will be revealed in future research. As mentioned in this review, Contactin-2/TAG-1 has been reported to be involved in various diseases such as gliomas, AD, MS, CIDP, and epilepsy. Future findings on the signal cascade of Contactin-2/TAG-1 may provide a potential target for therapeutic intervention of these diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by JSPS KAKENHI Grant Nos. JP15K08148 and JP15H02765 from the program Grants-in-Aid for Scientific Research of the MEXT, Japan to T.M.
References
- [1].Yamamoto M, Boyer AM, Crandall JE, Edwards M, Tanaka H. Distribution of stage-specific neurite-associated proteins in the developing murine nervous system recognized by a monoclonal antibody. J Neurosci 1986; 6:3576-94; PMID:3794790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron 1988; 1:105-16; PMID:3272160; https://doi.org/ 10.1016/0896-6273(88)90194-8 [DOI] [PubMed] [Google Scholar]
- [3].Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell 1990; 61:157-70; PMID:2317872; https://doi.org/ 10.1016/0092-8674(90)90223-2 [DOI] [PubMed] [Google Scholar]
- [4].Zuellig RA, Rader C, Schroeder A, Kalousek MB, Von Bohlen und Halbach F, Osterwalder T, Inan C, Stoeckli ET, Affolter HU, Fritz A, et al.. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem 1992; 204:453-63; PMID:1311675; https://doi.org/ 10.1111/j.1432-1033.1992.tb16655.x [DOI] [PubMed] [Google Scholar]
- [5].Hasler TH, Rader C, Stoeckli ET, Zuellig RA, Sonderegger P. cDNA cloning, structural features, and eucaryotic expression of human TAG-1/axonin-1. Eur J Biochem 1993; 211:329-39; PMID:8425542; https://doi.org/ 10.1111/j.1432-1033.1993.tb19902.x [DOI] [PubMed] [Google Scholar]
- [6].Zhou L, Barão S, Laga M, Bockstael K, Borgers M, Gijsen H, Annaert W, Moechars D, Mercken M, Gevaert K, et al.. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J Biol Chem 2012; 287:25927-40; PMID:22692213; https://doi.org/ 10.1074/jbc.M112.377465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gautam V, D'Avanzo C, Hebisch M, Kovacs DM, Kim DY. BACE1 activity regulates cell surface contactin-2 levels. Mol Neurodegener 2014; 9:4; PMID:24405708; https://doi.org/ 10.1186/1750-1326-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, Volbracht C, Schepers U, Imhof A, Hoffmeister A, et al.. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J 2012; 31:3157-68; PMID:22728825; https://doi.org/ 10.1038/emboj.2012.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell 2000; 101:425-33; PMID:10830169; https://doi.org/ 10.1016/S0092-8674(00)80852-1 [DOI] [PubMed] [Google Scholar]
- [10].Mörtl M, Sonderegger P, Diederichs K, Welte W. The crystal structure of the ligand-binding module of human TAG-1 suggests a new mode of homophilic interaction. Protein Sci 2007; 16:2174-83; PMID:17766378; https://doi.org/ 10.1110/ps.072802707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tzimourakas A, Giasemi S, Mouratidou M, Karagogeos D. Structure-function analysis of protein complexes involved in the molecular architecture of juxtaparanodal regions of myelinated fibers. Biotechnol J 2007; 2:577-83; PMID:17405182; https://doi.org/ 10.1002/biot.200700023 [DOI] [PubMed] [Google Scholar]
- [12].Wolfer DP, Henehan-Beatty A, Stoeckli ET, Sonderegger P, Lipp HP. Distribution of TAG-1/axonin-1 in fibre tracts and migratory streams of the developing mouse nervous system. J Comp Neurol 1994; 345:1-32; PMID:8089271; https://doi.org/ 10.1002/cne.903450102 [DOI] [PubMed] [Google Scholar]
- [13].Morino P, Buchstaller A, Giger R, Sonderegger P, Rager G. Differential expression of the mRNAs of the axonal glycoproteins axonin-1 and NgCAM in the developing chick retina. Brain Res Dev Brain Res 1996; 91:252-9; PMID:8852376; https://doi.org/ 10.1016/0165-3806(95)00184-0 [DOI] [PubMed] [Google Scholar]
- [14].Rager G, Morino P, Schnitzer J, Sonderegger P. Expression of the axonal cell adhesion molecules axonin-1 and Ng-CAM during the development of the chick retinotectal system. J Comp Neurol 1996; 365:594-609; PMID:8742305; https://doi.org/ 10.1002/(SICI)1096-9861(19960219)365:4%3c594::AID-CNE7%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- [15].Yoshihara Y, Kawasaki M, Tamada A, Nagata S, Kagamiyama H, Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1, and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J Neurobiol 1995; 28:51-69; PMID:8586965; https://doi.org/ 10.1002/neu.480280106 [DOI] [PubMed] [Google Scholar]
- [16].Wolfer DP, Giger RJ, Stagliar M, Sonderegger P, Lipp HP. Expression of the axon growth-related neural adhesion molecule TAG-1/axonin-1 in the adult mouse brain. Anat Embryol (Berl) 1998; 197:177-85; PMID:9543336; https://doi.org/ 10.1007/s004290050129 [DOI] [PubMed] [Google Scholar]
- [17].Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci 2002; 22:3016-24; PMID:11943804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Felsenfeld DP, Hynes MA, Skoler KM, Furley AJ, Jessell TM. TAG-1 can mediate homophilic binding, but neurite outgrowth on TAG-1 requires an L1-like molecule and beta 1 integrins. Neuron 1994; 12:675-90; PMID:7512353; https://doi.org/ 10.1016/0896-6273(94)90222-4 [DOI] [PubMed] [Google Scholar]
- [19].Rader C, Stoeckli ET, Ziegler U, Osterwalder T, Kunz B, Sonderegger P. Cell-cell adhesion by homophilic interaction of the neuronal recognition molecule axonin-1. Eur J Biochem 1993; 215:133-41; PMID:8344273; https://doi.org/ 10.1111/j.1432-1033.1993.tb18015.x [DOI] [PubMed] [Google Scholar]
- [20].Kunz S, Spirig M, Ginsburg C, Buchstaller A, Berger P, Lanz R, Rader C, Vogt L, Kunz B, Sonderegger P. Neurite fasciculation mediated by complexes of axonin-1 and Ng cell adhesion molecule. J Cell Biol 1998; 143:1673-90; PMID:9852159; https://doi.org/ 10.1083/jcb.143.6.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pavlou O, Theodorakis K, Falk J, Kutsche M, Schachner M, Faivre-Sarrailh C, Karagogeos D. Analysis of interactions of the adhesion molecule TAG-1 and its domains with other immunoglobulin superfamily members. Mol Cell Neurosci 2002; 20:367-81; PMID:12139915; https://doi.org/ 10.1006/mcne.2002.1105 [DOI] [PubMed] [Google Scholar]
- [22].Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4). J Cell Biol 1991; 115:1113-26; PMID:1720120; https://doi.org/ 10.1083/jcb.115.4.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buchstaller A, Kunz S, Berger P, Kunz B, Ziegler U, Rader C, Sonderegger P. Cell adhesion molecules NgCAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol 1996; 135:1593-607; PMID:8978825; https://doi.org/ 10.1083/jcb.135.6.1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Suter DM, Pollerberg GE, Buchstaller A, Giger RJ, Dreyer WJ, Sonderegger P. Binding between the neural cell adhesion molecules axonin-1 and Nr-CAM/Bravo is involved in neuron-glia interaction. J Cell Biol 1995; 131:1067-81; PMID:7490283; https://doi.org/ 10.1083/jcb.131.4.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fitzli D, Stoeckli ET, Kunz S, Siribour K, Rader C, Kunz B, Kozlov SV, Buchstaller A, Lane RP, Suter DM, et al.. A direct interaction of axonin-1 with NgCAM-related cell adhesion molecule (NrCAM) results in guidance, but not growth of commissural axons. J Cell Biol 2000; 149:951-68; PMID:10811834; https://doi.org/ 10.1083/jcb.149.4.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Milev P, Maurel P, Häring M, Margolis RK, Margolis RU. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and N-CAM. J Biol Chem 1996; 271:15716-23; PMID:8663515; https://doi.org/ 10.1074/jbc.271.26.15716 [DOI] [PubMed] [Google Scholar]
- [27].Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, Xu RX, Bagnard D, Schachner M, Furley AJ, et al.. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol 2008; 10:283-94; PMID:18278038; https://doi.org/ 10.1038/ncb1690 [DOI] [PubMed] [Google Scholar]
- [28].Namba T, Kibe Y, Funahashi Y, Nakamuta S, Takano T, Ueno T, Shimada A, Kozawa S, Okamoto M, Shimoda Y, et al.. Pioneering axons regulate neuronal polarization in the developing cerebral cortex. Neuron 2014; 81:814-29; PMID:24559674; https://doi.org/ 10.1016/j.neuron.2013.12.015 [DOI] [PubMed] [Google Scholar]
- [29].Kasahara K, Watanabe K, Takeuchi K, Kaneko H, Oohira A, Yamamoto T, Sanai Y. Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts. J Biol Chem 2000; 275:34701-9; PMID:10944523; https://doi.org/ 10.1074/jbc.M003163200 [DOI] [PubMed] [Google Scholar]
- [30].Kunz S, Ziegler U, Kunz B, Sonderegger P. Intracellular signaling is changed after clustering of the neural cell adhesion molecules axonin-1 and NgCAM during neurite fasciculation. J Cell Biol 1996; 135:253-67; PMID:8858178; https://doi.org/ 10.1083/jcb.135.1.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yan Y, Jiang Y. RACK1 affects glioma cell growth and differentiation through the CNTN2-mediated RTK/Ras/MAPK pathway. Int J Mol Med 2016; 37:251-7; PMID:26718491 [DOI] [PubMed] [Google Scholar]
- [32].Nawabi H, Castellani V. Axonal commissures in the central nervous system: how to cross the midline? Cell Mol Life Sci 2011; 68:2539-53; PMID:21538161; https://doi.org/ 10.1007/s00018-011-0691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron 1995; 14:1165-79; PMID:7541632; https://doi.org/ 10.1016/0896-6273(95)90264-3 [DOI] [PubMed] [Google Scholar]
- [34].Stoeckli ET, Sonderegger P, Pollerberg GE, Landmesser LT. Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron 1997; 18:209-21; PMID:9052792; https://doi.org/ 10.1016/S0896-6273(00)80262-7 [DOI] [PubMed] [Google Scholar]
- [35].Lustig M, Sakurai T, Grumet M. Nr-CAM promotes neurite outgrowth from peripheral ganglia by a mechanism involving axonin-1 as a neuronal receptor. Dev Biol 1999; 209:340-51; PMID:10328925; https://doi.org/ 10.1006/dbio.1999.9250 [DOI] [PubMed] [Google Scholar]
- [36].Stoeckli ET, Kuhn TB, Duc CO, Ruegg MA, Sonderegger P. The axonally secreted protein axonin-1 is a potent substratum for neurite growth. J Cell Biol 1991; 112:449-55; PMID:1991792; https://doi.org/ 10.1083/jcb.112.3.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Masuda T, Okado N, Shiga T. The involvement of axonin-1/SC2 in mediating notochord-derived chemorepulsive activities for dorsal root ganglion neurites. Dev Biol 2000; 224:112-21; PMID:10926753; https://doi.org/ 10.1006/dbio.2000.9813 [DOI] [PubMed] [Google Scholar]
- [38].Perrin FE, Rathjen FG, Stoeckli ET. Distinct subpopulations of sensory afferents require F11 or axonin-1 for growth to their target layers within the spinal cord of the chick. Neuron 2001; 30:707-23; PMID:11430805; https://doi.org/ 10.1016/S0896-6273(01)00315-4 [DOI] [PubMed] [Google Scholar]
- [39].Masuda T, Tsuji H, Taniguchi M, Yagi T, Tessier-Lavigne M, Fujisawa H, Okado N, Shiga T. Differential non-target-derived repulsive signals play a critical role in shaping initial axonal growth of dorsal root ganglion neurons. Dev Biol 2003; 254:289-302; PMID:12591248; https://doi.org/ 10.1016/S0012-1606(02)00087-8 [DOI] [PubMed] [Google Scholar]
- [40].Masuda T, Fukamauchi F, Takeda Y, Fujisawa H, Watanabe K, Okado N, Shiga T. Developmental regulation of notochord-derived repulsion for dorsal root ganglion axons. Mol Cell Neurosci 2004; 25:217-27; PMID:15019939; https://doi.org/ 10.1016/j.mcn.2003.10.005 [DOI] [PubMed] [Google Scholar]
- [41].Law CO, Kirby RJ, Aghamohammadzadeh S, Furley AJ. The neural adhesion molecule TAG-1 modulates responses of sensory axons to diffusible guidance signals. Development 2008; 135:2361-71; PMID:18550718; https://doi.org/ 10.1242/dev.009019 [DOI] [PubMed] [Google Scholar]
- [42].Dang P, Smythe E, Furley AJ. TAG1 regulates the endocytic trafficking and signaling of the semaphorin3A receptor complex. J Neurosci 2012; 32:10370-82; PMID:22836270; https://doi.org/ 10.1523/JNEUROSCI.5874-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Okamoto M, Namba T, Shinoda T, Kondo T, Watanabe T, Inoue Y, Takeuchi K, Enomoto Y, Ota K, Oda K, et al.. TAG-1-assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat Neurosci 2013; 16:1556-66; PMID:24056697; https://doi.org/ 10.1038/nn.3525 [DOI] [PubMed] [Google Scholar]
- [44].Denaxa M, Kyriakopoulou K, Theodorakis K, Trichas G, Vidaki M, Takeda Y, Watanabe K, Karagogeos D. The adhesion molecule TAG-1 is required for proper migration of the superficial migratory stream in the medulla but not of cortical interneurons. Dev Biol 2005; 288:87-99; PMID:16225856; https://doi.org/ 10.1016/j.ydbio.2005.09.021 [DOI] [PubMed] [Google Scholar]
- [45].Xenaki D, Martin IB, Yoshida L, Ohyama K, Gennarini G, Grumet M, Sakurai T, Furley AJ. F3/contactin and TAG1 play antagonistic roles in the regulation of sonic hedgehog-induced cerebellar granule neuron progenitor proliferation. Development 2011; 138:519-29; PMID:21205796; https://doi.org/ 10.1242/dev.051912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Baeriswyl T, Stoeckli ET. Axonin-1/TAG-1 is required for pathfinding of granule cell axons in the developing cerebellum. Neural Dev 2008; 3:7; PMID:18346270; https://doi.org/ 10.1186/1749-8104-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 1999; 24:1037-47; PMID:10624965; https://doi.org/ 10.1016/S0896-6273(00)81049-1 [DOI] [PubMed] [Google Scholar]
- [48].Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, et al.. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 2003; 162:1149-60; PMID:12963709; https://doi.org/ 10.1083/jcb.200305018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, et al.. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol 2003; 162:1161-72; PMID:12975355; https://doi.org/ 10.1083/jcb.200305078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, et al.. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet 2008; 82:165-73; PMID:18179895; https://doi.org/ 10.1016/j.ajhg.2007.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Derfuss T, Parikh K, Velhin S, Braun M, Mathey E, Krumbholz M, Kümpfel T, Moldenhauer A, Rader C, Sonderegger P, et al.. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc Natl Acad Sci U S A 2009; 106:8302-7; PMID:19416878; https://doi.org/ 10.1073/pnas.0901496106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tachi N, Hashimoto Y, Nawa M, Matsuoka M. TAG-1 is an inhibitor of TGFβ2-induced neuronal death via amyloid β precursor protein. Biochem Biophys Res Commun 2010; 394:119-25; PMID:20184861; https://doi.org/ 10.1016/j.bbrc.2010.02.127 [DOI] [PubMed] [Google Scholar]
- [53].Rickman DS, Tyagi R, Zhu XX, Bobek MP, Song S, Blaivas M, Misek DE, Israel MA, Kurnit DM, Ross DA, et al.. The gene for the axonal cell adhesion molecule TAX-1 is amplified and aberrantly expressed in malignant gliomas. Cancer Res 2001; 61:2162-8; PMID:11280781 [PubMed] [Google Scholar]
- [54].Yu F, Fu WM. Identification of differential splicing genes in gliomas using exon expression profiling. Mol Med Rep 2015; 11:843-50; PMID:25351872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kenwrick S, Leversha M, Rooke L, Hasler T, Sonderegger P. Localization of the human TAX-1 gene to 1q32.1: a region implicated in microcephaly and Van der Woude syndrome. Hum Mol Genet 1993; 2:1461-2; PMID:8242070; https://doi.org/ 10.1093/hmg/2.9.1461 [DOI] [PubMed] [Google Scholar]
- [56].Iijima M, Tomita M, Morozumi S, Kawagashira Y, Nakamura T, Koike H, Katsuno M, Hattori N, Tanaka F, Yamamoto M, et al.. Single nucleotide polymorphism of TAG-1 influences IVIg responsiveness of Japanese patients with CIDP. Neurology 2009; 73:1348-52; PMID:19776380; https://doi.org/ 10.1212/WNL.0b013e3181bd1139 [DOI] [PubMed] [Google Scholar]
- [57].Iijima M, Koike H, Katsuno M, Sobue G. Polymorphism of transient axonal glycoprotein-1 in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 2011; 16:52-5; PMID:21696500; https://doi.org/ 10.1111/j.1529-8027.2011.00308.x [DOI] [PubMed] [Google Scholar]
- [58].Stogmann E, Reinthaler E, Eltawil S, El Etribi MA, Hemeda M, El Nahhas N, Gaber AM, Fouad A, Edris S, Benet-Pages A, et al.. Autosomal recessive cortical myoclonic tremor and epilepsy: association with a mutation in the potassium channel associated gene CNTN2. Brain 2013; 136:1155-60; PMID:23518707; https://doi.org/ 10.1093/brain/awt068 [DOI] [PubMed] [Google Scholar]
- [59].Fukamauchi F, Aihara O, Wang YJ, Akasaka K, Takeda Y, Horie M, Kawano H, Sudo K, Asano M, Watanabe K, et al.. TAG-1-deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem Biophys Res Commun 2001; 281:220-6; PMID:11178983; https://doi.org/ 10.1006/bbrc.2001.4334 [DOI] [PubMed] [Google Scholar]
- [60].Savvaki M, Panagiotaropoulos T, Stamatakis A, Sargiannidou I, Karatzioula P, Watanabe K, Stylianopoulou F, Karagogeos D, Kleopa KA. Impairment of learning and memory in TAG-1 deficient mice associated with shorter CNS internodes and disrupted juxtaparanodes. Mol Cell Neurosci 2008; 39:478-90; PMID:18760366; https://doi.org/ 10.1016/j.mcn.2008.07.025 [DOI] [PubMed] [Google Scholar]
- [61].Chatzopoulou E, Miguez A, Savvaki M, Levasseur G, Muzerelle A, Muriel MP, Goureau O, Watanabe K, Goutebroze L, Gaspar P, et al.. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J Neurosci 2008; 28:7624-36; PMID:18650339; https://doi.org/ 10.1523/JNEUROSCI.1103-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bastakis GG, Savvaki M, Stamatakis A, Vidaki M, Karagogeos D. Tag1 deficiency results in olfactory dysfunction through impaired migration of mitral cells. Development 2015; 142:4318-428; PMID:26525675; https://doi.org/ 10.1242/dev.123943 [DOI] [PubMed] [Google Scholar]
- [63].Buchner DA, Geisinger JM, Glazebrook PA, Morgan MG, Spiezio SH, Kaiyala KJ, Schwartz MW, Sakurai T, Furley AJ, Kunze DL, et al.. The juxtaparanodal proteins CNTNAP2 and TAG1 regulate diet-induced obesity. Mamm Genome 2012; 23:431-42; PMID:22752552; https://doi.org/ 10.1007/s00335-012-9400-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


