Abstract
Objective
To determine if a neuroplasticity educational explanation for a manual therapy technique will produce a different outcome compared to a traditional mechanical explanation.
Methods
Sixty-two patients with chronic low back pain (CLBP) were recruited for the study. Following consent, demographic data were obtained as well as pain ratings for low back pain (LBP) and leg pain (Numeric Pain Rating Scale), disability (Oswestry Disability Index), fear-avoidance (Fear-Avoidance-Beliefs Questionnaire), forward flexion (fingertips-to-floor), and straight leg raise (SLR) (inclinometer). Patients were then randomly allocated to receive one of two explanations (neuroplasticity or mechanical), a manual therapy technique to their lumbar spine, followed by post-intervention measurements of LBP, leg pain, forward flexion, and SLR.
Results
Sixty-two patients (female 35 [56.5%]), with a mean age of 60.1 years and mean duration of 9.26 years of CLBP participated in the study. There were no statistically significant interactions for LBP (p = .325), leg pain (p = .172), and trunk flexion (p = .818) between the groups, but SLR showed a significant difference in favor of the neuroplasticity explanation (p = .041). Additionally, the neuroplasticity group were 7.2 times (95% confidence interval = 1.8–28.6) more likely to improve beyond the MDC on the SLR than participants in the mechanical group.
Discussion
The results of this study show that a neuroplasticity explanation, compared to a traditional biomechanical explanation, resulted in a measureable difference in SLR in patients with CLBP when receiving manual therapy. Future studies need to explore if the increase in SLR correlated to changes in cortical maps of the low back.
Keywords: Pain, brain, plasticity, education, manual therapy, straight leg raise, remapping
Introduction
In the last two decades, the profession of physical therapy (PT), including manual therapy, has seen a significant increase in the interest in pain science.[1,2] Original pain science interest centered on the understanding of pain biology and physiology,[3,4] clinical reasoning incorporating pain mechanisms[4–6] and ultimately, pain neuroscience education.[7–9] By far, the vast majority of this research has focused on chronic low back pain (CLBP).[8–10] In recent years, with the advances in brain scan technology,[11] the focus has shifted toward a greater understanding of the structural and functional changes in the brain of someone suffering from CLBP.[12]
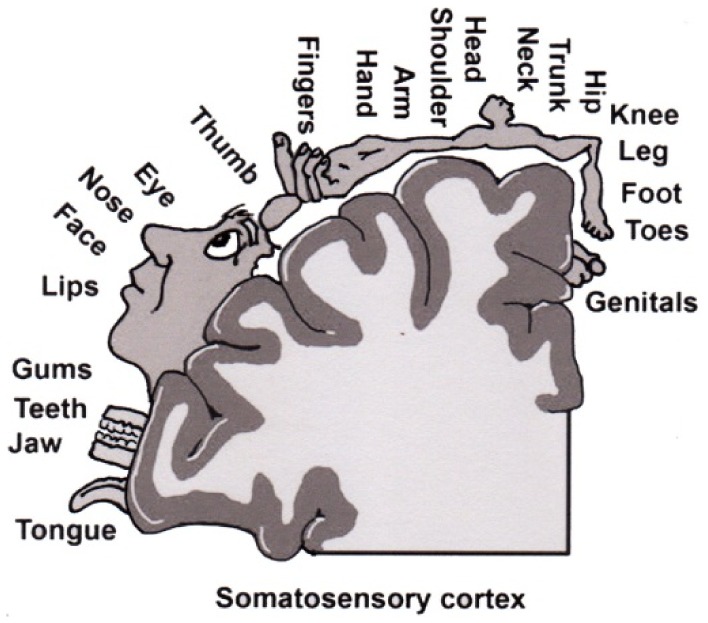
It is now well established that the physical body of a person is represented in the brain by a network of neurons.[11,13–15] This representation refers to the pattern of activity that is evoked when a particular body part is stimulated. The most famous area of the brain associated with representation is the primary somatosensory cortex (S1).[11,13–15] What is interesting is that these neuronal representations of body parts are dynamically maintained and it has been shown that patients with chronic pain display different S1 representations than people with no pain.[12,16–20] The interesting phenomenon associated with cortical restructuring is the fact that the body maps expand or contract, in essence increasing or decreasing the body map representation in the brain. Furthermore, these changes in shape and size of body maps seem to correlate to increased pain and disability.[12,21] Various factors have been linked to the development of this altered cortical representation of body maps in S1 including neglect and decreased use of the painful body part.[22] This reorganization of body maps occurs quickly. It has been shown that when four fingers are webbed together for 30 min, cortical maps associated with the fingers change.[15] This finding has significant clinical importance as it underscores the importance of strategies such as movement, tactile, and visual stimulation of the central nervous system and brain as a means to help maintain S1 representation.[23,24]
In manual therapy, traditional biomechanical models, used to explain to patients a proposed treatment or efficacy of a certain technique or approach, have focused heavily on biomechanical and anatomical models.[25–28] These models would imply that injury, disease, and muscle guarding may lead to altered movement patterns, asymmetrical loading, and resultant pain and dysfunction.[25–28] These biomechanical models have come under scrutiny, partly due to the advances in other fields such as use of diagnostic ultrasound, spinal imaging, and brain scans.[29–31] These advances, with the quest of understanding how a manual technique such as a spinal manipulation or mobilization results in increased range of motion or decreased pain and disability, have led to exciting ‘new’ areas of endeavor. For example, it is now well understood that immediately following a lumbar spine manipulation in someone with low back pain (LBP), there is an immediate neurophysiological effect on the muscles in the affected area.[32,33] Additionally, manual techniques result in endogenous mechanisms in the central nervous system and brain, thus mediating the pain experience.[31,34,35] Findings like these are exciting new frontiers to understand how and why a manual therapy approach helps people in pain. Given our knowledge of rapidly changing functional maps of the human body in pain, especially CLBP, it could be argued that a manual therapy technique may indeed also be seen as a form of sensory retraining,[23,24] apart from the neuromuscular and endogenous effects previously described. For example, in a recent case series of patients with CLBP, researchers asked patients to identify ‘where they were being touched’ on their low back (for 5 min), and immediately following the sensory discrimination session, the group’s mean pain rating for LBP decreased by 1.91, while forward flexion improved by 4.82 cm, exceeding the minimal detectable change (MDC) of 4.5 cm.[24] The aim of this study was to see if such a neuroplasticity explanation, compared to a traditional biomechanical explanation, would result in superior results in patients with CLBP in regards to pain and movement.
Methodology
Study design
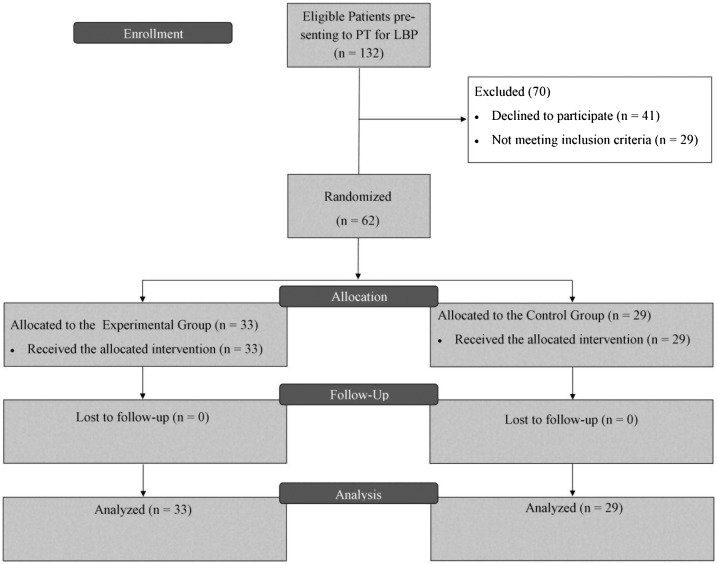
Institutional Review Board approval was obtained for the study. The study was registered as a clinical trial (NCT02757378). This was a randomized clinical trial (RCT) where participants were randomly assigned to either an experimental group (EG) or control group (CG) (Figure 1). All patients acknowledged their understanding and willingness to participate by providing signed consent. Participants were informed that the study aimed to determine the efficacy of manual therapy for CLBP.
Figure 1.
Study layout.
Randomization
Randomization was performed, using an alternating envelope system. Upon presenting at the clinical site, PTs drew an envelope which randomly assigned the patient to either the EG or CG. The envelopes contained identical information, except that the description of the manual therapy was different for the EG and CG.
Setting
All five PT’s collecting data were orthopedic residents. Each PT treated patients with CLBP, working in separate private practice outpatient clinics in Illinois and Iowa and obtained the necessary permission to participate in the study. Each PT was educated on the aims of the study, measurement tools used in the study, completion of forms, and delivery of the EG and CG words, prior to performing the manual therapy treatment. To ensure uniform application of the manual therapy, each PT, as a resident, reviewed the techniques while in class with a senior manual therapy instructor.
Participants and recruitment
The PTs screened all new patients with CLBP attending PT against the inclusion and exclusion criteria for participation in the study. Inclusion criteria were: adults over the age of 18; presenting at PT with a primary complaint of LBP; LBP being present for 6 months or more; fluent in English; and willing to participate in the study. Exclusion criteria included: medical precautions to the use of manual therapy (metal, skin lesions, etc.); prior spine surgery; and unable to lay prone for the treatment. The sample size was estimated using the Repeated Measures Analysis module in PASS 14 using LBP and a factorial design with one between factor (treatment and control) and one within factor (time). It was based on a Greenhouse-Geisser Corrected F Test for the interaction between the two variables. In order to detect an effect size difference of .45 between the two arms, a total of 48 participants (24 participants in each arm) were needed. The analysis was based on a two-sided test with the significance level at .05 and power at 70%.
Intervention
All patients received the same manual therapy techniques (central posterior–anterior mobilization), position (prone), duration (10 min) and grading of the manual technique (grade II), per Maitland.[36] The intervention tested in this RCT was the difference in the educational models used to explain the manual therapy techniques. The descriptions and explanations of the proposed techniques were different between the EG and CG (Table 1) but were equal in duration (5 min).
Table 1.
Biomechanical and anatomical explanation of manual therapy.
| Neuroplasticity (EG) | Biomechanical (CG) |
|---|---|
| Explanation | Explanation |
|
|
| Picture | Picture |
 |
 |
| Words during the treatment | Words during the treatment |
|
|
Outcome measures
Prior to the manual therapy techniques, all study subjects completed a demographics survey capturing age, gender, ethnicity, income, and duration of LBP. Additionally, subjects were asked in regards to any previous exposure to manual therapy and their perceived benefit from previous manual therapy (Likert scale). All patients additionally completed an Oswestry Disability Index (ODI) [37–39] and Fear-Avoidance Beliefs Questionnaire (FABQ) [40] to ascertain their level of disability and fear-avoidance at the time of enrollment into the study. Four measurements were taken prior to, and immediately following manual therapy techniques to determine the efficacy of the experimental intervention:
-
•
Pain: LBP and leg pain (if present) was measured using Numeric Pain Rating Scale (NPRS), as has been used in various spinal pain studies.[8,9,41] The MDC for the NPRS for LBP is reported to be 2.0.[42]
-
•
Lumbar flexion: Active trunk forward flexion, measured from the longest finger on the dominant hand to the floor.[10,43,44] MDC for active trunk forward flexion has been reported as 4.5 cm.[45]
-
•
Straight leg raise (SLR): We used the SLR as a neurodynamic measurement rather than a test of hamstring length. SLR was measured with an inclinometer placed on the tibial crest 5 cm distal to the inferior border of the patella on the most affected leg.[10,43,44] SLR for this study kept the ankle in neutral (90°) with no added dorsiflexion or plantar flexion, per previous studies.[10,43,44] MDC for SLR has been reported as a 5.7° difference.[45]
Patients completed the NPRS, underwent lumbar flexion and SLR testing immediately prior to receiving the manual therapy technique and immediately after manual therapy technique. Pre- and post-treatment measurements were performed by the therapists who provided the manual interventions. Following capture of the outcome measures and manual therapy, the PTs continued their treatment per their plan of care.
Statistical analysis
In order to ascertain any differences between the treatment and control, 2 (group: treatment and control) × 2 (time: pre and post) mixed factorial ANOVAs on four different outcome measures (LBP, leg pain, trunk flexion, SLR) were conducted. If an interaction between group and time was observed then simple main effects were conducted using a Bonferroni correction of .025 for two simple main effects tests for the within analysis (pre and post) of each group. If no interaction was observed, then main effects were analyzed. Chi-square analysis was used to compare the proportions of individuals in each treatment who exceeded the MDC for each dependent measure.
Results
Participants
Sixty-two patients (female 35 [56.5%]), with a mean age of 60.14 years and mean duration of 9.26 years of LBP, participated in the study (Table 2). A majority of the patients (n = 49 [79%]) had received manual therapy previously for their LBP. In general, patients had a positive experience of manual therapy for LBP, with an average score of 5.97/10 agreeing manual therapy had helped their LBP before (0 – do not agree; 10 – strongly agree).
Table 2.
Demographic information.
| Characteristic | Results |
|---|---|
| Age (years; mean) | 60.14 |
| Female (%) | 35 (56.5%) |
| White, non-Hispanic | 57 (91.9%) |
| Duration of LBP (years; mean) | 9.26 |
| Disability (ODI; mean) | 35.67 |
| Fear-avoidance – work subscale (FABQ-W; mean) | 17.28 |
| Fear-avoidance – physical activity (FABQ – PA; mean) | 16.12 |
| Have received manual therapy before for LBP (n; %) | 49 (79%) |
Outcome measures
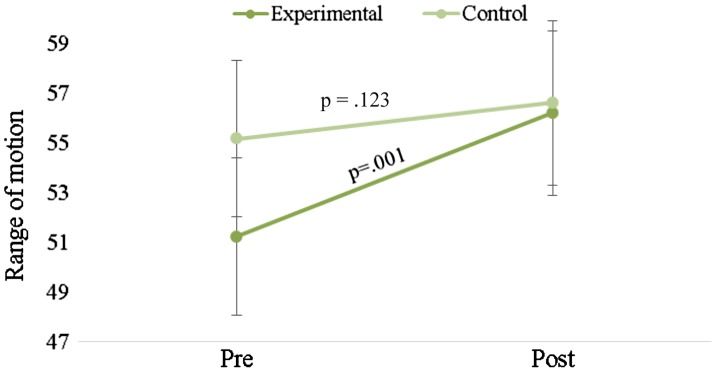
There were no statistically significant interactions for LBP (p = .325), leg pain (p = .172), and trunk flexion (p = .818) (Table 3). However, there was a significant interaction for SLR (p = .041) (Table 3, Figure 2). Simple main effects analyses indicate that the SLR improved for the EG (p = .001) but not for the CG (p = .123) (Figure 2). Descriptive data and main effect p values for each outcome variable are available in Table 3.
Table 3.
Means with standard deviations, including main effects of group and time, for each of the outcome variables.
| Variable | Group | Pre – means and SDs | Post – means and SDs | Interaction effect | Time main effect | Group main effect |
|---|---|---|---|---|---|---|
| LBP | Treatment | 3.8 ± 2.1 | 3.0 ± 2.4 | p = .325 | p = .004* | p = .180 |
| Control | 4.3 ± 2.4 | 4.0 ± 2.5 | ||||
| Leg pain | Treatment | 2.8 ± 2.4 | 2.1 ± 2.5 | p = .172 | p = .094 | p = .892 |
| Control | 2.6 ± 2.6 | 2.5 ± 2.2 | ||||
| Trunk flexion | Treatment | 25.9 ± 19.1 | 23.1 ± 19.8 | p = .818 | p = .004* | p = .853 |
| Control | 25.3 ± 15.4 | 22.1 ± 16.2 | ||||
| SLR | Treatment | 51.2 ± 18.1 | 56.2 ± 19.0 | p = .041* | p < .001* | p = .662 |
| Control | 55.2 ± 20.7 | 56.6 ± 21.0 |
Statistically significant at p < .05.
Figure 2.
Means and standard errors of the mean for the straight leg raise at the pre- and post-measurements for both the experimental and control groups.
The proportion of participants across the two groups who improved beyond the MDC was compared using chi-square analyses (Fisher’s exact test). There were no statistically significant differences for LBP (p = .055), leg pain (p = .676), and trunk flexion (p = 1.000); however, there was for SLR (p = .004). Participants in the treatment group were 7.2 times (95% confidence interval for odds ratio = 1.8–28.6) more likely to improve beyond the MDC on the SLR than participants in the CG (Table 4).
Table 4.
2 × 2 contingency table for the proportion of participants who improved beyond the MDC on the SLR.
| Yes | No | |||
|---|---|---|---|---|
| Group | Treatment | Count | 15 | 18 |
| % within group | 45.5 | 54.5 | ||
| Control | Count | 3 | 26 | |
| % within group | 10.3 | 89.7 | ||
| Total | Count | 18 | 44 | |
| % within group | 29.0 | 71.0 | ||
Discussion
The results of this study show that a neuroplasticity explanation, compared to a traditional biomechanical explanation, resulted in a measureable difference in the likelihood of improved SLR response in patients with CLBP when receiving manual therapy.
In the current study, with similar manual techniques applied to patients with CLBP, yet receiving different educational models for the manual techniques resulted in a difference in SLR, exceeding the MDC.[45] These results concur with various other pain science studies whereby pain neuroscience education resulted in immediate changes in neurodynamic tests such as SLR and the upper limb neurodynamic tests.[2,44,46–48] Coppieters et al. showed that altering the explanation of the SLR test by itself from describing the test as a test of ‘muscle’ vs. ‘nerve’ also significantly alters SLR, showcasing once again the power of words altering SLR.[49] In contrast, neither explanation resulted in a significant shift in LBP, leg pain or forward flexion. The results in regards to pain are expected. Given the complexity of pain, especially chronic pain,[1,50] it is not expected an education-alone session should result in immediate meaningful changes. This assumption is based on various pain neuroscience education studies showing no immediate effect on pain alone.[51–53] There is even evidence for an educational phenomenon referred to as ‘explain pain pain,’ whereby education about pain neuroscience results in a slight increase in pain after education, followed by a decrease over time.[47,54]
In regards to forward flexion, previous pain neuroscience education studies showed immediate changes in forward flexion[44,47,54] but not the current study. This study, however, was not a pain neuroscience study, but rather a comparison of different explanations for a manual therapy technique. In pain neuroscience education, a more elaborate discussion of pain science is undertaken.[55,56] The fact that SLR changed in the short amount of time and not flexion is intriguing. One potential explanation may be that SLR changes may be associated with the fact that neurodynamics is likely more affected by processes which may rapidly respond to education such as blood supply, muscle tone, etc.[57–60] In contrast, forward flexion, a common physical examination,[61] and often associated with LBP may need more extensive education, including addressing fear of movement.[61] In the current study, the mean FABQ-PA was 16.12, and an FABQ-PA > 14 is associated with a higher likelihood of not returning to work,[62,63] which would imply a ‘simple’ educational explanation for a manual technique would not be effective to alter flexion, but likely warrant a more comprehensive educational session.
The current study did not aim to specifically examine if cortical changes occurred in line with the education and manual techniques. Future studies will be needed to examine if these techniques and resultant increased SLR were in fact associated with cortical remapping. The results, however, do add to the body of growing evidence that manual therapy can be seen as a form of sensory discrimination, integration, and cortical remapping. For example, recent pain neuroscience education studies using single-case functional magnetic resonance imaging (fMRI) [41,47] have shown immediate cortical changes in the brain of patients with LBP, with one showing an immediate change in the motor cortex, as well as the patient’s SLR improving 7° following the education.[47] Other studies aiming to examine if tactile information via manual touch has a therapeutic effect in LBP, have shown to result in SLR changes,[23] immediate hypoalgesic effect,[23] and improved forward flexion exceeding the MDC.[24] Additionally, it has been shown there are immediate cortical changes in the brain following thoracic manipulation [64] as well as motor control exercises.[65,66]
Although a clear picture is yet to emerge in regards to manual therapy, cortical remapping and its influence on pain, function and movement, it does underscore the need for pain science to be hands-on vs. hands-off.[67] The interest in pain neuroscience education has likely driven the pendulum away from traditional manual approaches, which may in fact be detrimental.[68] A new systematic review on the efficacy of pain neuroscience education has highlighted that pain-educations sessions when combined with movement result in superior results when compared to education alone.[56] The current study underscores the need for combining education with manual therapy, ultimately strengthening the therapeutic alliance, which is defined as the working rapport or positive social connection between the patient and the therapist.[69–71] By virtue of its definition, therapeutic alliance is a complex blend of therapist technical skill, verbal and non-verbal communication, sense of warmth, trust, and collaboration.[69] One factor, heavily associated with therapeutic alliance, is the words clinicians chose in explaining a test or treatment. It is well established that word-choice by health care providers can have a positive or negative influence on their patients, which concurs with the current study.
This study contains various limitations. First, as described, the increased SLR cannot be directly attributed to cortical reorganization. The treatment techniques (Grade II) were arbitrarily chosen to not agitate the patient’s condition and not matched to presenting signs and symptoms, let alone specificity of the technique for the condition (a central posterior–anterior mobilization vs. other techniques). Even with an attempt to standardize the delivery of the education and manual therapy, there would have been differences in experience with the therapists delivering the treatment. A significant limitation is the fact that tests and treatments were performed with the attending therapists, thus the testing therapists were not blinded to the treatment. No attempt was made to differentiate if the education before the treatment or education during the treatment was responsible for the proposed change, or the combination of the two educational sessions, and future research should further explore this possibility. It is also important to point out that the MDC may not be clinically important or relevant; however, no minimal clinically important difference value has been reported for the SLR.[72,73]
Conclusion
The results of this study indicate that a neuroplasticity explanation, compared to a traditional biomechanical explanation, resulted in a measureable difference in SLR in patients with CLBP. The results also indicate no differences between the groups in regards to LBP, leg pain, and forward flexion. Future studies should investigate long-term effects of such education and may be also from a combination of therapies including neuroscience education.
Disclosure statement
None of these conflicts influence the subject matter or materials discussed in this manuscript.
Notes on contributors
Adriaan Louw, PT, PhD is the owner and CEO of International Spine and Pain Institute, Story City, IA, USA. He is an adjunct faculty in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA. He is an adjunct faculty: Department of Physical Therapy, School of Allied Health Sciences, University of Nevada, Las Vegas, NV, USA. He is the program director of Evidence in Motion Pain Certification and Pain Fellwoship.
Kevin Farrell, PT, PhD, OCS, FAAOMPT, is a professor and chair of the Orthopaedic Clinical Residency in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA.
Merrill Landers, PT, PhD, DPT, OCS, is the chair and professor in the Department of Physical Therapy, School of Allied Health Sciences, University of Nevada, Las Vegas, NV, USA. He is also Cyrus Chung Ying Tang Foundation Research Professor and fellow of the APTA Education Leadership Institute.
Martin Barclay, PT, DPT, OCS is a Resident in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA.
Elise Goodman, PT, DPT, OCS is a resident in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA.
Jordan Gillund, PT, DPT, OCS is a resident in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA.
Sara McCaffrey, PT, DPT, OCS is a resident in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA.
Laura Timmerman, PT, DPT, OCS is a resident in the Department of Physical Therapy Education, Residency Program, St. Ambrose University, Davenport, IA, USA.
Acknowledgements
The author Adriaan Louw, PT, PhD, acknowledges receiving payment for various speaking events (seminars, conferences, etc.) as well as royalties from books, from professionals and patients.
References
- [1].Moseley GL. Reconceptualising pain according to modern pain science. Phys. Ther. Rev. 2007;12:169–178. 10.1179/108331907X223010 [DOI] [Google Scholar]
- [2].Nijs J, Roussel N, Paul van Wilgen C, et al. . Thinking beyond muscles and joints: therapists’ and patients’ attitudes and beliefs regarding chronic musculoskeletal pain are key to applying effective treatment. Man. Ther. 2013;18:96–102. 10.1016/j.math.2012.11.001 [DOI] [PubMed] [Google Scholar]
- [3].Gifford LS. Pain, the tissues and the nervous system: a conceptual model. Physiotherapy. 1998;84:27–36. 10.1016/S0031-9406(05)65900-7 [DOI] [Google Scholar]
- [4].Gifford L, Butler D. The integration of pain sciences into clinical practice. J. Hand Ther. 1997;10:86–95. 10.1016/S0894-1130(97)80063-4 [DOI] [PubMed] [Google Scholar]
- [5].Jones MA. Clinical reasoning: the foundation of clinical practice. Part 1. Aust. J. Physiother. 1997;43:167–170. [PubMed] [Google Scholar]
- [6].Jones M. Clinical reasoning and pain. Man. Ther. 1995;1:17–24. 10.1054/math.1995.0245 [DOI] [PubMed] [Google Scholar]
- [7].Gifford L, Muncey H. Explaining pain to patients. Paper presented at: International Association on the Study of Pain1999; Vienna, Austria. [Google Scholar]
- [8].Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust. J. Physiother. 2002;48:297–302. 10.1016/S0004-9514(14)60169-0 [DOI] [PubMed] [Google Scholar]
- [9].Moseley GL. Joining forces – combining cognition-targeted motor control training with group or individual pain physiology education: a successful treatment for chronic low back pain. J. Man. Manipulative Ther. 2003;11:88–94. 10.1179/106698103790826383 [DOI] [Google Scholar]
- [10].Moseley GL, Hodges PW, Nicholas MK. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin. J. Pain. 2004;20:324–330. 10.1097/00002508-200409000-00007 [DOI] [PubMed] [Google Scholar]
- [11].Flor H. The functional organization of the brain in chronic pain. Prog. Brain Res. 2000;129:313–322. 10.1016/S0079-6123(00)29023-7 [DOI] [PubMed] [Google Scholar]
- [12].Flor H, Braun C, Elbert T, et al. . Extensive reorganisation of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 1997;224:5–8. 10.1016/S0304-3940(97)13441-3 [DOI] [PubMed] [Google Scholar]
- [13].Wand BM, Parkitny L, O’Connell NE, et al. . Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Man. Ther. 2011;16:15–20. 10.1016/j.math.2010.06.008 [DOI] [PubMed] [Google Scholar]
- [14].Penfield W, Boldrey E. Somatic, motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. 10.1093/brain/60.4.389 [DOI] [Google Scholar]
- [15].Stavrinou ML, Della Penna S, Pizzella V, et al. . Temporal dynamics of plastic changes in human primary somatosensory cortex after finger webbing. Cereb. Cortex. 2007;17:2134–2142. 10.1093/cercor/bhl120 [DOI] [PubMed] [Google Scholar]
- [16].Maihofner C, Handwerker HO, Neundorfer B, et al. . Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–1715. 10.1212/01.WNL.0000098939.02752.8E [DOI] [PubMed] [Google Scholar]
- [17].Moseley GL. I can’t find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain. 2008;140:239–243. 10.1016/j.pain.2008.08.001 [DOI] [PubMed] [Google Scholar]
- [18].Lotze M, Moseley GL. Role of distorted body image in pain. Curr. Rheumatol. Rep. 2007;9:488–496. 10.1007/s11926-007-0079-x [DOI] [PubMed] [Google Scholar]
- [19].Moseley GL. Distorted body image in complex regional pain syndrome. Neurology. 2005;65:773. 10.1212/01.wnl.0000174515.07205.11 [DOI] [PubMed] [Google Scholar]
- [20].Flor H, Elbert T, Muhnickel W, et al. . Cortical reorganisation and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp. Brain Res. 1998;119:205–212. 10.1007/s002210050334 [DOI] [PubMed] [Google Scholar]
- [21].Lloyd D, Findlay G, Roberts N, et al. . Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine. 2008;33:1372–1377. 10.1097/BRS.0b013e3181734a8a [DOI] [PubMed] [Google Scholar]
- [22].Marinus J, Moseley GL, Birklein F, et al. . Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637–648. 10.1016/S1474-4422(11)70106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Louw A, Schmidt SG, Louw C, et al. . Moving without moving: immediate management following lumbar spine surgery using a graded motor imagery approach: a case report. Physiother. Theory Pract. 2015;31:509–517. 10.3109/09593985.2015.1060656 [DOI] [PubMed] [Google Scholar]
- [24].Louw A, Farrell K, Wettach L, et al. . Immediate effects of sensory discrimination for chronic low back pain: a case series. N. Z. J. Physiother. 2015;43:58–63. [Google Scholar]
- [25].Twomey LT. A rationale for the treatment of back pain and joint pain by manual therapy. Phys. Ther. 1992;72:885–892. [DOI] [PubMed] [Google Scholar]
- [26].Edmondston SJ, Singer KP. Thoracic spine: anatomical and biomechanical considerations for manual therapy. Man. Ther. 1997;2:132–143. 10.1054/math.1997.0293 [DOI] [PubMed] [Google Scholar]
- [27].Edmondston SJ, Allison GT, Gregg CD, et al. . Effect of position on the posteroanterior stiffness of the lumbar spine. Man. Ther. 1998;3:21–26. 10.1054/math.1998.0312 [DOI] [PubMed] [Google Scholar]
- [28].Barrett CJ, Singer KP, Day R. Assessment of combined movements of the lumbar spine in asymptomatic and low back pain subjects using a three-dimensional electromagnetic tracking system. Man. Ther. 1999;4:94–99. 10.1054/math.1999.0175 [DOI] [PubMed] [Google Scholar]
- [29].Flynn TW. There’s more than one way to manipulate a spine. J. Orthop. Sports Phys. Ther. 2006;36:198–199. 10.2519/jospt.2006.0105 [DOI] [PubMed] [Google Scholar]
- [30].Flynn TW. Manual physical therapy: moving beyond the theoretical. J. Orthop. Sports Phys. Ther. 2004;34:659–661. 10.2519/jospt.2004.0111 [DOI] [PubMed] [Google Scholar]
- [31].Bialosky JE, Bishop MD, Price DD, et al. . The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man. Ther. 2009;14:531–538. 10.1016/j.math.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raney NH, Teyhen DS, Childs JD. Observed changes in lateral abdominal muscle thickness after spinal manipulation: a case series using rehabilitative ultrasound imaging. J. Orthop. Sports Phys. Ther. 2007;37:472–479. 10.2519/jospt.2007.2523 [DOI] [PubMed] [Google Scholar]
- [33].Puentedura EJ, Landers MR, Hurt K, et al. . Immediate effects of lumbar spine manipulation on the resting and contraction thickness of transversus abdominis in asymptomatic individuals. J. Orthop. Sports Phys. Ther. 2011;41:13–21. 10.2519/jospt.2011.3311 [DOI] [PubMed] [Google Scholar]
- [34].Bialosky JE, Bishop MD, George SZ, et al. . Placebo response to manual therapy: something out of nothing? J. Man. Manipulative Ther. 2011;19:11–19. 10.1179/2042618610Y.0000000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bialosky JE, Bishop MD, Robinson ME, et al. . Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys. Ther. 2009;89:1292–1303. 10.2522/ptj.20090058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maitland GD. Vertebral manipulation. 6th ed London: Butterworths; 1986. [Google Scholar]
- [37].Deyo RA, Battie M, Beurskens AJ, et al. . Outcome measures for low back pain research: a proposal for standardized use. Spine. 1998;23:2003–2013. [DOI] [PubMed] [Google Scholar]
- [38].Fritz JM, Irrgang JJ. A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys. Ther. 2001;81:776–788. [DOI] [PubMed] [Google Scholar]
- [39].Hakkinen A, Kautiainen H, Jarvenpaa S, et al. . Changes in the total Oswestry index and its ten items in females and males pre- and post-surgery for lumbar disc herniation: a 1-year follow-up. Eur. Spine J. 2007;16:347–352. 10.1007/s00586-006-0187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].George SZ, Fritz JM, Childs JD. Investigation of elevated fear-avoidance beliefs for patients with low back pain: a secondary analysis involving patients enrolled in physical therapy clinical trials. J. Orthop. Sports Phys. Ther. 2008;38:50–58. 10.2519/jospt.2008.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moseley GL. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Aust. J. Physiother. 2005;51:49–52. 10.1016/S0004-9514(05)70053-2 [DOI] [PubMed] [Google Scholar]
- [42].Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334. 10.1097/01.brs.0000164099.92112.29 [DOI] [PubMed] [Google Scholar]
- [43].Zimney K, Louw A, Puentedura EJ. Use of therapeutic neuroscience education to address psychosocial factors associated with acute low back pain: a case report. Physiother. Theory Pract. 2014;30:202–209. 10.3109/09593985.2013.856508 [DOI] [PubMed] [Google Scholar]
- [44].Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur. J. Pain. 2004;8:39–45. 10.1016/S1090-3801(03)00063-6 [DOI] [PubMed] [Google Scholar]
- [45].Ekedahl H, Jonsson B, Frobell RB. Fingertip-to-floor test and straight leg raising test: validity, responsiveness, and predictive value in patients with acute/subacute low back pain. Arch. Phys. Med. Rehabil. 2012;93:2210–2215. 10.1016/j.apmr.2012.04.020 [DOI] [PubMed] [Google Scholar]
- [46].Louw A, Puentedura EL, Mintken P. Use of an abbreviated neuroscience education approach in the treatment of chronic low back pain: a case report. Physiother. Theory Pract. 2012;28:50–62. 10.3109/09593985.2011.562602 [DOI] [PubMed] [Google Scholar]
- [47].Louw A, Puentedura EJ, Diener I, et al. . Preoperative therapeutic neuroscience education for lumbar radiculopathy: a single-case fMRI report. Physiother. Theory Pract. 2015;31:496–508. [DOI] [PubMed] [Google Scholar]
- [48].Meeus M, Nijs J, Van Oosterwijck J, et al. . Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: a double-blind randomized controlled trial. Arch. Phys. Med. Rehabil. 2010;91:1153–1159. 10.1016/j.apmr.2010.04.020 [DOI] [PubMed] [Google Scholar]
- [49].Coppieters MW, Ryan L, Chan KP, et al. . Do patients’ beliefs based on widespread medical information hinder accurate diagnosis? Paper Presented at: 11th World Congress on Pain2005; Sydney. [Google Scholar]
- [50].Melzack R. Pain and the neuromatrix in the brain. J. Dent. Educ. 2001;65:1378–1382. [PubMed] [Google Scholar]
- [51].Louw A, Diener I, Landers MR, et al. . Preoperative pain neuroscience education for lumbar radiculopathy: a multicenter randomized controlled trial with 1-year follow-up. Spine. 2014;39:1449–1457. 10.1097/BRS.0000000000000444 [DOI] [PubMed] [Google Scholar]
- [52].van Ittersum MW, van Wilgen CP, van der Schans CP, et al. . Written pain neuroscience education in fibromyalgia: a multicenter randomized controlled trial. Pain Pract. 2014;14:689–700. 10.1111/papr.2014.14.issue-8 [DOI] [PubMed] [Google Scholar]
- [53].Ryan CG, Gray HG, Newton M, et al. . Pain biology education and exercise classes compared to pain biology education alone for individuals with chronic low back pain: a pilot randomised controlled trial. Man. Ther. 2010;15:382–387. 10.1016/j.math.2010.03.003 [DOI] [PubMed] [Google Scholar]
- [54].Louw A, Diener I, Puentedura EJ. The short term effects of preoperative neuroscience education for lumbar radiculopathy: a case series. Int. J. Spine Surg. 2015;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Louw A, Diener I, Butler DS, et al. . The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch. Phys. Med. Rehabil. 2011;92:2041–2056. 10.1016/j.apmr.2011.07.198 [DOI] [PubMed] [Google Scholar]
- [56].Louw A, Zimney K, Puentedura E, et al. . The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract . 2016;Jun 28:1–24. [DOI] [PubMed] [Google Scholar]
- [57].von Piekartz HJ, Schouten S, Aufdemkampe G. Neurodynamic responses in children with migraine or cervicogenic headache versus a control group: a comparative study. Man. Ther. 2007;12:153–160. 10.1016/j.math.2006.06.004 [DOI] [PubMed] [Google Scholar]
- [58].Nee RJ, Butler D. Management of peripheral neuropathic pain: integrating neurobiology, neurodynamics and clinical evidence. Phys. Ther. Sport. 2006;7:36–49. 10.1016/j.ptsp.2005.10.002 [DOI] [Google Scholar]
- [59].Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man. Ther. 2008;13:213–221. 10.1016/j.math.2006.12.008 [DOI] [PubMed] [Google Scholar]
- [60].Nee RJ, Vicenzino B, Jull GA, et al. . Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: a randomised trial. J. Physiother. 2012;58:23–31. 10.1016/S1836-9553(12)70069-3 [DOI] [PubMed] [Google Scholar]
- [61].George SZ, Fritz JM, McNeil DW. Fear-avoidance beliefs as measured by the fear-avoidance beliefs questionnaire: change in fear-avoidance beliefs questionnaire is predictive of change in self-report of disability and pain intensity for patients with acute low back pain. Clin. J. Pain. 2006;22:197–203. 10.1097/01.ajp.0000148627.92498.54 [DOI] [PubMed] [Google Scholar]
- [62].Fritz JM, George SZ. Identifying psychosocial variables in patients with acute work-related low back pain: the importance of fear-avoidance beliefs. Phys. Ther. 2002;82:973–983. [PubMed] [Google Scholar]
- [63].Burton AK, Waddell G, Tillotson KM, et al. . Information and advice to patients with back pain can have a positive effect: a randomized controlled trial of a novel educational booklet in primary care. Spine. 1999;24:2484–2491. 10.1097/00007632-199912010-00010 [DOI] [PubMed] [Google Scholar]
- [64].Sparks C, Cleland JA, Elliott JM, et al. . Using functional magnetic resonance imaging to determine if cerebral hemodynamic responses to pain change following thoracic spine thrust manipulation in healthy individuals. J. Orthop. Sports Phys. Ther. 2013;43:340–348. 10.2519/jospt.2013.4631 [DOI] [PubMed] [Google Scholar]
- [65].Tsao H, Galea MP, Hodges PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131:2161–2171. 10.1093/brain/awn154 [DOI] [PubMed] [Google Scholar]
- [66].Tsao H, Danneels LA, Hodges PW. ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain. Spine. 2011;36:1721–1727. 10.1097/BRS.0b013e31821c4267 [DOI] [PubMed] [Google Scholar]
- [67].Lluch Girbes E, Meeus M, Baert I, et al. . Balancing, “hands-on” with “hands-off” physical therapy interventions for the treatment of central sensitization pain in osteoarthritis. Man. Ther. 2015;20:349–352. 10.1016/j.math.2014.07.017 [DOI] [PubMed] [Google Scholar]
- [68].Louw A, Puentedura E, Zimney K, et al. . Know pain; know gain? A perspective on pain neuroscience education in physical therapy. J. Orthop. Sports Phys. Ther. 2016;46:131–134. 10.2519/jospt.2016.0602 [DOI] [PubMed] [Google Scholar]
- [69].Crepeau EB, Garren KR. I looked to her as a guide: the therapeutic relationship in hand therapy. Disabil. Rehabil. 2011;33:872–881. 10.3109/09638288.2010.511419 [DOI] [PubMed] [Google Scholar]
- [70].Fuentes J, Armijo-Olivo S, Funabashi M, et al. . Enhanced therapeutic alliance modulates pain intensity and muscle pain sensitivity in patients with chronic low back pain: an experimental controlled study. Phys. Ther. 2014;94:477–489. 10.2522/ptj.20130118 [DOI] [PubMed] [Google Scholar]
- [71].Joyce AS, Ogrodniczuk JS, Piper WE, et al. . The alliance as mediator of expectancy effects in short-term individual therapy. J. Consult. Clin. Psychol. 2003;71:672–679. 10.1037/0022-006X.71.4.672 [DOI] [PubMed] [Google Scholar]
- [72].Dixon JK, Keating JL. Variability in straight leg raise measurements. Physiotherapy. 2000;86:361–370. 10.1016/S0031-9406(05)60630-X [DOI] [Google Scholar]
- [73].Boyd BS. Measurement properties of a hand-held inclinometer during straight leg raise neurodynamic testing. Physiotherapy. 2012;98:174–179. 10.1016/j.physio.2011.04.352 [DOI] [PubMed] [Google Scholar]