Abstract
Objective
The purpose of this study was to examine the effect of the Mulligan Concept (MC) Mobilization with movement (MWM) in the treatment of clinically diagnosed acute lateral ankle sprains in competitive athletes.
Methods
A prospective case series of 5 adolescent patients, ages ranging from 14 to 18 years (mean = 15.8 ± 1.64), that suffered an acute lateral ankle sprain (LAS). Patients were treated with the MC lateral ankle MWM. Mobilization was directed at the distal fibula or, using a modified MWM, 2–3 inches proximal to the distal fibula. Using paired t-tests and descriptive statistics (mean and standard deviation) results were analyzed.
Results
Treatment lasted an average of 9 days (mean = 9.2, ±SD 3.96) from intake to discharge. During that time frame, patients reported decreases in pain on the numeric pain rating scale (NRS), disability on the Disablement in the Physically Active (DPA) scale and an increase in function on the patient-specific functional scale (PSFS); and an immediate decrease in pain on the NRS within the first treatment. The minimal detectable change for the PSFS and NRS were exceeded from intake to discharge. Additionally, the minimally clinical important differences were exceeded on the NRS and DPA scale.
Discussion
The evidence presented in this Level-4 case series supports the use of the MC lateral ankle MWM to treat patients diagnosed with acute grade II LAS. Patients in this case series reported immediate decreases in pain and immediate increases in function. Therefore, further investigation of the MC lateral ankle MWM is warranted.
Keywords: Lateral ankle sprain (LAS), manual therapy, conservative care, mobilization with movement (MWM)
Introduction
Inversion ankle sprains are one of the most common injuries reported in the physically active population [1]. The National Collegiate Athletic Association conducted a 16 year observational study where up to 70% of all injuries affected the lower extremity [2]. Within the study, ankle injuries were the most prevalent injury, particularly identified in men and women’s basketball and soccer [2–5]. Ankle injuries were the most common injury in all of the 15 sports observed [3]. Ankle sprains are generally known to have a high occurrence rate in individuals under 35 years of age who participate in athletic and sporting events (e.g. basketball, soccer, football, running, or dance) [6,7].
The most commonly described ankle injury mechanism involves foot plantar-flexion (PF) combined with adduction and inversion resulting in possible lateral ankle ligament disruption [8]. These lateral ankle ligaments include the anterior talofibular ligament (ATFL), calcaneofibular ligament (CFL), and posterior talofibular ligament (PTFL) [9]. The lateral ankle sprain (LAS) is classified/graded based on severity [10]. There are many classification systems, most based on the number of ligaments involved in the injury [11]. However, a major shortcoming to the ligamentous grading process is that, unless the injury is treated with surgical intervention, there is no objective data allowing determination of damage to each ligament [11]. A three-category classification system including classic signs and symptoms of a LAS included the following characteristics: decreased range of motion (impairment), extent of edema, tenderness (pain), and joint stability[1] (Table 1), which are pertinent in the classification of acute LAS [12].
Table 1.
Ankle sprain grading system (Beynnon et al., 2006).
| Clinical grade | Description of grade level |
|---|---|
| Grade I (Mild) | Minimal swelling(edema) and tenderness; minimal or no function loss; no mechanical joint instability |
| Grade II (Moderate) | Moderate pain, swelling, and tenderness over involved structures; some loss of joint motion; joint stability is mild to moderately impaired |
| Grade III (Severe) | Complete ligament rupture with evident swelling, hemorrhage, and tenderness over involved structures; function lost; joint motion and instability evident as abnormal |
A history of ankle sprains is a common predisposing factor for the occurrence of an ankle sprain [10]; and without adequate treatment, ankle injuries may progress into chronic ankle instability (CAI), which can lead to further injury of the joint [6]. Further, many active individuals view ankle sprains as an inconsequential injury, thus up to 55% of the athletic population does not seek professional treatment following an ankle sprain [13]. Neglecting proper treatment often leads to repetitive ankle injuries (an indicator of CAI), ligamentous disruption, neurophysiological changes, and alteration in both ankle osteokinematic and arthrokinematic function [14–17]. The long-term effects of CAI can include posttraumatic ankle osteoarthritis and articular degeneration [18,19]. Chronic ankle instability could possibly become a widespread condition, due to the lack of significance placed on the care and treatment of acute ankle injuries.
In contrast to prior research of tissue-specific involvement, a growing trend is becoming more accepted that the LAS mechanism is thought to create minor displacement or positional fault at the distal fibula and tibia complex [8,20,21]. During the LAS mechanism of injury, the fibula is theorized to be subluxed anteriorly, causing a positional fault (arthrokinematic change) of the fibula at the talocrural joint [20,21]. In several studies, the presence of a distal fibular positional fault has been confirmed to have a direct relationship with lateral ankle instability and CAI [14,15,22,23]. The Mulligan Concept (MC) mobilization with movement (MWM) is a treatment paradigm theorized to correct positional faults and reduce patient-reported pain and dysfunction [20]. A MWM is a pain-free sustained accessory glide applied at a joint, with active and/or passive movement. Understanding the arthrokinematics of joint structure is an important foundational component in using the MC successfully. The treatment paradigm is expected to be pain free, provide immediate results and long-lasting effects (PILL) of decreased pain, increased range of motion, and increased function, referred to as the PILL effect [20]. The PILL effect is one of the core principles of the MC and an indication of proper treatment. If the PILL effect is not elicited after technique fine tuning (e.g. change in angle or intensity of MWM), the MC technique is considered a contraindication for continued treatment [20]. Mulligan concept taping techniques are often used in conjunction with therapy to reinforce the PILL effect, by taping the joint while matching the direction of the pain-free MWM.
The current management standards of LAS, based on severity, include various rehabilitation and treatment techniques, such as rigid immobilization (e.g. a cast), functional immobilization (e.g. a brace), progressive resistive exercise (PRE), and/or surgery coupled with modalities for pain relief [1,24]. The National Athletic Trainers’ Association (NATA) Position Statement: Conservative Management and Prevention of Ankle Sprains in Athletes recommends early use of modalities and mobilization techniques to treat grades I and II LAS [25]. Also, multiple research studies provide evidence supporting the use of MWMs in the treatment of subacute and chronic LAS [12,14–16]. While early mobilizations are recommended [12], there is a paucity of literature supporting the efficacy of the MC MWM to treat patients classified with grade I and II acute LAS. The purpose of this case series was to examine the effect of the Mulligan Concept MWM in the treatment of clinically diagnosed acute lateral ankle sprains in competitive athletes.
Methods
Participants
Five consecutive patients were recruited as a sample of convenience, who presented to the athletic training clinic, reporting acute pain at the lateral ankle were considered for participation in this study (Table 2). The inclusion criteria consisted of a primary complaint of a unilateral acute LAS and pain with ankle movement. Each patient was evaluated in the same manner to determine eligibility for inclusion: a detailed history, observation, palpation, orthopedic tests [25], and Ottawa Ankle Rules [26]. For the purpose of this case series, an acute injury was defined as an injury that was sustained within 72 h of initial evaluation. The study was approved by the school’s committee on human research, and all patients provided parental informed consent and assent from the minors, prior to data collection. The data collection and treatment was completed by the same certified athletic trainer, who had completed two Mulligan Concept courses and had 2 years of clinical practice using the concept. Patients were excluded from the study if they had any current evidence of a fracture in the lower limbs, or any open wounds in the area of treatment. Patients 101 and 104 both received radiographic imaging ruling out fractures prior to clinical assessment and treatment (Patient 101 and 104 received imaging based on parental concern). All patients were classified with a Grade-II sprain (Table 3).
Table 2.
Patient demographics.
| Patient demographics | Sex | Sport | Age | Height | Weight | # of Treatments | Days to Discharge | Days out of all sport activity | MWM Applied | ≈ Time from Injury to 1st Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| #101 | M | Basketball | 18 | 6’4 | 215 | 5 | 8 | 1 | Modified | 72hrs |
| #102 | M | Soccer | 14 | 5’9 | 145 | 4 | 6 | 0 | Traditional | 24hrs |
| #103 | M | Basketball | 17 | 6’3 | 205 | 4 | 7 | 2 | Traditional | 24hrs |
| #104 | F | Basketball | 15 | 5’3 | 178 | 4 | 16 | 2 | Modified | 48hrs |
| #105 | M | Soccer | 15 | 5’8 | 155 | 5 | 9 | 2 | Traditional | 24hrs |
Table 3.
Patient symptoms and classification at intake.
| Patient symptoms (Intake) | Ankle injury grade | Palpation of joint | Joint stability (Anterior Drawer) | Edema | Eccymosis | ROM (compared to uninjured limb) |
|---|---|---|---|---|---|---|
| #101 | II | Pain over ATFL and CFL | *Laxity | Moderate | Lateral ankle/lateral mid-foot | *DF 10°/*PF 22° |
| #102 | II | Pain over ATFL | *No Laxity | Minimal | N/A | *DF 16°/*PF 48° |
| #103 | II | Pain over ATFL | *No Laxity | Minimal | N/A | *DF 15°/*PF 45° |
| #104 | II | Pain over ATFL and CFL | *Laxity | Moderate | Lateral rear-foot | *DF 16°/*PF 42° |
| #105 | II | Pain over ATFL and CFL | *Laxity | Moderate | Lateral rear-foot | *DF 14°/*PF 38° |
Pain.
Outcome measures
Patient outcome measures were collected prior to the treatment intervention and used to identify progress, regression and treatment effects. The outcome measures included in this case series quantify pain, function, and disablement. The Numeric Pain Rating Scale (NRS) and the Patient Specific Functional Scale (PSFS), were collected during intake, pre/post treatment, and at discharge. The Disability in the Physically Active (DPA) Scale, was collected during intake, at the fourth treatment, and at discharge.
-
•
NRS: The Numerical Rating Scale (NRS) is a valid scale used to assess the patient’s pain (0 = no pain, 10 = extreme pain) [27]. The minimally clinical important difference (MCID) and the minimal detectable change (MDC) for the NRS is regularly reported as two points [27].
-
•
PSFS: The patient-specific functional scale (PSFS) is a valid scale used to assess the patient’s perception of function (0 = unable to perform, 10 = performs without issue) [28,29]. The MDC for the PSFS is regularly reported as an increase of three points for a single activity score [28,29], an MCID value was not found in the literature. During intake, the patient chose up to five activities based on their perception of difficulty to function. The patient and clinician then discussed which activity they viewed as pertinent to their sport (each patient chose an individual activity that could be performed in clinic), and that activity was used as the marker for assessing function pre/post for all treatments.
-
•
DPA scale: The disablement in the physically active (DPA) scale is a valid scale that is used to assess disablement over four dimensions: impairment, functional limitation, disability, and quality of life (0 = no disability, 64 = maximum disability) [30]. The DPA scale lists the MCID for acute conditions as a decrease of nine points [30], an MDC value was not found in the literature.
Intervention
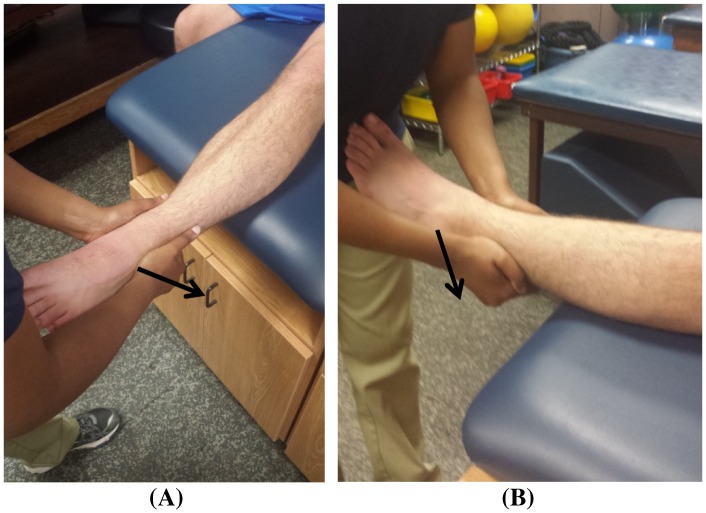
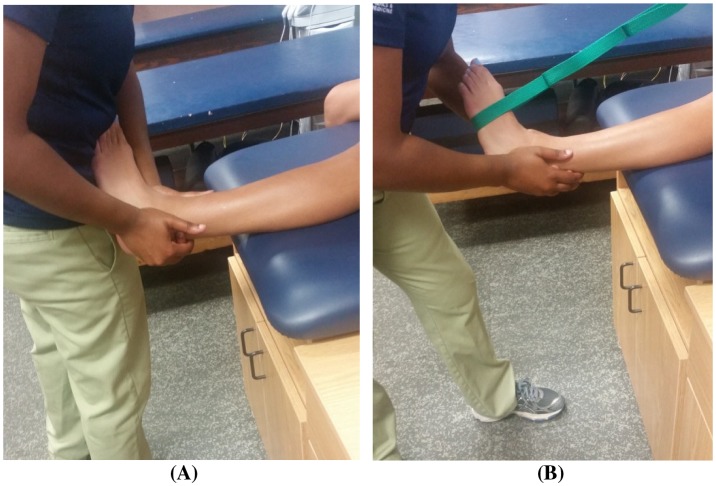
Patients were treated with traditional or modified MC LAS MWM, and patients were allowed to continue activities of daily living and athletic activities as tolerated. Patients were treated in a long-seated position on a treatment table, with the injured ankle suspended off the plinth. The clinician performed the treatment in a standing position by placing the thenar eminence on the anterolateral distal end of the fibula (lateral malleolus). The clinician applied a dorso-cranially (posterior-crainal) mobilization force (note: proper direction/mobilization will elicit a slight dorsiflexion and eversion of the patient’s foot) and used the opposite hand to support the ankle mortise (Figure 1) [20]. During the MWM, the patient was instructed to plantar flex and invert the foot. At the end of the patient’s range of motion (ROM), the clinician applied overpressure with her abdomen (overpressure can also be added with a band; Figure 2) [20]. The MWM remained painless throughout the entire application of the treatment, following the MC PILL principle. The MC guidelines were followed in all patients; however, patients who experienced pain (due to hand placement) during application of the traditional LAS MWM were treated with a modified LAS MWM. A modified MWM is indicated when soft tissue damage obstructs normal hand placement on the specified landmarks [20]. Similar to the traditional LAS MWM, the modified MWM is expected to be applied in a pain-free manner with immediate and long-lasting results (i.e. PILL effect). The modified MWM thenar eminence is placed approximately 2–3 inches proximal to the lateral malleolus with a similar dorso-cranial MWM (Figure 1) [31]. Outside of the hand placement modification, all other MC guidelines were followed. Three patients were treated with the traditional technique, and two were treated with the modification (Table 1). Each patient performed 3 sets of 10 MWM repetitions during one treatment session, with at least 30 s of rest between each set.
Figure 1.
MC LAS MWM hand placement (A) and modified hand placement on left ankle (B). Note: Arrow is in direction of the mobilization.
Figure 2.
MC LAS MWM with clinician overpressure (A) MC LAS MWM with band overpressure (B).
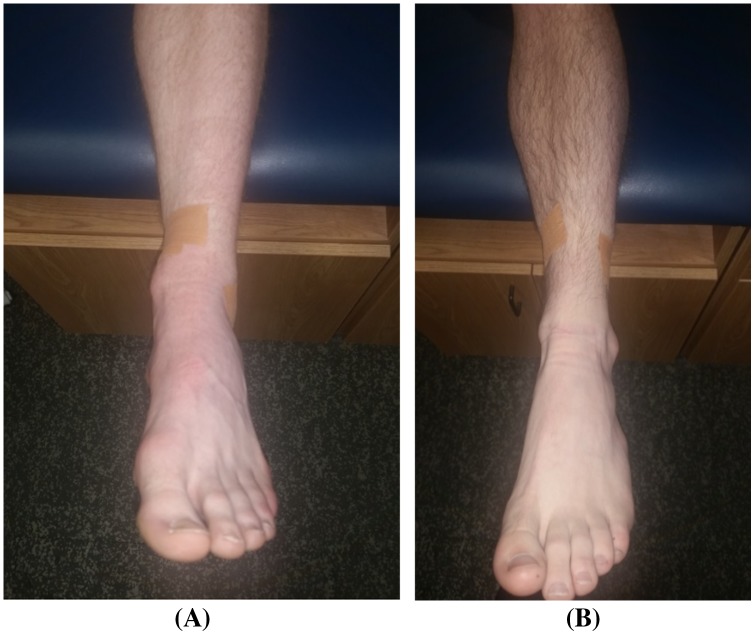
After each treatment, the clinician applied the fibular repositioning tape (FRT) by applying a strip of rigid Leukotape®P tape (BSN Medical, Inc-Charlotte, NC) directly to the skin in the direction of the MWM to reinforce the effects of the MWM. Patients were instructed to leave tape in place until the next treatment session (Figure 3), at which time the FRT was removed and the patient’s skin was cleaned and prepped for the subsequent treatment and FRT application. Each patient reported their PSFS activity after FRT application. All patients were treated with a 3″ wide tubular Cramer™ Compressionette (Cramer Products, Inc-Kansas City, MO) sleeve providing mild to moderate compression for the reduction of edema, worn during activities of daily living and sleep. Also, all patients except patient #101 were treated with natural (bagged) ice within the first 24 h of injury, by placement of ice directly on the area of perceived pain (lateral ankle).
Figure 3.
MC LAS MWM tape application (A) and modified tape application on left ankle (B).
Discharge criteria and follow-up
Patients were discharged from the study once they reached the predetermined criteria and maintained the outcomes a minimum of 24 h post treatment. The discharge criteria consisted of a PSFS score of nine or higher, NRS current pain of one or less, and a DPA scale score of 23 or less [27–30]. Patients were progressively released to activity as tolerated based on sport-specific return to competition criteria. After being discharged, patients could receive continued FRT application (Leukotape®P), without any other therapy, prior to each of their individual competitions, at their request; however, patients could not continue to receive FTR application without therapy if symptoms returned or re-injury occurred.
Data analysis
Descriptive statistics (mean ± SD) were calculated for patient demographics. A paired t-test was used to analyze the immediate pre/post treatment effects of the MC lateral ankle MWM on the patient’s current NRS pain rating. Additionally, paired t-tests were used to analyze the change in score from intake to discharge for the NRS, PSFS, and DPA Scale. Cohen’s d was calculated to determine the effect size of each outcome measure. For Cohen’s d, an effect size of 0.2–0.3 is considered a ‘small’ effect, 0.5 is a ‘moderate’ effect, and ≥ 0.8 a ‘large’ effect [32]. All data were analyzed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Five individuals with acute grade II LASs, whose ages range from 14 to 18 years (mean age = 15.8, standard deviation (SD) ± 1.64 years; males = 4, females = 1) participated in this study. Patients received an average of 4 treatments (mean = 4.4, SD ± .56) per patient over approximately 9 days (mean = 9.2, SD ± 3.96), from initial evaluation to patient discharge (Table 2).
NRS
The immediate effect of the MC lateral ankle MWM as assessed on the NRS after the first treatment (mean = 3.6 ± SD 2.88) was significantly lower than the pre-treatment score (mean = 5.6 ± 2.61, t(4) = 6.33, p = .003, two-tailed). The mean decrease in the NRS during the first treatment was 2 points, with a 95% CI ranging from 1.12 to 2.88. The mean difference satisfied the established MCID [27]. The Cohen’s d effect size (d = .73) indicated a moderate level of practical significance of the treatment, and the mean change indicated the treatment was clinically effective in one treatment. The NRS score at discharge was significantly lower (mean = 0.2 ± 0.45) than the initial score (mean = 5.6 ± 2.61, t(4) = 4.47, p = .011, two-tailed). The mean decrease was 5.4 points, with a 95% CI ranging from 2.05 to 8.75. The mean difference exceeded the established MCID [27]. The Cohen’s d effect size (d = 2.89) indicated a high level of practical significance of the treatment. Pain was reduced by a minimum of two points in all patients on the first visit and reduced by a minimum four points from intake to discharge. All patients were discharged with an average NRS score that was less than or equal to 1 and maintained discharge criteria at the 2 week and 4 week follow-up (Table 4).
Table 4.
Follow up data at 2 weeks and 4 weeks compared to Intake and Discharge.
| Intake | |
Comparison to intake scores | |||
|---|---|---|---|---|---|
| Patient # | NRS | PSFS | DPA | ROM | |
| #101 | 5 | 0 | 49 | DF 10°/PF 22° | MCID reached on NRS and PSFS after 1st treatment |
| #102 | 2 | 7 | 30 | DF 16°/PF 48° | MCID reached on NRS after 1st treatment |
| #103 | 7 | 3 | 25 | DF 15°/PF 45° | MCID reached on NRS and PSFS after 1st treatment |
| #104 | 9 | 3 | 32 | DF 16°/PF 42° | MCID reached on NRS and PSFS after 1st treatment |
| #105 | 5 | 3 | 38 | DF 14°/PF 38° | MCID reached on NRS and PSFS after 1st treatment |
| Discharge | Comparison to intake scores | ||||
| Patient # | NRS | PSFS | DPA | ROM | |
| #101 | 1* | 9* | 12* | DF 18°/PF 48° | MCID and/or MDC reached on all scales |
| #102 | 0* | 10* | 0* | DF 20°/PF 48 ° | MCID and/or MDC reached on all scales |
| #103 | 0* | 10* | 12* | DF 19°/PF 45° | MCID and/or MDC reached on all scales |
| #104 | 0* | 9* | 18* | DF 19°/PF 52° | MCID and/or MDC reached on all scales |
| #105 | 0* | 10* | 0* | DF 20°/PF 50° | MCID and/or MDC reached on all scales |
| 2 Week Follow-up | Comparison to discharge scores | ||||
| Patient # | NRS | PSFS | DPA | ROM | |
| #101 | 1 | 9 | 12 | Equal to opposite limb | No change from discharge |
| #102 | 0 | 10 | 0 | Equal to opposite limb | No change from discharge |
| #103 | 0 | 10 | 10 | Equal to opposite limb | Patient decreased on DPA scale |
| #104 | 0 | 9 | 20 | Equal to opposite limb | Patient increased on DPA scale, but within discharge criteria |
| #105 | 0 | 10 | 0 | Equal to opposite limb | No change from discharge |
| 4 Week Follow-up | Comparison to discharge scores | ||||
| Patient # | NRS | PSFS | DPA | ROM | |
| #101 | 1 | 9 | 12 | Equal to opposite limb | No change from discharge |
| #102 | 0 | 10 | 0 | Equal to opposite limb | No change from discharge |
| #103 | 0 | 10 | 12 | Equal to opposite limb | Returned to discharge scores |
| #104 | 0 | 10 | 16 | Equal to opposite limb | Patient decreased on DPA scale |
| #105 | 0 | 10 | 0 | Equal to opposite limb | No change from discharge |
MCID or MDC met or exceeded.
PSFS
The PSFS score at discharge (mean = 9.6 ± SD .55) was significantly higher than the initial scale score (mean = 3.2 ± 2.49, t(4) = –6.53, p = .003, two-tailed), which indicates a significant improvement of function. The mean difference in the PSFS score was −6.4, with a 95% CI ranging from −9.12 to −3.68. The mean difference exceeds the established MDC, and each patient sustained a reduction (at least 4 weeks post discharge) exceeding the MDC (Table 4) [28,29]. The Cohen’s d effect size (d = 3.6) indicated a high level of meaningfulness of the treatment. Function increased by a minimum of two points in all patients on the first visit and increased by a minimum three points from intake to discharge. All patients were discharged with a PSFS score of greater than or equal to 9 and maintained discharged criteria at the 2 and 4 week follow-up (Table 4).
DPA scale
The DPA scale score at discharge (mean = 8.4 ± SD 8.04) was significantly lower than the initial scale score (mean = 34.8 ± 9.20, t(4) = 4.85, p = .008, two-tailed). The mean decrease in the DPA scale score was 26.4 with a 95% CI ranging from 11.28 to 41.52. The mean difference exceeds the established MCID, and each patient sustained a reduction (from intake to discharge) exceeding the MCID [30]. The Cohen’s d effect size (d = 3.1) indicated a high level of meaningfulness of the treatment. All patients were discharged within a range expected of active, healthy individuals (score less than 23), as recorded in the established literature [30]. The discharge criteria were maintained at the 2 and 4 week follow-up (Table 4).
Discussion
The results of this case series indicate that a single treatment of MWM for a LAS led to an immediate reduction of pain in all patients (N = 5) and an increase in function four out the five patients. From intake to discharge, all reported scales illustrated statistical meaningfulness. Also, all of the patients in this case series experienced improvement that met or exceeded the MCID for NRS from initial to post first treatment. The patients also reported clinically significant improvements from initial exam to discharge in pain on the NRS, function on the PSFS, and disablement on the DPA Scale. The outcomes presented in this case series appear to be similar to those of O’Brien et al. [33], who reported an immediate decrease in pain and an increase in function and ROM beyond the natural course of healing. More importantly, in 5 treatments or less over the average of 9 days, patients with grade II ankle sprains were able to reach discharge criteria, return to competition, return to normal function (9 or higher on the PSFS), and report healthy disablement levels expected on the DPA Scale without suffering a return of symptoms or re-injury within the 4 week follow-up period. Conversely, surpassing the average 4 to 8 week return to full activity using traditional rehabilitation techniques, for treatment after injury [11].
The intervention produced long-lasting results with 100% of the patients remaining pain-free and competitively functional at 2 and 4 weeks post discharge (Table 4). After discharge, all patients continued to receive the MC FRT prior to each competition throughout their individual sporting activity, at their request; however, patients did not receive the FRT application or use any bracing during sport-specific practices or activities of daily living.
In this case series, patients were treated based on arthrokinematic changes [20], which contradicts many recommendations to focus on muscle strengthening, tissue healing, and protection of disrupted ligaments [1,24,31]. The Mulligan Concept LAS MWM addresses the positional fault theory [20] versus the traditional ligamentous damage theory [9]. The position of the distal fibula, after an acute lateral ankle sprain, is proposed to be subluxed (anterior or posterior), with the majority of subluxations being anterior [14,15,22,23]. The technique used potentially addresses arthrokinematic dysfunction of the ankle joint that is often neglected in ankle rehabilitation and may lead to CAI [14,16,18,23]. Although no radiographic exams were conducted to confirm fibula malalignment, application of the lateral ankle MWM resulted in pain-free movement and reduction in patient reported dysfunction that was maintained post-treatment. Another mechanism potentially explaining the benefit of MWMs is a neurophysiological component; it is likely that a MWM has a positive pain-altering effect, mediated by large A-Beta fibers stimulated by peripheral touch and transmitting non-painful contact stimuli to the central nervous system (CNS) faster than the smaller delta fibers transmit noxious stimuli [34]. Also observed in research is a pain relieving sympathetic nervous system response after a treatment of MWMs, similar to those after a spinal manipulation [35]. In addition to the resulting improvement in pain, the treatment is thought to improve function and ROM through the theorized correction of the positional fault caused by the LAS [12,31]. Moreover, the FRT application is theoretically used to support the correction of the arthrokinematic positional fault, continually providing a mobilizing force as the patient participates in activities.
In the past, clinicians have been encouraged to delay the complete physical exam following a LAS for 5–7 days after the initial trauma, due to pain and swelling thought to inhibit the physical examination [36]. In contrast, the application of MWMs is encouraged immediately once the PILL effect can be obtained after the initial injury [20]. Mobilizations with movement, in the MC, are utilized as part of the evaluation process to determine if the application is indicated; if the PILL effect cannot be created or sustained, then a different treatment is indicated [20]. When applied in these cases, the initial MWM was able to be applied pain-free within 72 h of injury and produced an immediate and clinically significant change in pain on the NRS.
Current standards of care related to the treatment of acute LAS recommend protection rest, ice, compression, elevation (PRICE), and other modalities within the early phase of the injury [25,37–39]; however not all ankle sprains are alike and an individualized plan should be created after a comprehensive assessment [25]. In this case study, patients were treated with a compression sleeve throughout the course of their treatment and reported using ice only on the day of injury (except patient 101). Specifically, focal compression directed to the soft tissue around the fibular malleolus appears to reduce edema, assisting with increased function over time, which is why compression was used in conjunction with the MC technique [40]. However, compression has yet to be identified as making a substantial impact on acute ankle sprain recovery, in high-quality randomized control trials [41]. Compression is identified as Category C Evidence by the NATA, meaning the recommendation is based on limited evidence consisting of case series, opinions, and usual practice [25]. It is possible that a traditional PRICE treatment could have had a positive effect on the recovery time seen within this case series. Currently, there is insufficient evidence available from randomized controlled trials to determine the relative effectiveness of PRICE for acute grade II ankle sprains [38,39,42]; however, based on the literature, it seems unlikely that PRICE treatment would explain the immediate benefit of improved function and ROM [27–36,38,39,42].
Limitations and future research
This study is limited by the small sample size and the limitation to an adolescent athletic population. The sample population in this case study does not represent the general population, which may make it difficult to translate these findings to a more diverse population. In addition, activity was not restricted during treatment, which could have improved or hindered outcomes. Initial treatment was not standardized, as this study was conducted in a true clinical setting and not a controlled lab. The effect of using a compressionette sleeve also confounds the effects of the MWM treatment, and effects cannot solely be attributed to the MC MWM. Furthermore, there was no control or comparison group to support the findings in this five person case series, and the clinician was not blinded. Further research is needed to determine if the MC lateral ankle MWM will have the same positive effect on other patient populations. Specific motor control testing (e.g. Y-balance test) should also be investigated evaluating changes in measure prior to MWM versus post MWM treatment. Patient outcome measures should be collected on larger populations with standardized treatments and controlled activity. Patients’ should also be evaluated for long-term ankle instabilities and/or osteoarthritis.
Conclusion
The outcomes of this case series provide evidence for the integration of the MC LAS MWM into treatment and rehabilitation protocols for patients with an acute grade II LAS. The patients in this case series reported immediate decreases in pain and immediate increases in functional activity while maintaining positive patient reported outcomes for 4 weeks post discharge. More importantly, the results in the case series demonstrate a quick return to activity (average of 4.4 treatments across 9 days) without a return of symptoms or re-injury 4 weeks post-discharge. Although the current results support the use of the MC lateral ankle MWM for acute LASs, more research is needed to establish this treatment as the standard of care in treating patients with LAS.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Robinetta Hudson is the head athletic trainer at Concordia Lutheran High School. She is focused on patient-centered care and the continued advancement of her clinical practice through the use of multiple treatment paradigms including but not limited to the Mulligan concept, reactive neuromuscular stabilization and primal reflex release techniques.
Russell T. Baker is a clinical assistant professor and the director of the MSAT program at the University of Idaho. His research interests include rehabilitation of acute and chronic musculoskeletal pathologies using manual therapy and patient reported outcome instrument psychometrics.
James May is a clinical assistant professor and DAT clinical education coordinator of athletic training at the University of Idaho.
Don Reordan is a physical therapist in Jacksonville, Oregon, an orthopedic clinical specialist, certified by the American Board of Physical Therapy Specialties and an accredited member of the Mulligan Concept Teachers Association.
Alan Nasypany is a clinical associate professor and DAT program director of athletic training at the University of Idaho.
References
- [1].Beynnon BD, Renström PA, Haugh L, et al. . A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401–1412. 10.1177/0363546506288676 [DOI] [PubMed] [Google Scholar]
- [2].Dick R, Hertel J, Agel J, et al. . Descriptive epidemiology of collegiate men’s basketball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J Athl Train. 2007;42:194–201. [PMC free article] [PubMed] [Google Scholar]
- [3].Agel J, Olson DE, Dick R, et al. . Descriptive epidemiology of collegiate women’s basketball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J Athl Train. 2007;42:202–210. [PMC free article] [PubMed] [Google Scholar]
- [4].Agel J, Evans TA, Dick R, et al. . Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2002–2003. J Athl Train. 2007;42:270–277. [PMC free article] [PubMed] [Google Scholar]
- [5].Dick R, Putukian M, Agel J, et al. . Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2002–2003. J Athl Train. 2007;42:278–285. [PMC free article] [PubMed] [Google Scholar]
- [6].van den Bekerom MJ, Kerkhoffs GJ, McCollum GA, et al. . Management of acute lateral ankle ligament injury in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21:1390–1395. 10.1007/s00167-012-2252-7 [DOI] [PubMed] [Google Scholar]
- [7].Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. . Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121:462–471. 10.1007/s004020100283 [DOI] [PubMed] [Google Scholar]
- [8].Petersen W, Rembitzki IV, Koppenburg AG, et al. . Treatment of acute ankle ligament injuries: a systematic review. Arch Orthop Trauma Surg. 2013;133:1129–1141. 10.1007/s00402-013-1742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roemer FW, Jomaah N, Niu J, et al. . Ligamentous injuries and the risk of associated tissue damage in acute ankle sprains in athletes: a cross-sectional MRI Study. Am J Sports Med. 2014;42:1549–1557. 10.1177/0363546514529643 [DOI] [PubMed] [Google Scholar]
- [10].Hiller CE, Refshauge KM, Herbert RD, et al. . Intrinsic predictors of lateral ankle sprain in adolescent dancers: a prospective cohort study. Clin J Sport Med. 2008;18:44–48. 10.1097/JSM.0b013e31815f2b35 [DOI] [PubMed] [Google Scholar]
- [11].Lynch SA. Assessment of the injured ankle in the athlete. J Athl Train. 2002;37:406–412. [PMC free article] [PubMed] [Google Scholar]
- [12].Loudon JK, Reiman MP, Sylvain J. The efficacy of manual joint mobilisation/manipulation in treatment of lateral ankle sprains: a systematic review. Br J Sports Med. 2014;48:365–370. 10.1136/bjsports-2013-092763 [DOI] [PubMed] [Google Scholar]
- [13].McKay GD, Goldie PA, Payne WR, et al. . Ankle injuries in basketball: injury rate and risk factors. Br J Sports Med. 2001;35:103–108. 10.1136/bjsm.35.2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hubbard TJ, Hertel J, Sherbondy P. Fibular position in individuals with self-reported chronic ankle instability. J Orthop Sports Phys Ther. 2006;36:3–9. 10.2519/jospt.2006.36.1.3 [DOI] [PubMed] [Google Scholar]
- [15].Hubbard TJ, Hertel J. Anterior positional fault of the fibula after sub-acute lateral ankle sprains. Man Ther. 2008;13:63–67. 10.1016/j.math.2006.09.008 [DOI] [PubMed] [Google Scholar]
- [16].Wikstrom EA, Hubbard TJ. Talar positional fault in persons with chronic ankle instability. Arch Phys Med Rehabil. 2010;91:1267–1271. 10.1016/j.apmr.2010.04.022 [DOI] [PubMed] [Google Scholar]
- [17].Gerber JP, Williams GN, Scoville CR, et al. . Persistent disability associated with ankle sprains: a prospective examination of an athletic population. Foot Ankle Int. 1998;19:653–660. 10.1177/107110079801901002 [DOI] [PubMed] [Google Scholar]
- [18].Valderrabano V, Hintermann B, Horisberger M, et al. . Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34:612–620. 10.1177/0363546505281813 [DOI] [PubMed] [Google Scholar]
- [19].Harrington KD. Degenerative arthritis of the ankle secondary to long-standing lateral ligament instability. J Bone Joint Surg Am. 1979;61:354–361. 10.2106/00004623-197961030-00006 [DOI] [PubMed] [Google Scholar]
- [20].Mulligan B. Manual Therapy: NAGS, SNAGS, MWMS, etc. 6th ed Wellington (NZ): Plane View Services; 2010. [Google Scholar]
- [21].Kavanagh J. Is there a positional fault at the inferior tibiofibular joint in patients with acute or chronic ankle sprains compared to normals? Man Ther. 1999;4:19–24. 10.1016/S1356-689X(99)80005-8 [DOI] [PubMed] [Google Scholar]
- [22].Mavi A, Yildirim H, Gunes H, et al. . The fibular incisura of the tibia with recurrent sprained ankle on magnetic resonance imaging. Saudi Med J. 2002;23:845–849. [PubMed] [Google Scholar]
- [23].Berkowitz MJ, Kim DH. Fibular position in relation to lateral ankle instability. Foot Ankle Int. 2004;25:318–321. 10.1177/107110070402500507 [DOI] [PubMed] [Google Scholar]
- [24].Prado MP, Mendes AM, Amodio DT, et al. . A comparative, prospective, and randomized study of two conservative treatment protocols for first-episode lateral ankle ligament injuries. Foot Ankle Int. 2014;35:201–206. 10.1177/1071100713519776 [DOI] [PubMed] [Google Scholar]
- [25].Kaminski TW, Hertel J, Amendola N, et al. . National athletic trainers’ association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528–545. 10.4085/1062-6050-48.4.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Plint AC, Bulloch B, Osmond MH, et al. . Validation of the Ottawa ankle rules in children with ankle injuries. Acad Emerg Med. 1999;6:1005–1009. 10.1111/acem.1999.6.issue-10 [DOI] [PubMed] [Google Scholar]
- [27].Childs J, Piva S, Fritz J. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334. 10.1097/01.brs.0000164099.92112.29 [DOI] [PubMed] [Google Scholar]
- [28].Chatman AB, Hyams SP, Neel JM, et al. . The patient-specific functional scale: measurement properties in patients with knee dysfunction. Phys Ther. 1997;77:820–829. 10.1093/ptj/77.8.820 [DOI] [PubMed] [Google Scholar]
- [29].Horn KK, Jennings S, Richardson G, et al. . The patient-specific functional scale: psychometrics, clinimetrics, and application as a clinical outcome measure. J Orthop Sports Phys Ther. 2012;42:30–42. 10.2519/jospt.2012.3727 [DOI] [PubMed] [Google Scholar]
- [30].Vela Li, Denegar CR. The disablement in the physically active scale, Part II: the psychometric properties of an outcomes scale for musculoskeletal injuries. J Athl Train. 2010;45:630–641. 10.4085/1062-6050-45.6.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mau H, Baker RT. A modified mobilization-with-movement to treat a lateral ankle sprain. Int J Sports Phys The. 2014;9:540–548. [PMC free article] [PubMed] [Google Scholar]
- [32].Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale (NJ): L. Erlbaum Associates; 1998. [Google Scholar]
- [33].O’Brien T, Vicenzino B. A study of the effects of Mulligan’s mobilization with movement treatment of lateral ankle pain using a case study. Man Ther. 1998;3:78–84. 10.1016/S1356-689X(98)80022-2 [DOI] [Google Scholar]
- [34].Vicenzino B, Hing W, Rivett D, et al. . Mobilisation with movement: the art and the science. Chatswood: Elsevier Australia; 2011. [Google Scholar]
- [35].Paungmali A, Vicenzino B, Smith M. Hypoalgesia induced by elbow manipulation in lateral epicondylalgia does not exhibit tolerance. J Pain. 2003;4:448–454. 10.1067/S1526-5900(03)00731-4 [DOI] [PubMed] [Google Scholar]
- [36].Witjes S, Gresnigt F, van den Bekerom MJ, et al. . The ANKLE TRIAL (ankle treatment after injuries of the ankle ligaments): what is the benefit of external support devices in the functional treatment of acute ankle sprain? A randomised controlled trial. BMC Musculoskelet Disord. 2012;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42:311–319. [PMC free article] [PubMed] [Google Scholar]
- [38].van den Bekerom MJ, Struijs PA, Blankevoort L, et al. . What is the evidence for rest, ice, compression, and elevation therapy in the treatment of ankle sprains in adults? J Athl Train. 2012;47:435–443. 10.4085/1062-6050-47.4.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bleakley CM, O’Connor S, Tully MA, et al. . The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain [ISRCTN13903946]. BMC Musculoskelet Disord. 2007;8:285. 10.1186/1471-2474-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wilkerson GB, Horn-Kingery HM. Treatment of the inversion ankle sprain: comparison of different modes of compression and cryotherapy. J Orthop Sports Phys Ther. 1993;17:240–246. 10.2519/jospt.1993.17.5.240 [DOI] [PubMed] [Google Scholar]
- [41].Hansrani V, Khanbhai M, Bhandari S, et al. . The role of compression in the management of soft tissue ankle injuries: a systematic review. Eur J Orthop Surg Traumatol. 2015;25:987–995. 10.1007/s00590-015-1607-4 [DOI] [PubMed] [Google Scholar]
- [42].Green T, Refshauge K, Crosbie J, et al. . A randomized controlled trial of a passive accessory joint mobilization on acute ankle inversion sprains. Phys Ther. 2001;81:984–994. [PubMed] [Google Scholar]