Abstract
Objective
In addition to lung volume restriction, persons with chronic tetraplegia demonstrate obstructive airway physiology evinced by pharmacologically-induced bronchodilation. We previously found independent evidence that anticholinergic agents (ipratropium bromide; IB) and beta-2 adrenergic agonists (albuterol sulfate; AS) were associated with significant bronchodilation in subjects with tetraplegia as determined via spirometry or body plethysmography. Direct comparison of these two classes of agents has received little attention.
Methods
Twelve subjects with chronic tetraplegia completed single dose treatment on alternate days with nebulized IB or AS. Patients underwent pre- and 30-minute post-bronchodilator spirometry, body plethysmography, and impulse oscillation system (IOS) in accordance with established protocols.
Results
Spirometry and specific airway conductance revealed significant bronchodilator responsiveness following both IB and AS. As determined by increases in specific airway conductance post-bronchodilator, IB tended toward greater bronchodilation than AS (71% vs. 47%). IOS revealed a greater reduction in central airway resistance (R20) following IB compared to AS (22% vs. 9%, P < 0.01). A greater number of subjects exhibited a clinically significant reduction in R20 following IB compared to AS (58% vs. 8%, P < 0.01).
Conclusion
Among subjects with tetraplegia, both IB and AS elicit significant bronchodilation, although the magnitude of the bronchodilator response is greater following IB. This lends support to theory of overriding cholinergic airway tone in tetraplegia. The IOS findings further suggest that the predominant site of action of IB is upon the larger central airways congruent with findings in able-bodied subjects.
Keywords: Spinal cord injury, Tetraplegia, Pulmonary function, Bronchodilator
Introduction
Significant bronchodilator responsiveness has been observed among spontaneously breathing subjects with tetraplegia using spirometric criteria, present in approximately forty-five percent of individuals following inhalation of either a short-acting anticholinergic agent or beta-2 adrenergic agonist.1,2 Greater sensitivity for the assessment of bronchodilator responsiveness following inhalation of these agents was later confirmed in separate studies utilizing body plethysmography to determine changes in specific airway conductance (sGaw).3–5 As opposed to individuals with paraplegia or able-bodied control subjects, all subjects with tetraplegia exhibited significant bronchodilation evidenced by mean increases in sGaw of greater than 40% following inhalation of either ipratropium bromide (IB) or albuterol sulfate (AS).3–5 Direct comparison of the relative efficacy of these two classes of bronchodilating agents among subjects with tetraplegia has never been assessed, although a distinction could have clinical relevance if greater bronchodilation to a given agent were found to more effectively reduce respiratory symptoms and/or pulmonary complications, both prevalent in this population.6–9
Certain considerations are taken into account when using spirometry or body plethysmography to assess bronchodilator responsiveness among subjects with tetraplegia. Greater within test variability and respiratory muscle weakness may hamper the interpretation of spirometry among these subjects if during repeated forced expiratory efforts flow limitation is not reached.10,11 Body plethysmography, although less effort dependent than spirometry, is limited by practical considerations; many wheelchairs will not fit into the body box apparatus, and transferring subjects might otherwise be difficult. In addition, both techniques necessitate taking maximal breaths, such that results may be confounded by volume history.12 The impulse oscillation system (IOS), which measures respiratory impedance across a wide range of frequencies after impulse pressure variations are applied at the mouth during quiet tidal breathing, offers a relatively simple, non-invasive, and effort independent alternative for the assessment of bronchodilator responsiveness.13 Application of IOS among subjects with spinal cord injury, however, has received little attention.5 By use of the IOS, it is thought that resistance measured at 5 Hertz (R5) reflects a composite of central and peripheral pulmonary resistances, whereas measurement at 20 Hertz (R20) reflects central pulmonary resistance.13 Measurement of relative changes in R5 and R20 might therefore be used to discern the predominant sites of action of bronchodilating agents. Similarly, it may be possible to compare the bronchodilator effects of IB and AS upon the smaller peripheral airways by determining relative changes in AX, which represents the area defined by integration of all values of pulmonary reactance from frequencies ranging from 5 Hz (X5) up to resonant frequency (Fres).13
The purpose of the present study was therefore to assess the relative magnitude of the bronchodilator responses among subjects with tetraplegia following inhalation of either IB or AS using spirometry, body plethysmography, and the IOS. We also sought to assess the applicability of impulse oscillation compared to conventional techniques, and to infer from impedance parameters the relative sites of action of IB and AS.
Methods
Twelve subjects (11 male, 1 female) with chronic tetraplegia (injury C4 to C8) participated in the study (Table 1). Subjects were selected who reported no history of pulmonary disease, atopy, or asthma, and all denied a history of recent or active pulmonary infections. All of the subjects were non-smokers, defined as never smokers or having quit smoking a minimum of 5 years earlier. None of the participants were receiving beta-2 adrenergic agonists or anticholinergic agents. Studies subjects were recruited from among outpatients followed at the spinal cord injury unit of The James J. Peters Veterans Affairs Medical Center. The study was approved by the institutional review board of The James J. Peters Veterans Affairs Medical Center, and informed consent was obtained prior to investigation.
Table 1.
Characteristics of study population.
| Tetraplegia (n = 12) | |
|---|---|
| Age (y) | 44 ± 9 |
| Sex | 11 ♂, 1 ♀ |
| Height (m) | 1.79 ± 0.06 |
| Weight (kg) | 78.1 ± 15.4 |
| BMI (kg/m2) | 24.3 ± 4.1 |
| DOI (y) | 14 ± 10 |
| Neurological Injury Level (range) | C4–C8 |
| AIS Impairment Scale (range) | A–B |
| Active Smokers | 0 |
Note: Data represent mean ± SD.
BMI, body mass index; DOI, duration of injury; C, cervical.
Measurements of respiratory impedance (pulmonary resistance and reactance) were performed during morning hours while subjects were seated in their wheelchairs using the IOS system (Viasys, Yorba Linda, CA, USA). With mouthpiece and nose clips attached, and with cheek support provided by the study investigator, sound-generated pressure oscillations in a range of 3 Hz to 35 Hz were superimposed at the mouth upon normal tidal breaths of study subjects. Each recording was of 30 seconds duration, and was considered acceptable if quality data encompassed at least five breaths or 20 seconds of the testing interval as defined by coherence values for pulmonary resistance measurements at 5 Hz (R5) and 10 Hz (R10) of at least 0.7 and 0.9, respectively.13 The mean of three pulmonary resistance measurements that were within 10% of each other was obtained for data analysis.
Subjects were then transferred to a variable-pressure, constant-volume whole body plethysmograph (model Vmax/6200 Body Plethysmograph, SensorMedics, Yorba Linda, CA, USA) for measurement of airway resistance (Raw) and specific airway conductance (sGaw). The technique for this procedure among subjects with spinal cord injury (SCI) has been previously reported,4 and conforms to the methods originally described by Du Bois et al.14,15 Because subjects with tetraplegia were unable to manually support their cheeks while within the body plethysmograph, all study participants were instructed to minimize use of their cheek muscles and to maintain an open airway while performing rapid and shallow panting maneuvers at approximately 2 cycles per second from a thoracic gas volume (Vtg) that approximated functional residual capacity.4 The specific airway conductance (sGaw) was defined as the reciprocal of Raw corrected for the Vtg. The mean of three acceptable sGaw measurements within 10% of each other was obtained for data analysis.
Following the initial plethysmographic measurements, subjects remained seated in the body box, and spirometry was performed in accordance with American Thoracic Society standards.16 Although forced expiratory maneuvers with back extrapolated volumes in excess of standard limits and/or with expiratory times < a 6-second duration have been reported among subjects with tetraplegia,10 these performance limitations were not encountered in our study subjects. Spirometric parameters were expressed as absolute values and percent predicted based upon the prediction equations of Morris et al.17
Post-bronchodilator studies were performed 30 minutes after administration of nebulized ipratropium bromide (IB), with repeat assessment of pulmonary function via IOS, body plethysmography, and spirometry performed in sequence as described above. On a separate study day one to three weeks later, same subjects underwent repeat baseline assessment of pulmonary function as described above, with post-bronchodilator studies completed 20 minutes after receiving nebulized albuterol sulfate (AS).
Values are presented as mean ± standard deviation (SD) unless otherwise specified. Individual pre- and post-bronchodilator comparisons for each agent (IB and AS) were made using paired t test with P < 0.05 considered statistically significant. Nonparametric frequency statistics were analyzed by χ2 test. For comparison of the relative bronchodilator responses between IB and AS across the three measurement techniques (spirometry, airway conductance, IOS), a Bonferroni correction was applied with P < 0.017 considered statistically significant. Statistical analyses were computed using SPSS version 22 (IBM SPSS Statistics version 22; IBM Corp., Armonk, NY, USA) and figures were generated by Prism (GraphPad Software, version 5.04 for Windows, San Diego, CA, USA).
Results
Ipratropium bromide
The bronchodilator responses to IB are summarized in Table 2. Following the administration of Ipratropium Bromide (IB), spirometry revealed statistically significant increases in FEV1 and FEF 25–75% (0.22 ± 0.16 L, P < 0.001; 0.58 ± 0.43 L/s, P < 0.001; respectively), but not FVC (0.12 ± 0.23 L, P = 0.11). Specific airway conductance (sGaw) increased significantly (0.10 ± 0.04 cmH20−1sec−1, P < 0.001), (Fig. 1A). Following IB, impulse oscillation (IOS) revealed a reduction in respiratory resistance parameters across all variables: R5 (–1.00 ± 0.47 cmH2O/L/s, P < 0.001), R20 (–0.78 ± 0.55 cmH2O/L/s, P < 0.001), R5–R20 (–0.26 ± 0.25 cmH2O/L/s, P < 0.01) and AX (–1.19 ± 1.29 cmH2O/L, P < 0.05), (Fig. 1B). An increase of at least 12% or 200 ml in FVC or FEV1, thus defining significant bronchodilator responsiveness as per ATS/ERS criteria,18 was witnessed in five of the twelve subjects following IB (42%) (Table 3). A greater than 40% increase in sGaw, the suggested criterion for significant bronchodilation using plethysmography,19 was seen in ten of twelve subjects (83%) following IB. A greater than 20% fall in respiratory resistance parameters, the threshold for identifying a significant fall in IOS parameters in the general population20 and SCI population,21 was found among subjects following IB as follows: R5 (9 of 12 subjects, 75%), R20 (7 of 12, 58%), and R5–R20 (6 of 12, 50%) (Table 3).
Table 2.
Pulmonary Function pre- and post-bronchodilator.
| Ipratropium Bromide (IB) |
Albuterol Sulfate (AS) |
|||
|---|---|---|---|---|
| Parameters | Pre | Post | Pre | Post |
| FVC (L) | 3.14 ± 0.81 | 3.36 ± 0.77 | 3.25 ± 0.94 | 3.32 ± 0.91 |
| FEV1 (L) | 2.60 ± 0.55 | 2.82 ± 0.59*** | 2.66 ± 0.63 | 2.80 ± 0.64* |
| FEF 25–75% (L/s) | 2.68 ± 0.74 | 3.25 ± 1.08*** | 2.78 ± 0.85 | 3.27 ± 1.17* |
| sGaw (cmH20−1sec−1) | 0.14 ± 0.03 | 0.24 ± 0.05*** | 0.17 ± 0.07 | 0.25 ± 0.14** |
| R5 (cmH2O/L/s) | 3.94 ± 0.87 | 2.94 ± 0.62*** | 3.94 ± 1.00 | 3.13 ± 0.67*** |
| R20 (cmH2O/L/s) | 3.29 ± 0.81 | 2.51 ± 0.46*** | 3.06 ± 0.59 | 2.76 ± 0.45** |
| R5-R20 (cmH2O/L/s) | 0.81 ± 0.50 | 0.55 ± 0.36** | 1.06 ± 0.72 | 0.45 ± 0.34** |
| AX (cmH2O/L) | 3.93 ± 3.81 | 2.73 ± 2.81* | 4.98 ± 3.87 | 2.10 ± 2.28** |
R5, resistance at 5Hz; R20, resistance at 20Hz.
*P < 0.05; **P < 0.01; ***P < 0.001.
Figure 1.
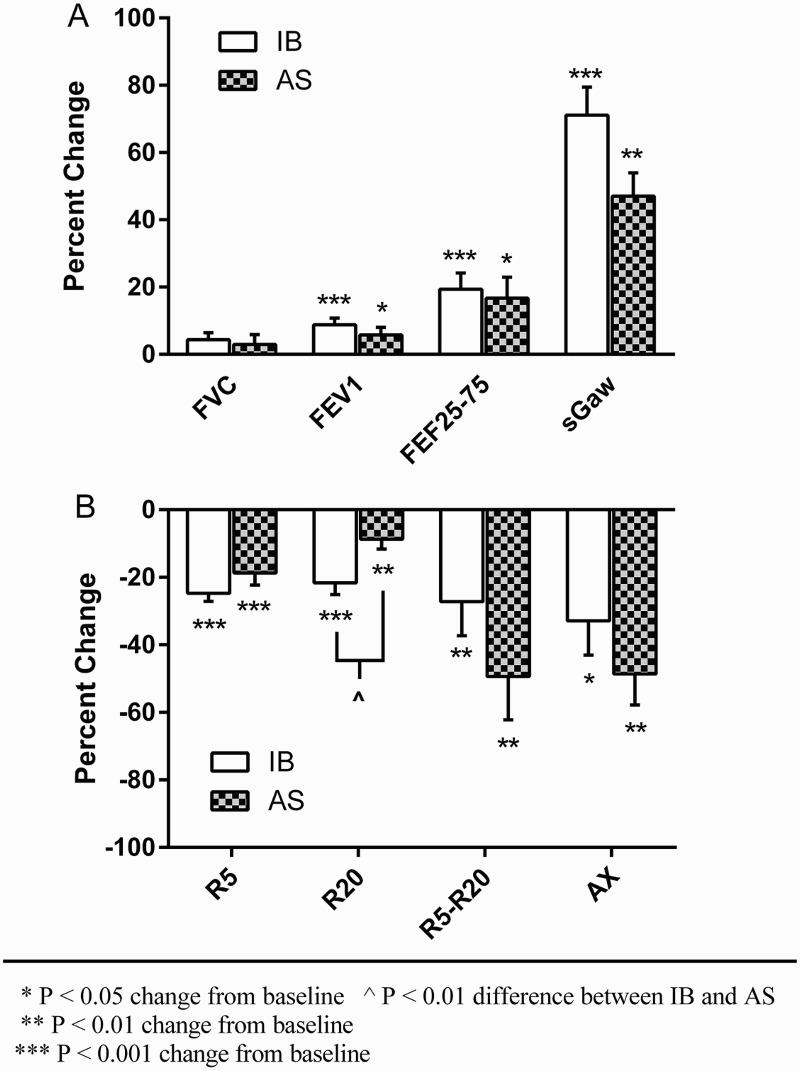
Percent change in (A) spirometry, specific airway conductance, and (B) IOS parameters following IB and AS administration.
Table 3.
Number of responders following administration of IB and AS.
| Parameters | IB (n = 12) | AS (n = 12) | P-Value |
|---|---|---|---|
| FVC or FEV1 | 5 (42) | 3 (25) | NS |
| sGaw | 10 (83) | 8 (67) | NS |
| R5 | 9 (75) | 7 (58) | NS |
| R20 | 7 (58) | 1 (8) | P < 0.01 |
| R5–R20 | 6 (50) | 8 (67) | NS |
Note: Responders equal the number (percent) of subjects with post-bronchodilator response attributed to action of the bronchodilator agent defined as: FVC or FEV1 ≥ 12% and 200 ml; sGaw ≥ 40%; R5, R20, R5–R20 ≥ 20%.
Albuterol sulfate
The bronchodilator responses to AS are summarized in Table 2. Following the administration of Albuterol Sulfate (AS), spirometry revealed statistically significant increases in FEV1 and FEF 25–75% (0.14 ± 0.18 L, P < 0.05; 0.49 ± 0.59 L/s, P < 0.05; respectively), but not FVC (0.07 ± 0.31 L, P = 0.47), (Figure 1a). Specific airway conductance (sGaw) increased significantly (0.08 ± 0.07 cmH20−1sec−1, P < 0.01). Following AS, impulse oscillation (IOS) revealed a reduction in respiratory resistance parameters as follows: R5 (–0.81 ± .59 cmH2O/L/s, P < 0.001), R20 (–0.30 ± 0.31 cmH2O/L/s, P < 0.01), R5-R20 (–0.61 ± 0.57 cmH2O/L/s, P < 0.01) and AX (–2.89 ± 3.02 cmH2O/L, P < 0.01), (Fig. 1b). An increase of at least 12% and 200 ml in FVC or FEV1 was seen in three of the twelve subjects following AS (25%). A greater than 40% increase in sGaw was witnessed in eight of twelve subjects (67%) following AS. A greater than 20% fall in respiratory resistance parameters following AS was found among subjects as follows: R5 (7 of 12, 58%), R20 (1 of 12, 8%), and R5–R20 (8 of 12, 67%) (Table 3).
Bronchodilator comparison
Specific airway conductance (sGaw) tended toward a larger percentage increase following IB compared to AS (71.2 ± 28.7% vs. 47.4 ± 23.5%; P = 0.036) (Fig. 1A). Resistance at R20 showed a larger percentage decrease following IB compared to AS (–21.6 ± 12.2% vs. -8.6 ± 10%; P < 0.01) (Fig. 1B). A significantly greater number of subjects exhibited a greater than 20% fall in R20 following IB as compared to AS (7 of 12, 58% vs. 1 of 12, 8%; P < 0.01), (Table 3). There was no statistical difference in the bronchodilator response between agents with regard to spirometric parameters.
Discussion
In previous independent investigations, bronchodilator responsiveness was found among subjects with chronic tetraplegia following inhalation of either a short-acting anticholinergic agent (ipratropium bromide; IB) or a short-acting beta-2 adrenergic agonist (metaproterenol sulfate). The initial studies involved simple spirometry, with mild restriction and normal FEV1/FVC ratios at baseline; unmasking of obstruction and bronchodilator responsiveness independent of smoking status was observed by increases in FEV1 of ≥ 12% and > 200 ml in 48% and 42% of subjects following inhalation of nebulized IB and metaproterenol sulfate, respectively.1,2 This degree of bronchodilation was not witnessed among patients with paraplegia, and suggested a unique physiology among persons with tetraplegia. It was hypothesized that these findings stemmed from interruption of sympathetic neurotransmission to the lungs at the level of the upper six thoracic nerve roots due to cervical injury, thereby mitigating bronchodilating influences upon cholinergic neurotransmission carried by intact vagal nerves. The resulting overriding cholinergic airway tone, and thus bronchoconstriction, might be overcome through the specific actions of an inhaled anticholinergic agent such as IB via competitive binding to muscarinic receptors abundantly found on airway smooth muscle, thus resulting in the bronchodilator responses observed.22 We further suspected that if the autonomic imbalance hypothesis was true, the bronchodilator response to anticholinergic agents might exceed the bronchodilation elicited non-specifically by beta-2 agonists.23 In the current investigation, our spirometric findings appear to support this hypothesis. Direct comparison of IB with albuterol sulfate (AS) among same subjects with tetraplegia revealed statistically significant increases among all subjects in FEV1 of 8.7% and 5.8%, respectively, suggesting greater bronchodilation after IB. Further, using ATS/ERS criteria for bronchodilator responsiveness,18 more subjects following IB nebulization (42%) exhibited bronchodilation compared to AS (25%). However, perhaps related to the small number of subjects, the difference in the increase in FEV1 between the two study drugs did not reach statistical significance.
A reasonable degree of certainty is conferred by our spirometric determinations among subjects who exhibited at least a 12% and 200 ml increase in FEV1 or FVC following bronchodilator administration; meeting this criterion confers specificity, making it unlikely that the bronchodilator response observed was related to intra-subject or intraday variability, but rather to pharmacologic intervention.19 A more sensitive measure of bronchodilation involves body plethysmography to assess specific airway conductance (sGaw), the reciprocal of airway resistance corrected for lung volume. An increase post-bronchodilator of at least 40% in sGaw is felt the minimum for significant bronchodilator responsiveness.19 In prior independent investigations, consistent significant mean increases in sGaw exceeding 40% among subjects with tetraplegia but not paraplegia who received nebulized IB or AS.3–5 In this study, same subject comparison of IB versus AS revealed significant increases in sGaw of 77% and 47%, respectively. The magnitude of the bronchodilator response tended to be greater following IB as compared to AS, thus further suggesting that IB may be a more effective bronchodilator among subjects with tetraplegia.
The IOS measurements reflect respiratory resistance, which includes not only airway resistance (as in sGaw), but also components of resistance imparted by lung parenchyma and chest wall.24 This technique offers advantages over spirometry and body plethysmography by requiring minimal effort, and is performed during normal tidal breathing.5 We found significant decreases following inhalation of IB and AS in total respiratory resistance (R5), central respiratory resistance (R20), peripheral respiratory resistance (R5–R20), and reactance area (AX). On a comparative basis, the fall in total respiratory resistance (R5) following IB compared to AS tended toward significance (P = 0.09), likely a reflection of the significantly greater fall in central respiratory resistance (R20) following nebulized IB (P < 0.01). These findings suggest a predominant site of action of anticholinergic agents in the larger central airways as has been previously reported by others,25,26 but the first so demonstrated among subjects with tetraplegia. Because larger airways are affected, a fall in resistance across all frequencies is expected and is reflected by our data.13 Peripheral respiratory resistance (R5–R20) fell more following AS compared to IB (49% versus 27%), as did reactance area (AX) (49% versus 33%). These findings did not reach statistical significance in our small sample, but suggest a predominant site of action of beta-2 agonists on peripheral airways, similar to that reported in able-bodied subjects.25 Recently, Ikeda et al.,27 by use of radioligand binding with intact tissue segments, found the distribution of M3 muscarinic receptors highest in bronchi and decreasing in density toward more peripheral airways, whereas B2-adrenoceptors were increased along the airways and lung parenchyma.27 The distribution of these receptors lends credence to IOS measurements in subjects with tetraplegia for determining the predominant sites of action for these two classes of bronchodilators.
Conclusion
The clinical implications of our findings are not known. Intuitively, it might be expected that bronchodilation elicited preferentially by IB might confer benefit to patients with tetraplegia beset by recurrent atelectasis or pneumonia to help facilitate pulmonary clearance and maximize lung function. It is also conceivable that co-administration of IB and AS to subjects with tetraplegia might confer greater bronchodilation than either agent alone given apparent differences in their predominant sites of action along the airway. Most research investigations among subjects with spinal cord injury, however, involve individuals who tend to be healthier than their sicker counterparts, and it is unclear if physiologic benefits would be elicited in a sicker cohort at higher risk for pulmonary complications. Important questions remain: should bronchodilators such as IB be administered for maintenance therapy among persons with higher level spinal cord injury, especially those prone to complications, and would these medications reduce complications if given prophylactically, improve quality of life, or reduce pulmonary symptoms? Larger studies and validation of a respiratory questionnaire for use among subjects with SCI are likely needed to answer these questions.
Acknowledgments
This research was supported by the Department of Veterans Affairs Rehabilitation Research and Development Service (#B9212-C, #B4162-C) and the James J. Peters VA Medical Center. The research performed in this manuscript was completed at the James J. Peter VA Medical Center (JJPVAMC) and approved by the JJPVAMC Institutional Review Board. All authors listed on this study had no conflict of interest.
Author declaration
On behalf of all authors, we agree to the rules, regulations and requirements of the publication process. All authors had significant contributions to the study design, performance, data analysis/interpretation, manuscript development and approval of the final document.
Disclaimer statements
Contributors None.
Funding None.
Conflict of Interest No author has a conflict of interest with the material presented.
Ethics approval None.
Disclosures None.
References
- 1.Spungen AM, Dicpinigaitis PV, Almenoff PL, Bauman WA.. Pulmonary obstruction in individuals with cervical spinal cord lesions unmasked by bronchodilator administration. Paraplegia 1993;31(6):404–7. [DOI] [PubMed] [Google Scholar]
- 2.Almenoff PL, Alexander LR, Spungen AM, Lesser MD, Bauman WA.. Bronchodilatory effects of ipratropium bromide in patients with tetraplegia. Paraplegia 1995;33(5):274–7. [DOI] [PubMed] [Google Scholar]
- 3.Schilero GJ, Grimm D, Spungen AM, Lenner R, Lesser M.. Bronchodilator responses to metaproterenol sulfate among subjects with spinal cord injury. J Rehabil Res Dev 2004;41(1):59–64. doi: 10.1682/JRRD.2004.01.0059 [DOI] [PubMed] [Google Scholar]
- 4.Schilero GJ, Grimm DR, Bauman WA, Lenner R, Lesser M.. Assessment of airway caliber and bronchodilator responsiveness in subjects with spinal cord injury. Chest 2005;127(1):149–55. doi: 10.1378/chest.127.1.149 [DOI] [PubMed] [Google Scholar]
- 5.Radulovic M, Schilero GJ, Wecht JM, Weir JP, Spungen AM, Bauman WA, et al. Airflow obstruction and reversibility in spinal cord injury: evidence for functional sympathetic innervation. Arch Phys Med Rehabil 2008;89(12):2349–53. doi: 10.1016/j.apmr.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Spungen AM, Grimm DR, Lesser M, Bauman WA, Almenoff PL.. Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord 1997;35(10):652–7. doi: 10.1038/sj.sc.3100489 [DOI] [PubMed] [Google Scholar]
- 7.Spungen AM, Grimm DR, Schilero G, Lenner R, Oei E, Bauman WA, et al. Relationship of respiratory symptoms with smoking status and pulmonary function in chronic spinal cord injury. J Spinal Cord Med 2002;25(1):23–7. doi: 10.1080/10790268.2002.11753597 [DOI] [PubMed] [Google Scholar]
- 8.DeVivo MJ, Krause JS, Lammertse DP.. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999;80(11):1411–9. doi: 10.1016/S0003-9993(99)90252-6 [DOI] [PubMed] [Google Scholar]
- 9.Shavelle RM, DeVivo MJ, Brooks JC, Strauss DJ, Paculdo DR.. Improvements in long-term survival after spinal cord injury? Arch Phys Med Rehabil 2015;96(4):645–51. doi: 10.1016/j.apmr.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 10.Kelley A, Garshick E, Gross ER, Lieberman SL, Tun CG, Brown R.. Spirometry testing standards in spinal cord injury. Chest 2003;123(3):725–30. doi: 10.1378/chest.123.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forner JV. Lung volumes and mechanics of breathing in tetraplegics. Paraplegia 1980;18(4):258–66. [DOI] [PubMed] [Google Scholar]
- 12.Skloot G, Togias A.. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clin Rev Allergy Immunol 2003;24(1):55–72. doi: 10.1385/CRIAI:24:1:55 [DOI] [PubMed] [Google Scholar]
- 13.Goldman MD, Saadeh C, Ross D.. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol 2005;148(1–2):179–94. doi: 10.1016/j.resp.2005.05.026 [DOI] [PubMed] [Google Scholar]
- 14.Dubois AB, Botelho SY, Bedell GN, Marshall R, Comroe JH Jr.. A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest 1956;35(3):322–6. doi: 10.1172/JCI103281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois AB, Botelho SY, Comroe JH Jr.. A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest 1956;35(3):327–35. doi: 10.1172/JCI103282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 17.Morris JF, Koski A, Johnson LC.. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 1971;103(1):57–67. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26(5):948–68. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 19.Van Noord JA, Smeets J, Clement J, Van de Woestijne KP, Demedts M.. Assessment of reversibility of airflow obstruction. Am J Respir Crit Care Med 1994;150(2):551–4. doi: 10.1164/ajrccm.150.2.8049845 [DOI] [PubMed] [Google Scholar]
- 20.Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22(6):1026–41. doi: 10.1183/09031936.03.00089403 [DOI] [PubMed] [Google Scholar]
- 21.Cirnigliaro CM, Lesser M, Moyer J, Kirshblum SC, Bauman WA, Spungen AM.. Reproducibility and effect of posture on impulse oscillation parameters in persons with spinal cord injury. J Spinal Cord Med 2012;35(1):28–34. doi: 10.1179/2045772311Y.0000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buels KS, Fryer AD.. Muscarinic receptor antagonists: effects on pulmonary function. Handb Exp Pharmacol 2012;(208):317–41. doi: 10.1007/978-3-642-23274-9_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadel JA, Barnes PJ.. Autonomic regulation of the airways. Annu Rev Med 1984;35:451–67. doi: 10.1146/annurev.me.35.020184.002315 [DOI] [PubMed] [Google Scholar]
- 24.Hellinckx J, Cauberghs M, De Boeck K, Demedts M.. Evaluation of impulse oscillation system: comparison with forced oscillation technique and body plethysmography. Eur Respir J 2001;18(3):564–70. doi: 10.1183/09031936.01.00046401 [DOI] [PubMed] [Google Scholar]
- 25.Hensley MJ, O'Cain CF, McFadden ER Jr., Ingram RH Jr.. Distribution of bronchodilatation in normal subjects: beta agonist versus atropine. J Appl Physiol Respir Environ Exerc Physiol 1978;45(5):778–82. [DOI] [PubMed] [Google Scholar]
- 26.Smith TC, DuBois AB.. The effects of scopolamine on the airways of man. Anesthesiology 1969;30(1):12–8. doi: 10.1097/00000542-196901000-00011 [DOI] [PubMed] [Google Scholar]
- 27.Ikeda T, Anisuzzaman AS, Yoshiki H, Sasaki M, Koshiji T, Uwada J, et al. Regional quantification of muscarinic acetylcholine receptors and beta-adrenoceptors in human airways. Br J Pharmacol 2012;166(6):1804–14. doi: 10.1111/j.1476-5381.2012.01881.x [DOI] [PMC free article] [PubMed] [Google Scholar]


