Abstract
Mesenchymal stem cells (MSCs) show considerable promise as a cellular immunotherapy for the treatment of a number of autoimmune and inflammatory disorders. However, the precise physiologically and therapeutically relevant mechanism(s) by which MSCs mediate immune modulation remains elusive. Dental pulp stem cells are a readily available source of MSCs that have been reported to show similar immune modulation in vitro as bone marrow MSCs. To test their potential in vivo, we used a clinically relevant humanized mouse model of GvHD in which only human T cells engraft. In this model, we found no effects on either T-cell proliferation, T-cell phenotype or disease progression. To determine if this lack of efficacy was related to a failure of engraftment or persistence of the cells, we used viability dependent radioactive cell tracking and showed that no cells were detectable after 24-h postinjection. Given the apparent failure of MSC to survive following intravenous injection, we hypothesized that their apoptosis may account for the widely reported therapeutic effect in numerous experimental models in vivo. To address this, we employed a well-established model of allergic airway inflammation to compare the efficacy of live and apoptotic MSCs in a fully immunocompetent model. In this model, both live and apoptotic dental pulp MSCs induced a robust immune suppressive reaction that was substantially greater with apoptotic cells. We propose that the mechanism of immune modulation following systemic application of MSCs is a result of cell entrapment and apoptosis occurring in the lungs.
Mesenchymal stem cells (MSCs) are a population of tissue-resident adult progenitor cells that are capable of differentiating into a number of mesodermal lineages in vitro. In addition to these ‘stem cell properties’, MSCs have been shown to exhibit broad and potent immunomodulatory effects in vitro and in vivo. It is hoped that the immunosuppressive properties of MSCs can be harnessed as a means of therapeutic immunomodulation for the treatments of autoimmune diseases, graft-versus-host disease (GvHD) and allograft rejection. Indeed, initial clinical investigations have reported promising results in the treatment of GvHD, MS and Crohn's disease [1–3], and there are currently a large number of safety and efficacy clinical trials currently ongoing to investigate the use of MSCs as a cellular immunotherapy.
MSC-like cells can be isolated from a number of anatomical locations and are able to undergo substantial ex vivo expansion. These properties, together with their hypo-immunogenic phenotype and lacking expression of MHC-II and classical co-stimulatory molecules, make MSCs a particularly attractive target for such a therapeutic strategy as it affords the banking of MSCs and subsequent use in an allogeneic ‘off the shelf’ manner. In vitro, MSCs have been shown to be capable of inhibiting T-cell proliferation and effector function, inhibiting monocyte differentiation and modulating NK cell function [4–8]. Moreover, these findings have been translated in vivo in a number of animal models of immune pathology including: collagen induced arthritis [9]; ovalbumin (OVA) induced allergic airway inflammation [10,11]; experimental autoimmune encephalitis [12]; systemic lupus erythematosus [13]; and autoimmune Type 1 diabetes mellitus [14]. Moreover, MSCs have been shown to prevent graft rejection in mouse models of heart and kidney transplantation [15,16].
Despite the surfeit of promising preclinical data and the increasing number of clinical studies, there is a need for a more in depth understanding of the precise mechanisms exerted by MSCs to modulate the immune system since this will inform the appropriate clinical deployment of MSCs as a therapy. Previous studies have provided a number of mechanistic explanations for the observed immunoregulatory effects of MSCs in vitro, highlighting the importance of IFN-γ licensing of MSCs [17–21] and implicating the role of several additional soluble factors, PGE2, HGF, TGF-β, IL-10 and galectins [22–24], and cell contact dependent mechanisms such as PD-L1 [25]. Significantly, however all these mechanisms are short range and act in the local manner and thus unable to explain the observed effects following systemic injection of MSCs.
We provide evidence to support the hypothesis that the observed immune modulatory effects of systemically administered MSCs are mediated indirectly as a consequence of cell apoptosis.
Results
MSCs inhibit T-cell proliferation in vitro
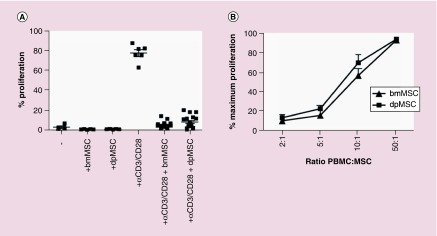
The immunomodulatory function in vitro of MSCs derived from bone marrow (bmMSC) is well established [26–28]. MSCs can be derived from many different anatomic locations and here we compared the immunomodulatory abilities of bmMSCs with dental pulp MSC (dpMSCs). dpMSCs are not only a more accessible source of MSCs, capable of multilineage differentiation [29], but have also been shown to exhibit greater proliferative potential ex vivo than bmMSC [30]. dpMSC were shown to have very similar immunomodulatory properties as bmMSC. They were unable to induce T-cell proliferation (Figure 1A) and were capable of inhibiting a-CD3/CD28-mediated T-cell proliferation in vitro in a dose dependent manner (Figure 1A & B).
Figure 1. . Dental pulp MSC inhibit T-cell proliferation in vitro.
(A) CFSE dilution of CD4+ T cells in direct co-culture with bmMSC (n = 4) or dpMSC (n = 4) or CD4+ T cells stimulated with αCD3/CD28 T-cell activation beads alone (n = 6) or in direct co-culture with bmMSC (n = 20) or dpMSC (n = 17). (B) Proliferation, as measured by CFSE dilution, of CD4+ T cells co-cultured with dpMSC (n = 6) or bmMSC (n = 4) at different ratios.
bmMSC: Bone marrow MSC; CFSE: Carboxyfluorescein succinimidyl ester; dpMSC: Dental pulp MSC; MSC: Mesenchymal stem cell.
MSCs are unable to directly modulate human T-cell function in vivo
To assess whether dpMSC could effectively modulate human T cells in vivo we utilized the hu-peripheral blood mononuclear cells (PBMC)-NSG model of GvHD – a model in which a near totally immune-depleted mouse is reconstituted only with human T cells [31].
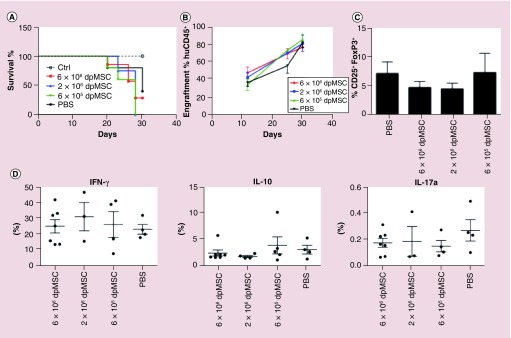
NOD/SCIDγ-/- (NSG) mice were reconstituted with 2 × 107 human PBMCs and engraftment and disease progression were monitored by flow cytometric analysis of peripheral blood chimerism (ratio of huCD45:mCD45) and weight loss respectively. To assess the ability of dpMSCs to modulate established disease, an intravenous (iv.) administration of either 6 × 105, 2 × 106 or 6 × 106 was given at the onset of GvHD-like symptoms (equivalent to ∼80% engraftment with human CD45+ cells) and was found to have no bearing on disease progression as determined by weight, huCD45 engraftment and ultimately survival (Figure 2A & B). Similarly, phenotypic analysis of human CD3+ T cells in the spleens of mice showed that MSC infusion at any of the doses tested had no impact on the phenotype of human T cells in vivo, in terms of the proportion of FoxP3+ Tregs (Figure 2C) and cytokine production (Figure 2D).
Figure 2. . Intravenous injection of dental pulp MSC does not inhibit disease onset or progression in humanized mouse model of graft-versus-host disease.
(A) Survival of NSG mice following iv. injection of 2 × 107 human PBMC alone (n = 5) or with subsequent injection of 6 × 106 dpMSC (n = 5), 2 × 106 dpMSC (n = 5) or 6 × 105 dpMSC (n = 4) (B) Engraftment, measured as the percent of human CD45+ cells relative to mouse CD45+ cells, in blood. (C) Proportion of FoxP3+ Tregs in spleens GvHD mice. (D) Intracellular cytokine staining of T cells isolated from spleens of huGvHD mice.
dpMSC: Dental pulp MSC; GvHD: Graft-versus-host disease; MSC: Mesenchymal stem cell.
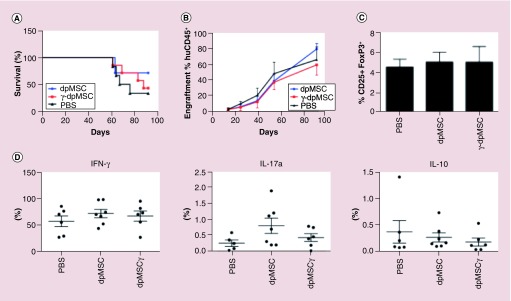
To determine, if the apparent failure of dpMSCs to directly modulate T-cell function following a single dose was a result of either insufficient longevity of the dpMSCs in vivo or failure to become licensed by IFN-γ [19], we assessed the effect of multiple doses of dpMSCs 5 × (4 × 106) either untreated or preconditioned with IFN-γ. We found that neither repeat doses of naive or IFN-γ-activated dpMSCs were able to suppress the xenogeneic T-cell response in the huPBMC-NSG model and again (Figure 3A & B), dpMSCs were unable to influence the phenotype of human CD3+ T cells in the spleen (Figure 3C & D).
Figure 3. . Repeat doses of activated dental pulp MSC does not inhibit disease onset or progression in humanized mouse model of graft-versus-host disease.
(A) Survival of NSG mice following iv. injection of 2 × 107 human PBMC (n = 7) and 5 subsequent injections of 4 × 106 naive (n = 7) or IFN-γ activated dpMSC (n = 7). (B) Engraftment, measured as the percent of human CD45+ cells relative to mouse CD45+ cells, in blood. (C) Proportion of FoxP3+ Tregs in spleens GvHD mice. (D) Intracellular cytokine staining of T cells isolated from spleens of huGvHD mice.
dpMSC: Dental pulp MSC; GvHD: Graft-versus-host disease; MSC: Mesenchymal stem cell; PBMC: Peripheral blood mononuclear cell.
In vivo tracking of dpMSCs by SPECT reveals that they die immediately after injection
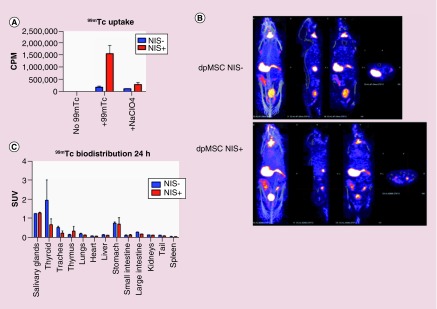
In order to understand more about the longevity of MSCs in vivo, we transduced dpMSCs with a retroviral construct encoding for human Sodium Iodide Symporter (NIS) (described previously in [1]), which resulted in dpMSCs expressing NIS and control cells NIS−. We then confirmed the functionality of NIS expressed by dpMSC by demonstrating specific uptake of 99mTc through NIS (Figure 4A). The specific uptake of 99mTc by NIS+ dpMSCs was blocked using sodium perchlorate (NaClO4) to further validate the uptake (Figure 4A). NSG mice were reconstituted with 2 × 107 human PBMCs and at the onset of GvHD symptoms, NIS- or NIS+ dpMSCs were injected intravenously (iv.). After 24 h, mice were injected iv. with 20 MBq of 99mTc to radiolabel the NIS+ cells in vivo and to understand more about the longevity of dpMSCs in vivo. We imaged these mice using NanoSPECT/CT 1-h postinjection of 99mTc. In both groups of mice (NIS- or NIS+) only the endogenously NIS expressing organs were observed and we could not identify any signals from the NIS+ dpMSCs (Figure 4B). In order to confirm our imaging data, mice were culled and 99mTc levels were measured in the numerous organs by direct scintillation (Figure 4C). The results from the biodistribution analysis also confirmed the imaging data that there was no significant difference between the radiolabel uptake by the organs in mice receiving either NIS- or NIS+ dpMSCs. The results presented here suggest that at least in this in vivo model dpMSCs die within 24-h postinjection.
Figure 4. . Viability dependent in vivo imaging of dental pulp mesenchymal stem cells.
(A) In vitro uptake of technetium 99m in NIS transduced (NIS+) and control (Nis-) dpMSC, error bars represent SEM of three technical replicates. (B) In vivo SPECT CT of dpMSC transduced with NIS (NIS+) and control (NIS-) at 24 h post iv. injection of dpMSC (images representative of three mice per group). (C) Ex vivo direct scintillation of organs from mice injected with NIS+ or NIS -ve MSC at 24 h (n = 3 per group).
dpMSC: Dental pulp MSC; MSC: Mesenchymal stem cell; NIS: Sodium iodide symporter; SEM: Standard error of the mean.
Apoptotic dpMSCs are very efficient in controlling lung inflammation
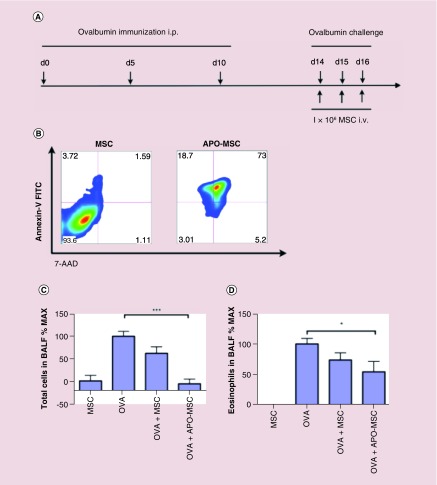
The finding that MSC fail to survive in any significant numbers does not necessarily contradict the finding that iv. administration of MSC exerts a therapeutic effect. Indeed, apoptotic cells and apoptotic cell debris are known to drive antigen presenting cells to adopt an immunosuppressive phenotype [32–34]. Thus, we hypothesized that via their apoptosis following iv. injection may account for the widely reported suppressive properties of MSC in vivo. To determine whether apoptotic dpMSCs retain their ability to modulate the immune system, we investigated their effect in a murine model of allergic lung inflammation induced by chicken egg OVA. This model was chosen for a number of reasons; first, unlike the humanized mouse model of GvHD (in which only human T cells engraft) the lung airway inflammation model is fully immunocompetent thus the APCs required to mediate the immunosuppressive effects of apoptotic cells would be present. Second, the model has previously been proposed as a useful tool to define MSC efficacy in vivo [35]. Third, as the name suggests, the pathology occurs primarily in the lung where, in line with previous reports, we would expect the MSC to become trapped following iv. injection [36], thus, providing the best opportunity to observe any immunomodulatory effect. Furthermore, this model has been well described and is characterized by a robust infiltration of eosinophils to the lung [37]. Mice were sensitized to OVA over a 2-week period followed by a nebulized OVA challenge every day for three consecutive days (Figure 5A). Immediately after each challenge, mice were injected with either 4 × 106 dpMSCs or an equivalent dose of dpMSCs that had undergone H2O2-induced apoptosis (Figure 5B). As expected, OVA sensitization and subsequent aerosol challenge resulted in robust allergic airway inflammation as determined by the number of eosinophils in the bronchoalveolar lavage fluid (Figure 5C). Mice treated with live dpMSCs exhibited a reduction in the number of inflammatory cells in the lung lavage that did not reach statistical significance. By contrast, mice treated with an equivalent dose of apoptotic dpMSCs displayed a significant inhibition of allergic airway inflammation both in terms of total cells (Figure 3A) and eosinophil number (Figure 3B). This data demonstrates that apoptosis of dpMSC alone, independently of any active immunomodulatory function, can account for the regulatory effects of dpMSCs in vivo.
Figure 5. . Apoptotic dental pulp MSC are effective at suppressing allergic airway inflammation.
(A) Schematic of ovalbumin allergic airway inflammation model. (B) Annexin-V viability staining of dpMSC, and apoptotic dpMSC following H202 treatment prior to injection (representative of two separate experiments). (C) Cellular infiltration in BAL fluid from female BALB/c mice sensitized to, and challenged with, OVA alone (n = 15) or mice that received co-injection of either live dpMSC (n = 15) or apoptotic dpMSC (n = 7). (D) Eosinophil infiltration in BAL as determined by histological analysis of eosin stained cyto-spin BAL samples.
BAL: Bronchoalveolar lavage; dpMSC: Dental pulp MSC; MSC; Mesenchymal stem cell; OVA: Ovalbumin.
Materials & methods
Cells
Deciduous teeth were collected from patients having given informed consent and stored overnight in DMEM 10% FCS. Pulp tissue was extracted and digested with 200 u/ml collagenase II (Worthington) at 37°C for 1 h. dpMSCs (also called SHED cells) were cultured in 10% DMEM/10%FCS at 37°C 5%CO2. SHED cells were confirmed to express CD90 and CD146 and capable of tri-lineage differentiation (osteoblast-like, chondrocyte-like and adipocyte-like) following appropriate stimulation (data not shown). PBMC were isolated form apheresis cones. CD4+ T cells were isolated by means of negative magnetic-bead selection against CD8 (Invitrogen), CD14, CD16, CD19, CD33 and CD56 (ebioscience) and biomag goat antimouse IgG magnetic-beads (Qiagen). CD4+ purity was consistently >95%. Teeth were collected with ethical approval, COREC AB/137374/1SSI 137374/225062/1, KCh REC 08/H0808/119.
Mice
NOD/scid/IL-2Rg-/-(NOD.cg-PrkdcscidIl2rgtm1Wjl/SzJ) were purchased from Charles River and maintained under pathogen-specific sterile conditions in the Biological Services Unit at King's College London. All procedures were performed in accordance with institutional guidelines, approved by the KCL ethics committee and the Home Office Animals Scientific Procedures Act under licenses, PPL7007866, PPL70/8279 and PPL70/7302 (1986). Mice were aged between 8 and 12 weeks at the time of experimental procedure. A total of 84 mice were used in this study.
Humanized mouse model of xeno-graft versus host disease
PBMC isolated from healthy donors were washed three-times in PBS prior to adoptive transfer into NOD/scid/IL-2Rg-/- mice via tail vein injection (2 × 107/mouse, in 200l of PBS). Tail bleeds were performed to monitor engraftment of human-CD45+ by flow-cytometry. Engraftment was defined as the ratio of human: mouse-CD45+ cells. Body weight was monitored and mice culled when they reached the humane end point of 15% decrease in body weight. MSC were expanded in vitro as previously described and were administered via tail vein injection in a volume of 200 μl.
MSC raidolabelling in vitro/vivo & NanoSPECT/CT imaging
MSCs where first transduced with a retrovirus encoding for human sodium iodide symporter (NIS) and a fluorescent reporter gene, mCherry, as described previously [43]. Then For assessing receptor mediated 99mTc uptake in vitro, transduced (NIS+) and nontransduced (NIS-) dpMSCs were washed in Hank's Balanced Salt solution (Gibco, Invitrogen) before 1 × 106 MSCs were incubated with 1 MBq of 99mTc (kindly provided by Department of Nuclear Medicine, Guy's and St. Thomas’ Hospital) for 15 min at 37°C. Cells were then pelleted and the supernatant carefully removed. The amount of 99mTc uptake was assessed using a Compugamma 1281 gamma counter (LKB Wallac). Included in same experiment was the NIS inhibitor, Sodium Perchlorate (100 μM per 1 × 106 cells).
For in vivo radiolabeling and imaging, mice that was discussed in previous section were iv. injected with 10× dpMSC NIS+ or NIS- dpMSC. The next day mice were imaged 1 h after intravenous injection of 20 MBq of 99mTc. Mice were imaged under inhaled isofluorane anaesthetics for 1 h using a NanoSPECT/CT preclinical imager (Bioscan) equipped with a multipinhole (nine pinholes, aperture 1.0 mm) collimator. Images were then reconstructed using the Invivoscope software (Bioscan). Mice were then culled and 99mTc biodistribution studies were performed at the end of the imaging. Standard uptake values of 99mTc were calculated by the formula: [CPM (organ)/weight (organ)]/[CPM (whole mouse)/weight (whole mouse)].
Ovalbumin induced model of allergic airway inflammation
Female BALB/c mice were immunized via intraperitoneal injection on days 0, 5 and 10 with 30 μg of OVA (grade V, Sigma) and 1 mg of 0.1 M Al(OH)3 diluted in saline (Sanofi/Aventis, Brazil). Sham animals received Al(OH)3 alone. All mice, including sham, were subsequently exposed to aerosolized OVA (3% solution) for 20 min periods, once per day, on days 14–16. Mice received 4 × 106 live or apoptotic MSC via tail vein injection immediately prior to aerosolized OVA challenge. MSC apoptosis was induced via overnight incubation with 5 mM H2O2 in DMEM 10% FCS.
Bronchoalveolar lavage
A total of 24 h after the last antigen challenge, mice were terminally anaesthetized with urethane (2 g/kg intraperitoneal; Sigma Chemical Co.) and a cannula was inserted into the exposed trachea and three 0.5 ml aliquots of sterile saline were injected into the lungs. The total number of cells in the lavage was counted with an improved Neubauer haemocytometer. For differential cell counts, cytospin preparations were prepared from aliquots of BAL fluid (100 μl) centrifuged at 1000 rpm for 1 min using a Shandon Cytospin 2 (Shandon Southern Instruments, PA, USA) at room temperature. Cells were stained with Diff Quick (DADE Behring, Germany) and a total of 100 cells were counted to determine the proportion of neutrophils, eosinophils and monocytes using standard morphological criteria.
Statistics
Statistical analysis was performed using GraphPad Prism V7.0. Statistical significance between two experimental groups was performed using two-tailed Student's t-test. Analysis of more than two groups was performed using one-way ANOVA controlling for multiple comparison false discovery rate using Benjamini Hochberg method. All significance is denoted as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
MSCs have been shown in multiple in vitro studies to have strong immunomodulatory effects, with the principal effector being the potent inhibition of T-cell function. These observations have provided the rationale for clinical translation of MSCs as a cellular immunotherapy. The physiological role of MSCs is to provide sources of cells to replace mesenchymal-derivatives in stromal tissues with high cell turnover (homeostasis) such as bone, or following stromal tissue damage to elicit repair. The natural ability of MSCs to modulate T-cell function would thus appear to be most likely related to their function in tissue repair where local, transient suppression of immune responses would benefit cell differentiation.
Following systemic administration, effective immune suppression by MSCs through a short-range, local response is inconsistent with the normal biology of MSCs, and with the anatomical fate of systemically administered MSCs [38]. While evidence exists that MSCs are able to undergo adhesion and transendothelial migration in vitro [39,40] there is no direct in vivo evidence that MSCs migrate through the circulation as part of their normal behaviour. Physiologically speaking (as distinct from therapy), it appears that MSCs are resident in all stromal tissues where they mediate tissue homeostasis and repair, without requiring mobilization and dissemination via the circulation. This is an important distinction since it makes it unlikely that MSCs are endowed with the complex molecular machinery required to mobilize, circulate, home and diapedise at sites of local inflammation, as would be required for direct immune.
The possibility that a short range endogenous in vivo mechanism of MSC action on immune cells can explain the observed effects following systemic injection seems unlikely and thus, alternative mechanisms need to be established. The obvious failure to detect substantial numbers of dpMSCs postsystemic injection either engrafted at sites of tissue disease and/or damage or distributed elsewhere in the body, is indicative of their short-term survival. We thus, hypothesized that such short-term survival is a result of rapid cell death and that this process may provide the stimulus that results in modification of immune-cell functions. To test this, we simply prekilled dpMSCs and injected these in a well-established mouse model of allergic lung inflammation and compared the resulting responses with the same number of live dpMSCs. Not only did the prekilled dpMSCs illicit a modulated immune response, the level of the effect was significantly greater than that observed with live dpMSCs. The most likely explanation for the greater effect is that apoptosis of live dpMSCs that become trapped in the lungs as a result of pulmonary first-pass effect occurs over time whereas injection of a bolus of prekilled dpMSCs accumulating in the lungs may provide a single, large response that compared with the relatively slower death of live dpMSCs. The effectiveness of this action may be enhanced in our model which involves lung inflammation and thus models involving more diffuse pathology need to be tested.
Conclusion
If, as we propose, the mechanism of immune modulation following systemic injection of MSCs is caused by apoptosis of cells trapped in the lungs then the injection of prekilled MSCs may provide a more effective and clinically much simpler and safer alternative. The therapeutic action of MSCs is often attributed to indirect effects results from secretion of multiple growth factors, cytokines and chemokines [41,42]. Clearly prekilled cells lack such secretion and could not replace any such effects. However, any effects on immune-cell modulation resulting from secretion by MSCs is predicated on their survival and engraftment, something that is not observed following systemic injection in mouse models and may only be an effective mechanisms where MSCs are applied locally.
Future perspective
The recent tightening of regulations regarding cell therapies offers new challenges for immune-modulatory MSC-based therapies. If, as we suggest here, the efficacy of these cells following systemic administration is based on apoptosis, then the clinical use of dead cells may offer a more straightforward route for approval that supports rapid clinical translation.
Summary points.
Mesenchymal stem cells inhibit T-cell proliferation in vitro
Dental pulp MSC (dpMSCs) do not support T-cell proliferation.
dpMSCs and bone marrow (bm)MSCs are equally effective at suppressing T-cell proliferation.
MSCs are unable to directly modulate human T-cell function in vivo
Despite their in vitro effects, dpMSCs do modulate T-cell function in vivo in a model of graft-versus-host disease.
Multiple doses of dpMSCs failed to modulate T-cell function.
In vivo tracking of dpMSCs by SPECT reveals that they die immediately after injection
dpMSCs do not survive for longer than 24 h following systemic administration.
Apoptotic dpMSCs are very efficient in controlling lung inflammation
Prekilled (dead) dpMSCs are as effective, if not more so that live MSCs at suppressing lung inflammation.
Footnotes
Financial & competing interests disclosure
This research was funded by the Medical Research Council (MRC) Centre for Transplantation, King's College London, UK – MRC grant no. MR/J006742/1. The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label Phase 2a proof-of-concept study. Lancet Neurol. 2012;11(2):150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a Phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]; • Mesenchymal stem cell (MSC) thereapy in steroid-resistant graft-versus-host disease (GvHD) in humans.
- 3.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 5.Fazekasova HH, Lechler RR, Langford KK, Lombardi GG. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J. Tissue Eng. Regen. Med. 2011;5(9):684–694. doi: 10.1002/term.362. [DOI] [PubMed] [Google Scholar]
- 6.Beyth SS, Borovsky ZZ, Mevorach DD, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 7.Di Nicola M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 8.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 9.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis & Rheumatol. 2007;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 10.Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI. Defining human mesenchymal stem cell efficacy in vivo . J. Inflamm. (Lond). 2010;7(1):51. doi: 10.1186/1476-9255-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrate efficacy of MSC in suppressing allergic airway inflammation – advocates the use of this model in assessing MSC in vivo efficacy.
- 11.Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299(6):L760–70. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 13.Yamaza T, Kentaro A, Chen C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell. Res. Ther. 2010;1(1):5. doi: 10.1186/scrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune Type 1 diabetes. J. Immunol. 2009;183(2):993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J. Immunol. 2008;181(6):3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 16.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 17.Polchert D, Sobinsky J, Douglas GW, et al. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008;38(6):1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 19.DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-γ-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng. Part A. 2009;15(10):2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 20.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 21.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007;149(2):353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meisel R. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 23.Najar M, Raicevic G, Id Boufker H, et al. Modulated expression of adhesion molecules and galectin-1: role during mesenchymal stromal cell immunoregulatory functions. Exp. Hematol. 2010;38(10):922–932. doi: 10.1016/j.exphem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell. Immunol. 2010;264(2):171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol. Cell Biol. 2010;88(8):795–806. doi: 10.1038/icb.2010.47. [DOI] [PubMed] [Google Scholar]
- 26.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Glennie S, Soeiro I, Dyson PJ, Lam EW-F, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 28.Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in “innate tolerance?”. Immunol. 2012;137(3):206–213. doi: 10.1111/j.1365-2567.2012.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl Acad. Sci. USA. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009;35(11):1536–1542. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Ali N, Flutter B, Sanchez Rodriguez R, et al. Xenogeneic graft-versus-host-disease in nod-scid il-2rγnull mice display a T-effector memory phenotype. PLoS ONE. 2012;7(8):e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren Y, Xie Y, Jiang G, et al. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J. Immunol. 2008;180(7):4978–4985. doi: 10.4049/jimmunol.180.7.4978. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y-J, Moon C, Lee SH, et al. Apoptotic cell instillation after bleomycin attenuates lung injury through hepatocyte growth factor induction. Eur. Respir. J. 2012;40(2):424–435. doi: 10.1183/09031936.00096711. [DOI] [PubMed] [Google Scholar]
- 34.Huynh M-LN, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 36.Riffo-Vasquez Y, Coates ARM, Page CP, Spina D. Mycobacterium tuberculosis chaperonin 60.1 inhibits leukocyte diapedesis in a murine model of allergic lung inflammation. Am. J. Respir. Cell Mol. Biol. 2012;47(2):245–252. doi: 10.1165/rcmb.2011-0412OC. [DOI] [PubMed] [Google Scholar]
- 37.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007;354(3):700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teo GSL, Ankrum JA, Martinelli R, et al. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30(11):2472–2486. doi: 10.1002/stem.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rüster B, Göttig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 40.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharif-Paghaleh E, Sunassee K, Tavaré R, et al. In vivo SPECT reporter gene imaging of regulatory T cells. PLoS ONE. 2011;6(10):e25857. doi: 10.1371/journal.pone.0025857. [DOI] [PMC free article] [PubMed] [Google Scholar]