Abstract
Background:
The Wnt signaling pathway involves secreted glycoproteins that bind to the Frizzled family receptors to activate intracellular signal transduction events that regulate cell proliferation, apoptosis, cell migration and many critical aspects of developmental biology.
Discussion:
Aberrant Wnt signaling underlies a wide range of pathologies in humans including tumor initiation, tumor growth, cell senescence, cell death, differentiation and metastasis. The inhibition of Wnt signaling offers a novel approach for anticancer therapeutics.
Conclusion:
Focusing on recent developments, we reviewed the small-molecule inhibitors targeting various components of Wnt signaling pathways and the progress from the discovery of lead compounds to highly potent inhibitors with significant therapeutic potential.
The Wnt signaling pathway is a complex network that regulates important biological processes such as embryonic development, cell fate determination, cell motility and stem cell renewal. Deregulation of the Wnt signaling proteins often leads to various pathologies including cancer. The Wnt proteins comprise 19 secreted glycoproteins that serve as extracellular Wnt signals. Upon binding to a Frizzled family receptor, Wnt proteins trigger intracellular signal transduction cascades by the canonical Wnt/β-catenin pathway or the β-catenin independent pathway which can be further divided into the planar cell polarity and the Wnt/Ca2+ pathway [1]. The Wnt/β-catenin cascade regulates gene transcription and is thought to be closely associated to cancer development [2–6]. Therefore, the majority of therapeutic development targeting the Wnt pathway involves interfering β-catenin associated pathways, and will be the focus of this review.
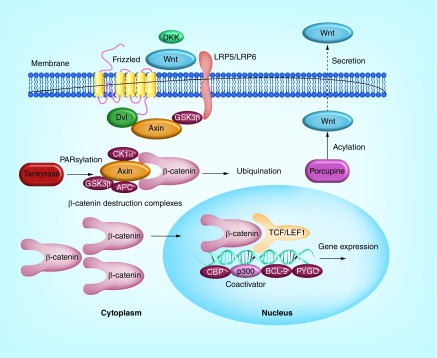
The hallmark of Wnt/β-catenin signaling is the involvement of a key mediator protein β-catenin, whose level is tightly controlled for its transcriptional regulation activities. Without Wnt signaling initiated by binding of a Wnt protein ligand to a Frizzled receptor, β-catenin in the cytoplasm is degraded by a destruction complex comprising Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK3) and casein kinase 1α (CK1α). Such degradation is carried out by CK1-mediated phosphorylation of β-catenin at Ser45 and GSK3-mediated phosphorylation at Ser33, Ser37 and Thr41, which constitutively targets β-catenin for ubiquitination and proteasome degradation. The binding of Wnt ligands to the cysteine-rich domain of Frizzled receptors leads to the disassembly of the destruction complex and accumulation of cytoplasmic β-catenin, which is imported into the nucleus where it interacts with a large number of binding partners such as the LEF/TCF DNA-binding transcription factors, E1A-associated protein p300, Pygopus (PYGO) and B cell lymphoma 9 (BCL-9) (Figure 1) [2,4–7].
Figure 1. . Diagram of the canonical Wnt/β-catenin signaling pathway.
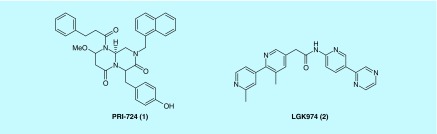
Aberrant Wnt signaling underlies a wide range of pathologies in humans including tumor initiation, tumor growth, cell senescence, cell death, differentiation and metastasis. The inhibition of Wnt signaling offers a novel approach for anticancer therapeutics [2,4–7]. To date, PRI-724 (1) [8] and LGK974 (2) (ClinicalTrials Identifier: NCT01351103) (Figure 2) as small molecule inhibitors of Wnt pathway are in clinical trials to treat cancer. There are a few published reviews on small-molecule inhibitors of Wnt pathway as potential new drugs for neoplastic diseases [9–13]. Herein, focusing on recent developments, we reviewed the small-molecule inhibitors targeting various components of Wnt signaling pathway and the progress from the discovery of lead compounds to highly potent inhibitors with significant clinical potential.
Figure 2. . Structures of PRI-724 (1) and LGK974 (2).
Approaches to disrupt Wnt signaling pathways
Targeting enzymes to disrupt Wnt signaling pathway
Inhibitors of porcupine (Porcn)
Porcupine, a membrane-bound O-acyltransferase, is essential to acylating Wnt proteins required for their biological activities. Compromised Porcn activity commonly results in developmental disorders including focal dermal hypoplasia, whereas hyperactivity of Porcn is associated with cancerous cell growth [14].
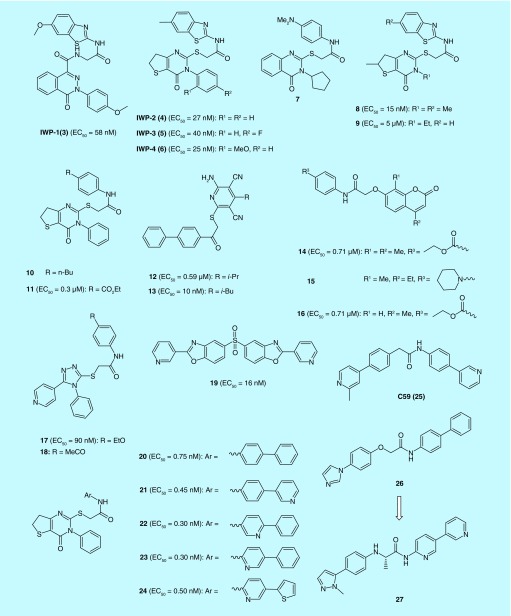
By screening a diverse synthetic chemical library, Chen et al. has discovered small molecules IWP1-IWP4 (3–6) that disrupt Wnt pathway responses. Further study confirmed that IWP compounds inhibited Porcn function thereby blocking palmitoylation of Wnt proteins [15]. Thirteen additional Porcn inhibitors were identified from the same screening that netted 3–6, as shown in (Figure 3). Five of them (7–11) possess similar molecular skeletons as 3–6. There are eight other inhibitors (12–19) representing different chemotypes [16]. Subsequent structure–activity relationship (SAR) studies of the pathalazinone and pyrimidinone inhibitors yielded new Porcn inhibitors (20–24) that suppress Wnt signaling at subnanomolar concentrations. Selected 24 has good stability in human plasma but compromised stability in rat and mouse plasma. The in vivo activities of 24 were also validated by their ability to disrupt well-established Wnt-dependent developmental processes of embryonic and juvenile zebrafish and the branching morphogenesis in cultured mouse embryonic kidneys [17].
Figure 3. . Structures of Porcn inhibitors and their EC50 values of Wnt pathway responses.
Liu et al. of Novartis developed a screen for small molecules that block Wnt secretion and this effort led to the discovery of LGK974 (2) [18]. Not only more potent than IWP compounds as a Porcn inhibitor, 2 also demonstrated promising in vivo efficacies and is currently in Phase I clinical trials to treat cancers (Clinical Trials Identifier: NCT01351103). C59 (25), an analog of 2, was evaluated for its activity and toxicity in cultured cells as well as in mice. 25 inhibited Porcn activity in vitro at nanomolar concentrations and blocked progression of mammary tumors in MMTV-Wnt1 transgenic mice while downregulating Wnt/β-catenin target genes. Surprisingly, mice exhibit no apparent toxicity. These results provided initial evidence that blocking Wnt signaling can be achieved by interfering Porcn function with small-molecule inhibitors as a therapeutically relevant approach [19].
Recently, Duraiswamy et al. identified 26 as Porcn inhibitor from cellular high-throughput screening. Classical SAR based cellular optimization resulted in the discovery of 27 with nanomolar activity and excellent bioavailability that demonstrated efficacy in a Wnt-driven murine tumor model. Finally, they also discovered that enantiomeric PORCN inhibitors show very different activity in their reporter assay, suggesting that such compounds may be useful for mode of action studies on the PORCN O-acyltransferase [20].
Inhibitors of tankyrase (TNKS)
Tankyrase (TNKS), including TNKS1 (also known as PARP5A and ARTD5) and TNKS2 (also known as PARP5B and ARTD6), is a another member of a poly(ADP-ribose) polymerase (PARP) at human telomeres which has wide-ranging roles in cellular processes such DNA repair and Wnt signaling. TNKS also mark the β-catenin destruction complex component axin for degradation and its inhibition enhances axin stability in β-catenin destruction complex and leads to the block of Wnt signaling. Thus, inhibition of TNKS activity is appearing as a promising strategy for targeting cancer by diverse mechanism [21].
Inhibitors by binding to the nicotinamide pocket of the PARP domain
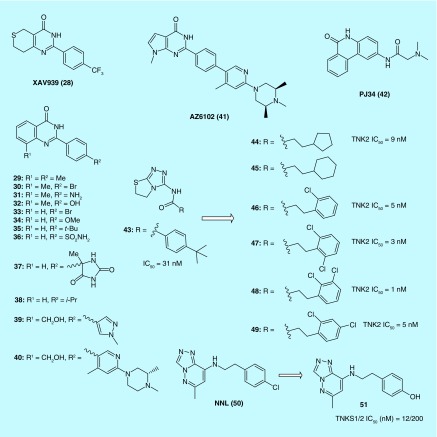
Also at Novartis, Huang et al. used a chemical genetic screen to identify XAV939 (28) as a Wnt signaling inhibitor (Figure 4). The authors further discovered that 28 disrupted Wnt signaling pathway by inhibiting TNKS. It is very interesting that parental H2170 and H358 cell (two kinds of non-small-cell lung cancer cells) displayed no sensitivity to 28 while resistant cells (made resistant to SU11274, a c-Met inhibitor and erlotinib, an EGFR inhibitor) were significantly inhibited (39%) by 28 [22].
Figure 4. . Structures of tankyrases inhibitors binding to the nicotinamide pocket of PARP domain.
Using 28 as a structural lead and the crystal structure [23] of TNKS2 with 28 as a template for structure-based design, Nathubhai et al. designed a series of 2-arylquinazolin-4-ones as potential inhibitors of TNKS, compounds 29–32 (IC50 = 6.7, 5.5, 3.3 and 5.5 nM) being more potent and selective than 28 (IC50 = 9.6 nM) against TNKS2. Methyl at the 8-position (the position where R1group is attached in compounds 29–30) appears to be optimal for potency, and a variety of substituents are tolerated at the para-position of the 2-phenyl ring [24].
On the basis of the same 2-phenyl-3,4-dihydroquinazolin-4-one scaffold, Haikarainen et al. [25] identified a set of TNKS inhibitors (33–37) which have similar potency as 28. Substitutions at the para position of the scaffold's phenyl ring were evaluated as a strategy to increase potency and improve selectivity. The compounds effectively inhibit Wnt signaling in HEK293 cells. The crystal structure of TNKS2 complex with all inhibitors showed that all inhibitors share a structure and binding mode similar to that of 28 that bind the nicotinamide site of the catalytic domain.
An AstraZenaca team [26] identified 38 with the same 2-phenylquinazolin-4-one scaffold as a TNKS1 inhibitor by an HTS assay. The result encouraged them to explore the series further and finally resulted in the discovery of AZ6102 (41) based on the crystal structure of TNKS1 complex with 39 and 40. 41 is a potent TNKS1/2 inhibitor that has 100-fold selectivity against other PARP family enzymes and shows 5 nM Wnt pathway inhibition in DLD-1 cells. It has demonstrated good pharmacokinetics in preclinical species and shows low Caco2 efflux to avoid a possible tumor resistance mechanism.
A recent report [27] provided details of the crystal structures of TNKS1 when the protein formed complexes with PJ34 (42) and 28. It was revealed that two molecules of 42 were bound to TNKS1, one in the Nicotinamide adenine dinucleotide donor pocket, the other bound in the adenosine portion of the pocket. In contrast to the crystal structure of TNKS1 in the absence of ligand binding, the TNKS1–42 complex maintained accessibility of the donor site to cocrystalized solvent molecules. Similarly, the crystal structure of TNKS1–28 was solved, providing a foundational understanding for the design of structure-based drugs targeting TNKS1 [28].
Shultz et al. of Novartis discovered [1,2,4]-triazol-3-ylamine derivatives, a group of novel nicotinamide isosteres using a combination of a parmacophore model and a biochemical HTS as an effective approach to speedy screening. Their study resulted in the identification of 43 as an efficient tankyrase inhibitor which showed remarkable selectivity compared with other PARP family members. Additional analogs (44–49) demonstrated high potency as well as favorable pharmcologocal and physicochemical properties [27].
Liscio et al. started hit compound NNL (50) and synthesized a small library of new triazolopyridazine derivatives. The absence of an amide moiety in an anticonformation as the classical pharmacophoric group of PARP inhibitors confers to this new class of derivatives with high TNKS’ specificity. Among them, 51 is the most active and selective derivative, endowed with low nanomolar values of IC50 on TNKS proteins and devoid of any PARPs cross-reactivity [29].
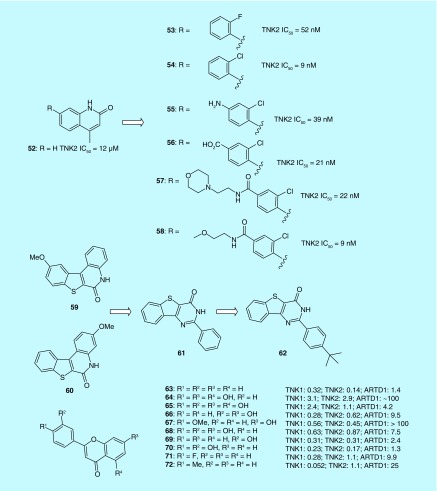
Larsson et al. used a fragment-based ligand design (FBLD) approach to identify novel inhibitors for TNKS2 in which the catalytic PARP domain was targeted. Chemical expansion from the nicotinamide-pocket-anchored fragment 52 along its 7-vector was achieved to exploit a novel chloro-O(sp2) interaction with the main chain carbonyl oxygen of Tyr1071. The study identified the nonplanar analog, 53 as being capable of docking into a pocket where TNKS and other PARP proteins have distinct features (Figure 5). Further structural optimization generated more potent analogs with improved solubility and PARP selectivity, as shown in compounds 54–58 [30].
Figure 5. . Structures of tankyrases inhibitors binding to the nicotinamide pocket of PARP domain.
Through pharmacophore screening of a compounds collection from the SPECS database, Liscio et al. identified the methoxy[l]benzothieno[2,3-c]quinolin-6(5H)-one (59 and 60) scaffold as nicotinamide mimetic. A method called tandem structure-based and scaffold hopping was adopted in this study in search of more potent and selective TNKS inhibitors. As a result, 61 (2-(phenyl)-3H-benzo [4,5] thieno[3,2-d]pyrimidin-4-one) was identified as a potent structural lead demonstrating excellent inhibition against TNK. From this virtual screening approach, the most promising compound 62 (2-(4-tert-butyl-phenyl)-3H-benzo [4,5] thieno[3,2-d]pyrimidin-4-one) was discovered which exhibited nanomolar potencies (IC50s TNKS1 = 21 nM and TNKS2 = 29 nM) and were found highly selective against a number of PARPs [31].
Narwal et al. screened a commercial flavonoid library and identified ten flavones derivatives (63–72) as TNKS inhibitors. These flavone derivatives were all found to bind to the nicotinamide binding site of TNKS2 with inhibitory activities ranging between between 50 nM and 1.1 μM as measured by IC50 values. In addition, the identified flavone inhibitors displayed up to 200-fold selectivity for TNKS over ARTD1. The authors proposed that further modification in the 4′ position may afford improved potency via additional interactions as demonstrated in the results of 72 [32].
Inhibitors by occupying the adjacent adenosine binding pocket
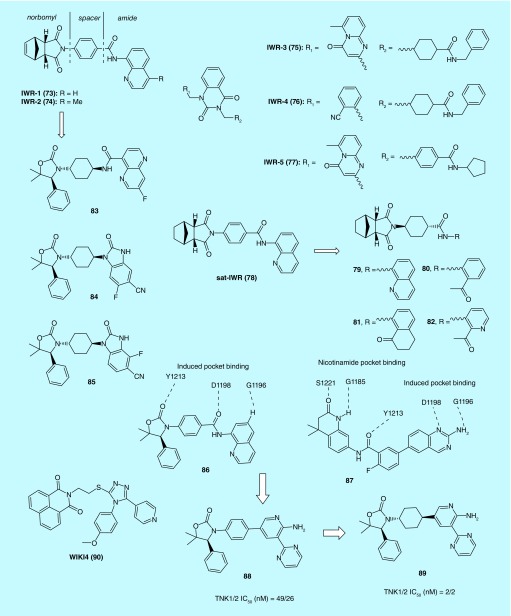
IWR-1-IWR-5 (73–77) compounds control levels of Wnt/β-catenin pathway response by inducing stabilization of Axin proteins via a direct interaction [15]. The crystal structure of human TNKS2 catalytic fragment (PARP) in complex with 73 was reported (Figure 6). The first crystal structure that do not bind to the widely utilized nicotinamide-binding site make the structure valuable for development of PARP inhibitors in general [33].
Figure 6. . Structures of tankyrases inhibitors occupying the adjacent adenosine binding pocket.
Lu et al., have conducted focused structure-activity relationship studies on IWR-1 (73) and IWR-2 (74). The norbornyl, spacer and amide regions can all be modified with compromised activity. Sat-IWR (78) and 73 were equally potent in the in vitro assays with EC50 of 0.2 μM [34]. Lanier et al. further optimized the structure of 73 and prepared a novel series of analogs. Among them, compounds 79–82 showed decreased IC50 values for inhibition of the Wnt pathway to compared 73 [35].
An Amgen team utilized the method of structure-based design and molecular modeling to modify 73 to yield new lead molecules within amide and benzimidazoline series including 83–85 that have good selectivity over PARP1/2. Amide containing lead 83 had moderate cellular potencies but poor oral exposure in mice. Disubstituted benzimidazoles (84 and 85) were found to be 40-fold more potent than 73 in inhibiting Axin in vitro. Moreover, in vivo pharmacology study showed plasma free drug concentration (Cmax) was significantly greater than the cellular IC50 values [36].
An overlay of the cocrystal structures of 86 and 87 bound to TNKS1 suggests that replacing the labile aminoquinazoline amide of 86 with a 2-aminopyridine might increase stability in plasma while maintaining two critical H-bond interactions with the protein. The aminopyridine moiety would serve as a simplified mimic of the aminoquinazoline group in 87. This rationale prompted the design and synthesis of a series of 2-aminopyridine oxazolidinones that showed improved cellular activities via inhibition of TNKS with excellent selectivity over other PARP family members. Notably, studies of the cocrystal structures of the 2-aminopyridine oxazolidinones with TNKS suggest a different binding mode from one that involves interactions with the nicotinamide binding pocket. In a subsequent animal study, compounds 88 and 89 were administered orally to mice bearing DLD-1 tumor. The in vivo efficacy was confirmed by measuring the Wnt-pathway biomarkers [37].
Using HTS, James et al. identified WIKI4 (90) as a Wnt/β-catenin signaling inhibitor. The compound inhibited Wnt/β-catenin signaling in both cancer cell lines and human embryonic stem cells. In addition, the inhibitory effect of 90 on the Wnt/β-catenin signaling pathway was shown to be mediated by blocking the enzymatic activity of TNKS2 [38]. Further studies determined the binding mode of 90 to TNKS2 by x-ray crystallography with a resolution of 2.4 Å, uncovering a novel binding mode of the compound to the adenosine subsite of the donor NAD+ binding groove of the catalytic domain. Thus, the scaffold of 90 may serve as a structural template from which to design and synthesize TNKS inhibitors with greater potency and selectivity [39].
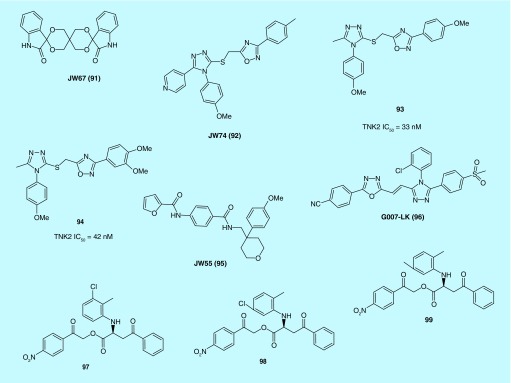
Waaler et al. identified JW67 (91) and JW74 (92) as efficient and specific inhibitors of the canonical Wnt signaling through a high-throughput screening (Figure 7). 91 and 92 rapidly reduced active β-catenin with a subsequent downregulation of Wnt target genes, Axin2 protein levels were significantly increased after treatment with the compounds. Long-term treatment with 92 inhibited the growth of tumor cells in both a mouse xenograft model of colorectal cancer cells (CRC) and in ApcMin mice [40]. 92 was also demonstrated to reduce cell growth and differentiation of osteosarcoma cells [41]. It is very interesting that neither 91 nor 92 is structurally similar to IWR-1 (73) or XAV939 (28) and shows efficacy in submicromolar concentrations in similar assays as a TNKS inhibitor, but 90 has amazing structural similarity with 17 and 18 as Porcn inhibitors.
Figure 7. . Structures of tankyrases inhibitors occupying the adjacent adenosine binding pocket.
Schulz et al. of Novartis based on the structure of 92 and utilizing composite parameter analysis helped identify several analogs including 93 and 94 with low nanomolar TNKS inhibition and submicromolar activity in cellular models of Wnt pathway signaling. A crystal structure determination of the lead compound 93 revealed a novel binding mode with a PARP family member, involving the adenosine binding pocket of TNKS1/2. This hitherto unknown mode of binding may have contributed to its selectivity against other PARP family members [42].
JW55 (95) is another TNKS inhibitor which was identified by Waaler et al. Similar to XAV939 (28), 95 inhibits auto-PARsylation of TNKS1 and TNKS2 in a biochemical assay. However, in contrast to 28, no evidence was found for the inhibition of PARA1 by 95, making this chemotype attractive for further development toward a TNKS1/2 selective inhibitor [43].
Also on the basis of JW74 (92), Voronkov et al. developed 1,2,3-triazole-based JW74 analogs from which G007-LK (96) emerged as a potent, ‘rule of 5’ compliant and metabolically stable TNKS1/2 inhibitor. The IC50 value of 96 against TNKS1 and 2 was determined to be 46 nM and 25 nM, respectively, with a cellular IC50 measured at 50 nM. Study of the crystal structure of 96 bound to TNKS2 showed distinct interactions in the extended adenosine binding pocket [44]. In vivo, 96 inhibited growth of APC-mutant CRC xenograft tumors. In particular, in a xenograft model derived from the COLO-320DM cell line, 96 was found to inhibit cell cycle progression and colony formation while driving cancer cell differentiation. This observation indicates that TNKS inhibition may disrupt the undifferentiated state of cancer cell maintained by β-catenin-dependent signaling. Despite its favorable pharmacokinetic properties, intestinal toxicity associated with G007-LK (96) may limit the clinical potential of the Wnt/β-catenin signaling inhibitor [45].
Kirubakaran et al. assessed five lead molecules identified from a screening of three compound databases by a calorimetric assay. One of the five compounds, 97 was observed to potently inhibit TNKS1 activity and to exert significant cytotoxicity and block cell growth, more so than 98 and 99. The morphological assessment, DNA damage and chromatin condensation and fragmentation results also confirmed that 97 has enhanced activity against MCF-7 cells [46].
Inhibitors that bind to both the nicotinamide pocket & the induced pocket
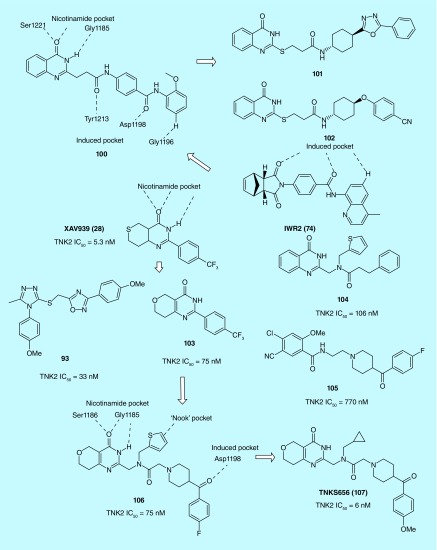
An Amgen team identified 100 as the most potent inhibitor of TNKS with an IC50 of 8 nM through a combination of substructure searching of the Amgen compound collection based on a minimal binding pharmacophore hypothesis and HTS (Figure 8). Interestingly, 100 appeared to possess not only the binding motif for the induced pocket but also the binding motif for the nicotinamide pocket [47]. Further structure- and property-based optimization of 100 leading to the identification of more potent and selective TNKS inhibitors 101 (IC50 = 0.1 nm) and 102 (IC50 = 0.1 nm) with improved pharmacokinetic properties in rodents, which are well suited as tool compounds for further in vivo validation studies [48].
Figure 8. . Structures of tankyrases inhibitors binding to both the nicotinamide pocket and the induced pocket.
A Novartis team optimized XAV939 (28) into a dihydropyran motif (103) with greater stability and efficiency but lower potency. This was achieved by a unique approach combining structure-based design and LipE-based structure–efficiency relationship. The dihydropyran core was incorporated into other activity-conferring elements from 93, 104 and 105. The results were two promising TNKS inhibitors, 106 and 107 with superb selectivity and pharmacological profiles. In particular, 107 showed excellent oral bioavailability in mouse, acting as an antagonist of Wnt signaling pathway in the MMTV-Wnt1 mouse xenograft model. Overall, the unique binding mode characterized by enthalpy-driven thermodynamics, the improved physicochemical properties and enhanced lipophilic efficiency make 107 a good candidate for further therapeutic development [49].
Inhibitors of p300 histone acetyl transferase (p300)
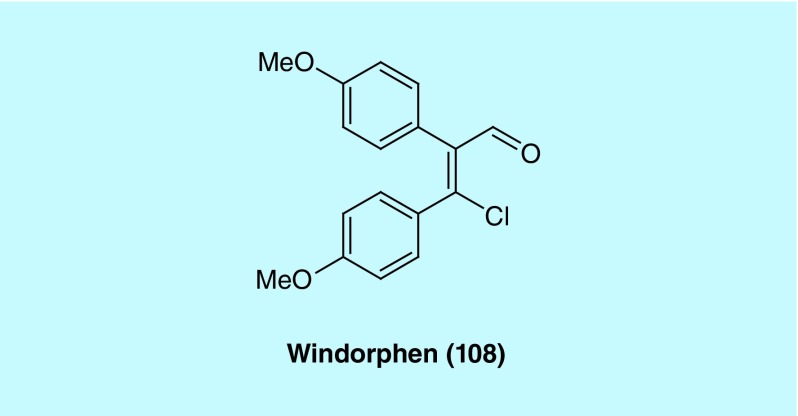
In a zebrafish-based in vivo screen for small molecules that specifically perturb embryonic dorsoventral patterning, Hao et al. discovered windorphen (Figure 9, 108) that selectively blocks the Wnt signal required for ventral development. 108 exhibits remarkable specificity toward β-catenin-1 function, indicating that the two β-catenin isoforms found in zebrafish are not functionally redundant. It was demonstrated that 108 selectively inhibited p300, a β-catenin-associated coactivator. In cancer cells where Wnt signaling is constitutively activated by mutations, 108 was shown to be strongly cytotoxic. Thus, this type of Wnt signaling inhibitors holds therapeutic potential [50].
Figure 9. . Structure of windorphen (108) as a p300 inhibitor.
Targeting protein–protein interactions & other types of inhibitors
Effect on β-catenin activity in nucleus
Inhibitors of T cell factor/β-catenin protein–protein interactions
The formation of the β-catenin/Tcf complex in the cell nucleus is the penultimate step of canonical Wnt signaling. Key molecular lesions in colorectal and other cancers cause β-catenin-dependent transactivation of Tcf-dependent genes [51,52]. Selective disruption of this signal represents an opportunity for rational cancer therapy.
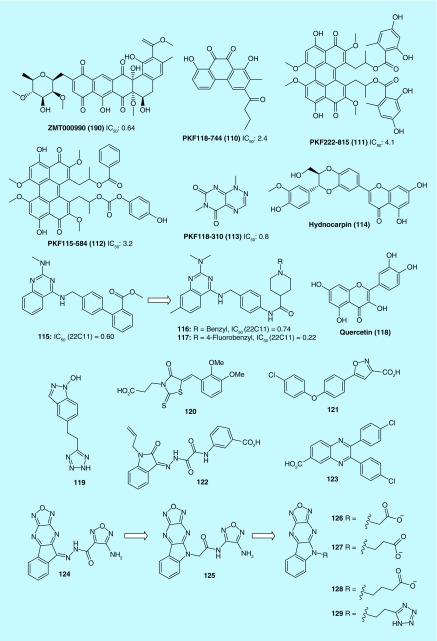
Lepourcelet et al. identified several antagonists (109–113) of the Tcf/β -catenin protein complex from an HTS screening of libraries of natural compounds (Figure 10). These compounds were found to disrupt Tcf/β-catenin complexes and inhibit β-catenin-driven cellular processes such as cell proliferation, reporter gene activation, expression of c-myc or cyclin D1 and duplication of the Xenopus embryonic dorsal axis [53]. Lee et al. discovered a natural lignan Hydnocarpin (114) from Lonicera japonica that acted as an inhibitor of Wnt/β-catenin signaling. In SW480 human colon cancer cells, the Wnt signaling suppression by 114 was also believed to be responsible for the arrest of cell proliferation [54].
Figure 10. . Structures of inhibitors of β-catenin/Tcf interaction and their IC50 value (μM).
A Wyeth team reported that 115 was a potent inhibitor of β-catenin/Tcf-4 through screening of their compound library. Replacement of the biphenyl moiety in 115 with an N-phenylpiperidine-4-carboxamide chain led to a series of new analogs that retained much of the potency as inhibitors of the β-catenin/Tcf-4 signaling pathway. These new compounds (e.g., 116) were also effective in suppressing β-catenin/Tcf-4-mediated cellular effects, as well as good solubility and desirable metabolic stability and oral bioavailability. However, neither compound 116 nor 117 demonstrated significant in vivo efficacy in HT29 and HCT116 xenograft models. Further optimization in in vivo potency and PK properties of this series will be needed [55].
Park et al. found that quercetin (118) potently inhibited the binding of the Tcf complexes to its specific DNA-binding sites, suggesting that its suppression of transcriptional activity of β-catenin/Tcf in cancer cells was mediated by its action through β-catenin. Additional studies by immunoprecipitation and Western blot confirmed that 118 disrupted the binding of β-catenin to Tcf-4 by reducing the nuclear level of β-catenin and Tcf-4 proteins [56]. The structural difference between flavone derivatives (63–72) as TNKS inhibitors and 118 is that 118 has an extra 3-position hydroxyl group substituent. Therefore, the 3-position hydroxyl group of 118 could play a crucial role in targeting the β-catenin/Tcf interaction.
Ji et al. identified 119, [57] 120–123 [58] and 124 [59] as new inhibitors for β-catenin/Tcf protein–protein interaction (PPI) with AlphaScreen and FP assays. The optimization of 124 resulted in chemically stable derivatives (125–129) that disrupt the β-catenin/Tcf PPI. The binding mode of new inhibitors was characterized by site-directed mutagenesis and structure–activity relationship studies. This series of inhibitors with a new scaffold exhibits dual selectivity for β-catenin/Tcf over β-catenin/cadherin and β-catenin/APC PPIs. 129 of this series suppress canonical Wnt signaling, downregulates the expression of Wnt target genes and inhibits the growth of cancer cells. 129 represents a solid starting point for the development of potent and selective β-catenin/Tcf inhibitors [59].
Inhibitors of β-catenin/LEF-1 PPIs
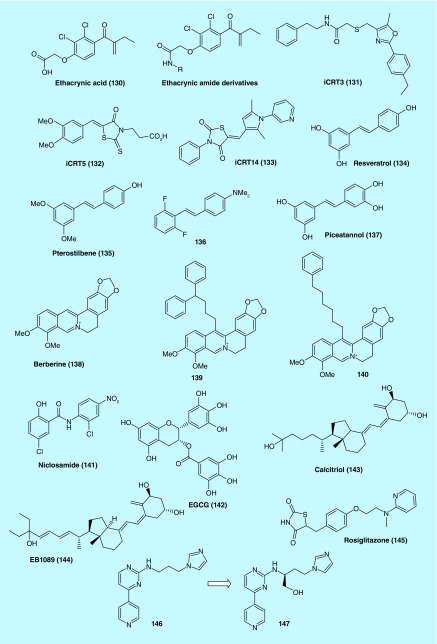
A cell-based Wnt reporter assay identified ethacrynic acid (Figure 11, EA, 130) as an inhibitor of Wnt signaling. 130 was previously known as a diuretic agent, yet in vitro assays in this study [60] demonstrated its inhibitory effect on Wnt/β-catenin signaling. Moreover, primary chronic lymphocytic leukemia (CLL) cells treated with 130 were observed to have downregulated Wnt/β-catenin target genes including LEF-1, cyclin D1 and fibronectin and are subject to cell death induced by 130. Immunoprecipitation study further revealed that the LEF-1/β-catenin complex was destabilized by binding of 130 to the LEF-1 protein. The ability of 130 to induce apoptosis in CLL cells through the drug's inhibition of Wnt/β-catenin signaling was abrogated by N-acetyl-l-cysteine (NAC), an agent that is known to react with the α, β-unsaturated ketone in 130, but not other anti-oxidants [60]. 130 significantly reduced cell viability in murine, as well as human pancreatic cancer cell lines [61]. Another report described the preparation and biological evaluation of a series of amides of ethacrynic acid [62]. A number of these derivatives were identified as having micromolar potency in their ability to inhibit Wnt signaling and CLL cell survival [63].
Figure 11. . Structures of inhibitors of various protein–protein interactions.
Inhibitors of β-catenin/CBP PPIs
Emami et al. identified ICG-001 (also known as PRI-724, Figure 2, 1) as a specific inhibitor of β-catenin/T cell factor signaling. 1 was found to bind to the cyclic AMP response element-binding protein and selectively induce apoptosis of colon cancer cells but not normal colon cells. 1 inhibited both in vitro colon cancer cells and in vivo xenograft tumor growth in C57BL6J-Apc Min/+ mouse and nude mouse models [62]. The compound was recently investigated in detail by Arensman et al. for its therapeutic potential for pancreatic cancer using pancreatic ductal adenocarcinoma (PDAC) cell line. When used alone, 1 was found to inhibit both anchorage-dependent and anchorage-independent growth of several PDAC cell lines. When used in combination with gemcitabine, inhibition of cell growth was enhanced. However, the synergistic effect was not mediated through PDAC apoptosis, but through increased induction of G1 cell-cycle arrest. These findings did not appear to involve the inhibition of Wnt/β-catenin-mediated transcription. DNA microarray analysis of the PDAC cells treated with 1 showed alteration of several gene expressions including SKP2 and CDKN1A that are known to regulate DNA replication and cell-cycle progression. Importantly, in an in vivo orthotopic xenograft model of PDAC, mice treated with 1 had significantly longer survival time, demonstrating the compound's potential as small molecule lead for therapeutic development for treatment of pancreatic cancer [64].
By inhibiting expression of β-catenin target gene
An RNAi-based chemical genetic screen identified iCRT3 (131), iCRT5 (132) and iCRT14 (133) inhibitors of Wnt/β-catenin signaling pathway. The inhibitors efficiently block Wnt/β-catenin-induced target genes and phenotypes in various mammalian and cancer cell lines. The data support for the specificity of these inhibitors in antagonizing the transcriptional function of nuclear β-catenin. Importantly, they are specifically cytotoxic to human colon tumor biopsy cultures as well as colon cancer cell lines that exhibit deregulated Wnt signaling [65]. Effect of these compounds on cell proliferation and apoptosis were tested in several triple negative breast cancer (TNBC) cell lines (BT-549, MDA-MB-231, HCC-1143 and HCC-1937) and the ER+ cell line MCF-7. 131 was the most potent compound for inhibiting proliferation and antagonizing Wnt signaling in TNBC cells. In addition, treatment with 131 resulted in increased apoptosis in vitro [66].
Zhang et al. confirmed that naturally occurring stilbene derivatives, resveratrol (134) and pterostilbene (135) could inhibit Wnt signaling and repress CRC cell proliferation at concentrations greater than 10 μM. To improve potency, a series of fluorinated N,N-dialkylaminostilbenes were prepared of which 136 emerged as a potent Wnt signaling inhibitor exhibiting nanomolar activity against the growth of CRC cells in vitro. In a xenograft nude mice model, 136 was efficacious at a dose of 20 mg/kg. The fluorinated analogs were proposed to act at the transcriptional level, downstream of Wnt/β-catenin signaling [67]. Schmeel et al. reported that piceatannol (137) acted as a strong inhibitor of Wnt/β-catenin signaling that induced apoptosis of myeloma cells, but not in normal cells [68].
Albring et al. investigated berberine (138) and its 13-arylalkyl derivatives (139 and 140) for their ability to inhibit Wnt/β-catenin signaling. HEK-293 and HCT116 cells treated with 138 were found to have reduced cytoplasmic β-catenin levels and increased E-cadherin expression. The arylalkyl derivatives of berberine (139 and 140) were significantly more potent than barberine with over 100-fold lower EC50 values. This study suggests that berberine derivatives may represent potential anticancer agents targeting the Wnt signaling [69].
Effects on activity of disheveled proteins in cytoplasm
Inhibitors of SFRP-1/Wnt PPIs
Wnt proteins bind to seven-transmembrane Frizzled receptors to mediate the important developmental, morphogenetic and tissue-regenerative effects of Wnt signaling. Dysregulated Wnt signaling is associated with many cancers.
Chen et al. reported that niclosamide (141) induced endocytosis of Frizzled1, downregulation of Dvl-2 and inhibition of Wnt3A-mediated β-catenin stabilization and LEF/TCF reporter activity [70]. The internalized Frizzled1 receptor then colocalized in vesicles containing Transferrin and agonist-activated β2-adrenergic receptor. This finding suggests that 141 may act as a negative mediator of the Wnt/Frizzled1 signaling by eliminating Frizzled1 and Disheveled (Dvl) proteins. In addition, 141 may also serve as a research tool with which to investigate the physiological consequences of Wnt signaling [70].
Gödeke et al. found that the naturally occurring epigallocatechin-3-gallate (EGCG, 142) is highly effective against hepatoblastoma (HB) cell growth through inhibition of Wnt signaling. Interestingly, 142 restored the expression of SFRP1, a tumor suppressor gene in HB cells where it was transcriptionally silenced. SFRP1 has been known to suppress Wnt signaling, thus restoration of its expression by 142 represents a viable therapeutic approach. The benign nature of 142 in normal cells should therefore warrant further preclinical studies to explore its potential utility as an adjuvant [71].
Inhibitor of Wnt/DKK-1 PPIs
DKK-1 is a secreted inhibitor of canonical Wnt signaling. Aguilera O et al. [72] reported that Calcitriol (1α,25-dihydroxyvitamin D3, 143), the most active vitamin D metabolite, increased expression of DKK-1 RNA and protein in human SW480-ADH colon cancer cells. Immunodeficient mice bearing SW480-ADH tumors were treated with EB1089 (144) and the tumors were found to have upregulated DKK-1 protein expression. Further studies of 32 human colorectal cancer cell lines revealed a correlation between the expression of VDR and DKK-1 RNA [72].
Treatment with thiazolidinediones (TZDs) rapidly increased DKK-1 protein levels and secretion in both fully differentiated adipose cells and pre-adipocytes undergoing differentiation. Serum levels of DKK-1 were also increased in several patients with Type 2 diabetes after treatment with rosiglitazone (145) for 90 days [73].
Gilbert et al. of Wyeth prepared a series of (hetero)arylpyrimidines agonists of the Wnt-β-catenin cellular messaging system from 146 (Figure 11) that was initially discovered from an HTS effort. These compounds were found to have significant activity in U2OS cells overexpressing Wnt-3a, TCF-luciferase, DKK-1 and tk-Renilla. Importantly, inhibition of GSK-3β was minimal when selected arylpyrimidines were used, suggesting that the agonists may act through Wnt-3a/DKK-1 to exert their effect. In a mouse calvaria model, the compounds 146 and 147 showed osteogenic activity in which nonphosphorylated β-catenin formation in bone was promoted [74].
Inhibitors of Dvl/Frizzled PPIs
Dvl is an essential protein in the Wnt signaling pathways; it uses its PDZ domain to transduce the Wnt signals from the membrane receptor Frizzled to downstream components.
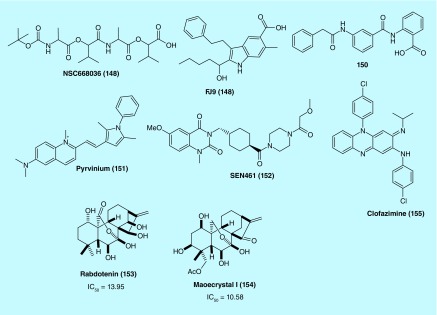
Shan et al. conducted a structure-based virtual screening of the NCI small-molecule library and identified NSC668036 (148) as a lead compound that showed binding activity toward the Dvl PDZ domain. Based on the structural features of 148 (Figure 12), molecular dynamics simulation was performed to characterize the binding of 148 to the PDZ domain. Moreover, Wnt3A-induced signaling was inhibited by 148. Thus, this compound could serve as a basis for rational design in order to obtain high-affinity inhibitors of the PDZ domain, which in turn can suppress Wnt signaling by disrupting the Frizzled–Dvl interaction [75].
Figure 12. . Structures of inhibitors of various Wnt signaling pathway.
Fujii et al. found that the PPI between the Frizzled-7 Wnt receptor and the PDZ domain of Disheveled can be disrupted by a small molecule FJ9 (149). Further, 149 downregulated the canonical Wnt signaling and inhibited growth of cancer cells. The compound was also found to inhibit tumor growth in a mouse xenograft model of H460 tumor cells. As the first nonpeptide inhibitor, 149 showed show promising therapeutic efficacy through disruption of PDZ protein–protein interactions [76].
Grandy et al. used a structure-based ligand screening and NMR spectrometry to identify a drug-like small molecule 150 as a Wnt signaling inhibitor. In prostate cancer cells (PC-3), 150 was effective in growth inhibition. The study also suggests that 150 inhibits the Wnt signaling by blocking the PDZ domain of Dvl [77].
Effect on Axin activity
By activating CK1α & disrupting the interaction of β-catenin/PYGO
β-catenin degradation occurs primarily via its association with a complex consisting of glycogen synthase kinase 3 (GSK3), casein kinase 1α (CK1α), adenomatous polyposis coli (APC) and Axin.
Using Xenopus laevis egg extract to screen for compounds that both stabilize Axin and promote β-catenin turnover, Thorne et al. identified pyrvinium (Figure 12, 151) as a potent inhibitor of Wnt signaling (EC50 of ˜10 nM). Mechanistically, 151 showed potent binding affinity (low nM range) towards all family members of CK1 and selective agonism for CK1α as evidenced in the loss of its inhibitory effect on Wnt signaling in CK1α knockdown cells. In addition, 151 induced the degradation of a Wnt transcriptional component, Pygopus (PYGO). Colon cancer cells treated with 151 showed suppressed Wnt signaling and proliferation. The study suggests that CK1α activation by small molecules such as 151 can be an effective approach to Wnt signaling inhibition a novel strategy for therapeutic development [78].
By activating Axin 1 gene expression
The scaffold protein Axin1, a key negative regulator of the Wnt signaling with tumor suppressor function, resulting in downstream effects coherent with inhibition of canonical Wnt signaling.
Rorbertis et al. discovered a primary hit series as Wnt signaling inhibitors in glioblastoma (GBM) cells after a screening campaign. Medicinal chemistry modification and optimization of the original structures led to the identification of an advanced lead compound SEN461 (Figure 12, 152). 152 was found to inhibit the Wnt/β-catenin signaling pathway in GBM cells. It was confirmed that 152 induced Axin stabilization, enhanced β-catenin phosphorylation and degradation and inhibited anchorage-independent growth in both human GBM cells and patient-derived primary tumor cells. Furthermore, 152 inhibited the growth of GBM xenografted tumor and antagnonized Wnt signaling in Xenopus embryos [79]. Targeting different sarcoma cell lines with 152 produced a less transformed phenotype, as supported by modulation of anchorage-independent growth in vitro. At the molecular level, 152 treatment enhanced the stability of Axin1 which in turn led to genetic phenocopies that showed impaired soft-agar growth. Axin stabilization by small molecules like 152 also inhibited sarcoma growth in female CD-1 nude mice bearing the xenograft tumor. These results indicate that inhibition of Wnt signaling by stabilizing Axin activity has clear relevance to clinical strategies for treatment of sarcomas [80].
Other types of inhibitors
Recently, Zhang et al. used a dual-luciferase reporter gene assay to screen a natural ent-kauranoid library to identify rabdoternin B (153) and maoecrystal I (154) (Figure 12) as two potent inhibitors of the Wnt signaling pathway. 153 and 154 were able to block Wnt signaling in a dose-dependent manner and showed strong toxicity against colon cancer cells including SW480, HCT116 and HT29 but not in CCD-841-CoN, a normal colonic epithelial cell line. Flow cytometry study demonstrated that these two compounds induced G2/M phase arrest in SW480 cells. At the molecular level, 154 downregulated c-myc, cyclin D1, survivin and Axin2 in colon cancer cells, key Wnt signaling target genes, providing evidence that 153 and 154 represent novel inhibitors of the canonical Wnt/β-catenin signaling pathway with clinical potential for treatment of colon cancer [81].
Koval et al. reported that an in silico screening of the library of US FDA-approved drugs came up with a number of potential antagonists of the Wnt–Frizzled ligand-receptor interaction. Of the 14 compounds tested by the TopFlash luciferase reporter assay, four compounds were identified as potent and specific inhibitors of the Wnt3a-mediated signal transduction. Subsequent functional studies using GTP-binding and β-catenin stabilization assays revealed that these compounds acted by inhibiting the signaling downstream of the Wnt-FZD interaction. For instance, an antileprosy drug clofazimine (Figure 12, 155) was found to suppress the growth of triple-negative brest cancer cells that are Wnt-signaling dependent. These results suggest that further investigation of 155 for its therapeutic potential in treatment of Wnt-dependent cancers may be a promising direction [82].
Future perspective
The Wnt signaling pathways have proven to be an attractive target for the discovery of anticancer drugs from studies over the past several decades. On one hand, the multiple components involved in the complex Wnt signaling network and the crucial roles of Wnt signaling in both normal biological processes and pathologies present enormous challenges to therapeutic development. On the other hand, as the saying goes, ‘challenges and opportunities coexist’, the various components associated with the Wnt pathways also provide a rich source of druggable targets for exploring small-molecule inhibitors acting on different constituents of the Wnt signaling network. Indeed, such increased opportunities have been evidenced in the wide-range of available inhibitors targeting various Wnt elements. For instance, the Porcupine inhibitor LGK974 (2) (Phase I), the β-catenin/CBP interaction inhibitor PRI-724 (1) (Phase I), the Wnt/Frizzled interaction inhibitor OMP-18R5 and OMP-54F28 (Monoclonal antibody, Phase I and II) [11] and the TNKS1/2 inhibitors XAV939 (28) and JW55 (95) (preclinical studies) all demonstrate the multitude of Wnt signaling targets. However, despite progress in the discovery of numerous inhibitors that target various points in the Wnt signaling cascade, few small molecule inhibitors currently exist that directly act on the Wnt ligand, which remains an under-explored direction that warrants continued investigation.
Several studies that just came out recently have continued to expand the scope of possible small molecules the can interfere with the Wnt pathways. Anthracene-9,10-dione dioximes potently inhibit β-catenin in vitro and the growth of several cancer cell lines; [83] pryanopyridones were demonstrated to be cell active and orally bioavailable TNKS inhibitors [84]. A highly specific, direct p300/β-catenin antagonist was recently reported [85]. Last but not least, an active β-catenin signaling intrinsic to melanoma was identified as the culprit of T-cell exclusion and resistance to anti-PD-L1/anti-CTLA-4 monoclonal antibody therapy, suggesting that the Wnt/β-catenin signaling pathway may become a new target for immune potentiation [86].
Key terms.
Wnt signaling pathway: A group of signal transduction pathways made of proteins that pass signals from outside of a cell through cell surface receptors to the inside of the cell.
Wnt/β-catenin pathway (or canonical Wnt pathway): One of the three best characterized Wnt signaling pathways, causes an accumulation of β-catenin in the cytoplasm and its eventual translocation into the nucleus to act as a transcriptional coactivator of transcription factors that belong to the TCF/LEF family.
Porcupine: Is a member of the membrane-bound O-acyltransferase family of proteins. It catalyzes the palmitoylation of Wnt proteins, a process required for their secretion and activity.
Tankyrases: Are proteins with poly(ADP-ribose) polymerase activity. They promote the degradation of Axin which in turn enhances β-catenin stability and Wnt signaling.
β-catenin destruction complex: Is likely a dynamic multiprotein assembly, its core components include β-catenin itself, the Ser/Thr kinases glycogen synthase kinase 3 (GSK-3) and casein kinase 1 (CK1), the scaffolding protein Axin and the APC protein. Without Wnt signaling, β-catenin is degraded by the destruction complex.
Protein–protein interactions: Refer to physical contacts established between two or more proteins as a result of biochemical events and/or electrostatic forces.
Executive summary.
Wnt signaling pathway is a complex network that regulates important biological processes. Deregulation of Wnt signaling pathway often leads to various pathologies including cancer. The inhibition of Wnt signaling offers a novel approach for anticancer therapy.
The enzymes porcupine, tankyrases and p300 histone acetyl transferase are important components of the Wnt signaling pathway. The inhibition of their activity is emerging as a promising strategy for anticancer therapeutics. The review summarizes a large number of recent successful examples of enzyme inhibitors that showed in vitro and in vivo activities against various types of cancers. Using computer-assisted drug design as a tool and the crystal structure of enzyme bound to small molecules as a template, this class of inhibitors of Wnt signaling pathway will continue to be optimized in the future.
The protein–protein interactions are emerging as an attractive class of targets for therapeutic intervention of cancer. Wnt signaling pathway involves the complex interactions of protein–protein including β-catenin/co-activator (Tcf, Lef-1, CBP, PYGO), Wnt/SFRP-1, Wnt/DKK-1, Dvl/Frizzled, Axin/CK1α and so on. The selective disruption of this protein–protein interaction suppresses Wnt signaling and further inhibits the growth of cancer cells. One successful example is the discovery of PRI-724 as an inhibitor of β-catenin/CBP which is in clinical trial. However, proteins have dynamic and complex behaviors, and their interactions often involve shallow interfaces. Targeting protein–protein presents numerous challenges.
The complex Wnt signaling pathway involved the multiple components have been proven an attractive target and also present enormous challenges for the discovery of anticancer drugs. A very recently study revealed a correlation between activation of Wnt/β-catenin signaling pathway and absence of a T-cell gene expression signature and implicated the active Wnt/β-catenin signaling as the underlying mechanism for T-cell exclusion and resistance to monoclonal antibody therapy. Therefore, the inhibition of Wnt/β-catenin signaling pathway may be a strategy to overcome resistance to immunotherapies.
Footnotes
Financial & competing interests disclosure
This work was supported by NIMHD RCMI program through grant 2G12MD007595; NIGMS grant number 1U54GM104940, and the Louisiana Cancer Research Consortium (LCRC). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 2.Stewart DJ. Wnt signaling pathway in non–small cell lung cancer. J. Natl Cancer Inst. 2014;106(1):djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 3.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer. 2012;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012;4(5):a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Ginther C, Kim J, et al. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012;10(12):1597–1606. doi: 10.1158/1541-7786.MCR-12-0155-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIH National Cancer Institute. Combination chemotherapy and bevacizumab with or without PRI-724 in treating patients with newly diagnosed metastatic colorectal cancer. www.cancer.gov/about-cancer/treatment/clinical-trials/search/view?cdrid=771168&version=HealthProfessional&protocolsearchid=9920214
- 9.Van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol. Med. 2005;11(11):496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2012;19(4):634. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le PN, Mcdermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015;146(0):1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13(7):513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H-J, Zhang X, Zheng JJ. Inhibiting the Wnt signaling pathway with small molecules. In: Gross KH, Khan M, editors. Targeting the Wnt Pathway in Cancer. Springer; NY, USA: 2011. pp. 183–209. [Google Scholar]
- 14.Chen Z, Li J, Li QS, et al. Suppression of PPN/MG61 attenuates Wnt/[beta]-catenin signaling pathway and induces apoptosis in human lung cancer. Oncogene. 2008;27(24):3483–3488. doi: 10.1038/sj.onc.1211006. [DOI] [PubMed] [Google Scholar]
- 15.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report on the small-molecule inhibitor of porcupine.
- 16.Dodge ME, Moon J, Tuladhar R, et al. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. J. Biol. Chem. 2012;287(27):23246–23254. doi: 10.1074/jbc.M112.372029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Moon J, Dodge ME, et al. The development of highly potent inhibitors for Porcupine. J. Med. Chem. 2013;56(6):2700–2704. doi: 10.1021/jm400159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Pan S, Hsieh MH, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl Acad. Sci. USA. 2013;110(50):20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Development of one of two small-molecule inhibitors of Wnt signaling pathways that are in clinical trials.
- 19.Proffitt KD, Madan B, Ke Z, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73(2):502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 20.Duraiswamy AJ, Lee MA, Madan B, et al. Discovery and optimization of a porcupine inhibitor. J. Med. Chem. 2015;58(15):5889–5899. doi: 10.1021/acs.jmedchem.5b00507. [DOI] [PubMed] [Google Scholar]
- 21.Riffell JL, Lord CJ, Ashworth A. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat. Rev. Drug Discov. 2012;11(12):923–936. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- 22.Huang S-MA, Mishina YM, Liu S. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]; • Discovered the first tankyrase inhibitor.
- 23.Karlberg T, Markova N, Johansson I, et al. Structural basis for the interaction between tankyrase-2 and a potent Wnt-signaling inhibitor. J. Med. Chem. 2010;53(14):5352–5355. doi: 10.1021/jm100249w. [DOI] [PubMed] [Google Scholar]
- 24.Nathubhai A, Wood PJ, Lloyd MD, Thompson AS, Threadgill MD. Design and discovery of 2-arylquinazolin-4-ones as potent and selective inhibitors of tankyrases. ACS Med. Chem. Lett. 2013;4(12):1173–1177. doi: 10.1021/ml400260b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haikarainen T, Koivunen J, Narwal M, et al. Para-substituted 2-phenyl-3,4-dihydroquinazolin-4-ones as potent and selective tankyrase inhibitors. ChemMedChem. 2013;8(12):1978–1985. doi: 10.1002/cmdc.201300337. [DOI] [PubMed] [Google Scholar]
- 26.Johannes JW, Almeida L, Barlaam B, et al. Pyrimidinone nicotinamide mimetics as selective tankyrase and Wnt pathway inhibitors suitable for in vivo pharmacology. ACS Med. Chem. Lett. 2015;6(3):254–259. doi: 10.1021/ml5003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shultz MD, Majumdar D, Chin DN, et al. Structure–efficiency relationship of [1,2,4]Triazol-3-ylamines as novel nicotinamide isosteres that inhibit tankyrases. J. Med. Chem. 2013;56(17):7049–7059. doi: 10.1021/jm400826j. [DOI] [PubMed] [Google Scholar]
- 28.Kirby CA, Cheung A, Fazal A, Shultz MD, Stams T. Structure of human tankyrase 1 in complex with small-molecule inhibitors PJ34 and XAV939. Acta Crystallographica Section F. 2012;68(Pt 2):115–118. doi: 10.1107/S1744309111051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liscio P, Carotti A, Asciutti S, et al. Design, synthesis, crystallographic studies, and preliminary biological appraisal of new substituted triazolo[4,3-b]pyridazin-8-amine derivatives as tankyrase inhibitors. J. Med. Chem. 2014;57(6):2807–2812. doi: 10.1021/jm401356t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson EA, Jansson A, Ng FM, et al. Fragment-based ligand design of novel potent inhibitors of tankyrases. J. Med. Chem. 2013;56(11):4497–4508. doi: 10.1021/jm400211f. [DOI] [PubMed] [Google Scholar]
- 31.Liscio P, Carotti A, Asciutti S, et al. Scaffold hopping approach on the route to selective tankyrase inhibitors. Eur. J. Med. Chem. 2014;87(0):611–623. doi: 10.1016/j.ejmech.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narwal M, Haikarainen T, Fallarero A, Vuorela PM, Lehtiö L. Screening and structural analysis of flavones inhibiting tankyrases. J. Med. Chem. 2013;56(9):3507–3517. doi: 10.1021/jm3018783. [DOI] [PubMed] [Google Scholar]
- 33.Narwal M, Venkannagari H, Lehtiö L. Structural basis of selective inhibition of human tankyrases. J. Med. Chem. 2012;55(3):1360–1367. doi: 10.1021/jm201510p. [DOI] [PubMed] [Google Scholar]; • Discovery of non-nicotinamide-binding site for tankyrase inhibitors
- 34.Lu J, Ma Z, Hsieh J-C, et al. Structure–activity relationship studies of small-molecule inhibitors of Wnt response. Bioorg. Med. Chem. Lett. 2009;19(14):3825–3827. doi: 10.1016/j.bmcl.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanier M, Schade D, Willems E, et al. Wnt inhibition correlates with human embryonic stem cell cardiomyogenesis: a structure–activity relationship study based on inhibitors for the Wnt response. J. Med. Chem. 2012;55(2):697–708. doi: 10.1021/jm2010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bregman H, Chakka N, Guzman-Perez A, et al. Discovery of novel, induced-pocket binding oxazolidinones as potent, selective, and orally bioavailable tankyrase inhibitors. J. Med. Chem. 2013;56(11):4320–4342. doi: 10.1021/jm4000038. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Guzman-Perez A, Acquaviva L, et al. Structure-based design of 2-aminopyridine oxazolidinones as potent and selective tankyrase inhibitors. ACS Med. Chem. Lett. 2013;4(12):1218–1223. doi: 10.1021/ml4003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James RG, Davidson KC, Bosch KA, et al. WIKI4, a novel inhibitor of tankyrase and Wnt/ss-catenin signaling. PLoS ONE. 2012;7(12):e50457. doi: 10.1371/journal.pone.0050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haikarainen T, Venkannagari H, Narwal M, et al. Structural basis and selectivity of tankyrase inhibition by a Wnt signaling inhibitor WIKI4. PLoS ONE. 2013;8(6):e65404. doi: 10.1371/journal.pone.0065404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waaler J, Machon O, Von Kries JP, et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71(1):197–205. doi: 10.1158/0008-5472.CAN-10-1282. [DOI] [PubMed] [Google Scholar]
- 41.Wessel Stratford E, Daffinrud J, Munthe E, et al. The tankyrase-specific inhibitor JW74 affects cell cycle progression and induces apoptosis and differentiation in osteosarcoma cell lines. Cancer Med. 2014;3(1):36–46. doi: 10.1002/cam4.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shultz MD, Kirby CA, Stams T, et al. [1,2,4]Triazol-3-ylsulfanylmethyl)-3-phenyl-[1,2,4]oxadiazoles: antagonists of the Wnt pathway that inhibit tankyrases 1 and 2 via novel adenosine pocket binding. J. Med. Chem. 2012;55(3):1127–1136. doi: 10.1021/jm2011222. [DOI] [PubMed] [Google Scholar]
- 43.Waaler J, Machon O, Tumova L, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72(11):2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 44.Voronkov A, Holsworth DD, Waaler J, et al. Structural basis and SAR for G007-LK, a lead stage 1,2,4-triazole based specific tankyrase 1/2 inhibitor. J. Med. Chem. 2013;56(7):3012–3023. doi: 10.1021/jm4000566. [DOI] [PubMed] [Google Scholar]
- 45.Lau T, Chan E, Callow M, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation–driven colorectal tumor growth. Cancer Res. 2013;73(10):3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 46.Kirubakaran P, Arunkumar P, Premkumar K, Muthusamy K. Sighting of tankyrase inhibitors by structure- and ligand-based screening and in vitro approach. Mol. Biosyst. 2014;10(10):2699–2712. doi: 10.1039/c4mb00309h. [DOI] [PubMed] [Google Scholar]
- 47.Bregman H, Gunaydin H, Gu Y, et al. Discovery of a class of novel tankyrase inhibitors that bind to both the nicotinamide pocket and the induced pocket. J. Med. Chem. 2013;56(3):1341–1345. doi: 10.1021/jm301607v. [DOI] [PubMed] [Google Scholar]
- 48.Hua Z, Bregman H, Buchanan JL, et al. Development of novel dual binders as potent, selective, and orally bioavailable tankyrase inhibitors. J. Med. Chem. 2013;56(24):10003–10015. doi: 10.1021/jm401317z. [DOI] [PubMed] [Google Scholar]
- 49.Shultz MD, Cheung AK, Kirby CA, et al. Identification of NVP-TNKS656: the use of structure–efficiency relationships to generate a highly potent, selective, and orally active tankyrase inhibitor. J. Med. Chem. 2013;56(16):6495–6511. doi: 10.1021/jm400807n. [DOI] [PubMed] [Google Scholar]
- 50.Hao J, Ao A, Zhou L, et al. Selective small molecule targeting β-catenin function discovered by in vivo chemical genetic screen. Cell Rep. 2013;4(5):898–904. doi: 10.1016/j.celrep.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashihara E, Kawata E, Nakagawa Y, et al. β-catenin small interfering RNA successfully suppressed progression of multiple myeloma in a mouse model. Clin. Cancer. Res. 2009;15(8):2731–2738. doi: 10.1158/1078-0432.CCR-08-1350. [DOI] [PubMed] [Google Scholar]
- 52.Scholer-Dahirel A, Schlabach MR, Loo A, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc. Natl Acad. Sci. USA. 2011;108(41):17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepourcelet M, Chen Y-NP, France DS, et al. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell. 2004;5(1):91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]; • Identified the inhibitors of β-catenin/T-cell factor interaction.
- 54.Lee M-A, Kim WK, Park HJ, Kang SS, Lee SK. Anti-proliferative activity of hydnocarpin, a natural lignan, is associated with the suppression of Wnt/β-catenin signaling pathway in colon cancer cells. Bioorg. Med. Chem. Lett. 2013;23(20):5511–5514. doi: 10.1016/j.bmcl.2013.08.065. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Venkatesan AM, Dehnhardt CM, et al. 2,4-Diamino-quinazolines as inhibitors of β-catenin/Tcf-4 pathway: Potential treatment for colorectal cancer. Bioorg. Med. Chem. Lett. 2009;19(17):4980–4983. doi: 10.1016/j.bmcl.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 56.Park CH, Chang JY, Hahm ER, Park S, Kim H-K, Yang CH. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005;328(1):227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 57.Yu B, Huang Z, Zhang M, Dillard DR, Ji H. Rational design of small-molecule inhibitors for β-catenin/T-cell factor protein–protein interactions by bioisostere replacement. ACS Chem. Biol. 2013;8(3):524–529. doi: 10.1021/cb300564v. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M, Catrow JL, Ji H. High-throughput selectivity assays for small-molecule inhibitors of β-catenin/T-cell factor protein–protein interactions. ACS Med. Chem. Lett. 2013;4(2):306–311. doi: 10.1021/ml300367f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catrow JL, Zhang Y, Zhang M, Ji H. Discovery of selective small-molecule inhibitors for the β-Catenin/T-Cell factor protein–protein interaction through the optimization of the acyl hydrazone moiety. J. Med. Chem. 2015;58(11):4678–4692. doi: 10.1021/acs.jmedchem.5b00223. [DOI] [PubMed] [Google Scholar]
- 60.Lu D, Liu JX, Endo T, et al. Ethacrynic acid exhibits selective toxicity to chronic lymphocytic leukemia cells by inhibition of the Wnt/β-catenin pathway. PLoS ONE. 2009;4(12):e8294. doi: 10.1371/journal.pone.0008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wall I, Schmidt-Wolf IGH. Effect of Wnt inhibitors in pancreatic cancer. Anticancer Res. 2014;34(10):5375–5380. [PubMed] [Google Scholar]
- 62.Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of β-catenin/cyclic AMP response element-binding protein transcription. Proc. Natl Acad. Sci. USA. 2004;101(34):12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identified a specific inhibitor of binding cyclic AMP response element-binding protein, which is also in clinical trial.
- 63.Jin G, Lu D, Yao S, et al. Amide derivatives of ethacrynic acid: synthesis and evaluation as antagonists of Wnt/β-catenin signaling and CLL cell survival. Bioorg. Med. Chem. Lett. 2009;19(3):606–609. doi: 10.1016/j.bmcl.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arensman MD, Telesca D, Lay AR, et al. The CREB-binding protein inhibitor ICG-001 suppresses pancreatic cancer growth.Mol. Cancer Ther. 2014;13(10):2303–2314. doi: 10.1158/1535-7163.MCT-13-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonsalves FC, Klein K, Carson BB, et al. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc. Natl Acad. Sci. USA. 2011;108(15):5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J. Transl. Med. 2013;11(1):280. doi: 10.1186/1479-5876-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Sviripa V, Kril LM, et al. Fluorinated N,N-Dialkylaminostilbenes for Wnt Pathway Inhibition and Colon Cancer Repression. J. Med. Chem. 2011;54(5):1288–1297. doi: 10.1021/jm101248v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmeel FC, Schmeel LC, Kim Y, Schmidt-Wolf IG. Piceatannol exhibits selective toxicity to multiple myeloma cells and influences the Wnt/beta-catenin pathway. Hematol. Oncol. 2014;32(4):197–204. doi: 10.1002/hon.2122. [DOI] [PubMed] [Google Scholar]
- 69.Albring KF, Weidemüller J, Mittag S, et al. Berberine acts as a natural inhibitor of Wnt/β-catenin signaling-Identification of more active 13-arylalkyl derivatives. Biofactors. 2013;39(6):652–662. doi: 10.1002/biof.1133. [DOI] [PubMed] [Google Scholar]
- 70.Chen M, Wang J, Lu J, et al. The anti-helminthic niclosamide inhibits Wnt/frizzled1 signaling. Biochemistry. 2009;48(43):10267–10274. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gödeke J, Maier S, Eichenmüller M, Müller-Höcker J, Von Schweinitz D, Kappler R. Epigallocatechin-3-gallate inhibits hepatoblastoma growth by reactivating the Wnt inhibitor SFRP1. Nutr. Cancer. 2013;65(8):1200–1207. doi: 10.1080/01635581.2013.828085. [DOI] [PubMed] [Google Scholar]
- 72.Aguilera O, Peña C, García JM, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1α, 25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28(9):1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 73.Gustafson B, Eliasson B, Smith U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: a link with osteogenesis. Diabetologia. 2010;53(3):536–540. doi: 10.1007/s00125-009-1615-1. [DOI] [PubMed] [Google Scholar]
- 74.Gilbert AM, Bursavich MG, Alon N, et al. Hit to lead studies on (hetero)arylpyrimidines – agonists of the canonical Wnt-β-catenin cellular messaging system. Bioorg. Med. Chem. Lett. 2010;20(1):366–370. doi: 10.1016/j.bmcl.2009.10.093. [DOI] [PubMed] [Google Scholar]
- 75.Shan J, Shi D-L, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44(47):15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- 76.Fujii N, You L, Xu Z, et al. An antagonist of dishevelled protein-protein interaction suppresses β-catenin-dependent tumor cell growth. Cancer Res. 2007;67(2):573–579. doi: 10.1158/0008-5472.CAN-06-2726. [DOI] [PubMed] [Google Scholar]
- 77.Grandy D, Shan J, Zhang X, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J. Biol. Chem. 2009;284(24):16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thorne CA, Hanson AJ, Schneider J, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat. Chem. Biol. 2010;6(11):829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identified a potent inhibitor of Wnt signaling by activating casein kinase 1-α.
- 79.De Robertis A, Valensin S, Rossi M, et al. Identification and characterization of a small-molecule inhibitor of Wnt signaling in glioblastoma cells. Mol. Cancer Ther. 2013;12(7):1180–1189. doi: 10.1158/1535-7163.MCT-12-1176-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Robertis A, Mennillo F, Rossi M, et al. Human sarcoma growth is sensitive to small-molecule mediated AXIN stabilization. PLoS ONE. 2014;9(5):e97847. doi: 10.1371/journal.pone.0097847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, Kong L-M, Zhan R, et al. Two natural ent-kauranoids as novel Wnt signaling inhibitors. Nat. Prod. Bioprospect. 2014;4(3):135–140. doi: 10.1007/s13659-014-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koval AV, Vlasov P, Shichkova P, et al. Anti-leprosy drug clofazimine inhibits growth of triple-negative breast cancer cells via inhibition of canonical Wnt signaling. Biochem. Pharmacol. 2014;87(4):571–578. doi: 10.1016/j.bcp.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Soldi R, Horrigan SK, Cholody MW, et al. Design, synthesis, and biological evaluation of a series of anthracene-9,10-dione dioxime β-catenin pathway inhibitors. J. Med. Chem. 2015;58(15):5854–5862. doi: 10.1021/acs.jmedchem.5b00460. [DOI] [PubMed] [Google Scholar]
- 84.De Vicente J, Tivitmahaisoon P, Berry P, et al. Fragment-based drug design of novel pyranopyridones as cell active and orally bioavailable tankyrase inhibitors. ACS Med. Chem. Lett. 2015;6(9):1019–1024. doi: 10.1021/acsmedchemlett.5b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higuchi Y, Nguyen C, Yasuda S-Y, Mcmillan M, Hasegawa K, Kahn M. Specific direct small molecule p300/β-catenin antagonists maintain stem cell potency. Curr. Mol. Pharmacol. 2015;8:1–8. doi: 10.2174/1874467208666150526155146. [DOI] [PubMed] [Google Scholar]
- 86.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic [bgr]-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]; •• Revealed a correlation between activation of Wnt/β-catenin signaling pathway and absence of a T-cell gene expression signature.