Abstract
Radiotherapy is a component of the standard of care for many patients with locally advanced nonmetastatic tumors and increasingly those with oligometastatic tumors. Despite encouraging advances in local control and progression-free and overall survival outcomes, continued manifestation of tumor progression or recurrence leaves room for improvement in therapeutic efficacy. Novel combinations of radiation with immunotherapy have shown promise in improving outcomes and reducing recurrences by overcoming tumor immune tolerance and evasion mechanisms via boosting the immune system's ability to recognize and eradicate tumor cells. In this review, we discuss preclinical and early clinical evidence that radiotherapy and immunotherapy can improve treatment outcomes for locally advanced and metastatic tumors, elucidate underlying molecular mechanisms and address strategies to optimize timing and sequencing of combination therapy for maximal synergy.
Keywords: : adoptive cell therapy, cancer immunotherapy, cancer vaccines, combination therapy, CTLA4, cytokines, immune checkpoint inhibitors, PD1/PDL1, radiation, sequence, timing
Radiotherapy (RT) exerts its effects on tumors indirectly by generating free radicals that result in DNA damage or directly by DNA double-strand breakage. In addition to DNA damage, radiation causes mitochondrial redox imbalances, lipid peroxidation of cell membranes and a variety of signaling cascades that lead to cells either succumbing to radiation-induced cell death or temporarily arresting cell division to repair the damage. Traditionally, tumor cells are understood to evade the cytotoxic effects of radiation by one or more of four classical processes, namely repair, repopulation, reoxygenation and reassortment, the so called four ‘R's of radiobiology [1]. Escape from immune-mediated tumor rejection has been proposed as a fifth process [2]. In addition to direct and indirect damage of DNA, RT has both immunostimulatory and immunosuppressive roles depending on the radiation dose, number of fractions, type of tumor and site of irradiation. On the one hand, RT‘s immunostimulatory effects take place in many ways, including increased expression of major histocompatibility complex antigens on the tumor cells; release of tumor-associated antigens (TAAs), leading to an in situ vaccine effect; and enhanced expression of immunostimulatory signals like calreticulin, ATP, heat shock proteins and high mobility group box proteins [3–5]. On the other hand, RT can suppress the immune system through depletion of hematopoietic progenitor cells; upregulation of PDL1); and proportional increases in Treg populations [6].
Tumors have the potential to evade the immune system through a complex series of processes collectively referred to as ‘immunoediting’. Downregulation of major histocompatibility complex antigens, loss of TAAs, anergy of T cells, methylation of TAA-processing complexes, disordered vascularity and increased infiltration of immunosuppressive cells (Tregs, myeloid-derived suppressor cells, cancer-associated fibroblasts and M2 macrophages) are all potential ways of evading immune detection and eradication [4]. Despite advances in surgery, chemotherapy and radiation for cancer treatment, the development of long-term resistance and tumor recurrence has remained a challenge, and the underlying mechanisms remain elusive. One common denominator, however, is thought to be the immune system.
The discovery of immune checkpoints that modulate immune function has led to exploration of new combinations of multimodality therapies for cancer. Immunotherapeutic agents act via mechanisms that depend on expression of immune checkpoints, like CTLA4, T-cell immunoglobulin and TIM3, PD1 and its ligand PDL1; on T-cell costimulatory molecules like OX40 (also known as CD134), 4-1BB (CD137) and the glucocorticoid-induced TNF receptor-related protein GITR; and on circulating serum cytokines such as TGF-β and IL-2, -7, -10 and -15. Radiation's unique effects on the immune microenvironment can be used to enhance the efficacy of co-administered immunotherapeutic agents. Together, RT and immunotherapy mediate their antitumor effects in a dynamic interplay between effector and regulatory cells [7,8]. RT can modulate the expression of tumor antigens and immune checkpoints and influence the circulating cytokine profile. RT-induced changes in the tumor milieu facilitate the action of immunotherapeutic agents. In turn, immunotherapy facilitates the action of RT by targeting and modulating various T-cell populations. This synergistic action varies, however, depending on when the RT and immunotherapies are given and on the presence of certain immune molecules in the tumor microenvironment. Hence, a better understanding of how radiation and immunotherapy influence the tumor immune profile will provide insights that will help to optimize strategies for timing and sequencing the two forms of therapy. Here we review preclinical and clinical studies of the effects of RT given in combination with immunotherapy agents, in the context of optimizing the timing and sequencing of the two complementary treatment modalities to enhance their effectiveness while minimizing treatment-related toxicity.
RT & cancer vaccines
Cancer vaccines have long been sought as a means of generating antitumor immune responses. However, effective vaccines have been difficult to develop because they are responsible for overcoming only part of the complex set of mechanisms driving tumor-mediated immunosuppression. Radiation, like therapeutic cancer vaccines, has been shown to release TAAs such as carcinoembryonic antigen (CEA) and mucin-1 [9] and to generate tumor-specific T cells [10]. However, repeated exposure to moderate doses of radiation may have detrimental effects on the immune system, resulting in the clearance of effector cell types required within the tumor microenvironment for potent antitumor activity [10]. In that context, administering therapeutic cancer vaccines before or concomitant with radiation doses may ultimately be futile because of the lack of an intact adaptive immune system at the tumor site. For this reason, it may prove most useful to administer vaccines after RT, thereby using the vaccines as a ‘booster’ for the immune cells generated by RT. Also, RT induces the production of the chemokine CXCL16 by tumors such as 4T1 breast cancer cells, which in turn attracts and recruits CD8+ effector T cells expressing the CXCR6 receptor [11]. How long this effect lasts, however, is unclear, because the experiments demonstrating those effects extended only to 72 h after the irradiation. Nevertheless, these findings provide a rationale for administering immunotherapeutic boosters and vaccines after RT to further expand adaptive immune responses.
Dendritic cell vaccines
Experimental data
One strategy for administering vaccines with radiation involves targeting and using dendritic cells (DCs), which are highly efficient at presenting antigens to immune cells. In preclinical experiments, Kim et al. demonstrated in a mouse model of a fibrosarcoma that intratumoral injections of DCs, given after irradiation to 15 Gy, led to immune-mediated cytotoxic activity against tumors even at distant metastatic locations [12].
Clinical data, perspectives & ongoing trials
The first cancer vaccine approved by the US FDA was sipuleucel-T (Provenge®), a DC-based vaccine, in April 2010 for the treatment of prostate cancer. Treatment involves obtaining DCs from patients by leukapheresis, loading them with the prostate cancer cell antigen prostatic acid phosphatase and GM-CSF, and injecting the activated product back into the patients. Because of concerns that continuing RT after the adoptive transfer might lead to loss of the antigen-presentation capabilities of the DCs, one of five currently ongoing trials, the randomized Phase II clinical trial NCT01807065, involves giving patients external beam RT in weeks 1–2 followed by intravenous sipuleucel-T on days 22, 36 and 50. Another Phase II trial (NCT01818986) involves giving sipuleucel-T concurrently with stereotactic ablative RT to metastatic sites in an effort to enrich the antigenic pool and immune response. A pilot study (NCT01833208) is evaluating whether giving a single high-dose RT fraction to at least one bone lesion at 2 days after the first administration of sipuleucel-T affects the vaccine's immunogenicity. A third Phase II trial (NCT02232230) involves giving RT to at least one metastatic site of advanced castrate-refractory metastatic prostate cancer, followed by sipuleucel-T 28 days later, and testing the effects on immune cell profiles at various time points after treatment. The fourth trial, a Phase II multicenter study of men with limited bone-metastatic castrate-resistant prostate cancer (NCT02463799), is investigating the effects of intravenous sipuleucel-T on weeks 6, 8 and 10, given with or without intravenous radium-223 on weeks 0, 4, 8, 12, 16 and 20. End points include antitumor effects and measures of immune activation through T-cell proliferation. Results from these studies, now pending, will help to evaluate outcomes based on the sequencing of RT relative to the vaccine administration.
Viral vaccines
Experimental data
Viral vaccines represent a unique strategy in which a virus is used not only to attack the tumor but also to produce immunostimulatory compounds and immune-cell recruitment. In one preclinical investigation, Chakraborty et al. studied the effects of an 8-Gy dose of RT combined with the plaque-forming units of a vaccine containing recombinant vaccinia and fowlpox vectors (rV-CEA/TRICOM and rF-GM-CSF) to treat an animal model of colon cancer expressing CEA antigen (MC38-CEA+) [13]. Tumors were implanted on day 0, followed by vaccine administration on day 8, RT on day 14 and three more rounds of vaccination on days 15, 22 and 29. Neither vaccine nor RT alone had significant therapeutic benefits, but the combination had significant antitumor activity, leading to cure rates in excess of 50%. In 2008, that same group tested the efficacy of this recombinant vaccine and RT regimen with an yttrium-90-radiolabeled murine antibody against CEA and again found superior survival after the combined therapy relative to vaccine or antibody alone. As was the case for the previous study, vaccine treatment was begun on day 8 and radiolabeled antibody was given on days 15, 22 and 29 [14]. These findings underscore the diversity in potential applications of RT and therapeutic cancer vaccines.
Clinical data, perspectives & ongoing trials
Three clinical trials have been conducted to date in which RT was combined with viral vaccines. One Phase II study from the US National Cancer Institute was designed to determine if a poxviral vaccine encoding prostate-specific antigen (PSA) would induce a PSA-specific T-cell response when combined with RT for men with localized prostate cancer. The combination, given with the costimulatory molecule B7.1 as well as local GM-CSF and low-dose systemic IL-2, was well tolerated and generated a PSA-specific immune response [15]. Of the 19 patients enrolled in the combination therapy group, 17 completed the vaccination regimen and 13 showed evidence of PSA-specific T cells versus none in the RT-only group (p < 0.0005). A Phase I study by the same group showed that 17 of 18 patients receiving combination RT, IL-2 and rV-PSA/rV-B7.1 tolerated the regimen well, and some patients exhibited high numbers of PSA-specific T cells [16]. Long-term follow-up of a different group of 26 patients given a poxvirus vaccine with external-beam RT, however, showed no differences in PSA control, overall survival or late toxicity relative to external-beam RT alone [17].
Protein & peptide vaccines
Experimental data
In other preclinical studies of RT in combination with cancer vaccines, Mondini et al. used the TC-1 murine model to study HPV-related head and neck cancer. They gave tumor-bearing mice intraperitoneal Shiga toxin-based E7 vaccine once before and once after a dose of 7.5-Gy RT [18]. The survival rate for the mice given the combination therapy was 70%; however, the study did not include control groups of vaccine only or RT only. Moreover, substituting the single-fraction 7.5-Gy dose with four consecutive daily fractions of 2.6 Gy given with the vaccine reduced the survival rate to 40%, a finding that could reflect the negative effect of repeated rounds of RT on the immune system. Another study with a different HPV cervical cancer model involved giving subcutaneous E7 protein + CpG-oligodeoxynucleotide subunit vaccine three-times, 1 week apart, after a single 20-Gy dose of RT [19]. That regimen led to clearance of established tumors and generation of long-term immune memory. Those investigators also reported that giving RT first, followed by vaccine, reduced the RD50 value (radiation dose yielding 50% complete response rate) by 16 Gy relative to RT alone.
Another preclinical study reported by Harris et al. provided direct evidence that giving active immunotherapy within a specific temporal window (3–5 weeks after RT) led to enhanced antitumor efficacy compared with giving the drug before or during radiation [20]. That study involved use of a double-transgenic murine model that spontaneously develops prostate cancer; the cancer cells were made to express the influenza hemagglutinin antigen (HA) as a TAA. The RT was given in a single fraction of 15 Gy to the pelvic site, followed 5 weeks later by the adoptive transfer of CD4+ T cells specific for HA and followed 2 weeks after that by vaccination with recombinant Vaccinia-HA. The RT + vaccine group showed significant increases in percentages of HA-specific CD4+ T cells in prostate-draining lymph nodes and in axillary lymph nodes as compared with the vaccine-only arm. Another experiment in which RT was given as three 10-Gy fractions on days 0, 5 and 10, with adoptive transfer of CD4+ T cells on days 6, 16, 33 or 94, showed that CD4+ transfer on day 6 or day 16 abrogated the subsequent therapeutic effect of Vaccinia-HA given 2 weeks after the adoptive transfer [21]. The peak CD4+ T-cell expansion was detected on day 33 (i.e., at about 5 weeks), but again the effect was diminished when the HA-specific CD4+ T cells were transferred on day 94. These findings are useful for establishing a time frame and demonstrate that using vaccine as a ‘booster’ may help to reactivate immune cells generated by RT.
Clinical data, perspectives & ongoing trials
Vitespen® (marketed as Oncophage®) is a vaccine based on isolated heat shock protein peptide complex 96, and serves as a tumor antigen for kidney carcinoma and melanoma [22,23]. A clinical Phase I trial for pediatric patients with glioma (NCT02722512) involves administering 4 weekly doses of the vaccine beginning 28 days after completion of RT. A similar Phase II trial using the same vaccine (NCT00905060, now completed) gave the protein intradermally after standard treatment with radiation and temozolomide.
Additional studies in which vaccines are combined with RT are reviewed elsewhere by Garnett-Benson et al. [24]. Collectively, these studies highlight the potential for achieving better outcomes when RT is given first, followed by the cancer vaccine. This pattern also seems to hold true for combination treatments that involve RT and adoptive immunotherapy agents, as discussed in the following section.
RT & adoptive immunotherapy
Although currently available adoptive immunotherapy strategies such as T-cell and natural killer (NK)-cell-based therapies have been studied as monotherapy or in combination with chemotherapy for leukemia, to date few have evaluated the effects of these strategies in combination with RT, especially for solid tumors. Current evidence from preclinical and clinical studies is summarized below.
T-cell therapies
Experimental data
Filatenkov et al. tested the effectiveness of combining cyclophosphamide and RT with or without autologous T-cell infusions in the 4T1 murine model of metastatic breast cancer [25]. The authors concluded that an RT regimen of 60 Gy (three fractions of 20 Gy each) before autologous T-cell infusions resulted in significant improvement of survival and remission rates relative to mice receiving only cyclophosphamide and RT. In another approach, Ménager et al. combined RT with an α-particle emitter, bismuth-213, with adoptive T-cell transfer (α-radioimmunotherapy [RIT]) [26]. Using an immunocompetent murine multiple myeloma model that expresses CD138 and ovalbumin, these investigators administered an anti-CD138 antibody coupled to bismuth-213 followed by adoptive T-cell transfer of CD8+ T cells specific to ovalbumin. The combination of RIT and adoptive T-cell transfer modestly extended median survival times (31 vs 27 days for T-cell transfer alone and 28 days for RIT alone). Data published in abstract form by DeSelm et al. showed potentiation of the antitumor effect when RT (in doses as low as 2 Gy) was given in the neoadjuvant setting 2 days before infusion of chimeric antigen receptor (CAR) T cells [27]. Moreover, another abstract published by Cortez et al. showed that RT given with CAR T cells targeted to EGFR in A431 epidermoid murine models caused increased targeted T-cell infiltration relative to either therapy alone [28]; the timing in those experiments involved giving the RT 9 days before the CAR T cells.
Clinical data, perspective & ongoing trials
In clinical studies, Dudley et al. reported that treating patients with metastatic melanoma with chemotherapy (cyclophosphamide and fludarabine) plus total body irradiation to 2 or 12 Gy followed by infusion of tumor-infiltrating lymphocytes led to higher objective response rates (52 and 72%) than treatment that did not include total body irradiation (49%) [29]. More recently, Rosenberg et al. found similar objective response rates with that treatment regimen in 93 patients, noting complete response rates of 12% for those given chemotherapy alone, 20% for chemotherapy plus 2-Gy total body irradiation, and 40% for those given chemotherapy plus 12 Gy [30]. In a case study of a patient with poorly differentiated adenocarcinoma (stage IIIC) that recurred after surgery who was given T-cell and DC therapy before and concurrently with RT [31], Sato et al. observed signs of tumor shrinkage and improved performance status after the concurrent treatment. These results emphasize the diversity in tested combinations of T-cell therapies and RT, and suggest that the effects of these regimens depend on the type, dose and timing of RT relative to T-cell administration.
NK-cell therapies
Experimental data
Like regimens involving T-cell therapy, very few studies have evaluated the effects of administering NK cells with RT. In one preclinical study, Heo et al. hypothesized that concurrent RT and NK-cell treatment of the non-small cell cancer cell line NCI-H23 would result in increased NK-cell cytotoxicity, perhaps via RT-induced increases in the expression of NKG2DLs-activating ligands [32]. That study highlights a potential mechanism underlying synergy between NK cells and RT. Finkel et al. performed several experiments with B16 melanoma cells to evaluate the immunogenic potential of irradiated cells and the mechanisms through which this might occur [33]. They found that NK-cell depletion just before RT and hyperthermia led to retardation of tumor growth, which suggests that immunization with B16 cells followed by RT and hyperthermia could induce a memory response, and that NK cells might hinder proper immune activation during the immunization. These results suggest that tumor cell irradiation could participate in promoting NK-cell activation. Although this study involved testing NK cells in conjunction with hyperthermia, these findings could set the stage for strategies in which RT is used for tumor immune activation before NK-cell therapy.
RT & cytokines
Some of the studies already discussed included IL-2 in the treatment regimens, highlighting the trend toward inclusion of cytokines to stimulate the innate and adaptive immune cells and treat certain tumors. Such cytokines include but are not limited to TGF-β, TNF-α, GM-CSF, IL-2, IL-7 and IL-15. However, use of these cytokines is still limited because of their toxic side effects, especially in combination therapy. Studies involving these agents are described below.
TGF-β
Experimental data
TGF-β represents a classic double-edged sword – it has tumor-suppressive properties during the early phase of carcinogenesis but acts to favor malignant progression in later stages. TGF-β suppresses immune-cell infiltration into tumors by modulating lymphocyte activation and macrophage function. Among the numerous approaches to inhibiting TGF-β signaling tested to date are monoclonal antibodies, antisense oligonucleotides, small molecule inhibitors, peptide aptamers and stabilized soluble proteins [34]. The time at which the TGF-β-modifying drugs are administered is an important factor. In rat breast cancer models, TGF-β levels were found to increase after cyclophosphamide and RT [35], an undesirable effect potentially leading to tumor resistance. Conversely, inhibition of TGF-β with neutralizing antibodies restored the drug sensitivity of the tumor, suggesting that giving TGF-β inhibitors with chemoradiation could enhance therapeutic efficacy. A small molecule inhibitor of TGF-β, LY364947, given with a TGF-β-neutralizing antibody was found to radiosensitize the breast cancer cell line MDA-MB231 by augmenting dsDNA damage [36]. Further support for the observation that inhibiting TGF-β before RT delays tumor growth comes from Vanpouille-Box et al., who reported that this combination had significant effects on both irradiated and nonirradiated metastatic 4T1 tumors [37]. In that study, tumor cells were injected subcutaneously in the right flank (day 1) and left flank (day 2) of mice; on days 13–17, RT to 6 Gy was delivered only to the right flank tumor followed by intraperitoneal injection of anti-TGF-β every other day from days 12 to 28. In another study, giving the anti-TGF-β antibody after 8 Gy of lung RT was shown to reduce RT-induced lung fibrosis, which was thought to be mediated through active TGF-β secretion by macrophages in thoracic tumors [38].
Clinical data, perspectives & ongoing trials
Clinical trials are evaluating the strategy of initial priming with TGF-β inhibitors followed by concurrent RT plus TGF-β inhibitors in two different patient populations. One such trial includes patients with non-small-cell lung cancer (NCT02581787), and the other, the ExIST trial (NCT02688712), is exploring this approach in patients with rectal cancer. Another recently completed trial (NCT01220271) also involved giving RT with concurrent TGF-β inhibitors and temozolomide to patients with newly diagnosed gliomas.
FMS-like tyrosine kinase 3 ligand
Experimental data
Binding between the FMS-like tyrosine kinase 3 ligand (Flt3L) and Flt3 (CD135) has been shown to stimulate hematopoietic cell differentiation [39]. In one preclinical study of Flt3L, a growth factor that induces and enhances DC function, Chakravarty et al. showed that 60 Gy of RT followed by 10 days of Flt3L, starting 1 day after RT, had great potential for limiting primary and metastatic tumors in a murine model of Lewis lung carcinoma [40]. Another group showed that treatment with Flt3L for 10 consecutive days after low-dose RT (2 or 6 Gy) promoted a systemic abscopal response in the 67NR murine model of mammary carcinoma [41]. An abscopal response refers to a systemic effect in response to local irradiation, for example, a reduction in size or extent of metastatic disease outside the scope of the radiation field. Additional confirmation that the success of this combination treatment depends on a functioning immune system is that the antitumor effect was lost in nude mice, as they lack T cells.
Clinical data, perspectives & ongoing trials
A clinical study is now underway combining Flt3L with stereotactic ablative body RT aimed at isolated lung lesions in patients with advanced non-small-cell lung cancer (NCT02839265).
TNF-α
Experimental data
The term TNF refers to a superfamily of numerous protein homologs that act primarily on two receptors: TNFR1, expressed on all cells and TNFR2, expressed only on immune cells and endothelial cells. TNF-α is predominantly produced by activated macrophages, T cells and NK cells. It acts to enhance the production of IL-1, IL-6, IL-8 and IFN-γ in NK cells; reactive oxygen species in macrophages; and nitric oxide synthase in endothelial cells, thereby augmenting the antitumor immune response [42,43]. Preclinical studies of human tumor cell lines have shown that TNF-α can intensify the effect of RT. One study showed that treating mice with human colon cancer xenograft tumors with four cycles of TNF-α, the first given 1 day before the first of 4 weekly RT for 4 weekly doses, led to better tumor control than RT alone [44]. In another study, human xenograft models of squamous cell carcinoma and malignant glioma showed increased RT-induced damage to vasculature in tumors that had been treated with TNF-α [45,46]. TNF-α gene therapy given before RT was also found in a preclinical study to enhance RT-induced cytotoxicity in a human prostate cancer xenograft model [47]. Similarly, TNF-α gene therapy combined with RT led to better local control in human esophageal cancer xenograft models than did either therapy alone [48].
Clinical data, perspectives & ongoing trials
In clinical studies, an early-1990s Phase I dose-escalation trial to explore the toxicity of TNF-α given once daily during RT indicated good response rates [49] but at the cost of severe side effects such as angina, cardiac arrhythmias, leukopenia, allergies and respiratory distress. These findings prompted the search for novel TNF-α delivery methods, one of which was TNFerade, a nonreplicating adenoviral vector containing human TNF-α DNA with a radiation-inducible promoter. Citrin et al. tested the feasibility of using TNFerade in combination with capecitabine and RT in a pilot study of patients with locally advanced rectal cancer [50]. The first dose of TNFerade was given with RT and followed with 5 weekly injections thereafter. This treatment led to a complete pathologic response in two of nine patients tested. Herman et al. reported findings from a Phase III clinical trial testing the combination of TNFerade with chemoradiation for patients with locally advanced pancreatic cancer [51]. In that trial, giving TNFerade intratumorally before the first fraction of RT along with standard treatment did not improve survival, a finding the authors attributed to ineffective delivery of TNFerade, inadequate activation of the radiation-inducible promotor and the presence of metastatic disease in most patients. These results suggest that TNF-α therapy should be given, perhaps intratumorally, before RT for maximum benefit in terms of local tumor control.
IL-2
Experimental data
IL-2 was the first immunotherapy agent to be approved for use in humans and is known to increase populations of CD8+ T cells, CD4+ helper T cells and Treg cells. The major limiting toxicity that has been reported is capillary leak syndrome. Attempts to overcome this toxicity have included conjugating IL-2 to L19, a small immunoprotein, which allows selective localization of IL-2 to the tumor by targeting the extradomain-B of fibronectin, a marker of tumor neoangiogenesis that is expressed only in the vasculature of solid tumors [52,53]. Rekers et al. used an F9 tumor model in which tumor-bearing 129/FvHsd mice received a single 10-Gy dose of RT followed by three rounds of L19–IL-2 cytokine treatment, or RT alone, or L19–IL-2 alone. The optimal schedule in terms of tumor growth delay was found to be giving RT 24 h before starting L9–IL-2 therapy. On the other hand, when these two therapies were combined with systemic IL-2 administration, the most effective schedule was presensitization with IL-2 followed by RT. That kind of sensitization could not be achieved by L19–IL-2, because the latter stimulates the immune system only in the immediate tumor area and not systemically, an important consideration given that NK cells (which require activation by IL-2) are lacking in the tumor stroma. Overall, the combination of RT and IL-2 led to better responses than did IL-2 alone. Zegers et al. [53] used three mouse models to study the antitumor effects of RT followed by L19–IL-2 treatment in light of extradomain-B expression: C51 colon carcinoma, with high levels; Lewis lung carcinoma, with intermediate levels; and 4T1 mammary carcinoma cells, with low levels. The highest response rates – up to 75% – were found in the C51 model; because depletion of CD8+ cytotoxic T cells negated the antitumor response, this effect was thought to be mediated through these cells.
Clinical data, perspectives & ongoing trials
From the clinical perspective, numerous trials are being conducted that test IL-2 in combination with RT for a variety of tumor types, but few results are available on the best sequencing of these two therapies. In one Phase I trial, Seung et al. studied the combination of IL-2 with stereotactic ablative RT in patients with metastatic melanoma and renal cell carcinoma. The treatment regimen included three rounds of radiation at 20 Gy per fraction, followed 3 days later by various numbers of cycles of IL-2 (600,000 IU/kg given intravenously every 8 h for up to 14 doses per cycle, with a 2-week rest period between cycles). At least a partial response was observed in >60% of patients [54]. Immune-cell profiling of samples from the patients who responded showed a higher frequency of proliferating CD4+ T cells with an activated memory phenotype. In summary, combinations of IL-2 and RT show promise, but the timing and sequence of the two therapies need further optimization, and specific consideration of agents and modes of administration is needed as well.
GM-CSF
Experimental data
GM-CSF is secreted by macrophages, NK cells and T cells, and stimulates presentation of tumor antigens by DCs (via Flt-3 ligand), which activates cytotoxic T-cell responses that mediate antitumor immunity.
Clinical data, perspectives & ongoing trials
Golden et al. published their observations of GM-CSF and RT leading to abscopal effects in a trial of patients with advanced solid tumors [55]. In that trial, GM-CSF was given subcutaneously daily for 14 days, beginning 1 week after the start of palliative RT (35 Gy given in ten fractions) to one of three distinct measurable sites of metastatic disease in combination with single-agent chemotherapy or hormonal therapy. Overall, >20% of patients demonstrated an abscopal response, defined as a 30% decrease in the longest diameter of an index off-target lesion. In another study, treatment with GM-CSF given 2 weeks after initiation of an accelerated 3-week course of RT was shown to reduce RT-induced mucositis in patients with laryngeal cancer without adversely affecting tumor control outcomes, presumably via improved mucosal wound healing as a result of proliferation of immune stem cells, surviving mucosal cells or both [56]. Another mechanism of GM-CSF administration is via GVAX, a vaccine shown to enhance immune responses in patients with non-small-cell lung cancer [57]. Currently, eight active clinical trials are studying the combination of GM-CSF and RT as concurrent or adjuvant therapy (NCT02946138, NCT00091052, NCT02663440, NCT03113851, NCT02623595, NCT01595321, NCT02648282 and NCT00727441).
IL-7
Experimental data
IL-7 is a product of stromal cells present in lymphoid tissue that is responsible for maturation of T cells and enriching the populations of both CD8+ cytotoxic T cells and CD4+ helper T cells. IL-7 has been used to restore lymphocyte counts in irradiated mice, where it causes preferential expansion of CD8+ T cells [58].
Clinical data, perspectives & ongoing trials
Radiation-induced lymphopenia has been linked with inferior survival in patients with high-grade glioma and other cancers [59,60]. Interest in IL-7 for treating radiation-induced lymphopenia stems from the observation that, unlike the compensatory increase in IL-7 and IL-15 noted in patients receiving total body irradiation to deplete their hematopoietic cells before bone marrow transplantation, RT for glioma causes lymphopenia but no compensatory increase in IL-7 and IL-15. One Phase I trial (NCT02659800) is exploring whether IL-7 can increase CD4 counts in patients with high-grade glioma experiencing lymphopenia after RT and temozolomide.
IL-15
Experimental data
IL-15 shares structural homology with IL-2 and results in increased CD8+ cytotoxic and CD4+ helper T-cell populations but has no effect on Treg cells. IL-15 has been investigated in preclinical models for its ability to synergize with RT. In two recent abstracts, Demaria et al. reported that concurrent RT and IL-15 plus adjuvant IL-15 produced the maximum antitumor response in mice bearing the TSA breast cancer cell line. The authors attribute this effect to increased tumor infiltration by NK cells and CD4+ and CD8+ cytotoxic lymphocytes [61,62].
RT & immune checkpoint inhibitors
As is true for studies of RT and cytokines, the effectiveness of combining radiation with immune checkpoint inhibitors has been shown in many models, but the question of the optimal timing of these treatment combinations remains unanswered. Interactions between myeloid cells (which are largely immunosuppressive) and antitumor cytotoxic T cells within tumors are complex. The data available to date seem to justify either simultaneous or delayed administration of checkpoint inhibitors after RT so that newly recruited T cells can destroy tumor cells, both at the primary site and systemically after being presented with novel tumor antigens. Studies of RT and immune checkpoint inhibitors are discussed briefly below.
CTLA4
Experimental data
CTLA4 is responsible for maintaining immune tolerance through inhibition of early stage T-cell development. CTLA4 inhibitors act by preventing the downregulation of helper T-cell activity while reducing Treg cell activity. Several studies have explored combinations of CTLA4 with RT in neoadjuvant, concurrent and adjuvant settings, yet the optimal combination remains elusive. Demaria et al. previously demonstrated that RT followed by CTLA4 blockade conferred improved survival relative to either treatment alone in a 4T1 breast cancer model [63]. Dewan et al. later showed that this effect holds for simultaneous delivery of immunotherapy and fractionated RT [64]. A review of preclinical studies since 2014 also reveals similar findings. For example, Son et al. demonstrated that CTLA4 inhibition enhanced antitumor immunity when immature DCs were intratumorally injected after an RT dose of 10 Gy in murine colon cancer models [65]. That study indicated that the RT/immature DC combination led to inhibition of distant tumors, and that the addition of anti-CTLA4 antibody significantly improved this effect by reducing Treg populations and increasing survival. Yoshimoto et al. also reported that RT-induced antitumor immunity has a crucial role in the therapeutic efficacy of RT, and suggested that modulating the immune microenvironment with monoclonal antibodies could further enhance efficacy [66]. In that study, they showed that lymphoma and Lewis lung carcinoma mouse models treated with a single RT dose of 30 Gy followed by anti-CTLA4 had substantially increased antitumor activity relative to control groups. Wu et al. also found that blocking CTLA4 on T cells after RT enhanced abscopal effects in a mouse model of mesothelioma [67]. That group showed that RT delayed growth of the primary tumor and that CTLA4 blockade significantly reduced Tregs while increasing cytotoxic T-cell populations in both primary and secondary tumors, thus leading to increased survival. Although all of these studies have reported enhanced antitumor effects from combining RT with anti-CTLA4, only one study to date has evaluated the effects of the timing of the CTLA4 blockade with RT. In that study, Young et al. showed that giving CTLA4 before RT followed by OX40 had the best effects, with clearance of CT26 tumors in all mice. Although giving CTLA4 either 1 day or 5 days after RT also provided a survival advantage, tumor clearance was present in only half of the mice so treated [68]. Across these studies, most, but not all, evidence suggests that immune activation via CTLA4 blockade synergizes optimally with a single or a few large fractions of radiation rather than multiple small fractions of radiation, possibly because repetitive radiation doses deplete newly arrived activated T cells that infiltrate the tumor after CTLA4 blockade [69].
Clinical data, perspectives & ongoing trials
Among the clinical studies conducted to date, Qian et al. reported findings from 75 patients with melanoma and brain metastasis showing that giving immunotherapy within 4 weeks of stereotactic radiosurgery (SRS) resulted in the highest lesion response rates [70]. However, that study was designed to compare only immunotherapy given <4 weeks versus >4 weeks after the SRS procedure. In a similar study, Kiess et al. retrospectively evaluated 46 patients undergoing single-fraction SRS to 21 Gy and found that giving the anti-CTLA4 agent ipilimumab either before or concurrently with SRS led to the longest survival benefit with the lowest rate of local recurrence [71]. Notably, however, the patients who received ipilimumab after SRS in this study had initially responded poorly to the SRS, and the ipilimumab had been given >2 months after the SRS. Jiang et al. reported early findings from a retrospective comparison of patients given SRS either within 5.5 months or ≥5.5 months after ipilimumab for brain metastases; that comparison showed that giving SRS closer to the last dose of ipilimumab led to the best intracranial control rates and that better control also correlated with higher absolute lymphocyte counts [72]. Schoenfeld et al. also reported a case review of 16 patients who had received whole-brain RT + SRS with ipilimumab for brain metastatic disease [73]. In that study, receipt of SRS before ipilimumab was associated with a median survival time of 26 months versus only 6 months for those who had received SRS after ipilimumab (p = 0.001) and 18 months for concurrent administration; however, the authors acknowledge that delays in the receipt of RT may have confounded the results. Knisely et al. reported a cohort of 77 patients of melanoma with brain metastasis of whom 35% received ipilimumab before or after SRS and found no difference in median survival time (p = 0.58) at 19.8 months for those given the drug before SRS versus 21.3 months for those given the drug after SRS [74]. Notably, however, the brain is an immune-privileged site, with low concentrations of lymphocytes, and these studies all involved very large RT fractions (∼20 Gy). These factors complicate the extrapolation of these findings to others tumors, particularly with treatment scenarios that involve more conventionally fractionated RT. Findings for patients with solid tumors have included anecdotal case reports suggesting that giving palliative RT to patients who had previously received ipilimumab could lead to good local response as well as systemic abscopal effects [75,76]. Barker et al. reviewed patients with unresectable stage III–IV melanoma who received nonbrain RT and found that the median survival time for those given induction ipilimumab followed by RT was 9 versus 39 months for those given ipilimumab maintenance therapy [77].
PD1 & PDL1
Experimental data
PD1 functions to restrict the activity of T cells in the tumor microenvironment. Increased expression of the PD1 ligand PDL1 is a resistance mechanism by which tumor cells evade the immune system. Radiation increases the expression of PDL1 in the tumor microenvironment and on CD8+ cytotoxic lymphocytes [78]. Dovedi et al. reported that RT increased PDL1 expression in syngeneic models of colorectal cancer (CT 26), breast cancer (4T1) and melanoma (4434 p16-negative BRAFV600E) [79]. Concurrent administration of anti-PDL1 antibody with RT led to a survival benefit, whereas sequential treatment did not. In another preclinical study, Wu et al. gave RT on days 1 and 14, and anti-PDL1 antibody daily on days 0–14 to mice orthotopically implanted with MB49 transitional cell bladder cancer [80]. This regimen, similar to concurrent regimens, resulted in longer tumor growth delays relative to RT or anti-PDL1 alone. Another group used Panc-02 murine pancreatic cell lines to study combinations of RT with anti-PDL1 antibody and gemcitabine (given on days 0 and 3) [81]. The anti-PDL1 antibody was administered on days 0, 3, 6 and 9 with RT or on days 4, 7, 10 and 13 with RT + gemcitabine. The gemcitabine-alone treatment group received the drug on days 0 and 3. The RT was given in one of five schedules, three high dose (one 12-Gy fraction, five 3-Gy fractions or one 20-Gy fraction) and two low dose (one 6-Gy fraction or five 2-Gy fractions). The best outcomes were obtained for the mice given high-dose RT with anti-PDL1 and gemcitabine. By using a mathematical model, these authors further concluded that a delay of 7 days between RT and receipt of the anti-PDL1 antibody abolished the radiosensitization effect.
Clinical data, perspective & ongoing trials
Currently, approximately 50 Phase I/II trials are underway to evaluate combinations of RT and PD1 inhibitors [82]. PDL1 upregulation has been found to be a tumor-escape mechanism when RT + anti-CTLA4 therapy is used [83], and it remains to be seen whether combining anti-CTLA4 + RT followed by anti-PDL1 treatment or triple therapy with anti-CTLA4, anti-PDL1 and RT would circumvent this resistance mechanism. Notably, anti-PDL1 therapy is relatively well tolerated; Ahmed et al. studied 26 patients of melanoma with brain metastasis treated with the anti-PDL1 agent nivolumab with SRS and found no undue toxicity from this regimen [84].
RT & immune costimulatory molecules
OX40
Experimental data
OX40 is a costimulatory molecule that increases the cytolytic activity of CD8+ T cells. In preclinical studies, Yokouchi et al. assessed the effect of an agonistic anti-OX40 monoclonal antibody, given with RT, in a mouse model of ovalbumin-transfected Lewis lung carcinoma [85]. In those experiments, anti-OX40 was given on the day of RT and also as adjuvant therapy on days 8, 11 and 14; this schedule was found to produce the longest overall survival and the most durable immunity compared with either RT or anti-OX40 given as single-modality therapy. Another group reported that adjuvant agonistic anti-OX40 given after RT or surgery in mice increased survival, presumably by increasing CD8+ T cells [86]. A previously mentioned study by Young et al. also evaluated the timing of OX40 administration and found that giving anti-OX40 1 day after RT resulted in optimal responses [68].
Clinical data, perspective & ongoing trials
Currently two clinical trials are underway to evaluate anti-OX40, one for metastatic prostate cancer (NCT01303705) and the other for metastatic breast cancer (NCT01862900); both are giving hypofractionated RT to metastatic disease sites with anti-OX40 antibody as adjuvant therapy. Initial immunologic analyses in a Phase I/II trial of patients with metastatic prostate cancer suggest that anti-OX40, RT and cyclophosphamide had no effect on the proliferation of peripheral blood lymphocytes but may increase the percentage of circulating activated CD8+ T cells [87].
Toll-like receptor agonists
Experimental data
Activation of the Toll-like receptor (TLR) can enhance the presentation of TAAs by DCs. Radiation is known to increase the release of TAAs, and TLR can potentiate the activity of RT. Several preclinical studies of TLR agonists have been published. One such study evaluated the immunogenicity of CBLB613, a TLR-2/6 agonist and a naturally occurring lipopeptide of Mycoplasma arginini [88]. Two other groups found that giving the TLR-7 agonist imiquimod 6 h before RT or 24 h after RT led to enhanced autophagy and CD8+ T-cell-mediated cell death in a mouse model of melanoma [89], and led to synergistic effects in a mouse model of cutaneous breast cancer [90]. Dovedi et al. tested 5 weekly doses of another TLR-7 agonist, R848, after RT in mice bearing B-cell lymphoma (A20) and T-cell lymphoma (EL-4) [91]. The combined therapy led to greater tumor regression than did either therapy given alone, and expansion of CD8+ T cells was suggested as an underlying mechanism. Another agent, a CpG oligodeoxynucleotide targeted to TLR-9, has been tested in a preclinical model of fibrosarcoma; the agent was given before and after RT total doses ranging from 10 to 90 Gy once a week for 6 weeks. A significant difference was found in the radiation dose resulting in 50% tumor cure (TCD50), with 23 Gy for the combination treatment versus 83.1 Gy for RT alone [92].
Clinical data, perspective & ongoing trials
When this article was written, nine active clinical trials were evaluating TLR agonists in combination with RT. In one Phase III trial of patients with stage IIB-IVA cervical cancer [93], chemoradiation was given concurrently with the TLR agonist Z-100, followed by adjuvant Z-100 until disease progression. The planned RT dose was 50 Gy, given in 1.8- to 2-Gy fractions five-times a week. A trend toward better overall survival was noted in the group receiving Z-100. Overall, both preclinical and clinical studies have indicated that TLR agonists require a priming dose followed by concurrent dosing with RT to produce the best antitumor response.
RT & steroids
Experimental data
Steroids such as glucocorticoids are commonly used to mitigate the side effects of immunotherapy, particularly immune checkpoint inhibitors [94]. Glucocorticoids are potent immune suppressants that function partly by inhibiting proliferation at the transcriptional level and triggering apoptosis and by interfering with IL-2 signaling [95–97]. However, because the goal of immunotherapy with RT is to activate the immune system against cancer through the production of new T cells, steroids could inhibit the antitumor immune response. Thus withholding steroids from patients receiving RT and immunotherapy may prove beneficial unless those patients experience immune-related adverse effects.
Glucocorticoids have several effects on T cells. In addition to triggering apoptosis, the synthetic glucocorticoid dexamethasone can lead to increased percentages of Tregs in patients with asthma and in healthy donors [98]. Dexamethasone was shown to upregulate the glucocorticoid-induced TNF receptor GITR on CD4+ T cells and its ligand GITRL on DCs; interactions between the two induce the CD4+ T cells to produce indoleamine 2,3-dioxygenase (IDO), which in turn controls inflammatory reactions and can lead to tumor cell tolerance [99]. Some evidence exists that glucocorticoid-induced apoptosis can be avoided through activation of T cells, including co-stimulation via CD80/CD86 on either CD28 or CTLA4; indeed, Wagner et al. found that co-incubating T cells with CD80- or CD86-transfected thymocytes and dexamethasone caused a large reduction in cells undergoing apoptosis [100]. However, when a CTLA4 fusion protein was added to block CD80 and CD86, the cells did undergo apoptosis, suggesting that signaling via CD28, CTLA4 or both is probably needed for this effect. Hinrichs et al., in exploring how steroids affect activated T cells used in adoptive T-cell immunotherapy, noted no difference in tumor growth after adoptive T-cell therapy in the presence or absence of dexamethasone. They also found that dexamethasone inhibited only naive antigen-specific T cells, not activated antigen-specific T cells, although IFN-γ secretion by the latter was reduced [101]. On the basis of this preclinical evidence, we recommend that steroids be avoided before RT and immunotherapy, because they may inhibit T-cell priming and activation.
Clinical data, perspective & ongoing trials
Clinically, numerous trials in melanoma in which ipilimumab was used with a prophylactic steroid regimen before SRS suggest that using steroids results in lower median survival rates [73,102,103]. However, giving steroids during treatment may not interfere with response rates, given that T cells may already be activated. Further preclinical and clinical studies are needed to address the unanswered questions as to the use of steroids with immunotherapy and RT, specifically, to address when steroids might be given without ablating the antitumor immune response and to identify the optimal interval after prior systemic steroid therapy when immunotherapy can be given without affecting antitumor response, as was done with ipilimumab for patients with brain metastases from melanoma [104].
Conclusion
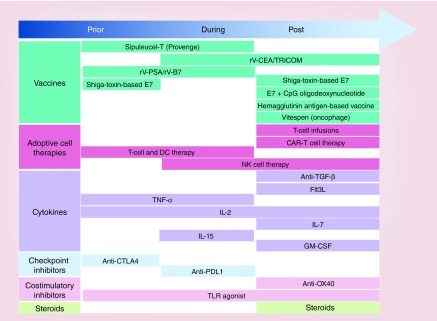
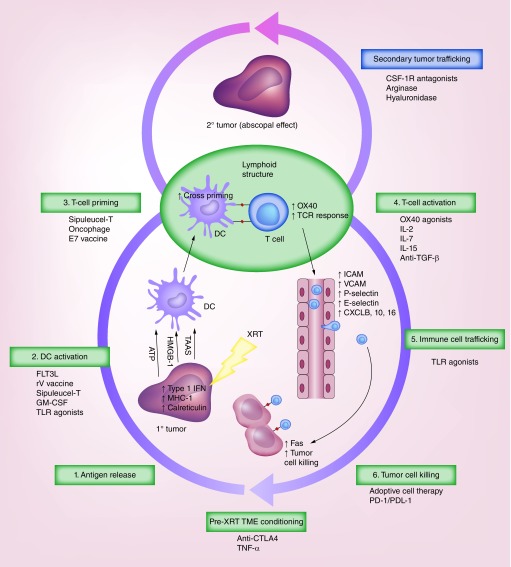
Figure 1 provides a summary of all the agents discussed in this article. Figure 2 illustrates the course of events involved in immune activation induced by RT, and how those events are affected by the various immunotherapies described in this article. One or more windows are apparent, particularly after RT, during which certain events take place, such as T-cell priming and stimulation. For example, OX40 expression on CD4+ T cells increases quickly after RT, which translates to a short time window in which to administer anti-OX40 agonists. Other changes, such as increased antigen presentation after RT, provide a rationale for the timing of delivering vaccines and DC-maturing factors during and especially after RT. Cytokine therapies that modulate T- and NK-cell activity should be given after RT to avoid radiation-induced T-cell apoptosis, whereas therapies that target Tregs, such as anti-CTLA4, may need to be given before RT to make the tumor microenvironment more favorable for proper antigen presentation. Furthermore, by facilitating trafficking of treatment-induced antigen-specific activated T cells deep within the primary tumor as well as to metastatic tumor sites, agents inducing stromal disruption such as arginase, hyaluronidase and CSF-1R antagonists may further enhance the benefits of the immunotherapy–RT combinations discussed here. Metastatic disease treated with RT may be an ideal scenario for exploring these questions in a systematic manner, because synergy at the treated site as well as distant sites (via induction of a systemic immune response) can be simultaneously evaluated. Furthermore, hypofractionated treatments, which tend to synergize optimally with some immunotherapy–RT combinations, are often used in this setting. Finally, the presence of multiple sites of disease in the same patient offers the opportunity to explore the relative benefits or pitfalls of different combination strategies that can initiate or re-initiate the cascade of events resulting in an effective immune response.
Figure 1. . Combinations of immunotherapy and radiotherapy with respect to timing and sequencing.
Figure 2. . Radiation effects on the tumor microenvironment and potential strategies for combining radiation with immunotherapy.
After radiation therapy, cancer cell antigens are released and recognized by dendritic cells, which upon activation cross present these antigens to T cells, which are then primed and activated. This cascade of events can be potentiated by using agents that enhance DC activation and T-cell priming and activation. Upon activation, circulating T cells infiltrate the tumor and engage cancer cell antigens to trigger immune-mediated cell death. Trafficking of activated T cells to both primary tumor and metastatic sites could be enhanced by stromal-disrupting agents.
Future perspective
The rational application of specific immunotherapies at specific times relative to RT hinges on a nuanced understanding of each therapy's effect on tumor cells and tumor microenvironmental immune cells and how they interact. This will help in the design and effectiveness of future clinical trials in which an enhanced immune response may be effectively triggered while avoiding chronic use of immunotherapies that can result in undesired toxicity. Recognizing that use of single immunotherapeutic agent strategies may risk development of tolerance or resistance, optimal combination of multiple immune-directed strategies with RT may need to be surveyed to maximize the chances of systemic antitumor immune activation. The ultimate goal of rationally designed combination therapy is, of course, better patient outcomes with reduced side effects.
Executive summary.
Radiation therapy & cancer vaccines
Experimental and clinical studies point to beneficial effects from delivering cancer vaccines after radiation therapy (RT).
Sipuleucel-T, a dendritic cell-based vaccine, has been extensively tested in combination with RT. Additional clinical studies are ongoing.
Viral-, protein- and peptide-based vaccine treatments after RT are in clinical trials.
RT & adoptive immunotherapy
Adoptive T-cell transfer in the setting of radiation works best when the T cells are given after RT.
Chimeric antigen receptor T cells have shown evidence of efficacy when given after RT in preliminary studies.
Natural killer cell therapy also has synergistic effects when given after radiation in preclinical studies.
RT & cytokines
TGF-β inhibition after RT can sensitize tumors and mitigate toxicity, and is currently being tested concurrently with RT in clinical trials.
Flt3 ligand treatment after RT generates a systemic antitumor immune response and off-target (abscopal) effects at distant sites of disease.
TNF-α has shown benefit when given before RT.
GM-CSF, like vaccines, has proven beneficial when given after RT.
Several studies are ongoing to clarify the timing and mechanism of interleukin administration with RT.
Interest is increasing in interleukin therapy for radiation-induced lymphopenia.
RT & immune checkpoint inhibitors or immune co-stimulatory molecules
Combination of RT and anti-CTLA4 has resulted in abscopal responses in patients with solid tumors. Most studies point to a benefit of anti-CTLA4 given after RT.
Anti-PDL1 therapy after RT improves local tumor control in preclinical studies.
Dual therapy with anti-CTLA4 and anti-PDL1 in addition to RT can enhance response rates.
OX40 given after RT has resulted in extended overall survival as well as local tumor control in various in vivo models.
Toll-like receptor agonists have shown benefit when given before RT and are currently being investigated in clinical trials.
RT, immunotherapy & steroids
Prophylactic administration of glucocorticoids before RT results in lower survival.
Further studies are needed on the future use of steroids with RT and immunotherapy given the dampening effect on antitumor immune responses.
Conclusion & future perspective
Optimal combinations of RT and immunotherapy depend on understanding the effect of each agent on the tumor, tumor microenvironment and immune response.
Acknowledgements
The authors thank C Wogan of the Division of Radiation Oncology at MD Anderson Cancer Center for editorial contributions. The authors would also like to thank D Aten of the Creative Services Division at MD Anderson Cancer Center. Furthermore, the authors would like to acknowledge T R Cushman for all his help with responding to the reviewers’ and editor's comments.
Footnotes
Financial & competing interests disclosure
This investigation was supported by the NIH grant CA155446 (to S Krishnan). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. There are no industry relationships to disclose.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Withers HR. Lett JT, Adler H. Advances in Radiation Biology (4th Edition) Academic Press; NY, USA © Copyright 2003 Elsevier Science B.V., Amsterdam: 1975. The four R's of radiotherapy; pp. 241–269. [Google Scholar]
- 2.Golden EB, Formenti SC. Is tumor (R)ejection by the immune system the ‘5th R’ of radiobiology? Oncoimmunology. 2014;3(1):e28133. doi: 10.4161/onci.28133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyranvand Nejad E, Welters MJ, Arens R, Van Der Burg SH. The importance of correctly timing cancer immunotherapy. Expert Opin. Biol. Ther. 2017;17(1):87–103. doi: 10.1080/14712598.2017.1256388. [DOI] [PubMed] [Google Scholar]
- 4.Reynders K, De Ruysscher D. Radiotherapy and immunotherapy: improving cancer treatment through synergy. Prog. Tumor Res. 2015;42:67–78. doi: 10.1159/000437185. [DOI] [PubMed] [Google Scholar]
- 5.Wattenberg MM, Fahim A, Ahmed MM, Hodge JW. Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy. Radiat. Res. 2014;182(2):126–138. doi: 10.1667/RR13374.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 7.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 8.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. 2017 doi: 10.1038/nrclinonc.2016.211. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 10.Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 2015;21(16):3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008;181(5):3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int. J. Cancer. 2004;109(5):685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]; • Dendritic cells, given after radiation, augmented antitumor immunity.
- 13.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty M, Gelbard A, Carrasquillo JA, et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol. Immunother. 2008;57(8):1173–1183. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 16.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin. Cancer Res. 2008;14(16):5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamrava M, Kesarwala AH, Madan RA, et al. Long-term follow-up of prostate cancer patients treated with vaccine and definitive radiation therapy. Prostate Cancer Prostatic Dis. 2012;15(3):289–295. doi: 10.1038/pcan.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondini M, Nizard M, Tran T, et al. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol. Cancer Ther. 2015;14(6):1336–1345. doi: 10.1158/1535-7163.MCT-14-1015. [DOI] [PubMed] [Google Scholar]
- 19.Ye GW, Park JB, Park YJ, Choi YS, Sin JI. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Mol. Ther. 2007;15(8):1564–1570. doi: 10.1038/sj.mt.6300149. [DOI] [PubMed] [Google Scholar]
- 20.Adler AJ, Marsh DW, Yochum GS, et al. CD4(+) T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 1998;187(10):1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris TJ, Hipkiss EL, Borzillary S, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68(12):1319–1329. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood CG, Mulders P. Vitespen: a preclinical and clinical review. Future Oncol. 2009;5(6):763–774. doi: 10.2217/fon.09.46. [DOI] [PubMed] [Google Scholar]
- 23.Eton O, Ross MI, East MJ, et al. Autologous tumor-derived heat-shock protein peptide complex-96 (HSPPC-96) in patients with metastatic melanoma. J. Transl. Med. 2010;8:9. doi: 10.1186/1479-5876-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnett-Benson C, Hodge JW, Gameiro SR. Combination regimens of radiation therapy and therapeutic cancer vaccines: mechanisms and opportunities. Semin. Radiat. Oncol. 2015;25(1):46–53. doi: 10.1016/j.semradonc.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Diverse vaccine approaches require further study to develop strategies for optimizing the effects of both radiotherapy (RT) and vaccine.
- 25.Filatenkov A, Baker J, Muller AM, et al. Treatment of 4T1 metastatic breast cancer with combined hypofractionated irradiation and autologous T-cell infusion. Radiat. Res. 2014;182(2):163–169. doi: 10.1667/RR13471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menager J, Gorin JB, Maurel C, et al. Combining α-radioimmunotherapy and adoptive T cell therapy to potentiate tumor destruction. PLoS One. 2015;10(6):e0130249. doi: 10.1371/journal.pone.0130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deselm C, Hamieh M, Sadelain M. Radiation sensitizes tumor cells to CAR T cell immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2016;96(2):S127. [Google Scholar]
- 28.Cortez M, Korngold A, Valdecanas D, et al. Using radiation therapy to improve CAR T-cell targeting of solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 2016;96(2):E600. [Google Scholar]
- 29.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato H, Suzuki Y, Yoshimoto Y, et al. An abscopal effect in a case of concomitant treatment of locally and peritoneally recurrent gastric cancer using adoptive T-cell immunotherapy and radiotherapy. Clinical case reports. 2017;5(4):380–384. doi: 10.1002/ccr3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heo W, Lee YS, Son CH, Yang K, Park YS, Bae J. Radiation-induced matrix metalloproteinases limit natural killer cell-mediated anticancer immunity in NCI-H23 lung cancer cells. Mol Med Rep. 2015;11(3):1800–1806. doi: 10.3892/mmr.2014.2918. [DOI] [PubMed] [Google Scholar]
- 33.Finkel P, Frey B, Mayer F, et al. The dual role of NK cells in antitumor reactions triggered by ionizing radiation in combination with hyperthermia. Oncoimmunology. 2016;5(6):e1101206. doi: 10.1080/2162402X.2015.1101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunier EF, Akhurst RJ. TGF β inhibition for cancer therapy. Curr. Cancer Drug Targets. 2006;6(7):565–578. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 35.Kakeji Y, Maehara Y, Ikebe M, Teicher BA. Dynamics of tumor oxygenation, CD31 staining and transforming growth factor-β levels after treatment with radiation or cyclophosphamide in the rat 13762 mammary carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997;37(5):1115–1123. doi: 10.1016/s0360-3016(96)00573-1. [DOI] [PubMed] [Google Scholar]
- 36.Bouquet F, Pal A, Pilones KA, et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo . Clin. Cancer Res. 2011;17(21):6754–6765. doi: 10.1158/1078-0432.CCR-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFβ is a master regulator of radiation therapy-induced anti-tumor immunity. Cancer Res. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-β antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(3):876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 39.Lau CM, Nish SA, Yogev N, Waisman A, Reiner SL, Reizis B. Leukemia-associated activating mutation of Flt3 expands dendritic cells and alters T cell responses. J. Exp. Med. 2016;213(3):415–431. doi: 10.1084/jem.20150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59(24):6028–6032. [PubMed] [Google Scholar]
- 41.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]; •• Preclinical study showing that RT given with Flt3L has abscopal effects.
- 42.Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16(1):35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Van Horssen R, Ten Hagen TL, Eggermont AM. TNF-α in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 44.Gridley DS, Hammond SN, Liwnicz BH. Tumor necrosis factor-α augments radiation effects against human colon tumor xenografts. Anticancer Res. 1994;14(3a):1107–1112. [PubMed] [Google Scholar]
- 45.Weichselbaum RR, Hallahan DE, Beckett MA, et al. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54(16):4266–4269. [PubMed] [Google Scholar]
- 46.Staba MJ, Mauceri HJ, Kufe DW, Hallahan DE, Weichselbaum RR. Adenoviral TNF-α gene therapy and radiation damage tumor vasculature in a human malignant glioma xenograft. Gene Ther. 1998;5(3):293–300. doi: 10.1038/sj.gt.3300594. [DOI] [PubMed] [Google Scholar]
- 47.Chung T, Mauceri HJ, Hallahan DE, et al. Tumor necrosis factor-α-based gene therapy enhances radiation cytotoxicity in human prostate cancer. Cancer Gene Ther. 1997;5(6):344–349. [PubMed] [Google Scholar]
- 48.Gupta VK, Park JO, Jaskowiak NT, et al. Combined gene therapy and ionizing radiation is a novel approach to treat human esophageal adenocarcinoma. Ann. Surg. Oncol. 2002;9(5):500–504. doi: 10.1007/BF02557275. [DOI] [PubMed] [Google Scholar]
- 49.Hallahan DE, Vokes EE, Rubin SJ, et al. Phase I dose-escalation study of tumor necrosis factor-α and concomitant radiation therapy. Cancer J. Sci. Am. 1995;1(3):204–209. [PubMed] [Google Scholar]
- 50.Citrin D, Camphausen K, Wood BJ, et al. A pilot feasibility study of TNFerade™ biologic with capecitabine and radiation therapy followed by surgical resection for the treatment of rectal cancer. Oncology. 2010;79(5-6):382–388. doi: 10.1159/000323488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herman JM, Wild AT, Wang H, et al. Randomized Phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J. Clin. Oncol. 2013;31(7):886–894. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rekers NH, Zegers CM, Yaromina A, et al. Combination of radiotherapy with the immunocytokine L19-IL2: additive effect in a NK cell dependent tumour model. Radiother. Oncol. 2015;116(3):438–442. doi: 10.1016/j.radonc.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Zegers CM, Rekers NH, Quaden DH, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin. Cancer Res. 2015;21(5):1151–1160. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- 54.Seung SK, Curti BD, Crittenden M, et al. Phase I study of stereotactic body radiotherapy and interleukin-2 – tumor and immunological responses. Sci. Transl. Med. 2012;4(137):137ra174. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 55.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 56.Mcaleese JJ, Bishop KM, A'hern R, Henk JM. Randomized Phase II study of GM-CSF to reduce mucositis caused by accelerated radiotherapy of laryngeal cancer. Br. J. Radiol. 2006;79(943):608–613. doi: 10.1259/bjr/55190439. [DOI] [PubMed] [Google Scholar]
- 57.Nemunaitis J. Vaccines in cancer: GVAX, a GM-CSF gene vaccine. Exp. Rev. Vaccines. 2005;4(3):259–274. doi: 10.1586/14760584.4.3.259. [DOI] [PubMed] [Google Scholar]
- 58.Faltynek CR, Wang S, Miller D, et al. Administration of human recombinant IL-7 to normal and irradiated mice increases the numbers of lymphocytes and some immature cells of the myeloid lineage. J. Immunol. 1992;149(4):1276–1282. [PubMed] [Google Scholar]; • Potential use of IL-7 as a rescue drug for radiation-induced lymphopenia.
- 59.Chadha AS, Liu G, Chen HC, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int. J. Radiat. Oncol. Biol. Phys. 2017;97(2):323–332. doi: 10.1016/j.ijrobp.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 60.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pilones K, Aryankalayil J, Formenti S, Demaria S. Intratumoral IL-15 potentiates radiation-induced anti-tumor immunity. J. Immun. Cancer. 2015;3(2):P239. [Google Scholar]
- 62.Therapeutic effect of local Interleukin-15 with radiotherapy in breast cancer. March, 2017. http://meetinglibrary.asco.org/content/178459-194
- 63.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 64.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son CH, Bae JH, Shin DY, et al. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J. Immunother. 2014;37(1):1–7. doi: 10.1097/CJI.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimoto Y, Suzuki Y, Mimura K, et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS One. 2014;9(3):e92572. doi: 10.1371/journal.pone.0092572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu L, Wu MO, De La Maza L, et al. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget. 2015;6(14):12468–12480. doi: 10.18632/oncotarget.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11(6):e0157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat. Rev. Clin. Oncol. 2016;13(8):516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122(19):3051–3058. doi: 10.1002/cncr.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015;92(2):368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang W, Rodriguez Y, Kim B, et al. Temporally-dependent intracranial control of melanoma brain metastasis by stereotactic radiation therapy in patients treated with immune checkpoint blockade. Int. J. Radiat. Oncol. Biol. Phys. 2015;93(3):S57. [Google Scholar]
- 73.Schoenfeld JD, Mahadevan A, Floyd SR, et al. Ipilmumab and cranial radiation in metastatic melanoma patients: a case series and review. J. Immunother. Cancer. 2015;3:50. doi: 10.1186/s40425-015-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012;117(2):227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(2):293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1(2):92–98. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Giving RT after ipilimumab improved median survival in patients with unresectable stage II–IV melanoma.
- 78.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dovedi SJ, Lipowska-Bhalla G, Cheadle E, et al. The antitumor immune response generated by radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. 2014. [DOI] [PMC free article] [PubMed]
- 80.Wu CT, Chen WC, Chang YH, Lin WY, Chen MF. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 2016;6:19740. doi: 10.1038/srep19740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azad A, Yin Lim S, D'costa Z, et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. 2017;9(2):167–180. doi: 10.15252/emmm.201606674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Both anti-CTLA4 and anti-PDL1 therapy, in addition to RT, can inhibit Treg populations and mitigate T-cell exhaustion for enhanced tumor response.
- 84.Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann. Oncol. 2016;27(3):434–441. doi: 10.1093/annonc/mdv622. [DOI] [PubMed] [Google Scholar]
- 85.Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99(2):361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gough MJ, Crittenden MR, Sarff M, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J. Immunother. 2010;33(8):798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovacsovics-Bankowski M, Chisholm L, Vercellini J, et al. Phase I/II clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: immunological analysis. Journal for ImmunoTherapy of Cancer. 2013;1(1):P255. [Google Scholar]
- 88.Singh VK, Ducey EJ, Fatanmi OO, et al. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat. Res. 2012;177(5):628–642. doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 89.Cho JH, Lee HJ, Ko HJ, et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget. 2017;8(15):24932–24948. doi: 10.18632/oncotarget.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dewan MZ, Vanpouille-Box C, Kawashima N, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin. Cancer Res. 2012;18(24):6668–6678. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dovedi SJ, Melis MH, Wilkinson RW, et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood. 2013;121(2):251–259. doi: 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- 92.Mason KA, Ariga H, Neal R, et al. Targeting Toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin. Cancer Res. 2005;11(1):361–369. [PubMed] [Google Scholar]
- 93.Sugiyama T, Fujiwara K, Ohashi Y, et al. Phase III placebo-controlled double-blind randomized trial of radiotherapy for stage IIB-IVA cervical cancer with or without immunomodulator Z-100: a JGOG study. Ann. Oncol. 2014;25(5):1011–1017. doi: 10.1093/annonc/mdu057. [DOI] [PubMed] [Google Scholar]
- 94.Bourke JM, O'sullivan M, Khattak MA. Management of adverse events related to new cancer immunotherapy (immune checkpoint inhibitors) Med. J. Aust. 2016;205(9):418–424. doi: 10.5694/mja16.00586. [DOI] [PubMed] [Google Scholar]
- 95.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 2003;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 96.Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J. Immunol. 1993;151(8):4081–4089. [PubMed] [Google Scholar]
- 97.Horst HJ, Flad HD. Corticosteroid-interleukin 2 interactions: inhibition of binding of interleukin 2 to interleukin 2 receptors. Clin. Exp. Immunol. 1987;68(1):156–161. [PMC free article] [PubMed] [Google Scholar]
- 98.Karagiannidis C, Akdis M, Holopainen P, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 2004;114(6):1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 99.Grohmann U, Volpi C, Fallarino F, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat. Med. 2007;13(5):579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 100.Wagner DH, Jr, Hagman J, Linsley PS, Hodsdon W, Freed JH, Newell MK. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7-2. J. Exp. Med. 1996;184(5):1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinrichs CS, Palmer DC, Rosenberg SA, Restifo NP. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J. Immunother. 2005;28(6):517–524. doi: 10.1097/01.cji.0000177999.95831.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, Phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009;15(17):5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 103.Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23(3):191–195. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 104.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, Phase II trial. Lancet Oncol. 2012;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]