Abstract
We present the case of a 51-year-old woman admitted to our intensive care unit following an intentional overdose of a calcium channel antagonist and a beta blocker. The resultant hypotension was reversed with glucagon, noradrenaline, calcium and high-dose insulin. Despite these interventions, she remained vasoplegic and received a delayed, standard dose of intralipid. Subsequently, the vasoplegia resolved rapidly, and the vasopressor was stopped. Here, we review the management of overdose of calcium channel and beta-adrenergic receptor blockers, concentrating on the pharmacology of lipid emulsion therapy. There remain some unanswered questions about lipid emulsion therapy: treatment with lipid therapy is usually advocated as soon as possible; this case report suggests that it remains efficacious even if its administration were delayed.
Keywords: Calcium channel blocker, lipid emulsion, toxicity, beta-adrenergic blocker, insulin
Introduction
Poison-induced cardiogenic shock (PICS) is common; overdoses of beta-blockers (BBs) and calcium channel blockers (CCBs) account for over 65% of deaths from all cardiovascular medications.1 BB toxicity is the commonest cause of PICS in America;2 CCB overdose is less frequent, but is the cardiovascular agent with the highest mortality rate in America,3 responsible for 48% of all deaths from cardiovascular agents.1 Management of patients following overdose of these drugs can be difficult; profound hypotension and bradycardia are seen, despite administration of standard treatments used for circulatory support.
In haemodynamically unstable patients, priorities include maintaining a patent airway with correction of oxygenation and ventilation, and intravenous fluids for correction of hypotension. Symptomatic bradycardia may be treated with atropine and cardiac pacing; calcium, glucagon and catecholamines, such as noradrenaline, may also be given. For patients who remain haemodynamically unstable after these initial therapies, second-line treatment options include high-dose insulin (HDI), lipid emulsion (LE) therapy and mechanical life support, including intra-aortic balloon pump, cardiopulmonary bypass and extracorporeal membrane oxygenation. The pharmacology of HDI is discussed below. There is increasing experience in using LE, but there is concern over lack of clarity of the mechanism of action, and its risks, which are discussed below. This case report suggests that it remains efficacious even if its administration was delayed.
Case report
A 51-year-old woman was admitted to our intensive care unit (ICU) from the emergency department (ED) following an intentional overdose of 280 mg of amlodipine, a calcium channel antagonist, and 140 mg of bisoprolol. She had a past medical history of hypertension, but was otherwise fit and well.
On admission to the ED, she was alert and orientated, but had a blood pressure of 90/60, with a heart rate of 50 per minute. The hypotension failed to respond to conservative treatment, and expecting the hypotension to persist, she was admitted to the ICU. On the advice of the National Poisons Information Service (NPIS), she was treated with an infusion of glucagon and noradrenaline, to maintain an adequate perfusion pressure. She also received boluses of calcium chloride and an infusion of HDI (1.5 units/kg/h). The NPIS also advised to consider LE if deteriorating.
She became increasingly vasoplegic, requiring a dose of noradrenaline of 0.8 µg/kg/min at 16 h after the overdose, and, as advised by the NPIS, she received a standard dose of intralipid at this time. She received a bolus of 1.5 ml/kg of 20% LE, followed by an infusion at 15 ml/kg/h. Subsequently, the vasoplegia resolved rapidly and the vasopressor was stopped 4 h after the initial LE dose. She thereafter developed hypotension again, and the vasopressor was restarted at 15 h after the initial LE. A second dose of LE was given 10 h later, after review on the following morning’s ward round and 24 h after the first dose, which was followed by permanent resolution of the hypotension (see Table 1). There was no significant change in the heart rate. The rate of glucagon infusion was not changed during the two LE infusions, but increasing glycaemic sensitivity to insulin allowed the latter to be weaned and stopped. The insulin infusion was continued into the second episode of vasoplegia, but hypoglycaemia necessitated the infusion be stopped, 6 h before the second LE dose. A focussed echocardiogram performed before the LE infusion did not demonstrate hypovolaemia or left ventricular systolic dysfunction, suggesting that vasoplegia was the predominant cause of the hypotension. Serum triglyceride levels were not measured.
Table 1.
Cardiovascular parameters 1 h preceding, and 1 h and 6 h following LE administration.
| 1st LE dose |
2nd LE dose |
|||||
|---|---|---|---|---|---|---|
| 1 h before | 1 h after | 6 h after | 1 h before | 1 h after | 6 h after | |
| Time after ingestion (h) | 16 h | 41 h | ||||
| HR (min−1) | 82 | 87 | 81 | 69 | 70 | 86 |
| Mean arterial BP (mmHg) | 76 | 77 | 72 | 68 | 71 | 80 |
| Noradrenaline rate (µg/kg/min) | 0.81 | 0.3 | 0 | 0.25 | 0.1 | 0 |
| Glucagon rate (mg/h) | 15.7 | 15.7 | 15.7 | 15.7 | 15.7 | 15.7 |
| Insulin rate (units/h) | 94 | 94 | 66 | 0 | 0 | 0 |
Discussion
Pharmacodynamics of beta-adrenergic and CCBs
BBs competitively inhibit beta-receptors, which then indirectly decrease the production of intracellular cyclic adenosine mono-phosphate (cAMP); calcium influx through L-type calcium channels in the myocardium is reduced, which reduces the heart rate and cardiac contractility.
CCBs directly block L-type calcium channels, which causes relaxation of vascular smooth muscle, and as a result, vasodilatation. Some CCBs, for example, verapamil and diltiazem, also inhibit sinoatrial and atrioventricular node activity. In addition, CCBs switch the heart to metabolise carbohydrate preferentially, instead of free fatty acid oxidation that occurs in the myocardium in the non-stressed state.
Elsewhere, calcium channel antagonism inhibits insulin secretion in the beta-islet cells of the pancreas, producing insulin resistance and hyperglycaemia.
Treatment of CCB and BB overdose with glucagon and calcium
Calcium salts are standard first-line agents in BB and CCB overdose, since the latter cause intracellular hypocalcaemia. In significant overdoses, calcium supplementation may provide a modest improvement in blood pressure, inotropy and conduction; however, patients are unlikely to respond to calcium as a single agent.4
Glucagon is an attractive antidote in BB toxicity as it activates adenyl cyclase, which exerts a chronotropic and inotropic effect on the myocardium by stimulating cAMP synthesis, despite the beta-adrenergic receptor blockade.4,5 It produces an immediate increase in systemic arterial pressure; its effect on cardiac contractility may be modest. These positive chronotropic and inotropic effects have been demonstrated in various animal models of beta-blockade, and may be superior to a phosphodiesterase inhibitor and beta-receptor agonist in reversing the beta-blockade.4 Glucagon is associated with several side effects, including nausea and vomiting, hypokalaemia and hyperglycaemia. It also displays tachyphylaxis.
Pharmacology and clinical effects of HDI
The mechanism of action of HDI is not fully elucidated, but probably acts via several different mechanisms. Insulin in high doses has strong positive inotropic properties, improving maximum elastance at end systole, left ventricular end diastolic pressure and coronary artery blood flow compared with glucagon and adrenaline,6 and stroke volume after 1 h of HDI.7 The inotropic response is associated with increases in intracellular calcium ion flux and sensitivity,8 and an improved response to catecholamines.9 HDI produces vasodilatation, which improves local microcirculation and systemic perfusion. The onset of the effects is delayed, but the improvement in haemodynamics is more sustained than other vasoactive agents, without the tachyphylaxis observed with adrenergic agents.9
Under normal conditions, the primary energy substrates for the heart are fatty acids. Under stressed conditions, carbohydrates become the primary energy source. HDI improves myocardial uptake of carbohydrates, and inhibits free fatty acid metabolism. Additionally, exogenous insulin administration can help to overcome the insulin resistance and insulin deficiency that occurs in CCB toxicity.
The optimum dose has not been established. A dose of between 1 and 10 units/kg/h has been reported as effective in allowing vasopressor support to be stopped,10 but doses as high as 22 units/kg/h have been used successfully.11
The major adverse events are hypoglycaemia and hypokalaemia. Glucose supplementation is likely to be required throughout therapy and for up to 24 h after discontinuation of HDI. The hypokalaemia reflects a shifting of potassium into the intracellular space, rather than a decrease in total body stores.
Pharmacology and clinical use LE
Invented by Arvid Wretlind, a Swedish doctor, intralipid was approved for parenteral nutrition in 1962. Interest in the use of LE as an antidote has grown since a chance observation 25 years later that LE increased the dose of bupivacaine required to produce asystole in rats.12 Measurement of partitioning suggested that the local anaesthetic (LA) partitioned into the lipid. Following further experience, LE is now a standard recommended treatment for LA toxicity, including refractory cardiac arrest.13 There have subsequently been reports of its successful use in a wide range of drug overdose situations, including CCB, BB, typical and atypical antipsychotics, and tricyclic and other antidepressants, whose one common feature would appear to be that they are all lipophilic.
Intralipid® 20% is presented as a non-pyrogenic fat emulsion and consists of 20% soybean oil, 1.2% egg yolk phospholipids, 2.25% glycerin and water for injection. It is an alkaline solution, with the addition of sodium hydroxide. The soybean oil consists of triglycerides of mostly unsaturated fatty acids, primarily linoleic, oleic, palmitic, linolenic and stearic acids. Intralipid® has an osmolality of around 350 mOsmol/kg water, equivalent to 260 mOsmol/l of emulsion. The fat particle size is around 0.5 µm and should not therefore be a risk for fat embolism production. It has a caloric value of 2 kcal/ml. It also contains a significant phosphorous load of approximately 1.5 mmol/l emulsion. It is stored in a multi-layered film which is biologically inert.
It is thought to be cleared from the plasma in a similar way to chylomicron clearance – through phospholipolysis by hepatic lipases, generating remnant particles which are cleared by the liver.14
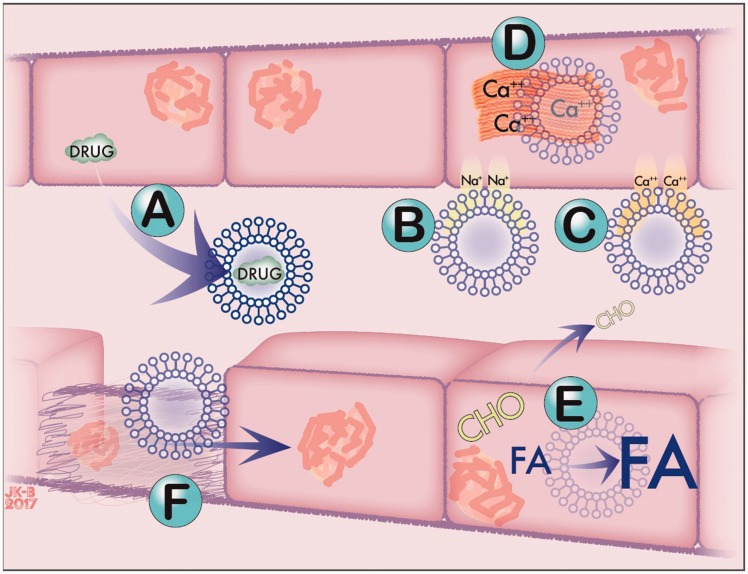
The mechanism of action of intralipid in treatment of drug toxicity is not clear. Proposed mechanisms include via lipid sink, improving cardiac fatty acid metabolism, changes in sodium or calcium channel function, or cardioprotection from cell damage (see Figure 1).
Figure 1.
Proposed mechanisms of action of LE. LE may repartition drug (A): lipophilic substances are drawn into ‘lipid sink’; a concentration gradient develops between tissue and blood, and the toxic drug moves from tissue into aqueous phase into lipid phase. LE may affect sodium channel function (B), or calcium channel function or flux (C), with an increase in intracellular calcium and inotropy (D). Alternatively, LE may revert the cell to fatty acid metabolism or provide FA substrate (E), which may increase inotropy or reverse vasodilatation. LE may provide cytoprotection or stimulate repair after ischaemic injury (F).
Lipid sink theory
The lipid sink theory suggests that when lipid droplets are administered intravenously, lipophilic molecules preferentially partition into the droplets. The sequestering of these pharmacologically active molecules is thought to allow pharmaceutical agents to be redistributed from tissues of critical organs such as the heart and brain to the bloodstream. The observed reversal of toxicity from lipophilic drugs is consistent with this theory. However, this theory may only partially account for its observed effects, and may not be the sole mechanism of reversal of LA systemic toxicity. The effects of lipid infusions tend to be observed within a few minutes, but pharmacokinetic modelling of bupivacaine cardiotoxicity predicts that LE reduces the concentration in heart tissue by only 11% after 3 min of initiating therapy.15 Additionally, the un-entrapped (non-lipid bound) and free (non-protein bound) bupivacaine plasma concentrations after a non-toxic dose of bupivacaine may not be altered by LE16 suggesting that the lipid sink effect may be less important than previously considered, and that reversal of hypotension with LE may therefore be due to mechanisms other than partitioning of lipophilic drugs. LA may instead be redistributed to other tissues with administration of LE.16
Inotropy
LE appears to have an inotropic effect, even in the absence of cardiodepressant agents. There are few human studies quantifying the change in inotropy, but in one study on normal rat heart, echocardiography demonstrated that the left ventricular ejection fraction increased by 7% after the administration of LE.17 The mechanism is not clear, but changes in metabolism in cardiac myocytes, ionic channel modulation or cardioprotective mechanisms may be responsible. The inotropic effect of LE may be of less importance in this case, as the hypotension was considered to be primarily from vasodilatation.
Cardiac fatty acid metabolism
Under normal conditions, myocytes preferentially metabolise fatty acid as its primary energy source; however, in stressed conditions, it reverts to metabolism of carbohydrate, adding weight to the use of HDI in patient care information system (PCIS).
Alternatively, infusion of triglycerides and phospholipids in LE may allow myocytes under toxic conditions to revert to preferential fatty acid metabolism. Carnitine is required in the transportation of fatty acids within eukaryotic cells. Selective inhibition of the carnitine palmitoyl transferase 1 enzyme abolishes LE-induced improvement in contractility,18 suggesting that the increased inotropy with LE is at least partly dependent on improvement in FA availability or metabolism. Patients with carnitine deficiency may be more at risk from cardiac toxicity from lower doses of bupivacaine,19 which is itself an inhibitor of carnitine transport.20 The addition of high-concentration lipids may therefore overwhelm the blockade to allow for preferential myocyte FA metabolism as an energy source.
LE causes an increase in vascular resistance, which is thought to be as a result of FA stimulation of an acute inflammatory response, and is associated with acute endothelial dysfunction. Impairment in vasodilatation occurs as a result of changes in locally-derived vascular mediators.21 The changes may be prolonged; an infusion of LE is associated with a reduction in vasodilatation even 48 h later.22
Intracellular calcium
Non-esterified fatty acids accumulate at sites of tissue injury and necrosis, but their physiological role is not clear. The presence of the fatty acids is associated with increased intracellular calcium concentration, which may account for the increase in inotropy,23 in addition to the metabolic changes outlined above. Long-chain unsaturated and saturated fatty acids, many of which are contained within LE, induce significant increases in voltage-dependent calcium currents (ICa) in cardiac myocytes.24 The mechanism is not clear; LE may directly stimulate calcium flux, possibly by acting at lipid sites near the channels or directly on the channel protein itself. This effect appears to be independent of any cellular second messenger system, and is therefore particularly important in reducing the toxicity from CCB, and could potentially be responsible for the short, direct cardiotonic effect seen with LE.
Changes in sodium channel function
Recent work has suggested that LE may in addition affect sodium channel function. This effect may be of less relevance in this case, but may explain the positive outcome observed in cases where sodium channel antagonists are prominent intoxicants. LE partially reverses bupivacaine-induced blockade on specific cardiac sodium channels in cell culture. In addition, recovery of inactivated channels after bupivacaine-induced block is faster in the presence of lipids,25,26 independent of the repartitioning of the LA in the presence of LE.
Cardioprotection from cell damage
Ischaemia and reperfusion can both damage myocardial cells, the latter from inflammatory changes and induction of oxidative stress during restoration of perfusion. Intralipid appears to elicit some protection against myocardial ischaemia-reperfusion injury when administered at the time of reperfusion, with one study of rats demonstrating an absolute reduction in infarct size of 37%.27 The mechanism is thought to be related to LE-induced inhibition of the opening of the mitochondrial permeability transition pore (mPTP), which is associated with excitotoxicity and apoptosis after cellular stress, and LE-induced protection of the heart by recruiting reperfusion pathways.
It is suggested that a combination of mechanisms was responsible for the improvement observed with LE in this case. At the time the first dose of LE was used (22 h post-ingestion), using mean half-lives of the drugs taken (amlodipine 30–50 h and bisoprolol 9–12 h) the effective doses of the drugs taken would have been meant that the predominant residual drug would have been amlodipine, and at the time of the second dose of LE, the bisoprolol would probably be exerting minimal biological action. Anecdotal evidence suggests that elimination of amlodipine may be delayed following overdose,28 while the pharmacokinetics of bisoprolol are independent of the dose up to at least 100 mg.29 One published case of an overdose of between 50 and 100 mg of amlodipine reports plasma concentrations falling from 88 ng/ml at 2.5 h, to 79 ng/ml 35 h later, a reduction of only 10%.28 The cardiovascular dysfunction was therefore predominantly from the amlodipine. Amlodipine is lipophilic, suggesting that LE may be working primarily through repartitioning of the drug (the ‘lipid sink’ theory), and through changes in calcium flux. The hypotension was thought to be as a result of vasodilatation rather than myocardial dysfunction; the effect of LE on vascular reactivity is probably more relevant in this case than the inotropic effects. LE produced improvement within a few minutes, suggesting that its cardioprotective mechanisms are less important here.
The optimum dose of intralipid is not clear. Current guidelines advise 1.5 ml/kg as an initial bolus, followed by 0.25 ml/kg/min over 30–60 min, or a total dose of 9–17.5 ml/kg30 but larger doses are usually well tolerated. In one reported case, there were no cardiorespiratory complications in a patient who received 2 l of 20% LE in error, during treatment for an amlodipine overdose.31 Even this dose is probably well within safe limits; rat studies suggest that the median lethal dose (LD50) is 67 ml/kg.32
While the precise mechanism of action and optimum dose remains unclear, there is increasing experience of its beneficial effects reversing the toxicity from a widening range of drug classes. However, there is increasing concern that the threshold for the use of LE is too low33: while undoubtedly of benefit in some cases, there is reported concern over publication bias, and the lack of a clear mechanism of action or optimal dose. LE is often given simultaneously with other treatments, making its efficacy difficult to determine. In one review of nine patients treated with LE for drug toxicity, six developed complications that were thought to be associated with the lipid infusion. Two patients developed pancreatitis, and four patients developed lipaemia sufficient to interfere with interpretation of laboratory studies. Three patients developed acute respiratory distress syndrome (ARDS).34 While causality from this paper was not determined, there is a temporal and biological association, and therefore a potential risk.
Amlodipine has a relatively long half-life. LE has previously been reported to be effective in amlodipine overdose.35 If LE is not given immediately after ingestion of a toxic dose, for example over concerns about adverse effects, this case report suggests that it may still be of benefit later.
Conclusions
We have reviewed the management of calcium channel and BB overdose, and specifically the use of insulin and LE. We wanted to raise awareness of the use of LE; if concerns about its adverse effects prevent its use at the time of the overdose, it may still be of use even after a few days if the patient remains compromised. The mechanism of action of LE remains unclear. Further elucidation would enable the use of LE to be targeted towards drugs whose pharmacodynamic effects are susceptible to modulation by LE, while minimising any adverse effects.
HDI has a number of beneficial effects, including a prolonged improvement in inotropy, increased sensitivity to catecholamines, and improvement in the availability of carbohydrates, the primary energy source in a myocardium in stressed conditions. It has few side effects.
Authors’ note
The authors do not advocate the treatment options discussed here. This article is a discussion on their observations on one particular case.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and β-blocker toxicity. Toxicol Rev 2004; 23: 223–238. [DOI] [PubMed] [Google Scholar]
- 2.Mowry JB, Spyker DA, Cantilena LR, et al. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol 2013; 51: 949–1229. [DOI] [PubMed] [Google Scholar]
- 3.Watson WA, Litovitz TL, Klein-Schwartz W, et al. 2003 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2004; 22: 335–404. [DOI] [PubMed] [Google Scholar]
- 4.Calcium and beta receptor antagonist overdose: a review and update of pharmacological principles and management. Available at: http://www.medscape.com/viewarticle/430202 (accessed 25 June 2016).
- 5.Kerns W. Management of beta-adrenergic blocker and calcium channel antagonist toxicity. Emerg Med Clin N Am 2007; 25: 309–331. [DOI] [PubMed] [Google Scholar]
- 6.Kline JA, Tomaszewsi CA, Schroeder JD, et al. Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharm Exp Ther 1993; 267: 744–750. [PubMed] [Google Scholar]
- 7.Klein LJ, van Campen CMC, Sieswerda GT, et al. Effects of high-dose insulin infusion on left ventricular function in normal subjects. Neth Heart J 2010; 18: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Lewinski D, Bruns S, Walther S, et al. Insulin causes Ca2+-dependent and Ca2+-independent positive inotropic effects in failing human myocardium. Circulation 2005; 111: 2588–2595. [DOI] [PubMed] [Google Scholar]
- 9.Kline JA. Insulin for calcium channel toxicity. Ann Emerg Med 2014; 63: 92–93. [DOI] [PubMed] [Google Scholar]
- 10.Holger JS, Stellpflug SJ, Cole JB, et al. High-dose insulin: a consecutive case series in toxin-induced cardiogenic shock. Clin Toxicol 2011; 49: 653–658. [DOI] [PubMed] [Google Scholar]
- 11.Stellpflug SJ, Harris CR, Engebretsen KM, et al. Intentional overdose with cardiac arrest treated with intravenous fat emulsion and high-dose insulin. Clin Toxicol 2010; 48: 227–229. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg GL, VadeBoncouer T, Ramaraju GA, et al. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesia 1998; 88: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 13.AAGBI. Management of severe local anaesthetic toxicity. Available at: http://www.aagbi.org/sites/default/files/la_toxicity_2010_0.pdf (accessed 15 November 2016).
- 14.Crawford SE, Borensztajn J. Plasma clearance and liver uptake of chylomicron remnants generated by hepatic lipase lipolysis: evidence for a lactoferrin-sensitive and apolipoprotein E-independent pathway. J Lipid Res 1999; 40: 797–805. [PubMed] [Google Scholar]
- 15.Kuo I, Akpa BS. Validity of the lipid sink as a mechanism for the reversal of local anesthetic systemic toxicity: a physiologically based pharmacokinetic model study. Anesthesia 2013; 118: 1350–1361. [DOI] [PubMed] [Google Scholar]
- 16.Litonius E, Tarkkila P, Neuvonen PJ, et al. Effect of intravenous lipid emulsion on bupivacaine plasma concentration in humans. Anaesthesia 2012; 67: 600–605. [DOI] [PubMed] [Google Scholar]
- 17.Feldman S, Carillion A, Riou B, et al. Positive inotropic effect of lipid emulsion Intralipid® in healthy rat myocardium. Eur J Anaesthesiol 2013; 30: 204. [Google Scholar]
- 18.Van de Velde M, Wouters PF, Rolf N, et al. Long-chain triglycerides improve recovery from myocardial stunning in conscious dogs. Cardiovasc Res 1996; 32: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg GL, Laurito CE, Geldner P, et al. Malignant ventricular dysrhythmias in a patient with isovaleric acidemia receiving general and local anesthesia for suction lipectomy. J Clin Anesth 1997; 9: 668–670. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg GL, Palmer JW, VadeBoncouer TR, et al. Bupivacaine inhibits acylcarnitine exchange in cardiac mitochondria. Anesthesia 2000; 92: 523–528. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 1997; 100: 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umpierrez GE, Smiley D, Robalino G, et al. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab 2009; 94: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothschild L, Bern S, Oswald S, et al. Intravenous lipid emulsion in clinical toxicology. Scand J Trauma Resusc Emerg Med 2010; 18: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A 1992; 89: 6452–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mottram A, Valdivia C, Makielski J. Fatty acids antagonize bupivacaine induced I (NA) blockade. Clin Toxicol 2011; 49: 729–733. [DOI] [PubMed] [Google Scholar]
- 26.Nadrowitz F, Stoetzer C, Foadi N, et al. The distinct effects of lipid emulsions used for lipid resuscitation on gating and bupivacaine induced inhibition of the cardiac sodium channel Nav1.5. Anesth Analg 2013; 117: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 27.Rahman S, Li J, Bopassa JC, et al. Phosphorylation of GSK-3β mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesia 2011; 115: 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanek EJ, Nelson CE, DeNofrio D. Amlodipine overdose. Ann Pharmacother 1997; 31: 853–856. [DOI] [PubMed] [Google Scholar]
- 29.Leopold G. Balanced pharmacokinetics and metabolism of bisoprolol. J Cardiovasc Pharmacol 1986; 8: S16–S20. [DOI] [PubMed] [Google Scholar]
- 30.Lipid rescue resuscitation. Available at: www.lipidrescue.org (2012, accessed 14 June 2016).
- 31.West PL, McKeown NJ, Hendrickson RG. Iatrogenic lipid emulsion overdose in a case of amlodipine poisoning. Clin Toxicol 2010; 48: 393–396. [DOI] [PubMed] [Google Scholar]
- 32.Hiller DB, Di Gregorio G, Kelly K, et al. Safety of high volume lipid emulsion infusion: a first approximation of LD50 in rats. Reg Anesth Pain Med 2010; 35: 140–144. [DOI] [PubMed] [Google Scholar]
- 33.Höjer J, Jacobsen D, Neuvonen PJ, et al. Lipid rescue – efficacy and safety still unproven. Basic Clin Pharmacol Toxicol 2016; 119: 345–348. [DOI] [PubMed] [Google Scholar]
- 34.Levine M, Skolnik AB, Ruha AM, et al. Complications following antidotal use of intravenous lipid emulsion therapy. J Med Toxicol 2014; 10: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meaney CJ, Sareh H, Hayes BD, et al. Intravenous lipid emulsion in the management of amlodipine overdose. Hosp Pharm 2013; 48: 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]