Abstract
Study Design:
Literature review.
Objective:
The aim of this study was to provide an overview of the available intraoperative monitoring techniques and the evidence around their efficacy in vertebral column resection.
Methods:
The history of neuromonitoring and evolution of the modalities are reviewed and discussed. The authors’ specific surgical techniques and preferred methods are outlined in detail. In addition, the authors’ experience and the literature regarding vertebral column resection and surgical mitigation of neurologic alarms are discussed at length.
Results:
Risk factors for signal changes have been identified, including preoperative neurologic deficit, severe kyphosis, increased curve magnitude, and significant cord shortening. Even though no evidence-based treatment algorithm exist for signal changes, strategies are discussed that can help prevent alarms and address them appropriately.
Conclusion:
Through implementation of multimodal intraoperative monitoring techniques, potential neurologic injuries are localized and managed in real time. Intraoperative monitoring is a valuable tool for improving the safety and outcome of spinal deformity surgery.
Keywords: neuromonitoring, MEP, EMG, SSEP, VCR, PSO, spinal deformity, spine surgery
Introduction
Within the past 2 decades, the management of spinal deformities has changed significantly. The advent and widespread use of pedicle screw fixation, in combination with advancements in posterior and 3-column osteotomy techniques, has permitted more aggressive deformity correction. Unfortunately, neurologic complications have been associated with increased complexity, large corrections, staged procedures, and significant blood loss. Although relatively infrequent, iatrogenic neurologic deficit can be a devastating complication. Intraoperative neuromonitoring was developed in an effort to mitigate the risks to the sensitive neural elements during spine surgery.
In order for intraoperative monitoring to be useful in detecting and preventing neurologic problems, it must provide sufficient warning of impending deficit to allow time for correction. Additionally, the safety, efficacy, and cost-effectiveness profile must be desirable. Paralleling the advancements in technique and instrumentation, there has been a steady evolution in utilization of various neuromonitoring methods. The purpose of this article is to provide a summary of the neuromonitoring modalities and discuss their efficacy in the prevention of postoperative neurologic deficits relating to spinal deformity correction. The incidence and etiology of neurologic complications in 3-column osteotomies and the recommendations for the utilization of intraoperative monitoring in this setting will be reviewed at length.
Neuromonitoring Modalities
Somatosensory-Evoked Potentials (SSEPs)
Somatosensory-evoked potentials primarily monitor the integrity of the dorsal columns by stimulating the peripheral nerves electrically and recording the potential over the cortex and subcortex during spinal reconstructive surgery. By measuring and analyzing repeated small amplitude signals from peripheral nerves and averaging the data into one signal, SSEPs provide a measurable display of amplitude and latency.1 Typically, the ulnar nerve is used as a reference for the upper extremity and the posterior tibial nerve for the lower extremity. This stimulus is carried along the dorsal nerve roots to the dorsal column spinal tracts and the somatosensory cortex, where they are recorded (Figure 1).2 Approximately 300 to 500 electrical stimuli are delivered to provide an averaged waveform. Significant decrease in amplitude, and/or a significant increase in latency, is associated with potential changes in neurologic status of the patient. The surgeon is typically notified when there is unilateral or bilateral amplitude loss of at least 50% to 60% or 10% increase in latency.3
Figure 1.
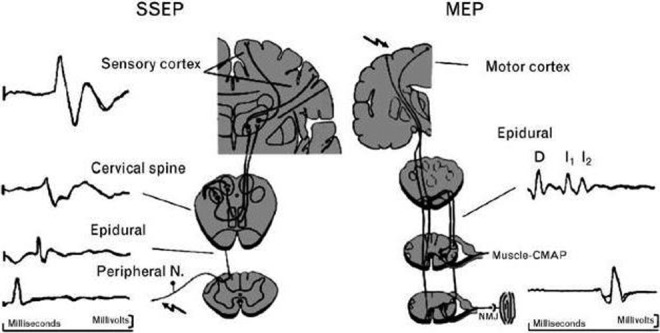
Neuroanatomic pathways of somatosensory-evoked potential (SEEP) and motor-evoked potential (MEP). The SSEP is produced by stimulation of a peripheral nerve. The electrical signal travels through the dorsal columns of the spinal cord and crosses to the contralateral side after synapsing at the cervicomedullary junction. It ascends to another synapse in the thalamus and finally to the primary sensory cortex where the response is recorded. The MEP is produced by stimulation of the motor cortex in the brain. An electrical signal descends in the corticospinal tract to reach the anterior horn cells of the spinal cord. After synapsing, the signal travels along a peripheral nerve and across the neuromuscular junction to produce a muscle response. MEP signals can be recorded from the epidural space (D and I waves) or in the muscle (compound muscle action potential). Reprinted with permission from Sloan TB, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anesthesiol. 2008;21(5):560-564.
Somatosensory-evoked potentials are the primary modality used to detect sensory changes. Although unable to detect motor change, they have a high specificity for detecting impending spinal cord injury. By directly analyzing the spinal cord sensory tracts, SSEPs provide reliable information for surgeon notification. However, there are some limitation to the technology. Because signals are averaged, they are not performed in real time, which does delay the identification of changes. Furthermore, the surgeon has to be aware that SSEPs are sensitive to systemic issues and agents (hypotension, hypothermia, and use of halogenated anesthetic agents), which may lead to inadvertent neuromonitoring changes unrelated to surgical events. Experienced technologists must be able to identify technical versus surgical causes of data change. Last, SSEPs have only limited effectiveness in identifying isolated nerve root injuries due to multilevel innervation of spinal nerve roots. SSEPs are a crucial component to intraoperative neuromonitoring, but are most effective when used in a multimodal fashion in conjunction with motor-evoked potentials and electromyography.
Transcranial Motor-Evoked Potentials (tcMEPs)
Motor-evoked potentials were developed to directly monitor the motor pathways, that is, the corticospinal tracts. Early attempts to monitor motor pathways by stimulating the spinal cord were discovered to be primarily sensory in nature. The only true motor potential is one that begins in the motor cortex itself. Stimulation of the motor cortex and recording on peripheral muscles allows for testing of the entire motor pathway (Figure 1).2 In events such as anterior spinal artery syndrome, SSEP responses would fail to identify changes in spinal cord function because the dorsal columns would remain intact. The advent of safe and effective MEP monitoring allowed detection of such events and increased the sensitivity of neuromonitoring modalities.
Electrodes placed on the scalp stimulate the motor cortex with a pulse train of high-voltage, short-duration signal.1 In contrast to the white matter–mediated SSEPs, the tcMEPs directly evaluate the spinal cord pyramidal tracts to provide motor information. The tcMEP can be measured in the epidural space or as a compound muscle action potential in the effector muscle.
A single universal warning criterion for motor-evoked potentials has yet to be determined. There are currently 3 different warning criteria that are primarily used to indicate a significant change in the patient’s motor function. The first criterion is referred to as the “Threshold Technique.” This technique uses a 100 volt increase in stimulus without recovery of the response’s amplitude as a means of quantifying significant change. Stimulus is increased when the amplitude of myogenic responses decrease below 65% of baseline values.4 Next, the “Amplitude Technique” allows for increase of stimulus up to maximum, as long as the amplitude of the recorded response is maintained within acceptable limits. Finally, the “All or None Technique” allows for increase of stimulus to maximum and a significant decrease in amplitude, as long as a reproducible response remains recordable. tcMEPs are both sensitive and specific for diagnosing intraoperative spinal cord injury and poor spinal cord perfusion. The concurrent use of tcMEPs with SSEPs provides the surgeon with highly reliable information about the status of the surgical patient’s neurologic status, both sensory and motor.
Descending Neurogenic-Evoked Potentials (dNEPs)
During the 1990s, prior to the advent of MEP monitoring, attempts were made to record motor-evoked potentials from peripheral nerves or muscles via stimulation of the spinal cord. The provided stimulus is either indirect with placement of needles into consecutive spinous processes or direct with insertion of an epidural catheter onto the dura through a laminotomy defect within operative spinal levels. Typically, the responses are recorded from the sciatic nerve at the popliteal fossa. Initially, dNEPs were believed to record motor activity, but findings from Minahan et al suggest the findings correlate more with antidromic dorsal column signal.5 Although they do not provide motor data, the dNEPs are still useful, especially in patients with poor motor data (as seen in patients with severe cerebral palsy). Additionally, dNEPs can help localize the area of spinal cord deficit by systematically stimulating at multiple points along the spinal column, allowing for precise mapping of the injury level.6 When stimulating above the point of spinal cord injury, recordings in the lower extremities are absent. When stimulation is applied distal to the point of injury to the cord, lower extremity data is recordable. This mapping can direct surgical intervention to the region of interest and potentially prevent or limit neurologic injury.
Similar to SSEPs, dNEPs may be unable to detect neurologic injury when the dorsal columns are intact. Also, dNEPs specifically monitor spinal cord function and are not used to detect nerve root injury. When used in a multimodal fashion, dNEPs are a useful adjunct to the neuromonitoring armamentarium.
Spontaneous Electromyography
Spontaneous electromyography detects the spontaneous electrical activity of muscles. Bipolar needles are used either intramuscularly or subdermally to detect neurotonic discharges from muscles during spine surgery.7 Proper needle placement is essential for accurate EMG recording, with the electrodes being placed into the “belly” of each recorded muscle. Baseline EMG values are recorded prior to surgical start and continuous recordings are made throughout the case. EMG is based on the premise that muscles at rest should be relatively electrically quiet—without spontaneous activity. Observed activity in an effector muscle group can indicate irritation or stretching of the lumbar root associated with that muscle. This information can provide useful feedback to the surgeon.
Unlike other modalities, spontaneous electromyography can provide real-time information about nerve root function. For example, significant burst pattern changes leading to nonrepetitive asynchronous potentials or train activity consisting of multiple and/or repetitive synchronous stimuli may indicate nerve root damage during the case.8 However, in most cases, burst patterns actually indicate temporary nerve root irritation from manipulation, electrocautery, or tugging rather than permanent injury. Classically, high-frequency discharges are more likely associated with true injury.9 Still the sustained repetitive stimuli recorded may be secondary from traction and compression of nerve roots, which in itself requires immediate identification and rectification during the case.
Unfortunately, electrically quiet muscle may not always be a normal finding. The absence of expected EMG activity should be questioned. In cases of chronic nerve root damage, the nerve root may be nonresponsive. Additionally, dosage and timing of surgical muscle relaxation must be considered. Nonetheless, spontaneous EMG is a useful adjunct to monitoring during a spinal procedure, but it should always be performed alongside multimodality testing. The passive nature of spontaneous EMG is complimented by the active testing protocols of SSEP and MEP monitoring.
Triggered EMG (tEMG)
Calancie et al first described the use of electrical stimulation of pedicle screws for evaluation of placement in an animal model.10 Since that time, tEMGs have been shown to be an effective tool for detecting pedicle breaches.1 tEMGs are obtained by stimulating the center of the tulip head of the pedicle screws with a monopolar probe while using a needle electrode in the paraspinal musculature as an anode. The EMG response was recorded from the effector muscles of each spinal level tested.
Stimulation begins at zero and is slowly increased until a sustained EMG response is recorded. The stimulation level needed to create an EMG response is called the “threshold.” Lower thresholds suggest that the cortical bone of the pedicle is breached, which could allow the pedicle screw to irritate the nerve root. There is no universally accepted set of thresholds, but values of 6 mA to 15 mA have been discussed in previous literature (Figure 2).11–14 Essentially, tEMG is an impedance test used to stimulate nerve roots. Loss of the structural integrity of the cortical pedicle leads to less impedance to the nerve root and lower electrical stimulation values required to detect a response in recording muscles. Responses lower than a predetermined threshold value suggests potential screw malposition. Danesh-Clough et al demonstrated the efficacy of tEMGs by placing 91 pedicle screws in 6 sheep using a threshold of 10 V with the result showing a sensitivity of 94% and specificity of 90% using this technique.15 Unfortunately, because thoracic nerve roots are nonspecifically innervated, it is difficult to truly define a threshold value.
Figure 2.
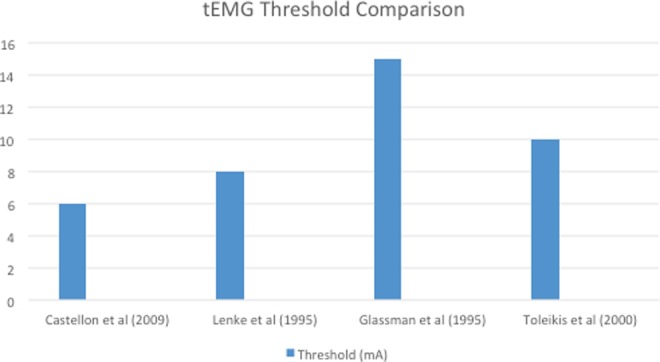
Comparison of published tEMG thresholds for pedicle screw stimulation. There is no universally accepted threshold, but values of 6 mA to 15 mA are published in the literature.
Although a useful tool, tEMG should always be used in addition to careful palpation of the pedicle tract and radiographic evaluation. Also, similar to other modalities that use muscle recording, it is important to consider the level of neuromuscular blockade, which can falsely elevate the stimulation threshold giving the appearance of a correctly placed screw. Last, the surgeon has to understand the different stimulation thresholds in different levels of the spine (thoracic vs lumbar).
Neurologic Complications in 3-Column Osteotomies
The treatment of severe spinal deformity presents a difficult challenge to the spine surgeon, often requiring osteotomy to correct sagittal and coronal deformities and imbalance. Although vertebral column resection (VCR) offers the greatest potential correction of these rigid deformities, it is a technically demanding procedure with increased estimated blood loss (EBL), operative time, and significant neurologic risk. Nevertheless, VCR is an accepted treatment for the correction of the most rigid, severe spinal deformities.
In his initial study of 70 posterior vertebral column resection (PVCR) patients, Suk et al reported 4 transient nerve root injuries (5.7%) and 2 permanent, complete spinal cord injuries (2.9%). The significantly high rate of complications in this landmark study was attributed to preoperative cord compromise and preexisting diminished blood supply to the thoracic cord that was unable to tolerate the additional stress of such an extensive surgery.16 In follow-up studies, Suk et al reported an 8% rate of transient nerve root injury in patients undergoing PVCR for a fixed, lumbosacral deformity and only one complete paralysis in patients undergoing PVCR for rigid scoliosis.17,18 Later reports by Lenke et al on pediatric patients and another on pediatric and adult patients undergoing VCR reported no spinal cord deficits in either study.19,20
In order to appropriately respond to neuromonitoring alerts, it is prudent to understand the etiology of potential neurologic deficits. Authors have speculated on the etiology of these neurologic complications and the effect of blood supply to the thoracic cord. Several studies in dog models have demonstrated how segmental thoracic artery ligation and cord shortening maneuvers may cause neurologic dysfunction. Fujimaki et al explored how many ligations of bilateral segmental arteries can cause ischemic spinal cord dysfunction by dividing 15 dogs into 5 groups: no ligation (sham), 3-level ligation (T11-T13), 4-level ligation (T10-T13), 5-level ligation (T10-L1), and 7-level ligation (T9-L2). Doppler spinal cord blood flow was measured at T12.21 Spinal cord–evoked and motor-evoked potentials were recorded at 10 hours postoperatively and clinical neurologic function measured at 1 week postoperatively. The authors concluded that 5-level bilateral segmental artery ligation within the dog T9-L2 region (approximating the human critical vascular zone of T4-T9) is sufficient to cause spinal cord ischemia and neurologic dysfunction. In humans where the artery of Adamkiewicz is typically the dominant supply, artery ligation may result in an “all or none” response, irrespective of laterality and number of ligated segmental arteries.
In another dog model, Kawahara et al described the dangers of spinal column shortening on spinal cord function during VCR.22 The authors performed total spondylectomy of T13 on 46 dogs, with instrumentation 2 levels above and 2 levels below, and gradually shortened the spinal column by a compression maneuver between the screws inserted into T12 and L1 until the lower endplate of T12 touched the upper endplate of L1, to a maximum of 20 mm. Morphologic changes of the dural sac and spinal cord were noted at specific amounts of shortening. From 7.2 to 12.5 mm, dura matter deformation occurred in an accordion-like fashion without spinal cord changes. At >12.5 mm of shortening, dura matter buckling and subsequent spinal cord kinking occurred. Based on these results, the authors defined 3 phases of acute spinal shortening. Phase 1 is the safety range involving shortening within one third of the vertebral segment and no deformity of dural sac or spinal cord. Phase 2 is the warning range (shortening between one third and two thirds of vertebral segment) where the dural sac shrinks without spinal cord changes. Phase 3 is the dangerous range (shortening greater than two thirds of vertebral segment) where buckled dural sac is associated with spinal cord deformity. Spinal cord–evoked potentials were also measured and abnormalities were noted in 33% dogs at 15 mm of shortening and 67% dogs at 20 mm of shortening. Clinical neurologic function paralleled the monitoring changes. Based on these results, the authors concluded that phases 1 and 2 of spinal cord shortening do not compromise spinal cord function, while phase 3 shortening increases this risk. Interestingly, spinal cord blood flow was actually shown to increase in phases 1 and 2, possibly through a local vasodilatory effect. Interestingly, this may provide the explanation for the clinical phenomenon of improvement in neuromonitoring changes with decompression and shortening. When the shortening progresses into phase 3, the cord kinks and spinal cord blood flow respectively decreases. This landmark study provides guidelines for the degree of cord shortening while minimizing neurologic impairment.
In addition to understanding the potential etiology of neurologic deficits, it is important to preoperatively identify which patients are at particularly high risk. In a 2014 study, Xie et al identified risk factors for postoperative neurologic deficits in 76 patients with severe rigid spinal deformities treated with PVCR.23 While none of the 76 patients suffered permanent paraplegia or nerve root injury, 6 had postoperative change in neurologic status on physical exam that resolved within 6 months. Preexisting neurologic dysfunction was noted to be the greatest risk factor for postoperative neurologic deficit (odds ratio [OR] = 49.322). Other risk factors were potential intraspinal and brainstem anomalies (OR = 18.423), scoliosis associated with hyperkyphosis (OR = 11.883), and level of VCR (OR = 8.769), which itself correlated with number of segmental vessels ligated and thus spinal cord perfusion. The authors noted that previous neurological dysfunction may be indicative of preexisting chronic ischemia or “sick spinal cord,” which could increase the patient’s susceptibility to neurologic deficits secondary to distraction of the neural elements or tension on the local vasculature. Similarly, intraspinal and brainstem anomalies like Chiari malformation or tethered cord could cause fixation of the cord and increased susceptibility to ischemic injury from mechanical traction on segmental vessels. In cases of a “sick spinal cord” or preexisting chronic ischemia, intraoperative positioning and hemodynamics must be optimized to prevent further undue insult to an already compromised cord.
Kim et al also identified risk factors for postoperative neurologic deficits after 3-column osteotomy.24 Their study included 233 patients, though only 152 underwent PVCR and 81 underwent pedicle subtraction osteotomy (PSO) and data on complication risk factors was combined for the 2 groups. The authors corroborated with multiple logistic regression analysis that preoperative neurologic deficit (OR = 3.04) and resection of 2 or more vertebrae (OR = 4.73) both increased the risk of postoperative neurologic deficit (P < .05); patients with both of these risk factors experienced a 29-fold increase in neurologic complication rate. Independent variable analysis further noted that preoperative kyphosis (OR = 4.46), a diagnosis of tuberculous kyphosis (OR = 4.23), fusion extent of >5 segments (OR = 3.20), insertion of titanium mesh (OR = 3.64), operative time >200 minutes (OR = 4.47), and EBL >3000 mL (OR = 3.98) all increased the risk of postoperative transient or permanent neurological deficit though these results did not remain significant with multiple logistic regression analysis. When all complications were included, multiple logistic regression analysis demonstrated that only preoperative neurologic deficit (OR = 3.64), preoperative kyphosis (OR = 3.01), and fusion extent of >5 segments (OR = 1.99) increased the overall complication rate.
Given the established risk of neurologic complication from VCR, it is essential to utilize intraoperative neuromonitoring to provide real-time data on the functional status of the spinal cord and to direct surgical decision making. Prior studies have demonstrated that MEP recordings are most effective at detecting spinal cord ischemia and have the highest sensitivity for detecting neurologic injury.25,26 Jarvis et al reported on neuromonitoring changes in 37 three-column posterior spinal osteotomies (7 PSO, 15 complete VCR, 15 partial VCR) performed in the thoracic spine of 28 pediatric patients and provided a strategy for how to respond to these changes to minimize poor neurologic outcomes.27 In his study, neuromonitoring alerts were defined as loss of SSEP or tcMEP to <50% of baseline amplitude and were classified chronologically as type I (prior to decompression), type II (during decompression), or type III (after osteotomy closure). Twenty-one alerts were noted in 18 patients (15 type II, 6 type III); all were MEP alerts and only 3 were SSEP alerts with no isolated SSEP alerts, confirming the minimal utility of SSEP. Larger curve magnitude was associated with alerts (combined sagittal/coronal curve of 188.6°, range 111° to 308°, for alert patients, vs 139.0°, range 67° to 230°, for nonalert patients, P = .0009); severe kyphosis was also associated with alerts (102.3° for alert patients vs 69.9° for nonalert patients, P = .006). Furthermore, complete VCR had a statistically significant highest incidence of alerts (12 of 15, 80%, P = .02), compared to partial VCR (6 of 15, 40%) and PSO (3 of 7, 43%).
For all MEP alerts, the authors recommended ruling out equipment, technical, or anesthetic causes, as well as increasing mean arterial pressure (MAP) to a minimum of 70 mm Hg while optimizing oxygenation. For failures of the type II alerts to improve with elevated MAPs, decompression was completed and the osteotomy was closed. This successfully shortened the decompressed spinal cord, improved its perfusion, and led to improvement in MEPs. For failures of the type III alerts to improve with elevated MAPs, reopening of the closed osteotomy, assuring absence of compression, and closure of the osteotomy with either less correction or a larger cage/graft led to improvement in MEPs. Moreover, implementation of their algorithm clinically led to <14% transient postoperative neurological deficits, which recovered at a mean of 24.2 days, and no permanent deficits were noted.27
Cho, Lenke, and colleagues evaluated 90 consecutive VCR procedures and found a 16.7% incidence of spinal cord monitoring signals being completely lost or meeting warning criteria. However, with various intraoperative responses including raising the MAP, removing any traction on the patient, or reversing previously applied correction maneuvers, all patients had return of data to acceptable limits without any major neurologic deficits sustained.28
Jarvis et al demonstrates the effectiveness of MEP changes in guiding surgical intervention. The authors identified high-risk steps of the procedure and methods to successfully improve monitoring changes.27 There are numerous possible mechanisms for neurologic injury within the lengthy and technically challenging PVCR procedure and protection of the spinal cord should be targeted to these specific mechanisms. Careful nerve root retraction during pedicle resection and a comprehensive knowledge of surgical anatomy during osteotome placement mitigates neurologic risk during bony resection. Wide laminectomies, preserving the anterior longitudinal ligament to prevent translation, placing an anterior mesh cage, and placing H-shaped strut allografts between intact spinous processes to prevent dural compression may prevent spinal column subluxation, dural buckling, or compression by residual bone and soft tissue.29 Furthermore, limiting segmental artery ligation to less than 5 levels and limiting shortening to less than two thirds of the segmental-level height will decrease the risk of neurologic deficit.21,22 Although segmental ligation of 5 levels would be an extreme clinical scenario, it is inadvisable to have multiple consecutive bilateral segmental ligations in any case. Finally, while some neurologic complications may be inevitable in a procedure as expansive as VCR, the use of continuous neuromonitoring data with rapid surgical response may help mitigate neurologic deficit.
Efficacy of Neuromonitoring and Responding to Positive Alarms
There are numerous neuromonitoring tests in the surgeon’s armamentarium to assist in performing safe spinal deformity surgery, with promising results previously discussed. Each neuromonitoring modality has potential benefits and drawbacks, but the use of multimodal neuromonitoring can be quite successful at alerting the surgeon to potential neurologic injury and guiding prompt intraoperative correction. A frequently used combination involves monitoring both SSEPs and MEPs. Hamilton et al reviewed over 100 000 spine surgery procedures in the Scoliosis Research Society morbidity and mortality database and analyzed neuromonitoring results, which were used in 65% of the cases. In this retrospective review, the combined SSEP and MEP had a sensitivity and specificity for new spinal cord deficit (61 cases) of 0.43 and 0.98, respectively, and the positive and negative predictive values of 0.21 and 0.99, respectively. For new nerve root deficits (128 cases), the combined SSEPs and MEPs monitoring had the sensitivity and specificity of 0.13 and 0.99, respectively, suggesting that normal intraoperative neuromonitoring findings correlate very highly with uninjured spinal cord or roots. The authors stress the combination of surgical judgment and neuromonitoring as only 39% to 44% of the new postoperative neurologic deficits were detected intraoperatively in their series. Although neuromonitoring changes allowed for quicker responses to intraoperative neurologic deficits, still more than half of neurologic deficits in the adult and pediatric revision cases were unidentified intraoperatively. Auspiciously, the complete and partial recovery rates for new postoperative spinal cord neurologic deficits are 88.7%.30
One of the most controversial aspects of neuromonitoring involves the thresholds required to prompt intraoperative action by the surgeon. Although thresholds widely depend on the institution and surgeon, some guidelines do exist for specific neuromonitoring methods. For example, the SSEP criteria for surgeon notification are an intraoperative unilateral or bilateral amplitude loss of at least 60% or 10% increase in latency.3 Using a similar threshold in a study identifying motor tract injury during cervical spine surgery, the SSEP is highly specific (100%) to injury, but not nearly as sensitive (25%). The tcMEP warning criteria of amplitude loss of at least 60% in the same study showed 100% specificity and sensitivity.24 The tEMG has sensitivity of 94% and specificity of 90% from an animal study using pedicle screws with a threshold of 10 V.15 Unfortunately, there is significant variability in the potentials elicited by a threshold stimulus for reliable and reproducible assessment of the motor system. Any change in the morphology of the complex polyphasic deflections of the compound muscle action potential can be considered a significant change in the tcMEP. Therefore, loss of signal rather than decreased amplitude may be more specific for motor injury.
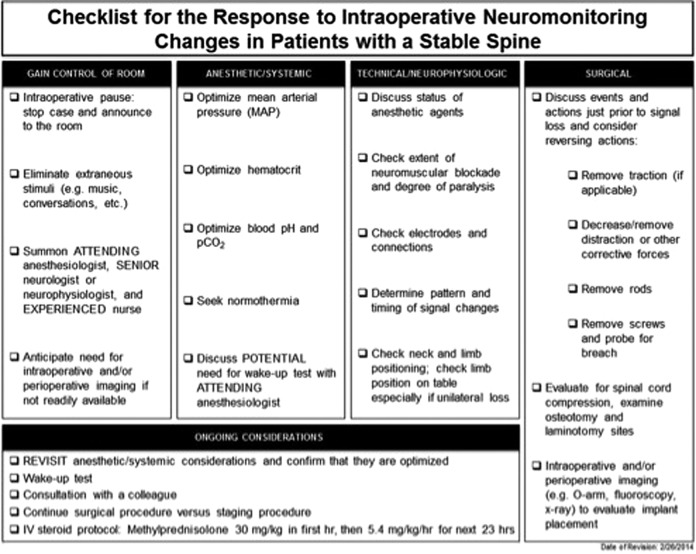
No evidence-based protocol exists for reacting to findings of a potentially new intraoperative neurologic deficit detected by neuromonitoring. Vitale et al recently published a consensus-based best practice guideline for positive neuromonitoring warning signs in pediatric deformity (Figure 3).31 The first step in the algorithm involves optimizing systemic issues that could cause monitoring changes, such as controlling the mean arterial pressure, hematocrit, body temperature, and blood pH. Once the systemic issues are evaluated and optimized, the status of anesthesia agent, electrode connection, and patient positioning are assessed. Finally, if the aforementioned measures did not resolve the signal changes, the guideline recommends removing traction, reducing the degree of surgical deformity correction, or removal of rods and screws to evaluate possible spinal cord/nerve compression. Last, intraoperative imaging like the O-arm and/or fluoroscopy should be used to identify the source of pathology.
Figure 3.
A consensus-based checklist to guide surgeon responses to intraoperative neuromonitoring (IONM) changes in patients with a stable spine. Reprinted with permission from Vitale MG, Skaggs DL, Pace GL, et al. Best practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize response. Spine Deformity. 2014;2(5):333-339.
Limitations of Neuromonitoring and Anesthetic Concerns
Intraoperative neuromonitoring may provide a reliable assessment of potential neurologic injury during spine surgery. However, each of the neuromonitoring modalities has inherent limitations, many of which involve the use of anesthetic agents and muscle relaxants. Unfortunately, these classes of medications can lead to invalid signal interpretation depending on the type of agent and the neuromonitoring modality. For example, the halogenated inhalation anesthetics and muscle relaxants have opposite effects on the SSEP and tcMEP signals. The inhalation anesthetics can affect the SSEP signals, but have limited effect on tcMEP signals.32 On the other hand, the use of muscle relaxants permit a more reliable recording on the SSEP signals, but can easily misinterpret the tcMEP findings.33 In order to limit such undesirable interactions, total intravenous anesthesia (TIVA) is more commonly used in major adult deformity spinal cases in conjunction with SSEP and MEP signals, which provides a more accurate assessment of the entire spinal cord in real time.
At the senior author’s institution, balanced anesthesia, such as sevoflurane or desflurane, along with propofol and remifentanyl infusion are used as the standard protocol for monitoring. For cases relying on the use of MEPs, TIVA with propofol, remifentanyl, and sufentanil are used to prevent any potential interactions with the neuromonitoring modalities. Halogenated inhalation agents are only used in exceptional cases when patients have reactive airway disease or difficult blood pressure control while on TIVA. Generally, our anesthesia team administers a small bolus of muscle relaxants, like rocuronium, during intubation and does not start an infusion until a baseline response is obtained. After baseline signals are recorded, rocuronium is infused to maintain the patient at 2 twitches on train of 4. In situations of neuromonitoring data collection error or issue, the muscle relaxant infusion is stopped until the problem is resolved. Despite the potential interference with certain neuromonitoring signals, muscle relaxants decreases the “jump” as well as the risk of bite injuries on stimulation.
Contrary to our practice, Martin et al recently published a randomized controlled trial demonstrating no clinically significant differences in SSEPs and MEPs while using either TIVA or volatile anesthesia during posterior spinal fusions.34 There is limited literature comparing TIVA and inhaled anesthesia technique during adult spinal deformity cases, but the study by Martin et al demonstrates possible advantages of inhaled anesthesia, without clinical compromise, including rapid awakening postoperatively and the feasibility of a rapid wake-up test in less than 5 minutes. An integral aspect of this study was the minimalistic administration of inhaled agents to maintain amnesia, while limiting any unwanted interaction with the neuromonitoring device. The current recommendation for anesthetic agent of choice during complex spinal deformity correction is still unknown. We strongly advocate for constant communication with the anesthesia team perioperatively to determine the safest and most effective anesthetic approach for each particular case.
Author’s Preferred Techniques
At the senior author’s institution, the approach to intraoperative neuromonitoring signal loss begins with localizing the area of concern. It is important to understand the difference in potential problems at the cord, conus, and nerve root levels. Moreover, we routinely use the peripheral upper extremity recording sites to predict if the changes were secondary to a surgical technique or systemic issues. Warning criteria for SSEP testing is defined as a 10% increase in latency or 50% to 60% decrease in amplitude. MEP warning criteria is defined as a 60% decrease in amplitude following a 100 V increase in baseline stimulus. dNEP warning criteria is a 10% increase in latency or 80% decrease in amplitude. Each of these criteria attempt to identify a “significant” deterioration in neurologic status, within a time period that allows for intervention and prevention of neurologic loss.
The surgical steps taken prior to the warning criteria event are sequentially reversed to identify a potential cause of the change. If the signals return to acceptable limits after a particular step is reversed, the source has likely been identified and corrected accordingly. Often, signals typically return after reducing the amount of deformity correction. If the signals do not return despite reversing the recent surgical steps, intraoperative imaging (fluoroscopy, O-arm, X-ray) is used to identify any implant-related complications. All the while, MAP and hematocrit are augmented to optimize blood flow to the neural axis. The patient is evaluated for proper positioning, normothermia, and optimal blood pH at the same time. All technical causes of data changes are ruled out by the monitoring team and surgeon.
In deformity cases requiring thoracic pedicle screw instrumentation, it is the senior author’s preference to remove the screw with the lowest threshold. A sounding device is then used to palpate the walls and floor of the pedicle screw tract to ensure no breach is found. If the tract is well-positioned, the screw is replaced and the surgery continues. However, if a cortical breach is found, the trajectory of that screw is redirected and the pedicle screw with the next lowest stimulation value is removed and assessed in similar fashion. At the senior author’s previous institution, lumbar thresholds have been more reliably defined as <2.8 mA for an almost certain pedicle wall defect, <4.0 mA for a strong likelihood of a pedicle wall defect, 4-8 mA for a possible pedicle wall defect, and >8 mA for an intact pedicle.35
Vertebrectomy cases usually require sacrifice of a thoracic root. Unfortunately, the anterior spinal artery is often intimately associated with these roots, particularly at T10 and T12 on the left. Ligation of the anterior spinal artery or its branches can lead to cord ischemia and loss of neurologic function. In order to prevent inadvertent ligation of the artery, we clamp the thoracic nerve root with an aneurism clip for 5 minutes to observe for neuromonitoring changes. Without observed changes, the artery is not likely associated with significant cord blood flow and it is safe to ligate the root.
A true spinal cord or nerve deficit is more likely with deterioration in both the SSEPs and the MEPs. Because neuromonitoring data is not in real-time, the alarms we receive may not be temporally associated with recent surgical maneuver. Therefore, determining the precise level of injury may be difficult. dNEP cord mapping with an epidural electrode is invaluable in these situations. The level of concern is identified by measuring dNEPs after performing a small laminotomy and placing the probe directly on the dura of the spinal cord. This allows a low-intensity stimulation (5-40 mA) compared to spinous process needles and other dNEP methods.6
Recommendations for Use
Before the advent of intraoperative evoked potential monitoring, the only available method for assessing spinal cord function was the Stagnara Wake-up test. For years and still today, the wake-up test is the gold standard at assessing neurologic function. Unfortunately, because the test is performed after the desired surgical correction, the specific cause of neurologic insult is unknown. The wake-up test is unable to provide timely, accurate monitoring of the spinal cord and nerve roots. The introduction of SSEP, tcMEP, dNEP, and EMG intraoperative neuromonitoring modalities has allowed for earlier detection of possible neurologic deficit and subsequent prevention of deficits postoperatively. However, with a cooperative, hemodynamically stable patient and a concerted effort with the surgical and anesthetic teams, the wake-up test is still an invaluable adjunct in patients at increased risk of neurologic deficits.
In 2009, the Scoliosis Research Society (SRS) released an information statement on neuromonitoring to its members supporting the use of intraoperative spinal cord monitoring to prevent neurologic complications. After extensive review of the literature and clinical experience of its members, the SRS concluded that the use of neurophysiological monitoring during spinal deformity correction and instrumentation is validated and efficacious. The majority of SRS members routinely used spinal cord monitoring during deformity cases and stress the importance of a multimodal approach. Furthermore, the SRS believes that intraoperative neuromonitoring multimodality allows for real-time neurologic assessment and should be considered the preferred method for detecting an impending spinal cord insult.36
It is important to understand that a solitary neuromonitoring modality or inexperienced use of multimodal neuromonitoring can be of little benefit. The understanding of the available electrophysiologic techniques as well as their potential shortcomings may allow for more effective implementation. It is the surgeon and electrophysiologist’s experience with both the surgical procedures and the available modalities that increases efficacy. An electrophysiologist’s thorough knowledge of the surgical steps and specific surgical maneuvers employed throughout the case may allow for early prediction of when changes may occur. The correlation of neuromonitoring events with surgical maneuvers is paramount for prompt recognition and rectification. Finally, it is only through open communication between the surgeon, anesthesia team, and the electrophysiologist that the detection and reversal of potential neurologic injury can be avoided. Communication and interdisciplinary respect are the keys to rapid intervention and successful outcomes.
Conclusion
There is a large body of evidence supporting the effectiveness of intraoperative neurophysiologic monitoring during spinal deformity surgery. Although monitoring is no longer considered investigational, current use is dictated by surgeon preference, technical availability, and coordination of care between specialties. Multimodality monitoring provide a real-time assessment of spinal cord and nerve root function and permit more aggressive surgical corrections than may have been undertaken. Intraoperative monitoring cannot replace a thorough understanding of neural anatomy and meticulous surgical technique, but can be a valuable tool for improving the safety and outcome of spinal deformity surgery. Finally, a thorough lower extremity motor neurologic exam should be performed following every spinal deformity corrective surgery to confirm neurologic status of the patient prior to leaving the operating room.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Lenke reports board membership in OREF and GSO; personal consultancy fees from DePuy Synthes Spine, K2M, and Medtronic; personal expert testimony fees from Fox Rothschild, LLP; grants from AO Spine, Scoliosis Research Society, DePuy Synthes Spine, Setting Scoliosis Straight Foundation, EOS, and philanthropic research funding; royalties from Medtronic; and personal travel accommodation/meeting expenses from AOSpine, Broadwater, Seattle Science Foundation, Scoliosis Research Society, and the Spinal Research Foundation. Dr Lehman reports grants from Department of Defense Peer Reviewed Orthopaedic Research Program (PRORP); personal fees and nonfinancial support from DePuy Synthes Spine; personal fees and nonfinancial support from Stryker; and personal fees and nonfinancial support from Medtronic.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ronald A. Lehman, MD http://orcid.org/0000-0002-5799-9885
Lawrence G. Lenke, MD http://orcid.org/0000-0002-5595-4958
References
- 1. Devlin VJ, Schwartz DM. Intraoperative neurophysiologic monitoring during spinal surgery. J Am Acad Orthop Surg. 2007;15:549–560. [DOI] [PubMed] [Google Scholar]
- 2. Sloan TB, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anesthesiol. 2008;21:560–564. doi:10.1097/ACO.0b013e32830f1fbd. [DOI] [PubMed] [Google Scholar]
- 3. Stecker MM. A review of intraoperative monitoring for spinal surgery. Surg Neurol Int. 2012;3(suppl 3):S174–S187. doi:10.4103/2152-7806.98579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–2449. [DOI] [PubMed] [Google Scholar]
- 5. Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic “motor” evoked potentials. Clin Neurophysiol. 2001;112:1442–1450. [DOI] [PubMed] [Google Scholar]
- 6. Padberg AM, Raynor BL, Thuet ED, Bolon SM. Spinal cord and nerve root monitoring In: Bridwell K, ed. The Textbook of Spinal Surgery. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2011:6–40. [Google Scholar]
- 7. Skinner SA, Transfeldt EE, Savik K. Surface electrodes are not sufficient to detect neurotonic discharges: observations in a porcine model and clinical review of deltoid electromyographic monitoring using multiple electrodes. J Clin Monit Comput. 2008;22:131–139. [DOI] [PubMed] [Google Scholar]
- 8. Chung I, Grigorian AA. EMG and evoked potentials in the operating room during spinal surgery In: Schwartz M, ed. EMG Methods for Evaluating Muscle and Nerve Function. InTech; 2011:325–340. [Google Scholar]
- 9. Romstöck J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000;93:586–593. [DOI] [PubMed] [Google Scholar]
- 10. Calancie B, Lebwohl N, Madsen P, Klose KJ. Intraoperative evoked EMG monitoring in an animal model: a new technique for evaluating pedicle screw placement. Spine (Phila Pa 1976). 1992;17:1229–1235. [DOI] [PubMed] [Google Scholar]
- 11. Castellon AT, Meves R, Avanzi O. Intraoperative neurophysiologic spinal cord monitoring in thoracolumbar burst fractures. Spine (Phila Pa 1976). 2009;34:2662–2668. [DOI] [PubMed] [Google Scholar]
- 12. Lenke LG, Padberg AM, Russo MH, Bridwell KH, Gelb DE. Triggered electromyographic threshold for accuracy of pedicle screw placement: an animal model and clinical correlation. Spine (Phila Pa 1976). 1995;20:1585–1591. [DOI] [PubMed] [Google Scholar]
- 13. Glassman SD, Dimar JR, Puno RM, Johnson JR, Shields CB, Linden RD. A prospective analysis of intraoperative electromyographic monitoring of pedicle screw placement with computed tomographic scan confirmation. Spine (Phila Pa 1976). 1995;20:1375–1379. [PubMed] [Google Scholar]
- 14. Toleikis JR, Skelly JP, Carlvin AO, et al. The usefulness of electrical stimulation for assessing pedicle screw placements. J Spinal Disord. 2000;13:283–289. [DOI] [PubMed] [Google Scholar]
- 15. Danesh-Clough T, Taylor P, Hodgson B, Walton M. The use of evoked EMG in detecting misplaced thoracolumbar pedicle screws. Spine (Phila Pa 1976). 2001;26:1313–1316. [DOI] [PubMed] [Google Scholar]
- 16. Suk SI, Kim JH, Kim WJ, Lee SM, Chung ER, Nah KH. Posterior vertebral column resection for severe spinal deformities. Spine (Phila Pa 1976). 2002;27:2374–2382. [DOI] [PubMed] [Google Scholar]
- 17. Suk SI, Chung ER, Lee SM, Lee JH, Kim SS, Kim JH. Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976). 2005;30:E703–E710. [DOI] [PubMed] [Google Scholar]
- 18. Suk SI, Chung ER, Kim JH, Kim SS, Lee JS, Choi WK. Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976). 2005;30:1682–1687. [DOI] [PubMed] [Google Scholar]
- 19. Lenke LG, O’Leary PT, Bridwell KH, Sides BA, Koester LA, Blanke KM. Posterior vertebral column resection for severe pediatric deformity: minimum two-year follow-up of thirty-five consecutive patients. Spine (Phila Pa 1976). 2009;34:2213–2221. [DOI] [PubMed] [Google Scholar]
- 20. Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;(468):687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujimaki Y, Kawahara N, Tomita K, Murakami H, Ueda Y. How many ligations of bilateral segmental arteries cause ischemic spinal cord dysfunction? An experimental study using a dog model. Spine (Phila Pa 1976). 2006;31:E781–E789. [DOI] [PubMed] [Google Scholar]
- 22. Kawahara N, Tomita K, Kobayashi T, Abdel-Wanis ME, Murakami H, Akamaru T. Influence of acute shortening on the spinal cord: an experimental study. Spine (Phila Pa 1976). 2005;30:613–620. [DOI] [PubMed] [Google Scholar]
- 23. Xie JM, Zhang Y, Wang YS, et al. The risk factors of neurologic deficits of one-stage posterior vertebral column resection for patients with severe and rigid spinal deformities. Eur Spine J. 2014;23:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SS, Cho BC, Kim JH, et al. Complications of posterior vertebral resection for spinal deformity. Asian Spine J. 2012;6:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quraishi NA, Lewis SJ, Kelleher MO, Sarjeant R, Rampersaud YR, Fehlings MG. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred two cases with independent evaluation. Spine (Phila Pa 1976). 2009;34:1504–1512. [DOI] [PubMed] [Google Scholar]
- 26. Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86:1248–1253. [DOI] [PubMed] [Google Scholar]
- 27. Jarvis JG, Strantzas S, Lipkus M, et al. Responding to neuromonitoring changes in 3-column posterior spinal osteotomies for rigid pediatric spinal deformities. Spine (Phila Pa 1976). 2013;38:E493–E503. [DOI] [PubMed] [Google Scholar]
- 28. Cho SK, Lenke LG, Bolon SM, et al. Can intraoperative spinal cord monitoring reliably help prevent paraplegia during posterior vertebral column resection surgery? Spine Deform. 2015;3:73–81. [DOI] [PubMed] [Google Scholar]
- 29. Hamzaoglu A, Alanay A, Ozturk C, Sarier M, Karadereler S, Ganiyusufoglu K. Posterior vertebral column resection in severe spinal deformities: a total of 102 cases. Spine (Phila Pa 1976). 2011;36:E340–E344. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton DK, Smith JS, Sansur CA, et al. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine (Phila Pa 1976). 2011;36:1218–1228. [DOI] [PubMed] [Google Scholar]
- 31. Vitale MG, Skaggs DL, Pace GI, et al. Best practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize response. Spine Deform. 2014;2:333–339. [DOI] [PubMed] [Google Scholar]
- 32. Malhotra NR, Shaffrey CI. Intraoperative electrophysiological monitoring in spine surgery. Spine (Phila Pa 1976). 2010;35:2167–2179. [DOI] [PubMed] [Google Scholar]
- 33. Owen JH. The application of intraoperative monitoring during surgery for spinal deformity. Spine (Phila Pa 1976). 1999;24:2649–2662. [DOI] [PubMed] [Google Scholar]
- 34. Martin DP, Bhalla T, Thung A, et al. A preliminary study of volatile agents or total intravenous anesthesia for neurophysiological monitoring during posterior spinal fusion in adolescents with idiopathic scoliosis. Spine (Phila Pa 1976). 2014;39:E1318–E1324. [DOI] [PubMed] [Google Scholar]
- 35. Raynor BL, Lenke LG, Bridwell KH, Taylor BA, Padberg AM. Correlation between low triggered electromyographic thresholds and lumbar pedicle screw malposition: analysis of 4857 screws. Spine (Phila Pa 1976). 2007;32:2673–2678. [DOI] [PubMed] [Google Scholar]
- 36. Scoliosis Research Society. Neuromonitoring Information Statement. Milwaukee, WI: Scoliosis Research Society; January 2009. [Google Scholar]