Abstract
Older adults do not sleep as well as younger adults. Why? What alterations in sleep quantity and quality occur as we age, and are there functional consequences? What are the underlying neural mechanisms that explain age-related sleep disruption? This review tackles these questions. First, we describe canonical changes in human sleep quantity and quality in cognitively normal older adults. Second, we explore the underlying neurobiological mechanisms that may account for these human sleep alterations. Third, we consider the functional consequences of age-related sleep disruption, focusing on memory impairment as an exemplar. We conclude with a discussion of a still-debated question: do older adults simply need less sleep, or rather, are they unable to generate the sleep that they still need?
Normative aging is associated with a reduced ability to initiate and maintain sleep. Moreover, deficits in sleep physiology, including those of non-rapid eye movement (NREM) sleep and its associated neural oscillations, are especially prominent in later life. Though sleep disruption is a common signature of “normal aging”, the underlying neural mechanisms explaining age-related sleep impairment are only now being revealed.
This review focuses on physiological changes associated with normative human aging. First, we characterize associated alterations in sleep structure and oscillatory activity in later life. Second, we describe emerging neurobiological mechanisms that may account for these sleep alterations. Third, we consider the functional consequences of age-related sleep disruption, focusing on memory impairment. We conclude with the exploration of a still-unresolved question: are older adults unable to generate the sleep that they need or do they simply need sleep less.
What about Sleep Changes with Age?
Both the macro-level structure of sleep, such as sleep duration and sleep stages, and the micro-level architecture of sleep, including the quantity and quality of sleep oscillations, change as we progress into our older age.
Macro Sleep Changes
Advancing into the fifth decade of older age and beyond are a collection of well-characterized changes in sleep architecture (Figure 1A): (1) advanced sleep timing (i.e., earlier bedtimes and rise times), (2) longer sleep-onset latency (i.e., longer time taken to fall asleep), (3) shorter overall sleep duration, (4) increased sleep fragmentation (i.e., less consolidated sleep with more awakenings, arousals, or transitions to lighter sleep stages), (5) more fragile sleep (i.e., higher likelihood of being woken by external sensory stimuli), (6) reduced amount of deeper NREM sleep known as slow wave sleep (SWS), (7) increased time spent in lighter NREM stages 1 and 2, (8) shorter and fewer NREM-REM sleep cycles, and (9) increased time spent awake throughout the night (Conte et al., 2014; Feinberg and Carlson, 1968; Kales et al., 1967; Klerman and Dijk, 2008; Landolt et al., 1996; Ohayon et al., 2004; Redline et al., 2004; Van Cauter et al., 2000; Vienne et al., 2016; Webb and Campbell, 1979; Zepelin et al., 1984). This is not to suggest a lack of individual variability in the degree of sleep disruption. It is clear that some older adults show little sleep impairment, while others show dramatic alterations, despite chronological age being similar (Redline et al., 2004; Vitiello, 2009), a topic that we will return to throughout this review.
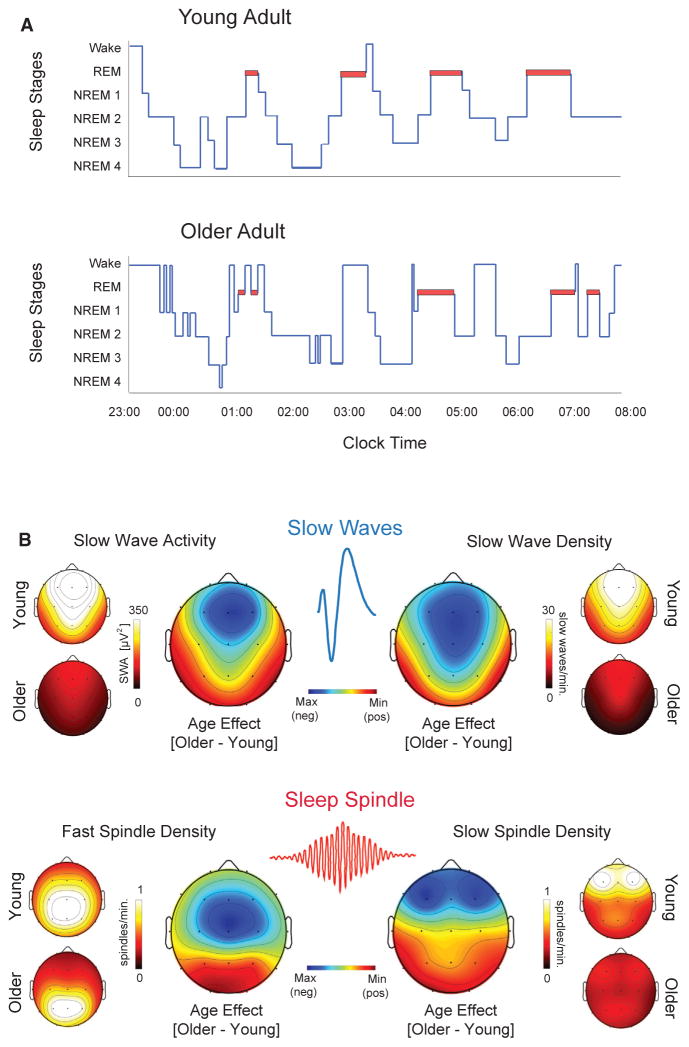
Figure 1. Schematic of Age-Related Changes in Sleep Architecture and NREM Sleep Oscillations.
(A) Prototypical sleep stage architecture across a 9 hr sleep period in a younger adult (top) and an older adult (bottom), using classic sleep staging criteria (Rechtschaffen and Kales, 1968). Relative to younger adults, older adults demonstrate: longer sleep latency, a greater number of transitions to lighter stages of sleep and wakefulness, more time spent awake after sleep onset, more fragmented sleep, and less time in slow wave sleep, especially within the early sleep cycles.
(B) Upper: Representative topographical head plots of EEG-quantified differences between younger and older adults in slow wave activity (left upper) and density (right upper). A similar sleep spindle density for fast sleep spindles (13.5–15 Hz; bottom left) and slow sleep spindles (12–13.5 Hz; bottom right) is shown in the bottom image. The hotter colors represent higher values. The center rainbow topoplots in each image represent the subtracted difference between younger and older adults, with darker blue representing larger deficits in older relative to younger adults. For both slow waves and sleep spindles, older adults demonstrate the largest regional oscillation impairments over frontal EEG derivations. The data are adapted from previous reports (Mander et al., 2013, 2014, 2015, 2016b).
Though age-related reductions in REM sleep time have been reported, these are subtler relative to changes in NREM sleep. Often, REM sleep impairments only emerge as adults progress into their 80s and beyond (Ohayon et al., 2004; Van Cauter et al., 2000) or as a symptom of degenerative dementias (Brayet et al., 2015; Hita-Yañez et al., 2012; Petit et al., 2004) (discussed in Box 1).
Box 1. Sleep, Neuropathology, and Abnormal Aging.
Beyond normative aging, sleep disruption is especially pervasive in neurodegenerative dementias. To date, the majority of pathological evidence concerns β-amyloid (Aβ) protein and tau neurofibrillary tangles that are characteristic of Alzheimer’s disease (AD). Subjective and objective measures of poor sleep correlate with cortical Aβ burden, as well as cerebrospinal fluid measures of Aβ and phosphorylated tau (Liguori et al., 2014; Mander et al., 2015; Spira et al., 2013; Sprecher et al., 2015). Similar associations have been observed in animals, with Aβ accumulation in rodent models of AD predicting greater sleep fragmentation (Roh et al., 2012). Recent studies in humans have further established that Aβ is selectively associated with the loss of <1 Hz NREM sleep oscillations (Mander et al., 2015). This association between Aβ and <1 Hz NREM oscillations appears to be unique and distinct from general age-related reductions in the broader SWA range of 0.6–4.8 Hz; with the latter linked, instead, to gray matter atrophy within the medial prefrontal cortex (Dubé et al., 2015; Mander et al., 2013; Varga et al., 2016). Aβ further correlates with reduced REM sleep amount in healthy older adults (Mander et al., 2015) and patients with AD (Liguori et al., 2014). This link may be connected to degeneration of REM-regulating cholinergic neurons projecting from the basal forebrain to the cortex (Brayet et al., 2015; Gagnon et al., 2008; Hassainia et al., 1997; Moraes et al., 2006; Petit et al., 2004), though direct evidence remains lacking. In addition to Aβ, tau-associated neurofibrillary tangles within the medial temporal lobe are believed to represent an early stage in AD disease progression (Bouras et al., 1994; Delacourte et al., 2002; Jack et al., 2010). Tau regional aggregation is relevant given the role of the hippocampus in generating ripples that are time locked to the expression of NREM sleep spindles and slow waves, hypothesized to support sleep-dependent declarative memory processing (Diekelmann and Born, 2010; Staresina et al., 2015). Fitting this overlap, tau within the rodent brain is associated with disrupted NREM oscillations, leading to abnormally long (slow) hyperpolarized down state and a reduction in successful transitions to the depolarizing up state of the slow wave (Menkes-Caspi et al., 2015). In human studies, tau levels within cerebrospinal fluid are associated with diminished slow wave sleep in AD patients (Liguori et al., 2014). Further supporting this tau-NREM oscillation hypothesis, both patients with mild cognitive impairment (MCI) and AD have significantly fewer posterior NREM fast sleep spindles relative to healthy older adults, with the degree of spindle reduction predicting the severity of memory impairment (Gorgoni et al., 2016; Rauchs et al., 2008). However, studies are needed to verify which neuropathological factor explains this loss of posterior spindles and, if tau, whether this association is specific to AD or present in other tauopathies. Beyond the medial temporal lobe, AD post-mortem studies have established that neurofibrillary tangles within the preoptic area of the hypothalamus correlate with the severity of prior fragmented sleep (Lim et al., 2014). Interestingly, tau deposition is also present in other sleep-regulating areas such as the locus coeruleus and basal forebrain and can be observed even in cognitively normal older adults (Braak and Del Tredici, 2016; Braak et al., 2011; Stern and Naidoo, 2015). This leads to the currently untested hypothesis that tau within these regions may trigger sleep abnormalities years before degenerative disease onset and, if such sleep disruption is specific, could serve as an early diagnostic biomarker (Holth et al., 2016). It is additionally becoming clear that the link between degenerative dementia conditions and sleep disruption is bi-directional. Work in mice has revealed an interacting mechanism, such that Aβ levels increase with time spent awake, while NREM sleep predicts the clearance of Aβ (Kang et al., 2009; Xie et al., 2013). In cognitively normal older human adults, those with greater initial levels of sleep fragmentation go on to suffer a more rapid subsequent rate of cognitive decline and a higher risk of developing AD over an ensuing six-year period (Lim et al., 2013a, 2013b). These bi-directional relationships are observed before the onset of disease and, furthermore, independently of sleep disorders that also increase dementia risk, such as insomnia or sleep apnea (Osorio et al., 2011; Yaffe et al., 2011). It suggests that inadequate sleep is not only a predisposing risk factor contributing to degenerative disease processes, but represents a novel treatment opportunity and/or even preventative strategy in this context (Mander et al., 2016a). In summary, dementia-related neuropathologies are associated with forms of sleep disruption that are uniquely distinct from typical age-related sleep disruption or, in some cases, a significantly more severe form of those same sleep impairments. The former is especially relevant in the context of sleep as a non-invasive and possible early biomarker distinguishing normal from abnormal aging.
The frequency of diurnal naps also increases in later life: 10% of adults ages 55–64, and 25% ages 75–84, report the occurrence of daytime naps (Foley et al., 2007). Across these older age groups, roughly half of these naps are unplanned. This is consistent with the finding that 1 in 4 older adults report experiencing daytime sleepiness severe enough to impair daytime plans on a regular basis (Foley et al., 2007) and may reflect many of the characteristic nighttime sleep abnormalities described above (e.g., more fragmented sleep, less total sleep time, and less slow wave sleep).
Excessive daytime sleepiness and daytime napping are not, however, a universal feature of old age. For some portion of older adults, daytime sleep propensity and daytime ratings of subjective sleepiness diminish with the transition from midlife into older adulthood (Dijk et al., 2010). One factor that appears to determine whether older adults are prone to daytime napping and excessive daytime sleepiness is the presence of comorbid conditions such as chronic pain, depression, sleep disorders, and frequent nighttime urination breaks (Foley et al., 2007; Vitiello, 2009).
Nevertheless, daytime sleep propensity can be higher in healthy older relative to younger adults in the evening, when the circadian alerting signal is otherwise at its peak in young adults (Münch et al., 2005). Therefore, discrepancies in reported daytime sleepiness in older adults—increases or decreases—appear to depend, at least in part, on the time of day and/or circadian preference of the individuals being compared. This is of further relevance given the fact of advances in circadian preference in older age, wherein older individuals shift to earlier wake times and earlier bed times (Monk, 2005).
Micro Sleep Architecture Changes with Age
Adding to gross alterations in sleep architecture with advancing age are equally large changes in the signature electrical oscillations of sleep, measured with electroencephalography (EEG). Most prominent are those involving NREM sleep and two of its constituent oscillations, slow waves and sleep spindles (Figure 1B).
One quantification of slow waves involves measuring spectral power in the 0.5–4.5 Hz range during NREM sleep or slow wave sleep, also known as slow wave activity (SWA). Significant reductions in SWA are already observed in middle-aged adults, relative to young adults, and this SWA impairment becomes especially prominent in older adults (Dijk et al., 1989; Landolt and Borbély, 2001; Landolt et al., 1996; Mander et al., 2013).
Importantly from a mechanistic and detection perspective, this age-related decrease in SWA is not evenly expressed in terms of head topography or sleep cycle across the night. Rather, maximal age-related decrements in absolute SWA are observed over the prefrontal cortex (PFC) derivations and in the first NREM sleep cycles, with reductions of 75%–80% on average relative to young adults (Figure 1B) (Dijk et al., 1989; Landolt and Borbély, 2001; Landolt et al., 1996; Mander et al., 2013).
Slow wave activity is intimately bound with the homeostatic drive to sleep following continued wakefulness; the longer an individual remains awake, the greater the pressure to sleep and the greater the amount of subsequent SWA during sleep (Borbély, 1982). In young adults, SWA is highest in the first NREM cycle of the night and then exponentially declines in intensity across successive NREM sleep cycles, reflecting a homeostatic dissipation of sleep pressure (Landolt and Borbély, 2001; Landolt et al., 1996) (Figure 2A).
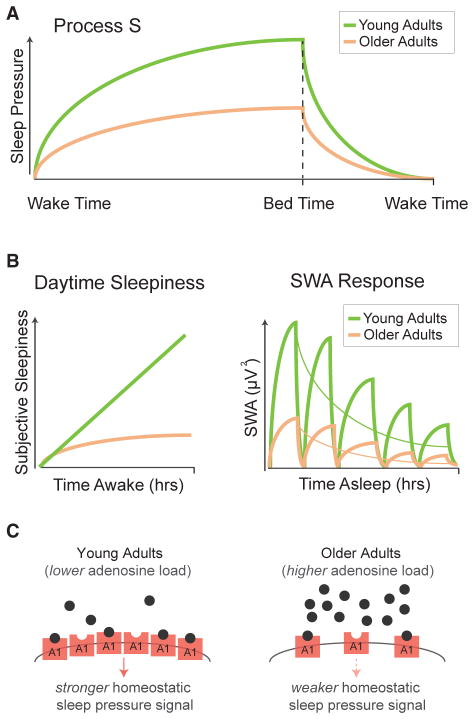
Figure 2. Age-Related Changes in Homeostatic Sleep Regulation.
(A) In healthy, young adults, the increasing homeostatic drive to sleep (or “Process S”; green line in schematic graphic) is partially driven by increases in extracellular adenosine building up with accruing time awake (Porkka-Heiskanen et al., 1997). During sleep, adenosine, and consequential sleep pressure, decreases and does so in proportion to the intensity of slow wave activity during sleep (Borbély, 1982). In older adults (orange line), the homeostatic buildup of sleep pressure across a day (Process S) is slower and weaker in older adults relative to younger adults. Furthermore, the dissipation of homeostatic sleep drive is also shallower across a night of sleep in older relative to younger adults.
(B) Left: Schematic of daytime subjective sleepiness, illustrating that older adults (orange) do not accumulate the same increase in subjective sleepiness across the day as younger adults (green). An illustration of the prototypical homeostatic discharge of SWA across sleep cycles at night is shown on the right. Younger adults express larger relative amounts of SWA in all sleep cycles, relative to older adults. Moreover, older adults show a shallower exponential decay of SWA across the night, relative to younger adults.
(C) Schematic representation of the proposed age-related differences in extracellular adenosine signaling of sleep pressure (Process S) with accumulating time spent awake. Relative to younger adults, older adults demonstrated higher levels of adenosine accumulation with increasing time awake. However, due to a reduction in adenosine A1 receptors in aging, we hypothesize that an adenosine insensitivity develops. Therefore, despite higher levels of adenosine in older relative to younger adults, there is potentially a weaker adenosine-associated signaling in the aging brain, resulting in a lower sleep pressure signal in older relative to younger adults.
This process of homeostatic sleep regulation, including SWA, is also altered as a function of aging. First, the exponential slope of SWA dissipation across the night is shallower in older relative to younger adults (Landolt and Borbély, 2001; Landolt et al., 1996) (Figure 2A), suggesting less of a homeostatic discharge in aging. Second, homeostatic increases in slow wave sleep time and SWA in response to prolonged prior wakefulness (that is, sleep deprivation), or selective slow wave sleep suppression, are blunted in older relative to younger adults (Landolt and Borbély, 2001; Munch et al., 2004) (Figure 2B). This latter finding has been interpreted as an impairment in the homeostatic regulation of SWA in older adults (Dijk et al., 1999). Similar to the topographically selective changes in SWA, the most dramatic changes in these homeostatic SWA features in aging are observed over the prefrontal cortex regions (Dijk et al., 2010; Munch et al., 2004).
Underlying these changes in SWA are impairments in the expression of two unique features of NREM slow waves. Both the amplitude as well as the density of slow waves are significantly reduced in middle-aged adults (Carrier et al., 2011). As adult individuals progress into older age, the reduction in slow wave amplitude and the decrease in the number of slow waves (density) continues (Dubé et al., 2015). Importantly, however, these age-related decreases in slow wave amplitude and density are not uniform across the entire topography of the head. The largest age-related changes in slow wave amplitude and density occur over the frontal lobe (Figure 1B) (Carrier et al., 2011). Moreover, these age-related differences in slow waves are maximal at times when the expression of NREM sleep oscillations are maximal, showing the greatest relative impairments during the first 1–2 NREM sleep cycles (Carrier et al., 2011). The slope, or steepness, of slow waves also becomes shallower in older adults relative to younger adults (Carrier et al., 2011). These morphological changes in waveform shape suggest that aging may diminish the synchronized neuronal en masse firing that gives rise to these sleep oscillations, caused by a disruption in the slow wave depolarized up and/or hyperpolarized down states that shape the slow wave (Beenhakker and Huguenard, 2009).
Parenthetically, the topographic specificity of age-related changes in slow waves can be overlooked by EEG studies that measure activity only at the center of the scalp, as is typical in standard sleep recordings. This is relevant, since assessment from only these EEG derivations may therefore contribute to weak effect sizes and apparent failures to replicate age-related changes in sleep-dependent memory associated with age-related cognitive decline (Scullin and Bliwise, 2015).
Another qualitative change involves the mean frequency of the slow waves, showing a slowing in average frequency of about 0.1 Hz in older relative to younger adults. Unlike density and amplitude, this effect is observed across most EEG derivations of the head (Carrier et al., 2011). Interestingly, the exact opposite of this typical slowing in slow wave frequency in cognitively normative older adults is observed in older adults with significant deposition of cortical β-amyloid burden and who also have worse memory retention (Mander et al., 2015). This raises the possibility that slow wave frequency represents a potential new, non-invasive sleep biomarker distinguishing normal from abnormal aging in the context of Alzheimer’s disease pathophysiology (Mander et al., 2016a).
Expression of the other defining oscillation of NREM sleep, the sleep spindle, also undergoes marked changes in later adult life (Carrier et al., 2011; De Gennaro and Ferrara, 2003; Mander et al., 2014; Martin et al., 2013). Sleep spindles reflect transient bursts of waxing and waning oscillatory activity in the 12–15 Hz range and are generated through an interaction between corticothalamic networks and the reticular nucleus of the thalamus (De Gennaro and Ferrara, 2003; Steriade et al., 1987). Spectral power in the frequency range of sleep spindles (e.g., 12–15 Hz) is reduced in middle-aged and older adults, relative to younger adults (De Gennaro and Ferrara, 2003; Dijk et al., 1989; Landolt et al., 1996). Furthermore, the magnitude of this spindle effect increases across the night, with the largest age-related impairments (up to 50%) being observed in the final sleep cycles of the night, where faster frequency spindles predominate in young adults (Landolt et al., 1996).
The age-related reduction in spectral power in the frequency range of sleep spindles (12–15 Hz) is, in part, related to impairments in the amount of sleep spindles generated. The number, or density per unit time, of sleep spindles declines significantly as adult age advances, with the greatest reductions occurring over frontal EEG derivations (De Gennaro and Ferrara, 2003; Mander et al., 2014; Martin et al., 2013). Unique features of the spindle waveform are similarly affected as we grow older, and these too appear to contribute to the overall decrease in signal power associated with sleep spindles. For example, the duration, as well as the peak and mean amplitude of sleep spindles all decrease in older relative to younger adults (De Gennaro and Ferrara, 2003; Mander et al., 2014; Martin et al., 2013). As with slow waves, these changes are topographically specific across the head and temporally specific across the night. Maximal impairments in spindle density and amplitude occur over frontal lobe regions (Figure 1B). In contrast, age-related reductions in spindle duration are maximal over posterior EEG derivations (Martin et al., 2013). All three of these age-related disruptions in sleep spindles are most prominent during the last sleep cycles of the night (De Gennaro and Ferrara, 2003; Mander et al., 2016b; Martin et al., 2013).
It is important to note that age-related declines in sleep spindles can be observed even when the sleep stage from which they arise does not. For example, stage 2 NREM sleep duration does not change appreciably with age, yet sleep spindle expression within stage 2 NREM sleep deteriorates significantly in older adults (De Gennaro and Ferrara, 2003; Fogel et al., 2016; Mander et al., 2014, 2016b; Martin et al., 2013). Furthermore, the characteristic reduction in total NREM sleep in older individuals is related to the selective loss of NREM stages 3 and 4, or slow wave sleep, wherein slow waves are principally expressed (Feinberg and Carlson, 1968). Therefore, even when older adults have the same total amount of NREM sleep time, significant differences in the density and amplitude of slow waves within that same sleep-time period can be observed (Carrier et al., 2011; Dubé et al., 2015). That is, the measure of NREM sleep stage duration alone does not capture all of the information regarding age-related differences in the expression of slow waves.
More generally, these findings support a model in which age-related changes in gross macro-level sleep architecture (e.g., time and sleep stages) can, and, often are, mechanistically distinct from micro-level changes in physiological sleep oscillations. We will return to the relevance of this dissociation below in discussing both the functional consequences of age-related sleep changes, and how this distinction informs the debate of whether or not older adults need less sleep.
Sleep, Aging, and Gender
Though reliable macro and micro sleep differences exist between younger and older adults, not all older adults suffer the same degree of sleep disruption. Instead, there is large inter-individual variability. This means that age per se is not the sole determinant of sleep disruption in later life. Rather, factors that interact with the aging process must confer vulnerability or resilience to age-associated declines in sleep quantity and quality.
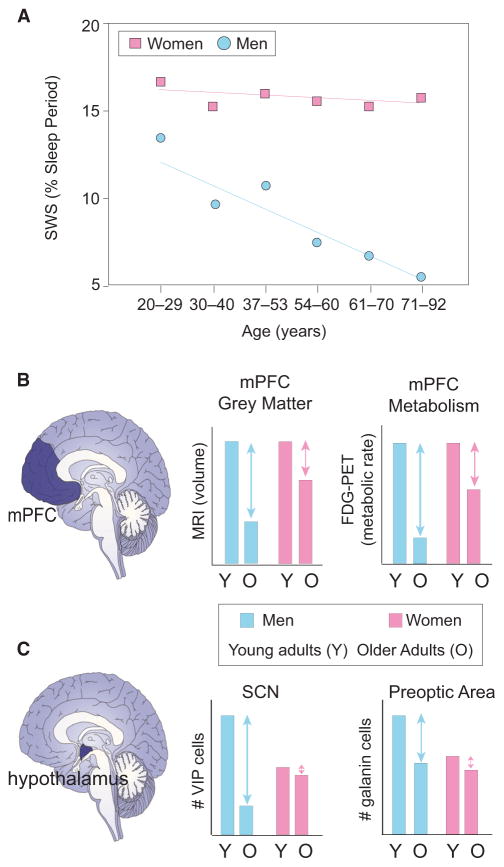
In the context of older age, one such interacting factor is gender. Men experience far greater relative disruption and impairment in NREM sleep than women later in life. In a study comparing over 2,500 older adults between the ages of 37 and 92 (Redline et al., 2004), increasing age was reliably associated with the same features described above: decreased slow wave sleep time, reduced sleep efficiency, increased NREM stage 1 sleep time, increased number of arousals, and modest decreases in REM sleep time. However, when stratified by gender, differences emerged (Figure 3A). Men over the age of 70 demonstrated a highly significant 50% reduction in slow wave sleep, relative to men under the age of 55, together with a concomitant increase in lighter NREM stages 1 and 2. In contrast, women showed no such significant decline in slow wave sleep or increase in lighter NREM sleep time relative to their younger gender group. Compared between genders, men over the age of 70 had more than a 3-fold deficit in slow wave sleep amount compared with age-matched women. Meta-analyses have replicated this gender-specific difference in slow wave sleep in older age (Ohayon et al., 2004). Interestingly, the moderate reduction in REM sleep time in the oldest participants (>70 years old) was common to both males and females, suggesting a gender-independent deterioration of this sleep stage.
Figure 3. Gender Alters the Impact of Age on Sleep and Related Brain Structure and Function.
(A) Representative changes in percent of the sleep period consisting of slow wave sleep in women (pink squares) and men (blue circles) across the lifespan (adapted from Ehlers and Kupfer, 1997; Redline et al., 2004).
(B) Schematic of changes in the medial prefrontal cortex gray matter volume and resting metabolic rate in men and women (adapted from Kakimoto et al., 2016), a region known to underlie the source generation of NREM slow waves. Relative to younger adults (Y), older (O) adult men (blue) exhibit significantly more gray matter loss and reduced metabolic rate than women (pink), proposed as one candidate mechanism that contributes to these gender-dependent differences in slow wave sleep (described in A).
(C) Changes in multiple hypothalamic nuclei in older adult (O) males (blue) and females (pink), relative to younger adult (Y) males (blue) and females (pink). Older adult males exhibit significantly greater relative loss of VIP neurons within the SCN and galanin-expressing neurons within the preoptic area of the hypothalamus. These cell changes offer one potential underlying cause of gender-specific changes in circadian and homeostatic sleep-wake regulation in older males than females.
Gender also impacts age-associated changes in slow wave sleep homeostasis. Older adult men demonstrate significantly less homeostatic slow wave sleep rebound during recovery sleep following sleep deprivation than equivalent-age older women (Reynolds et al., 1986). As with the comparison of basic sleep stages, both elderly men and women show similar homeostatic rebounds in REM sleep during post-deprivation recovery nights (Reynolds et al., 1986). Therefore, gender-dependent and gender–independent effects emerge in older age, further suggesting a model in which some homeostatic mechanisms of sleep remain equivalent and somewhat intact in cognitively normal older adult men and women, such as REM sleep, while others show strong gender-dependent differences, such as slow wave sleep.
Precisely when this gender effect on sleep emerges during the adult lifespan remains less clear. Young males and females in their 20s often do not to show such dramatic NREM sleep differences, though there is modest evidence of this divergence in those in their mid 30s (Ehlers and Kupfer, 1997; Van Cauter et al., 2000). A similarly timed divergence can be seen in the EEG spectral measure of SWA, which is ~50% lower in men in their 30s relative to their 20s, yet 25% in women in their 30s compared with their 20s (Ehlers and Kupfer, 1997). If and when gender-dependent changes in distinct features of NREM oscillations, such as the slope of slow waves or the density of sleep spindles, are present in later life remains unexplored, as does the topographical nature of such changes and when they occur across the night.
Despite gender-specific changes in NREM sleep quantity and quality being more severe in men, a paradoxical and, as yet unexplained, finding is that women are more likely to suffer subjective complaints of poor sleep as they get older, relative to older men (Ohayon et al., 2004). Whether this is due to report bias on the basis of gender, or is explained by an underlying physiological mechanism, remains unclear.
What Are the Neurobiological Mechanisms of Age-Related Sleep Impairment?
Neurophysiological and neurochemical changes that encompass the brainstem ascending arousal system, thalamus, and hypothalamus, together with select cortical regions, contribute to age-related sleep impairments. That large variability in sleep disruption is observed across equivalent age older adults, and the lack of correlation between different sleep features that are impaired in older individuals, indicate that some underlying mechanisms of these changes are independent, while others are interdependent.
Though there are a collection of central brain mechanisms that may underlie age-related changes in sleep, other factors also appear to contribute; e.g., presence of sleep disorders, obesity, medication use, nocturnal urinary frequency, chronic pain, hormonal changes, neurodegeneration, psychiatric conditions, and medical comorbidities that are especially prominent with advancing age (Lord et al., 2014; Vitiello, 2009). Moreover, processes of abnormal brain aging caused by degenerative neuropathology, such as β-amyloid and neurofibrillary tangles, are yet another important factor that may be antecedents of these signature sleep disruptions in later life (discussed in Box 1; Mander et al., 2016a).
Sleep Structure and Stages
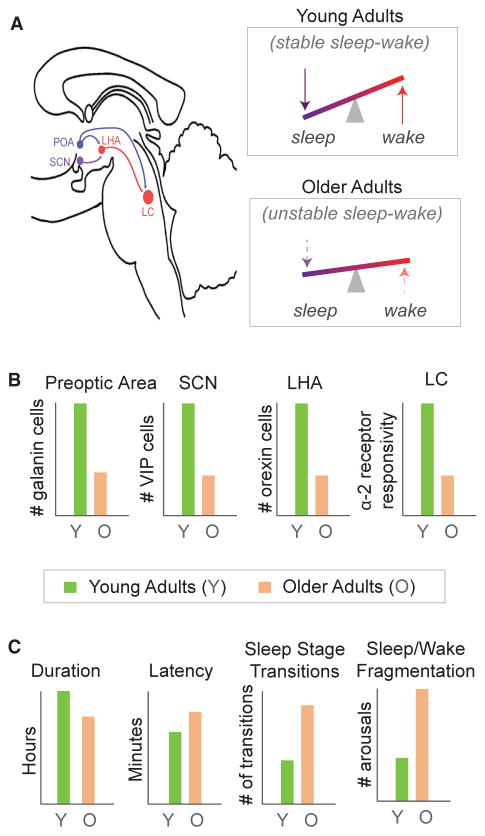
Age-related changes in sleep structure and sleep stages have constantly been linked to abnormalities in sleep-wake maintenance systems, resulting in instability (Figure 4). In young adults, consolidated sleep at night and wakefulness during the day emerges from a balance between brainstem, midbrain, and hypothalamic nuclei, which form a bi-stable sleep-wake flip-flop switch (Luppi et al., 2016; Saper et al., 2010). A primary promoter of sleep initiation and maintenance involves a small group of cells in the hypothalamic preoptic area, a proportion of which express the inhibitory neuropeptide galanin (Saper et al., 2010). The number of galanin-expressing neurons within the preoptic area of the hypothalamus significantly decline with age, and the severity of this cell loss at post-mortem examination predicts the severity of sleep fragmentation in older adults measured in the years prior (Lim et al., 2014). In pathological conditions where cell loss is more dramatic, such as Alzheimer’s disease, these relationships become even stronger (Lim et al., 2014).
Figure 4. Neuropathological Associations with Age-Related Impairments in Sleep Regulation and Sleep Architecture.
(A) Schematic of sagittal view of brainstem and hypothalamic flip-flop model of sleep and wake regulating nuclei (Saper et al., 2010). The wake-promoting LHA (red) and LC (red) help to maintain stable periods of wake. The sleep promoting preoptic area (POA; blue) sends inhibitory projections to the LHA and the LC and, through this inhibition, helps to initiate and maintain stable sleep. The SCN (purple) further modulates this signal across a 24 hr period by fostering higher orexigenic activity in the LHA, promoting wakefulness during the day and decreases in influence permitting sleep at night. Due to age-related changes in these nuclei, the strength of sleep and wake promoting signals is compromised, resulting in an unstable brain state (lower right for older adults, relative to upper in younger adults).
(B) Representation of age-related pathological differences in the nuclei that contribute to the flip-flop circuit of sleep-wake regulation. Relative to younger adults (green), older adults (orange) show reduced galanin-expressing preoptic area neurons, reduced VIP neurons in the SCN, reduced orexin-expressing neurons in the LHA, and reduced α-2 adrenoreceptor responsivity within the LC. These pathological changes may therefore contribute to greater sleep and wake instability in older adults, depicted in (A) bottom right.
(C) Emerging from this proposed instability associated with these age-related changes within brainstem and hypothalamic sleep and wake regulatory centers is a collection of macro-level sleep alterations. These include decreased sleep duration, increased sleep latency, increased number of sleep stage transitions toward lighter sleep stages and wake, and greater fragmentation of consolidated periods of sleep and wake in older (orange) relative to younger (green) adults.
Wake-promoting nuclei of the ascending brainstem arousal system and the wake-promoting hypocretin/orexin-expressing neurons within the lateral hypothalamic area (LHA)—which collectively help maintain stable wake states (Saper et al., 2010)—also undergo age-related deterioration. In rodent models, hypocretin/orexin-expressing neurons are reduced by 40% in older relative to younger adult rats (Kessler et al., 2011). In human post-mortem studies, the effect size is smaller though still significant, with a 10% reduction observed in older age (Hunt et al., 2015). Larger effects have been observed in patients with tauopathies, indicating that the greater loss of hypocretin/orexin-expressing neurons in such conditions may be partially responsible for the worsening of sleep-wake instability (Kasanuki et al., 2014).
Downstream targets of these wake-promoting systems also contribute to the typical deterioration of stable sleep-wake structure in later life. Older adult primates have fewer hypocretin/orexin-expressing axons that project to the sleep-wake regulating target of adrenergic centers in the locus coeruleus (LC) (Downs et al., 2007). Further implicating the adrenergic system, older rodents show less of the typical slow wave sleep increase in response to the α-2 adrenoreceptor agonist clonidine than younger rodents and less of the wake-promoting response to the antagonist yohimbine (de Sarro et al., 1988). However, whether these effects are also present in older adult humans is still unknown.
While several hypothalamic and brainstem nuclei that regulate sleep and wake brain states appear to be impacted by aging, it is of note that not all sleep and wake regulatory nuclei are affected, indicating specificity. For example, serotonin-expressing neurons of the dorsal raphe nucleus (Klöppel et al., 2001) and histamine-expressing neurons in the tuberomammilary nucleus of the hypothalamus (Ishunina et al., 2003) show minimal age-related changes in human post-mortem examinations and do not predict age-related sleep disruption.
Beyond sleep-wake regulatory changes, reduced sleep time at night in older human adults is associated with thinning of gray matter within regions in the lateral frontal and superior temporal cortices. A similar relationship between shorter sleep time and reduced gray matter volume in the lateral prefrontal cortex has been observed in a large cohort of community-dwelling older adults (Lim et al., 2016) and further predicts the severity of sleep fragmentation. Whether or not these associations are a cause or consequence remains less certain. Nevertheless, it is clear that selective yet systemic age-related changes in the structure and function of subcortical and cortical brain regions contribute to the deterioration of sleep and wake regulation across the adult lifespan.
Homeostatic Sleep Drive
The homeostatic pressure to sleep, including slow wave sleep and its associated EEG feature of slow wave activity, is regulated, in part, by increasing extracellular adenosine, a metabolic byproduct that accumulates with time spent awake (Dworak et al., 2010; Porkka-Heiskanen et al., 1997). Despite older adults generally having a lower homeostatic sleep response to continued time awake, extracellular adenosine appears to be higher in older relative to younger rodents. These age-related differences are particularly prominent in sleep/wake regulating centers of the brainstem and basal forebrain regions (Mackiewicz et al., 2006; Murillo-Rodriguez et al., 2004). This finding would at first appear to contradict the aforementioned lower homeostatic sleep drive commonly observed in human studies of aging, since higher adenosine would predict a higher, not lower, homeostatic drive to sleep.
One mechanism reconciling this discrepancy is the apparent widespread loss of adenosine A1 receptors that occurs with advancing age (Cheng et al., 2000; Ekonomou et al., 2000; Pagonopoulou and Angelatou, 1992), present in cortical, thalamic, hippocampal, and striatal regions. Significant A1 receptor loss is observed even when controlling for cell loss or overall regional brain volume or weight (Ekonomou et al., 2000; Meyer et al., 2007; Pagonopoulou and Angelatou, 1992) and is accompanied by reduced A1 receptor gene expression (Cheng et al., 2000). Therefore, the reduction of A1 receptors may decrease sensitivity to the higher extracellular adenosine concentration, despite the increased buildup of adenosine levels reflecting a higher sleep need in older adults (Figure 2C). Reduced receptor sensitivity would therefore prevent the otherwise greater homeostatic pressure from being translated into a greater homeostatic sleep drive, which would otherwise manifest as an increase in slow wave sleep time and slow wave activity (Figures 2A and 2B).
Nevertheless, it is unlikely that changes in adenosine alone fully explain age-related changes in the expression of homeostatic sleep drive. Indeed, additional explanatory factors are beginning to emerge and include age-related changes in glial function that may modulate adenosine A1 receptor-dependent signaling (Halassa et al., 2009) and upregulate central inflammation (Ingiosi et al., 2013).
In addition to changes in homeostatic sleep drive, age-related diminution of the arousal promoting circadian signal from the suprachaismatic nucleus (SCN) in the hypothalamus may further exacerbate disruptions in stable sleep-wake timing in old adults (Figures 4A and 4B). In post-mortem histology analyses, hypothalamic SCN neurons have been found to degenerate with advancing age, particularly those expressing vasoactive intestinal peptide (VIP) (Wang et al., 2015). Moreover, the severity of VIP degeneration retrospectively predicts the degree of blunting in the magnitude of the circadian rhythm in motor activity, measured using actigraphy. Such a decrease reflects a diminished circadian amplitude that may weaken the evening circadian up-swing in the alerting signal present in younger adults (Munch et al., 2005), one that leads to the common feature of older adults having an earlier preference to go to bed.
Sleep Oscillations
The mechanisms explaining age-related impairments in distinct sleep oscillations are now being revealed, and with them, the potential foundations for future therapeutic intervention. Structural brain atrophy has consistently been identified as one factor governing age-related impairments in NREM sleep oscillations. In young adults, measures of slow wave expression, including amplitude, density, and spectral power of slow waves, are strongly associated with structural gray matter density and volume in select cortical regions in the PFC (Saletin et al., 2013), the same region over which NREM slow waves show maximal expression and within which EEG source generators have been identified (Murphy et al., 2009).
Atrophy within similar PFC brain regions, measured by both gray matter volume and cortical thickness, predicts the severity of impairment in NREM slow wave features in older adults (Dubé et al., 2015; Mander et al., 2013; Varga et al., 2016). This association with gray matter atrophy is especially pronounced in medial and lateral PFC regions (Figure 5). While these relationships can be observed when measuring NREM slow wave impairments across a whole night, they are maximal during the first NREM sleep cycle, perhaps unsurprisingly, considering this is the same phase of sleep when SWA is highest (Dubé et al., 2015). These results are consistent with the fact that NREM slow wave power and amplitude are maximal over frontal EEG derivations where the biggest increases are also observed following sleep deprivation (Carrier et al., 2011; Landolt and Borbély, 2001; Mander et al., 2013; Munch et al., 2004). That the EEG source localization of slow waves predominates within midline frontal regions is also consistent with this postulate (Murphy et al., 2009).
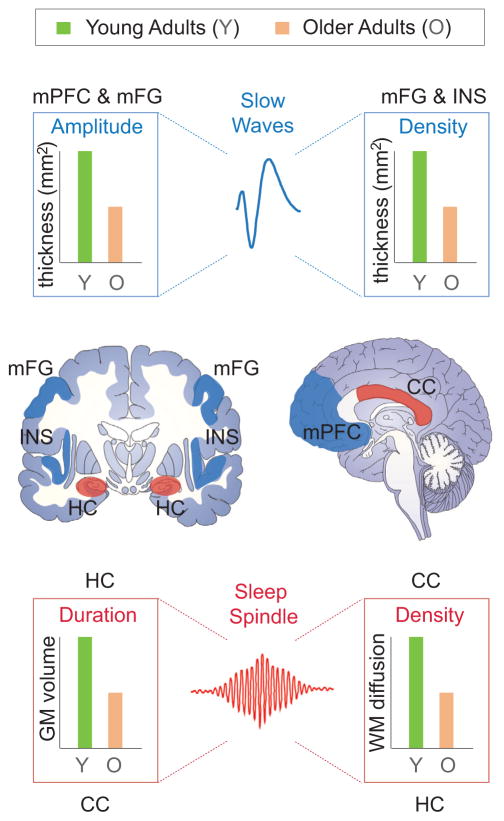
Figure 5. Structural Brain Changes and Age-Related Impairments in NREM Sleep Oscillations.
Upper and middle: Age-related deficits in NREM slow wave amplitude and density are significantly associated with the degree of reduced gray matter volume and thickness (middle; blue regions) in the medial prefrontal cortex (mPFC), middle frontal gyrus (mFG), and insula in older (orange) relative to younger (green) adults (adapted from Dubé et al., 2015; Mander et al., 2013; Varga et al., 2016). Impairments in NREM sleep spindle duration and density (bottom right bar graph) reductions in older (orange) relative to younger (green) adults are associated with the magnitude of decreased hippocampal gray matter volume and white matter integrity within the body and splenium of the corpus callosum (middle; red regions; adapted from Fogel et al., 2016; Mander et al., 2016b) (lower image).
Structural deterioration in other brain regions known to atrophy with age, such as the precuneus, temporal lobe, and hippocampus, do not predict age-related impairments in slow wave activity (Mander et al., 2013). This would suggest that age-related changes in NREM sleep oscillations are not simply a consequence of generalized, non-specific whole-brain atrophy (Mander et al., 2013), but regionally specific cortical atrophy in frontal regions (Dubé et al., 2015; Mander et al., 2013; Varga et al., 2016). However, cortical thinning of gray matter in posterior brain regions, including the superior parietal cortex, does mediate age-related changes in slow wave amplitude (Dubé et al., 2015), inferring that atrophy within specific regions beyond the PFC can indirectly contribute to age-related disruptions in slow wave expression.
The decline in the density of slow waves associated with advancing older age is predicted by the loss of gray matter in a network that is partially overlapping with PFC regions predicting SWA and amplitude (Dubé et al., 2015). Though reduced gray matter in the PFC does predict fewer slow waves in older adults, so too do regions beyond, including the insula cortex, regions of the inferior and superior temporal cortex, and a region of the inferior parietal cortex (Dubé et al., 2015). This was true when assessing slow wave density across the entire night or in the first cycles of the night where slow waves are most prominent in younger adults and most deficient in older adults (Carrier et al., 2011; Dubé et al., 2015; Landolt and Borbély, 2001; Munch et al., 2004).
The fact that atrophy in largely distinct cortical regions is associated with selectively different age-related changes in slow wave features (density and amplitude) intimates that similarly distinct mechanisms could underlie age-related changes in slow wave generation (e.g., density) and propagation/expression (e.g., amplitude) (Dubé et al., 2015), though these slow wave features are themselves interdependent.
Deficiencies in sleep spindles—the other dominant oscillation of NREM sleep—in older adults are also associated with structural brain integrity. The nature and anatomy of these links are less well understood, though it is distinct from that which predicts changes in slow waves (Figure 5). Subcortical reductions in gray matter within the hippocampus predict the degree of reduced number (density) of sleep spindles in older adults, expressly over the frontal lobe (Fogel et al., 2016). While sleep spindle generation has classically been considered in the context of thalamo-cortical systems, fMRI studies in young adults have shown reliable associations between sleep spindles and activity within the hippocampus (Andrade et al., 2011; Bergmann et al., 2012; Schabus et al., 2007). Moreover, sleep spindles are functionally linked to the temporal burst firing of sharp-wave ripple events in the hippocampus in young and middle-aged adults, further implicating this region in spindle regulation (Fell et al., 2001). Therefore, age-related atrophy of cell bodies within the hippocampus is fitting with impairments in the functional expression of sleep spindles.
Though oscillations, including sleep oscillations, are generated by neuronal cell bodies, their network propagation, or transmission, depends on the integrity of white matter fiber tracts. In young adult humans and rodents, white matter integrity in fiber tracts that form cortico-thalamic loops is associated with the quantitative and qualitative expression of sleep spindles (Piantoni et al., 2013; Steriade et al., 1987). In agreement with these findings, preliminary human data indicate that age-related deterioration in white matter integrity within the body and splenium of the corpus callosum predicts the severity of impaired sleep spindle expression in older adults (Mander et al., 2016b). These effects remain significant when controlling for age, reemphasizing the notion that processes associated with aging, but not an individual’s age, per se, are the more significant determinant of these links between brain deterioration and sleep deterioration.
Gender Differences in Sleep
The biological causes of gender-dependent differences in sleep impairment—men suffering worse NREM sleep deterioration than women—are somewhat less well characterized. However, four lines of evidence provide a set of tenable, non-mutually exclusive, explanatory candidates.
First, gender-specific changes in the circadian alerting signal may account for greater sleep fragmentation, less consolidated sleep, and higher daytime nap propensity in older men. Human post-mortem histopathology evidence has demonstrated far larger relative losses in the number of VIP-expressing neurons in the SCN in men relative to women across the lifespan (Zhou et al., 1995). Such findings are consistent with loss of VIP-expressing neurons in the SCN of aged rodents, a reduction that correlates with age-associated sleep and circadian disturbances (Figure 4) (Chee et al., 1988). These findings support the human post-mortem study linking loss of VIP-expressing neurons within the SCN to a blunting of the circadian rhythm of motor activity in older adults (Wang et al., 2015). Nevertheless, that age-related changes in the integrity of VIP-expressing neurons causally contributes to sex differences in sleep timing, organization, and/or consolidation in humans remains unproven at present.
Second, the preoptic region of the hypothalamus, which powerfully regulates NREM sleep pressure and thus amount, is sexually dimorphic. In young adults, males have greater numbers of galanin-expressing neurons within the preoptic area of the hypothalamus than women (Swaab and Fliers, 1985). However, human post-mortem histopathology studies have demonstrated that by late life, this gender difference was diminished, indicating a more rapid relative decline in galanin-expressing neurons in this preoptic hypothalamic region in males than females. This may explain the more severe relative impairment in slow wave sleep duration and quality in males than females with advancing age (Garcia-Falgueras et al., 2011; Redline et al., 2004). A similar male-specific decline in hypocretin/orexin-expressing neurons in the hypothalamus has also been observed in older adult rodents (Brownell and Conti, 2010). As these neurons are critical for maintaining stable sleep and wake states (Saper et al., 2010), such gender-specific changes may further contribute to male-selective declines in NREM sleep.
Third are structural changes within the LC, a region that plays a key role in slow wave sleep homeostasis (Cirelli et al., 2005). In vivo MRI measures have identified age-related declines in neuromelanin-sensitive cells in the LC, and this reduction in the LC cell mass is greater in older males than older females (Clewett et al., 2016). This gender-dependent age-related decline in noradrenergic brainstem signals has the potential to weaken slow wave sleep homeostasis, causing more dramatic NREM sleep disruption in older males relative to females.
Fourth, older adult men experience more accelerated gray matter atrophy in the core NREM slow wave generating region of the medial prefrontal cortex (Kakimoto et al., 2016; Murphy et al., 2009), which is the same cortical region in which atrophy predicts the severity of SWA decline in the elderly in general (Figure 5) (Mander et al., 2013). Moreover, older adult males suffer greater reductions in metabolic activity in the medial prefrontal regions similar to the medial prefrontal SWA-source generating regions, implicating a functional impairment that may even precede the greater medial prefrontal gray matter atrophy observed in men (Kakimoto et al., 2016). Independently or collectively, these regionally and functionally specific possibilities all lead to falsifiable predictions regarding the mechanisms of gender differences in sleep deterioration in later life.
There is currently no consensus mechanism explaining these age-related sex differences in the deterioration of sleep/wake regulatory brain centers, though candidate intrinsic and extrinsic pathways are emerging. For example, age-related decreases in the expression of growth hormone and testosterone have been tied to the deterioration of consolidated sleep, reductions in the amount and intensity of slow wave sleep, and decreases in the number of galanin-expressing neurons within the preoptic area of the hypothalamus in humans (Garcia-Falgueras et al., 2011; Latta et al., 2005; Van Cauter et al., 2000). Supporting an effect of sex, older males not only show a greater decline in slow wave sleep, relative to females, but in men this reduction correlates with decreased growth hormone secretion that begins in midlife (Van Cauter et al., 2000), while no such relationship is observed in older women (Latta et al., 2005).
Another intrinsic hormonal candidate is testosterone. The pulsatile secretion of testosterone increases with transitions into slow wave sleep in young men (Veldhuis et al., 2000). This association, and testosterone levels in general, decreases with age, and low testosterone levels in older men is associated with decreased sleep efficiency and greater sleep fragmentation (Barrett-Connor et al., 2008; Harman et al., 2001; Veldhuis et al., 2000). Testosterone has been proposed to underlie the sex differences in atrophy associated with galanin-expressing neurons within the preoptic hypothalamic area (Garcia-Falgueras et al., 2011). In rodent studies, galanin neuronal density is reduced by castration and restored with testosterone treatment (Park et al., 1997). In human studies, both castrated men and male to female transsexual individuals have galanin-expressing neurons within the preoptic area of the hypothalamus reduced to a number similar to that observed in women (Garcia-Falgueras et al., 2011).
Extrinsic influences include lifestyle factors and behavioral choices. One such example is alcohol intake, which was higher in men than women in the study of gender differences in age-related cortical atrophy and hypometabolism (Kakimoto et al., 2016). Alcohol has marked effects on the quantity and quality of sleep (Thakkar et al., 2015). Indirect effects of alcohol are also possible: greater alcohol intake is associated with greater medial prefrontal atrophy (Rando et al., 2011), and the integrity and functionality of the medial prefrontal cortex is critical for NREM slow wave generation (Dubé et al., 2015; Mander et al., 2013; Murphy et al., 2009; Varga et al., 2016). Nevertheless, all these proposed mechanisms remain speculative.
Are There Functional Consequences of Age-Related Sleep Impairment?
Age-related sleep disruption is not epiphenomenal. Rather, these alterations have significant downstream consequences for brain and body health. This is perhaps not surprising considering that sleep in young adults supports every major physiological system within the body, including immune, metabolic, thermoregulatory, endocrine, and cardiovascular function (Irwin, 2015); and numerous cognitive and affective neural processes, such as learning and memory, emotional regulation, attention, motivation, decision making, and motor control (Walker, 2009). While it is outside the scope of the current review to describe all links between sleep impairment and this diverse array of brain and body dysfunction in the elderly, as an exemplar, we devote the next section to reviewing the evidence linking age-related changes in sleep to impairments in learning and memory processing in older adults.
Sleep before and after learning plays a causal role in memory processing (Walker, 2009). NREM sleep quantity and oscillatory quality prior to learning support the restoration of next-day hippocampal-dependent encoding capacity and thus initial learning (Antonenko et al., 2013; Mander et al., 2014; Van Der Werf et al., 2009). Features of NREM sleep after encoding, including (but not limited to) NREM slow waves, have consistently been linked to the subsequent offline consolidation of hippocampal-dependent memory processing. In doing so, sleep after learning stabilizes new memory representations, thereby minimizing long-term forgetting (Diekelmann and Born, 2010). These same processes have since been characterized in older adults and shown to be interacting and deficient.
Sleep, Aging, and Initial Memory Encoding
A common feature of age-related cognitive decline is an impaired ability to form new hippocampal-dependent memories, such as new facts or new person-name associations (Miller et al., 2008; Sperling et al., 2003). A meta-analysis of studies of self-reported measures of sleep in older adults has established an association between poor and shorter sleep and the extent of impairment on numerous cognitive tests, including several requiring verbal memory encoding (Lo et al., 2016). Objective, rather than subjective, measures of sleep in older adults support these associations. The degree of actigraphy-measured sleep disturbance in older adults predicts increasingly worse scores on standardized neuropsychological tests, including memory subcomponents that depend upon the hippocampus. Moreover, these associations remain significant when controlling for cardiovascular conditions, history of medication use, indices of depression, anxiety, and stress (Cavuoto et al., 2016), suggesting sleep-specific associations with impaired memory encoding, beyond age-related cofactors.
Qualitative sleep EEG changes in aging have provided more detailed mechanistic explanations of impaired sleep-dependent memory encoding in the elderly. In young adults, the density of NREM fast-frequency sleep spindles over PFC derivations—the same region over which reductions in fast sleep spindles are most dramatic in older adults (Mander et al., 2014; Martin et al., 2013)—predicts the restoration of hippocampal encoding activity and episodic learning capacity in younger adults (Mander et al., 2011, 2014). In older adults, the magnitude of impairment in fast-frequency spindle density significantly predicts the failure to which the hippocampus will engage in episodic associative memory encoding the following day, which, in turn, mediates the success of learning ability (Mander et al., 2014) (Figure 6A).
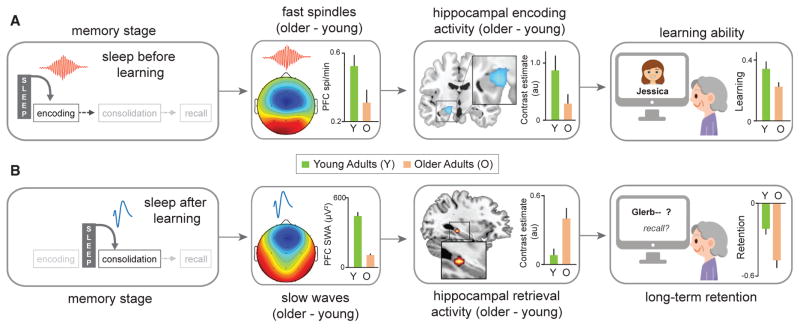
Figure 6. Hippocampus-Dependent Memory Decline Is Associated with Age-Related Sleep Disruption.
(A) Sleep, including NREM fast sleep spindles, before learning supports the initial act of memory encoding (e.g., learning a face-name association) through a mediating influence on learning-dependent hippocampal activity. Age-related disruption of fast-frequency NREM sleep spindles over the prefrontal cortex attenuates this hippocampal encoding activity and thus the sleep-dependent learning restoration benefit (adapted from Mander et al., 2014).
(B) Sleep, specifically NREM slow waves, after learning supports the subsequent offline consolidation of new memories, promoting hippocampal-neocortical memory transformation, resulting in hippocampal-independent retrieval the next day, minimizing overnight forgetting. Age-related impairment in NREM slow wave activity in the prefrontal cortex predicts worse overnight hippocampal-neocortical memory transformation, resulting in persistent, rather than progressively hippocampal-independent next-day retrieval activity. This is associated with accelerated forgetting and thus worse long-term memory retention (adapted from Mander et al., 2013).
Interestingly, this triadic relationship (impaired fast sleep spindles, decreased hippocampal encoding activity, and impaired learning) was independent of the gross structural properties of the hippocampus, suggesting a sleep-functional, rather than atrophy-structural, association with the inability to effectively form new memories in the elderly (Mander et al., 2014). Such evidence does not, however, negate the possibility that structural alterations within the hippocampus in older adults are a consequence of associated sleep impairment, nor that other non-mutually exclusive consequences to the hippocampus contribute to memory encoding deficits. For example, rodent models have established that sleep deprivation suppresses adult hippocampal neurogenesis (Guzman-Marin et al., 2007). Nevertheless, how such a relationship relates to the recognized reduction in the structural integrity of the human hippocampus in aging remains unexplored.
Functionally, chronic sleep restriction in rats, which is more representative of age-related sleep impairment than acute total sleep deprivation, adds to age-related calcium signaling impairments within the hippocampus of older animals (de Souza et al., 2012). This represents another viable route through which impairments in hippocampal-dependent learning can be affected by sleep impairments in later life. Additionally, increasing levels of adenosine within the hippocampus in middle-aged rats, presumably associated with deficient sleep and consequent increased time awake (Porkka-Heiskanen et al., 1997), predicts impairment in long-term potentiation of hippocampal neurons (Rex et al., 2005). Moreover, age-related memory deficits in rats, which may be associated with age-related sleep deterioration, can be reversed with caffeine or adenosine (A2A) receptor antagonists (Prediger et al., 2004; Rex et al., 2005). Therefore, intra-hippocampal structural and functional changes associated with aging suggest numerous pathways through which the canonical sleep disruption of later life can impair hippocampal-dependent learning ability and contribute to cognitive decline.
That NREM sleep offers a causal contribution to deficits in the formation of new hippocampal-based memories in later adult life has also been established. Experimentally applied acoustic-tone disruption of deep NREM sleep in older adults, during which slow waves coupled with sleep spindles predominate, significantly decreases next-day hippocampal encoding activity and associated learning, despite sleep duration and efficiency remaining intact (Van Der Werf et al., 2009). Additionally, enhancing the intensity of the slow oscillation in young adults using transcranial direct current stimulation causally renews post-sleep hippocampal-dependent learning ability (Antonenko et al., 2013). Such findings are of importance practically and clinically considering that older adults are often prescribed medications for sleep-unrelated conditions (e.g., hypertension) that can themselves disrupt NREM sleep (Novak and Shapiro, 1997) and thus negate a functional sleep-dependent memory benefit.
Sleep, Aging, and Memory Consolidation
Sleep after initial encoding supports the subsequent long-term consolidation of recent hippocampal-dependent memory representations. This benefit involves a coupled dynamic between three NREM oscillations: cortical slow oscillations (<1 Hz), sleep spindles, and hippocampal sharp-wave ripples (Diekelmann and Born, 2010; Staresina et al., 2015). It is through this coordination of oscillations that the long-term reorganization of memory representations is hypothesized to be achieved, transforming memories from a hippocampal- to a more cortical-dependent form, aiding in memory stabilization and averting interference from ongoing encoding within the hippocampus (Diekelmann and Born, 2010; Walker, 2009).
Across multiple levels of analysis, growing evidence suggests that sleep disruption mediates the effects of age-related long-term memory impairment for which consolidation is key. Self-reported sleep quality the night after word-list learning in older adults is associated with memory retention 1 week later, with number of awakenings predicting progressively worse remembering (Mary et al., 2013). While reductions in objective measures of slow wave sleep time in middle-aged (age 48–55) adults predicts worse offline consolidation and thus memory retention (Backhaus et al., 2007), the same relationship between this same gross measure of slow wave sleep does not reliably predict long-term memory retention in older adults (Scullin, 2013). A similar lack of prediction between the amount of time spent in slow wave sleep and post-sleep memory retention has been observed in older adults following a nap (Baran et al., 2016), demonstrating that the lack of such an association in older adults cannot simply be explained by time-of-day circadian recall factors. Nevertheless, slow wave sleep in older adults does predict post-sleep memory accuracy in the context of a reduction in false memories using a word learning paradigm (Lo et al., 2014), which may indicate a qualitative, more than quantitative, association between the global measure of slow wave sleep time and memory in older adults.
Other differences also appear to contribute to this heterogeneity of relationships with basic sleep stages. Offline consolidation of a hippocampus-dependent visuospatial memory task correlates with the severity of impairment in REM sleep time in elderly individuals, rather than the classic NREM sleep stage association in younger adults; though this effect is only observed in older adults who initially encoded the task effectively before the consolidation phase (Sonni and Spencer, 2015). This finding first suggests a connection between sleep stages and hippocampal-dependent memory consolidation in older adults that may encompass both NREM and REM (the latter, potentially in a compensatory manner), relative to younger adults. Second, these findings emphasize the careful need to train older adults to some degree of initial encoding competence prior to sleep, since the variability of initial learning of hippocampal-dependent memory representations may cause variability in whether or not offline sleep-dependent consolidation can be accomplished.
Robust and consistent relationships between sleep and memory consolidation are, however, observed when assessing the more detailed measure of physiological sleep oscillations, and these associations are topographically specific. The degree of impaired SWA across older adults predicts worse overnight memory consolidation, resulting in greater next-day forgetting (Figure 6B) (Mander et al., 2013; Varga et al., 2016). Though this association was observed globally across all electrode channels averaged, frontal electrodes showed the strongest relationship between SWA and overnight memory consolidation (Mander et al., 2013). This is consistent with the age-dependent sensitivity of this region to declining SWA in older adults (Mander et al., 2013). Identification of this SWA association in older adults appears to require assessing a full night of sleep, potentially because of the full homeostatic expression of SWA, since similar relationships do not reach significance when assessed using a daytime nap (Baran et al., 2016).
More than being associated with the end-outcome of failed long-term memory retention, age-related impairments in overnight NREM sleep oscillatory activity further predict an impoverished degree of neural transformation of hippocampal-neocortical transformation of episodic memory representations. Age-related deficits of SWA over PFC are associated with the continued next-day reliance of memory retrieval on hippocampal activity (Mander et al., 2013), rather than the adaptive development of increasing hippocampal-independent retrieval that is observed in young healthy adults (Takashima et al., 2006). Moreover, these age-related SWA impairments further diminish the development of hippocampal-prefrontal functional connectivity that is otherwise stronger during post-sleep retrieval in young adults, another proxy associated with hippocampal-neocortical memory consolidation (Baran et al., 2016; Mander et al., 2013).
Beyond encoding and consolidation, what remains to be established is the impact of age-related sleep impairment upon the final act of memory retrieval, years or even decades later. Recent evidence in young adults has demonstrated that sleep prior to attempted recall of memories predicts probability of successful retrieval (Dumay, 2016). That is, sleep also facilitates accessibility to potentially available memories. Whether or not sleep impairments in older adults contribute to canonical failures of memory recall in older adults is unknown (Burke and Light, 1981; Craik, 1977). Finally, though beyond the scope of our focus on hippocampal-dependent memory processing, age-related sleep impairments also compromise certain aspects of procedural skill memory, the underlying neurobiological mechanisms of which remain unclear (e.g., Backhaus et al., 2015; Fogel et al., 2013; King et al., 2016; Peters et al., 2008; Spencer et al., 2007; Tucker et al., 2011; Wilson et al., 2012).
Sleep Restoration, Aging, and Memory
The vast majority of studies reporting an association between sleep, aging, and memory are correlational in nature. However, several studies have sought to enhance the sleep of older adults, and with it, memory. These studies are informative in at least two ways. First, they establish a causal contribution of sleep to memory in the elderly, as has been shown in young adults. Second, therapeutic interventions that restore sleep may deliver preventative benefits that reduce the risk and/or severity of cognitive decline in aging, or mechanisms and/or processes that lead to mild cognitive impairment or Alzheimer’s disease (Mander et al., 2016a), or body ill-health consequences, such as hypertension or chronic pain (Neikrug and Ancoli-Israel, 2010). However, it is important to note that these methods have not been investigated using longitudinal study designs, and thus the longitudinal utility of such methods remains unknown.
In young adults, transcranial direct current stimulation (tDCS) in the <1 Hz slow oscillation frequency range during slow wave sleep increases slow oscillation power and almost doubles overnight memory retention (Marshall et al., 2006). In older adults, multiple studies have shown that enhancing the slow oscillation using tDCS during an afternoon nap leads to a memory enhancement; one showing heightened SWA in the slow oscillation frequency range (<1 Hz) and word-pair performance (Westerberg et al., 2015), and another showing enhanced slow oscillation and fast sleep spindle EEG power that led to a benefit in a visual memory task (Ladenbauer et al., 2016). Auditory closed-loop stimulation during slow wave sleep has also been shown to enhance slow oscillation power and associated hippocampus-dependent memory consolidation in young adults (Ngo et al., 2013). Preliminary findings in older adults have reported increased slow oscillation power during blocks of auditory stimulation that is associated with enhanced next-day declarative memory recall (Papalambros et al., 2017). It is of note, however, that some studies implementing varied forms of brain stimulation have failed to replicate the beneficial enhancement of sleep physiology and/or memory consolidation (Eggert et al., 2013; Passmann et al., 2016; Sahlem et al., 2015). Therefore, brain stimulation methods offer potential promise as intervention tools in the context of aging, but they require further refinement and demonstration of efficacy and reproducibility before being realistic options at present.
Pharmacological methods using classic GABA-targeting hypnotics for selective NREM sleep enhancement have so far proved less promising in the context of aging and often fail to trigger any corresponding sleep-dependent memory benefit in the elderly, sometimes even causing amnestic effects (Feld et al., 2013; Hall-Porter et al., 2014; Mednick et al., 2013; Vienne et al., 2012). Little is currently known regarding the impact of more contemporary non-GABA-targeting sleep medications on enhancing sleep and/or cognition in the elderly populations at risk for dementia.
Overall, several novel, non-pharmacological approaches are emerging that may represent candidate methods for sleep restoration in the elderly and, with such restoration, improvements in those mental and physical functions that rely on sleep and are causally deficient in the elderly as a result.
Do Older Adults Need Less Sleep?
It is clear that sleep changes markedly as we transition into old age. However, a fundamental question remains debated: do older adults get less sleep simply because they need less sleep, or do older adults need more sleep than they get, being incapable of generating that which they still need? We close this review by briefly exploring the arguments that favor and do not favor the claim that older adults need less sleep.
In Favor
Three core observations support the hypothesis that older adults do not require as much sleep as younger adults and therefore do not have a significant unmet sleep need. First, older adults sleep significantly less than younger adults when offered extremely long, enforced periods of sleep opportunity (time in bed >12 hr/day) (Klerman and Dijk, 2008). This has been considered to reflect an ad-lib sleep need that is lower in older relative to younger adults. Second, following sleep-deprivation or experimental slow wave sleep suppression, healthy older adults exhibit a smaller rebound in slow wave sleep time and SWA compared to younger adults (Dijk et al., 2010; Münch et al., 2004). This suggests a lower buildup of homeostatic sleep pressure in older relative to younger adults. Third, and related, older adults suffer less subjective and objective waking sleepiness following selective slow wave sleep deprivation than do younger adults; a result that even holds true under rested conditions (Dijk et al., 2010). Furthermore, older adults suffer less relative impairment on sleep-sensitive vigilance tasks under sleep deprivation conditions, relative to younger adults (Adam et al., 2006). These findings collectively suggest that older adults demonstrate many of the phenotypic signs of a reduced homeostatic sleep drive, indicative of a reduced sleep need (Dijk et al., 2010; Klerman and Dijk, 2008). That is, sleep need decreases with age.
Not in Favor
Four sets of findings support the alternative hypothesis: older adults need more sleep than they are normally able to obtain.
First, evidence does not favor the interpretation of a lower homeostatic sleep drive in older adults, but rather, impaired sensitivity to a still present homeostatic drive. Extracellular adenosine levels, which are a major molecular marker of homeostatic sleep drive (Porkka-Heiskanen et al., 1997), actually increases with advancing adult age in many sleep regulatory centers (Mackiewicz et al., 2006). A lower homeostatic sleep drive would predict the opposite. This discrepancy appears to be due to a widespread loss of A1 adenosine receptors in the aged brain (Meyer et al., 2007). Compounding this adenosine receptor downregulation, neuronal loss in sleep regulatory centers that are influenced by this system and involved in sleep homeostasis may further impair the regulation of sleep drive (Lim et al., 2014). Thus, instead of reduced sleep need, aging could alternatively be characterized as a desensitization to a still present homeostatic sleep drive and a reduction in the neural apparatus for regulating a still present sleep need.
Second, while healthy older adults report being less subjectively sleepy, such measures are confounded by prior sleep history. The rise in the subjective sleepiness in the first days of chronic sleep restriction in young adults will often re-normalize back to lower sleepiness in later deprivation days, despite objective performance continuing to decline (Van Dongen et al., 2003). Therefore, reports of less subjective sleepiness in older adults may not provide reliable indicators of a well-slept or even acutely sleep-deprived brain, because older adults may be suffering from the same renormalizing effect of chronic sleep restriction.
Third, although older adults show smaller relative impairments in objective performance on vigilance tasks when placed under conditions of sleep deprivation than younger adults (Adam et al., 2006), this too can be an artifact of baseline. Older adults often perform significantly worse than younger adults under well rested conditions to begin with (Harrison et al., 2000; Philip et al., 2004; Webb, 1985; Webb and Levy, 1982), so much so that some studies have indicated that rested older adults show levels of cognitive impairment similar to that of sleep-deprived younger adults (Chee and Choo, 2004; Harrison et al., 2000). Thus, older adults may already be impaired at baseline in numerous cognitive domains and, as a result, may not have much further to fall in terms of performance impairment when placed under conditions of sleep deprivation, while younger adults do. This floor effect in the elderly may complicate any interpretation of differences in sleep need between younger relative to older adults.
Fourth, older adults do, however, demonstrate significantly worse performance on other sleep-dependent cognitive functions, such as learning and long-term memory consolidation. This is not simply because they are older. Rather, sleep-dependent learning and memory impairments in older adults remain when statistically accounted for age, instead being explained by the degree of impairment in NREM sleep oscillations. Thus, the deterioration in sleep quantity and quality in older relative to younger adults has cognitive consequences; consequences that would suggest that older adults are not capable of generating the quality of sleep that they still appear to need to optimally maintain specific cognitive functions. Together, these findings argue against the suggestion that sleep need is reduced in older adults. That is, sleep need remains, but sleep regulating and/or generating capacity declines. It is important to note that this does not mean older adults need more sleep time, per se, but rather that sleep quality, and the associated capacity to express consolidated NREM sleep rich in sleep oscillations, may be deficient.
In conclusion, some evidence indicates that sleep need is reduced in older adults, including that older adults innately get less sleep, show less intense rebound sleep following deprivation, report less subjective sleepiness under sleep restriction conditions, and suffer a smaller increase in lapses of attention after sleep deprivation and restriction. However, alternative explanations of these findings leave open the possibility that sleep need remains high while sleep-generating capacity is impaired, for which there is now significant supportive empirical data. While there remains no complete consensus surrounding this debate, the current evidence appears to most parsimoniously support the hypothesis that older adults do not have a reduced sleep need, but rather, an impaired ability to register and/or generate that unmet sleep need.
Acknowledgments
This work was supported by awards R01–AG031164, R01–AG054019, and R01-MH093537 to M.P.W. from the NIH.
References
- Adam M, Rétey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–57. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Sämann PG, Czisch M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011;31:10331–10339. doi: 10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D, Diekelmann S, Olsen C, Born J, Mölle M. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37:1142–1151. doi: 10.1111/ejn.12118. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus W, Kempe S, Hummel FC. The effect of sleep on motor learning in the aging and stroke population - a systematic review. Restor Neurol Neurosci. 2015;34:153–164. doi: 10.3233/RNN-150521. [DOI] [PubMed] [Google Scholar]
- Baran B, Mantua J, Spencer RM. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci. 2016;28:792–802. doi: 10.1162/jocn_a_00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E Osteoporotic Fractures in Men Study Group. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93:2602–2609. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62:612–632. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012;59:2733–2742. doi: 10.1016/j.neuroimage.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994;4:138–150. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Potential pathways of abnormal tau and α-synuclein dissemination in sporadic Alzheimer’s and Parkinson’s diseases. Cold Spring Harb Perspect Biol. 2016;8:8. doi: 10.1101/cshperspect.a023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Brayet P, Petit D, Frauscher B, Gagnon JF, Gosselin N, Gagnon K, Rouleau I, Montplaisir J. Quantitative EEG of rapid-eye-movement sleep: A marker of amnestic mild cognitive impairment. Clin EEG Neurosci. 2015;47:134–141. doi: 10.1177/1550059415603050. [DOI] [PubMed] [Google Scholar]
- Brownell SE, Conti B. Age- and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neurosci Lett. 2010;472:29–32. doi: 10.1016/j.neulet.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Light LL. Memory and aging: the role of retrieval processes. Psychol Bull. 1981;90:513–514. [PubMed] [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, Filipini D. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33:758–766. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- Cavuoto MG, Ong B, Pike KE, Nicholas CL, Bei B, Kinsella GJ. Objective but not subjective sleep predicts memory in community-dwelling older adults. J Sleep Res. 2016;25:475–485. doi: 10.1111/jsr.12391. [DOI] [PubMed] [Google Scholar]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee CA, Roozendaal B, Swaab DF, Goudsmit E, Mirmiran M. Vasoactive intestinal polypeptide neuron changes in the senile rat suprachiasmatic nucleus. Neurobiol Aging. 1988;9:307–312. doi: 10.1016/s0197-4580(88)80070-8. [DOI] [PubMed] [Google Scholar]
- Cheng JT, Liu IM, Juang SW, Jou SB. Decrease of adenosine A-1 receptor gene expression in cerebral cortex of aged rats. Neurosci Lett. 2000;283:227–229. doi: 10.1016/s0304-3940(00)00961-7. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Lee TH, Greening S, Ponzio A, Margalit E, Mather M. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging. 2016;37:117–126. doi: 10.1016/j.neurobiolaging.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte F, Arzilli C, Errico BM, Giganti F, Iovino D, Ficca G. Sleep measures expressing ‘functional uncertainty’ in elderlies’ sleep. Gerontology. 2014;60:448–457. doi: 10.1159/000358083. [DOI] [PubMed] [Google Scholar]
- Craik FI. Age differences in human memory. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. New York: Van Nostrand Reinhold; 1977. pp. 384–420. [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- de Sarro GB, Bagetta G, Ascioti C, Libri V, Nisticó G. Micro-infusion of clonidine and yohimbine into locus coeruleus alters EEG power spectrum: effects of aging and reversal by phosphatidylserine. Br J Pharmacol. 1988;95:1278–1286. doi: 10.1111/j.1476-5381.1988.tb11765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza L, Smaili SS, Ureshino RP, Sinigaglia-Coimbra R, Andersen ML, Lopes GS, Tufik S. Effect of chronic sleep restriction and aging on calcium signaling and apoptosis in the hippocampus of young and aged animals. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:23–30. doi: 10.1016/j.pnpbp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Delacourte A, Sergeant N, Wattez A, Maurage CA, Lebert F, Pasquier F, David JP. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp Gerontol. 2002;37:1291–1296. doi: 10.1016/s0531-5565(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol Aging. 1989;10:677–682. doi: 10.1016/0197-4580(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33:211–223. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2007;28:1286–1295. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Dubé J, Lafortune M, Bedetti C, Bouchard M, Gagnon JF, Doyon J, Evans AC, Lina JM, Carrier J. Cortical thinning explains changes in sleep slow waves during adulthood. J Neurosci. 2015;35:7795–7807. doi: 10.1523/JNEUROSCI.3956-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumay N. Sleep not just protects memories against forgetting, it also makes them more accessible. Cortex. 2016;74:289–296. doi: 10.1016/j.cortex.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimulat. 2013;6:938–945. doi: 10.1016/j.brs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently? J Sleep Res. 1997;6:211–215. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Pagonopoulou O, Angelatou F. Age-dependent changes in adenosine A1 receptor and uptake site binding in the mouse brain: an autoradiographic study. J Neurosci Res. 2000;60:257–265. doi: 10.1002/(SICI)1097-4547(20000415)60:2<257::AID-JNR15>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Carlson VR. Sleep variables as a function of age in man. Arch Gen Psychiatry. 1968;18:239–250. [Google Scholar]
- Feld GB, Wilhelm I, Ma Y, Groch S, Binkofski F, Mölle M, Born J. Slow wave sleep induced by GABA agonist tiagabine fails to benefit memory consolidation. Sleep. 2013;36:1317–1326. doi: 10.5665/sleep.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernández G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, Jbabdi S, Benali H, Karni A, Maquet P, et al. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp. 2013;35:3625–3645. doi: 10.1002/hbm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S, Vien C, Karni A, Benali H, Carrier J, Doyon J. Sleep spindles: A physiological marker of age-related changes in grey matter in brain regions supporting motor skill memory consolidation. Neurobiol Aging. 2016;49:154–164. doi: 10.1016/j.neurobiolaging.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Petit D, Latreille V, Montplaisir J. Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharm Des. 2008;14:3430–3445. doi: 10.2174/138161208786549353. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Ligtenberg L, Kruijver FP, Swaab DF. Galanin neurons in the intermediate nucleus (InM) of the human hypothalamus in relation to sex, age, and gender identity. J Comp Neurol. 2011;519:3061–3084. doi: 10.1002/cne.22666. [DOI] [PubMed] [Google Scholar]
- Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, Mangiaruga A, D’Atri A, Tempesta D, Ferrara M, et al. Parietal fast sleep spindle density decrease in Alzheimer’s disease and amnesic mild cognitive impairment. Neural Plast. 2016;2016:8376108. doi: 10.1155/2016/8376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Porter JM, Schweitzer PK, Eisenstein RD, Ahmed HA, Walsh JK. The effect of two benzodiazepine receptor agonist hypnotics on sleep-dependent memory consolidation. J Clin Sleep Med. 2014;10:27–34. doi: 10.5664/jcsm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults–a model for healthy aging? Sleep. 2000;23:1067–1073. [PubMed] [Google Scholar]
- Hassainia F, Petit D, Nielsen T, Gauthier S, Montplaisir J. Quantitative EEG and statistical mapping of wakefulness and REM sleep in the evaluation of mild to moderate Alzheimer’s disease. Eur Neurol. 1997;37:219–224. doi: 10.1159/000117446. [DOI] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE ε4 genotype. Curr Alzheimer Res. 2012;9:290–297. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- Holth JK, Patel TK, Holtzman DM. Sleep in Alzheimer’s disease–beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2016;2:4–14. doi: 10.1016/j.nbscr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt NJ, Rodriguez ML, Waters KA, Machaalani R. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol Aging. 2015;36:292–300. doi: 10.1016/j.neurobiolaging.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Ingiosi AM, Opp MR, Krueger JM. Sleep and immune function: glial contributions and consequences of aging. Curr Opin Neurobiol. 2013;23:806–811. doi: 10.1016/j.conb.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, van Heerikhuize JJ, Ravid R, Swaab DF. Estrogen receptors and metabolic activity in the human tuberomamillary nucleus: changes in relation to sex, aging and Alzheimer’s disease. Brain Res. 2003;988:84–96. doi: 10.1016/s0006-8993(03)03347-x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto A, Ito S, Okada H, Nishizawa S, Minoshima S, Ouchi Y. Age-related sex-specific changes in brain metabolism and morphology. J Nucl Med. 2016;57:221–225. doi: 10.2967/jnumed.115.166439. [DOI] [PubMed] [Google Scholar]
- Kales A, Wilson T, Kales JD, Jacobson A, Paulson MJ, Kollar E, Walter RD. Measurements of all-night sleep in normal elderly persons: effects of aging. J Am Geriatr Soc. 1967;15:405–414. doi: 10.1111/j.1532-5415.1967.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanuki K, Iseki E, Kondo D, Fujishiro H, Minegishi M, Sato K, Katsuse O, Hino H, Kosaka K, Arai H. Neuropathological investigation of hypocretin expression in brains of dementia with Lewy bodies. Neurosci Lett. 2014;569:68–73. doi: 10.1016/j.neulet.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Kessler BA, Stanley EM, Frederick-Duus D, Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience. 2011;178:82–88. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Saucier P, Albouy G, Fogel SM, Rumpf JJ, Klann J, Buccino G, Binkofski F, Classen J, Karni A, Doyon J. Cerebral activation during initial motor learning forecasts subsequent sleep-facilitated memory consolidation in older adults. Cereb Cortex. 2016:bhv347. doi: 10.1093/cercor/bhv347. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep–implications for insomnia. Curr Biol. 2008;18:1118–1123. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S, Kovacs GG, Voigtlander T, Wanschitz J, Flicker H, Hainfellner JA, Guentchev M, Budka H. Serotonergic nuclei of the raphe are not affected in human ageing. Neuroreport. 2001;12:669–671. doi: 10.1097/00001756-200103260-00010. [DOI] [PubMed] [Google Scholar]
- Ladenbauer J, Külzow N, Passmann S, Antonenko D, Grittner U, Tamm S, Flöel A. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. Neuroimage. 2016;142:311–323. doi: 10.1016/j.neuroimage.2016.06.057. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Borbély AA. Age-dependent changes in sleep EEG topography. Clin Neurophysiol. 2001;112:369–377. doi: 10.1016/s1388-2457(00)00542-3. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–212. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Latta F, Leproult R, Tasali E, Hofmann E, L’Hermite-Balériaux M, Copinschi G, Van Cauter E. Sex differences in nocturnal growth hormone and prolactin secretion in healthy older adults: relationships with sleep EEG variables. Sleep. 2005;28:1519–1524. doi: 10.1093/sleep/28.12.1519. [DOI] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013a;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013b;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain. 2014;137:2847–2861. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, Bennett DA. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep. 2016;39:227–235. doi: 10.5665/sleep.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Sim SK, Chee MW. Sleep reduces false memory in healthy older adults. Sleep. 2014;37:665–671. 671A. doi: 10.5665/sleep.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. doi: 10.1016/j.sleep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Lord C, Sekerovic Z, Carrier J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol (Paris) 2014;62:302–310. doi: 10.1016/j.patbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Peyron C, Fort P. Not a single but multiple populations of GABAergic neurons control sleep. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.03.002. Published online March 12, 2016 http://dx.doi.org/10.1016/j.smrv.2016.03.002. [DOI] [PubMed]
- Mackiewicz M, Nikonova EV, Zimmermann JE, Romer MA, Cater J, Galante RJ, Pack AI. Age-related changes in adenosine metabolic enzymes in sleep/wake regulatory areas of the brain. Neurobiol Aging. 2006;27:351–360. doi: 10.1016/j.neurobiolaging.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21:R183–R184. doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex. 2014;24:3301–3309. doi: 10.1093/cercor/bht188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016a;39:552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Zhu A, Lindquist JR, Villeneuve S, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust W, Walker MP. Degeneration of white matter pathways in older adults explains the failure of sleep spindles to promote motor memory consolidation. Sleep. 2016b;39:A22. doi: 10.1523/JNEUROSCI.3033-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J. Topography of age-related changes in sleep spindles. Neurobiol Aging. 2013;34:468–476. doi: 10.1016/j.neurobiolaging.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Mary A, Schreiner S, Peigneux P. Accelerated long-term forgetting in aging and intra-sleep awakenings. Front Psychol. 2013;4:750. doi: 10.3389/fpsyg.2013.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkes-Caspi N, Yamin HG, Kellner V, Spires-Jones TL, Cohen D, Stern EA. Pathological tau disrupts ongoing network activity. Neuron. 2015;85:959–966. doi: 10.1016/j.neuron.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Meyer PT, Elmenhorst D, Boy C, Winz O, Matusch A, Zilles K, Bauer A. Effect of aging on cerebral A1 adenosine receptors: A [18F] CPFPX PET study in humans. Neurobiol Aging. 2007;28:1914–1924. doi: 10.1016/j.neurobiolaging.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- dos Moraes WS, Poyares DR, Guilleminault C, Ramos LR, Bertolucci PH, Tufik S. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep. 2006;29:199–205. doi: 10.1093/sleep/29.2.199. [DOI] [PubMed] [Google Scholar]
- Münch M, Knoblauch V, Blatter K, Schröder C, Schnitzler C, Kräuchi K, Wirz-Justice A, Cajochen C. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–1410. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- Münch M, Knoblauch V, Blatter K, Schröder C, Schnitzler C, Kräuchi K, Wirz-Justice A, Cajochen C. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26:1307–1319. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–370. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Novak M, Shapiro CM. Drug-induced sleep disturbances. Focus on nonpsychotropic medications. Drug Saf. 1997;16:133–149. doi: 10.2165/00002018-199716020-00005. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Pirraglia E, Agüera-Ortiz LF, During EH, Sacks H, Ayappa I, Walsleben J, Mooney A, Hussain A, Glodzik L, et al. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonopoulou O, Angelatou F. Reduction of A1 adenosine receptors in cortex, hippocampus and cerebellum in ageing mouse brain. Neuro-report. 1992;3:735–737. doi: 10.1097/00001756-199209000-00003. [DOI] [PubMed] [Google Scholar]
- Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub A, Paller KA, Zee PC. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Human Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00109. http://dx.doi.org/10.3389/fnhum.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Baum MJ, Tobet SA. Sex difference and steroidal stimulation of galanin immunoreactivity in the ferret’s dorsal preoptic area/anterior hypothalamus. J Comp Neurol. 1997;389:277–288. [PubMed] [Google Scholar]
- Passmann S, Külzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, Flöel A. Boosting slow oscillatory activity using tDCS during early nocturnal slow wave sleep does not improve memory consolidation in healthy older adults. Brain Stimulat. 2016;9:730–739. doi: 10.1016/j.brs.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res. 2008;17:23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N, Charles A, Bioulac B. Age, performance and sleep deprivation. J Sleep Res. 2004;13:105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Piantoni G, Poil SS, Linkenkaer-Hansen K, Verweij IM, Ramautar JR, Van Someren EJ, Van Der Werf YD. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci. 2013;33:227–233. doi: 10.1523/JNEUROSCI.2030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol. 2004;499:147–154. doi: 10.1016/j.ejphar.2004.07.106. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Denise P, Desgranges B, Eustache F, Gruber G, Anderer P. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. 2008;19:1159–1162. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System of Sleep Stages in Human Subjects. Los Angeles: UCLA Brain Information Services; 1968. [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Rex CS, Kramár EA, Colgin LL, Lin B, Gall CM, Lynch G. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Kupfer DJ, Hoch CC, Stack JA, Houck PR, Berman SR. Sleep deprivation in healthy elderly men and women: effects on mood and on sleep during recovery. Sleep. 1986;9:492–501. doi: 10.1093/sleep/9.4.492. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlem GL, Badran BW, Halford JJ, Williams NR, Korte JE, Leslie K, Strachan M, Breedlove JL, Runion J, Bachman DL, et al. Oscillating square wave transcranial direct current stimulation (tDCS) delivered during slow wave sleep does not improve declarative memory more than sham: A randomized sham controlled crossover study. Brain Stimulat. 2015;8:528–534. doi: 10.1016/j.brs.2015.01.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletin JM, van der Helm E, Walker MP. Structural brain correlates of human sleep oscillations. Neuroimage. 2013;83:658–668. doi: 10.1016/j.neuroimage.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28:105–114. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10:97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonni A, Spencer RM. Sleep protects memories from interference in older adults. Neurobiol Aging. 2015;36:2272–2281. doi: 10.1016/j.neurobiolaging.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, Elger CE, Axmacher N, Fell J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18:1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus. 2015;4:25. doi: 10.1186/s40064-014-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernández G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Sharma R, Sahota P. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49:299–310. doi: 10.1016/j.alcohol.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. J Am Geriatr Soc. 2011;59:603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJ. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Varga AW, Ducca EL, Kishi A, Fischer E, Parekh A, Koushyk V, Yau PL, Gumb T, Leibert DP, Wohlleber ME, et al. Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation. Neurobiol Aging. 2016;42:142–149. doi: 10.1016/j.neurobiolaging.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Godschalk M, Mulligan T. Older men manifest multifold synchrony disruption of reproductive neurohormone outflow. J Clin Endocrinol Metab. 2000;85:1477–1486. doi: 10.1210/jcem.85.4.6546. [DOI] [PubMed] [Google Scholar]
- Vienne J, Lecciso G, Constantinescu I, Schwartz S, Franken P, Heinzer R, Tafti M. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep. 2012;35:1071–1083. doi: 10.5665/sleep.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienne J, Spann R, Guo F, Rosbash M. Age-related reduction of recovery sleep and arousal threshold in Drosophila. Sleep. 2016;39:1613–1624. doi: 10.5665/sleep.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV. Recent advances in understanding sleep and sleep disturbances in older adults: growing older does not mean sleeping poorly. Curr Dir Psychol Sci. 2009;18:316–320. [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, Buchman AS, Bennett DA, Hu K, Saper CB. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78:317–322. doi: 10.1002/ana.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb WB. A further analysis of age and sleep deprivation effects. Psychophysiology. 1985;22:156–161. doi: 10.1111/j.1469-8986.1985.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Webb WB, Campbell SS. The first night effect revisited with age as a variable. Waking Sleeping. 1979;3:319–324. [PubMed] [Google Scholar]
- Webb WB, Levy CM. Age, sleep deprivation, and performance. Psychophysiology. 1982;19:272–276. doi: 10.1111/j.1469-8986.1982.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Florczak SM, Weintraub S, Mesulam MM, Marshall L, Zee PC, Paller KA. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging. 2015;36:2577–2586. doi: 10.1016/j.neurobiolaging.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging. 2012;33:991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepelin H, McDonald CS, Zammit GK. Effects of age on auditory awakening thresholds. J Gerontol. 1984;39:294–300. doi: 10.1093/geronj/39.3.294. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]