Abstract
The de novo generation of hematopoietic cells occurs during midgestation when a population of endothelial cells called hemogenic endothelium transitions into hematopoietic progenitors and stem cells. In mammalian embryos, the newly formed hematopoietic cells form clusters in the lumens of the major arteries in the embryo proper and in the vascular plexus of the yolk sac. Small clusters of hematopoietic cells that are independent of the vasculature (referred to here as extravascular islands) were shown to form in the mesentery during vascular remodeling of the vitelline artery. Using three-dimensional imaging of whole mouse embryos we demonstrate that extravascular budding of hematopoietic clusters is a more widespread phenomenon that occurs from the vitelline and the umbilical arteries both proximal to the embryo proper and distal in the extraembryonic yolk sac and placenta. Furthermore, we show that there are several mechanisms by which hematopoietic clusters leave the arteries, including vascular remodeling and extrusion. Lastly, we provide static images suggesting that extravascular islands contribute to the formation of new blood vessels. Thus, extravascular islands may represent a novel mechanism of vasculogenesis whereby established vessels contribute endothelial and hematopoietic cells to developing vascular beds.
Keywords: Hematopoiesis, Mesenteric blood islands, Yolk sac, Mouse embryo, Runx1, Ly6a, Vasculogenesis
Graphical abstract
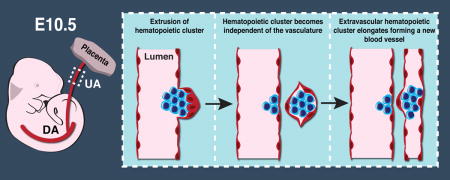
INTRODUCTION
During embryogenesis, definitive hematopoietic progenitors and stem cells (HSPCs) are derived de novo from a transient population of endothelial cells called hemogenic endothelium (HE). HE is located in the large diameter arteries including the vitelline artery, umbilical artery and dorsal aorta, and is also found in the heart, yolk sac and the head (Li et al., 2012, Nakano et al., 2013, Frame et al., 2015). HE cells are the precursors to hematopoietic cells, and as such transition into the full repertoire of definitive hematopoietic cells including erythro-myeloid progenitors, lymphoid progenitors, and hematopoietic stem cells (HSCs) during a process termed the endothelial to hematopoietic transition (EHT). Live-imaging studies demonstrated that during the EHT, HE cells bend away from the endothelial layer eventually releasing contact with neighboring endothelial cells and acquiring hematopoietic morphology in a cell-division independent process (Kissa and Herbomel, 2010, Lam et al., 2010, Boisset et al., 2010). The EHT is dependent upon expression of the transcription factor Runx1 (Lancrin et al., 2009, Chen et al., 2009, Boisset et al., 2010, North et al., 1999, Kissa and Herbomel, 2010, Yokomizo et al., 2001). Runx1 expression initiates a hematopoietic transcriptional program and inhibits an endothelial program in HE allowing the EHT to progress (Lancrin et al., 2012, Lichtinger et al., 2012, Hoogenkamp et al., 2009). Runx1 is widely considered the master regulator of hematopoiesis and is the most reliable marker of HE identity.
After the EHT, the newly formed hematopoietic cells take different paths in different species. In zebrafish, hematopoietic cells bud away from the lumen of the artery into the sub-aortic space, and enter the circulation via intravasation through the axial vein (Murayama et al., 2006, Kissa et al., 2008). In chick embryos the EHT occurs both into the aortic lumen and also out of the aorta into the subaortic mesenchyme (Jaffredo et al., 2000, Jaffredo et al., 1998). In mammalian embryos the newly formed hematopoietic cells bud into the lumen of the artery where they remain briefly attached to the endothelium forming clusters of hematopoietic cells (Yokomizo and Dzierzak, 2010, Garcia-Porrero et al., 1995). Hundreds of hematopoietic clusters line the major arteries of the midgestation mouse embryo and dozens can be found within the vascular plexus of the yolk sac (Yokomizo and Dzierzak, 2010, Frame et al., 2015). Hematopoietic clusters are heterogeneous, as individual cells within a single cluster can differ in gene expression and function (Yokomizo and Dzierzak, 2010). For example, the Ly6a-GFP transgene (Ly6a encodes Sca-1) was shown to mark a subset of hematopoietic cluster cells that are enriched for HSCs and lymphoid progenitors (Li et al., 2014, de Bruijn et al., 2002).
Clusters of hematopoietic cells wrapped in endothelial cells were observed in the mesentery of chick embryos one hundred years ago (Miller, 1913). These structures became know as mesenteric blood islands owing to their separation from the cardiovascular system. Mesenteric blood islands were observed in the vicinity of the aortic arches and along the aorta as far as the superior mesenteric artery (the structure that supersedes the vitelline artery) in 100 to 110 hour chick embryos (Miller, 1913). In the 1990’s mesenteric blood islands were also observed in mouse embryos between embryonic day (E) 9.5 and E11.5 (Garcia-Porrero et al., 1998, Garcia-Porrero et al., 1995). At the time, the origin of mesenteric blood islands was unknown, but immunohistochemistry of E11 mouse sections illustrated that mesenteric blood islands expressed the endothelial/hematopoietic markers CD31, CD34 and vWf, similar to intra-arterial clusters (Garcia-Porrero et al., 1998). Further histological analysis of sectioned mouse embryos revealed three types of mesenteric blood islands: type I consisting of tightly arranged, highly basophilic, electron-dense cells with no clear endothelial component, type II characterized by undifferentiated hemocytoblasts that are tightly wrapped by endothelial cells, and type III consisting of erythroblasts and hemocytoblasts that are loosely arranged and circumscribed by a single layer of endothelial cells (Garcia-Porrero et al., 1995). The vitelline artery was later identified as the source of mesenteric blood islands (Zovein et al., 2010). During midgestation the vitelline artery undergoes extensive remodeling, and during this process clusters of hematopoietic cells leave the artery and migrate through the mesentery as aggregates of endothelial and hematopoietic cells (Zovein et al., 2010). However, the extravascular emergence of hematopoietic clusters was called into question when studies using three-dimensional imaging of whole mouse embryos only found hematopoietic clusters within arterial lumens (Yokomizo et al., 2011)
Using confocal microscopy, we examined hematopoiesis in midgestation mouse embryos by mapping the expression of hematopoietic and endothelial-specific proteins. We provide additional evidence that extravascular clusters of endothelial and/or hematopoietic cells (referred to here as extravascular islands) emerge from the vitelline artery during vascular remodeling. We also demonstrate that the umbilical artery is another source of extravascular islands. Furthermore, we observed extravascular islands in both the embryo proper as well as in the extraembryonic yolk sac and placenta. We propose two mechanisms by which extravascular islands leave the umbilical and vitelline arteries including extrusion and vascular remodeling. We also demonstrate that extravascular islands elongate and contribute endothelial and hematopoietic cells to newly forming blood vessels. Finally, we show that neither the hematopoietic nor endothelial component of extravascular islands express the transcription factor Prox1, and are therefore unlikely to contribute to the lymphatic vascular system.
MATERIALS AND METHODS
Mice
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All of the animals were handled according to approved institutional animal care and use committee (IACUC) protocol #803789 of the University of Pennsylvania. Tg(Ly6a-GFP) mice [B6.Cg-Tg(Ly6a-GFP)G5Dzk/J] were described previously (Ma et al., 2002). Tg(Ly6a-GFP) and wild type conceptuses were generated by crossing B6C3F1 females with heterozygous Tg(Ly6a-GFP) males. B6C3F1 females were ordered from Charles River and Jackson Laboratory.
Confocal Microscopy
Embryos were prepared as previously described (Yokomizo et al., 2012). A Zeiss LSM 710 AxioObserver inverted confocal microscope with ZEN 2011 software was used to acquire Z-projections and single optical projections. Images were processed using Fiji software (Schindelin et al., 2012). 3-dimensional reconstructions were produced using Volocity software (PerkinElmer). The following primary antibodies were used; rat anti-mouse CD31 (Mec 13.3, BD Pharmingen, San Diego, CA), rat anti-mouse CD117 (2B8, eBioscience, San Diego, CA), chicken anti-GFP (polyclonal, Thermo Fisher Scientific, Waltham, MA), rabbit anti-mouse Prox1 (AngioBio, San Diego, CA) and rabbit anti-human/mouse Runx (EPR3099, Abcam, Cambridge, MA). Secondary antibodies used were goat anti-rat Alexa Fluor 647 (Invitrogen, Carlsbad, CA), goat-anti rat Alexa Fluor 555 (Abcam), donkey anti-rat Alexa Fluor 555 (Abcam), goat anti-chicken Alexa Fluor 647 (Jackson ImmunoResearch, West Grove, PA) and goat anti-rabbit Alexa Fluor 488 (Invitrogen).
RESULTS AND DISCUSSION
Extravascular islands are localized near the umbilical and vitelline arteries both proximal to the embryo proper and distal in the placenta and yolk sac
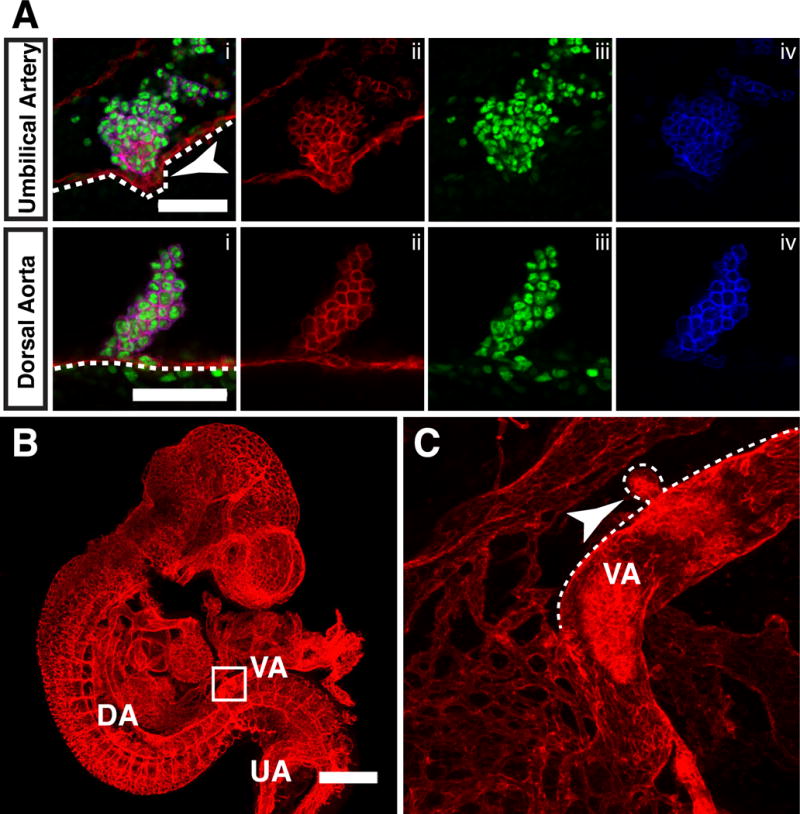
To localize extravascular islands in midgestation (E10.5–E11.5) embryos we immunostained for CD31 to mark the entire vasculature, Runx1 to mark hemogenic endothelial (HE) and hematopoietic cells, and Kit to identify hematopoietic cluster cells (Fig. 1A). We also analyzed Tg(Ly6a-GFP) embryos to determine if endothelial and hematopoietic cells in extravascular islands express Ly6a-GFP, which would suggest lymphoid/HSC potential (Chen et al., 2011, Li et al., 2014) (Fig. 1A). In our analysis of whole embryos, we observed all three types of the previously described extravascular islands (referred to in Garcia-Porrero et al. as mesenteric blood islands) (Garcia-Porrero et al., 1995). In addition, we identified a fourth type (Type IV) that consists of a sphere of endothelial cells without associated hematopoietic cells (Fig. 1B–C). By analyzing whole, as opposed to sectioned embryos, we were able to pinpoint the location of the extravascular islands to the region between the vitelline and umbilical arteries in the embryo proper (Fig. 2A–B, arrowheads).
Figure 1. Four types of extravascular islands.
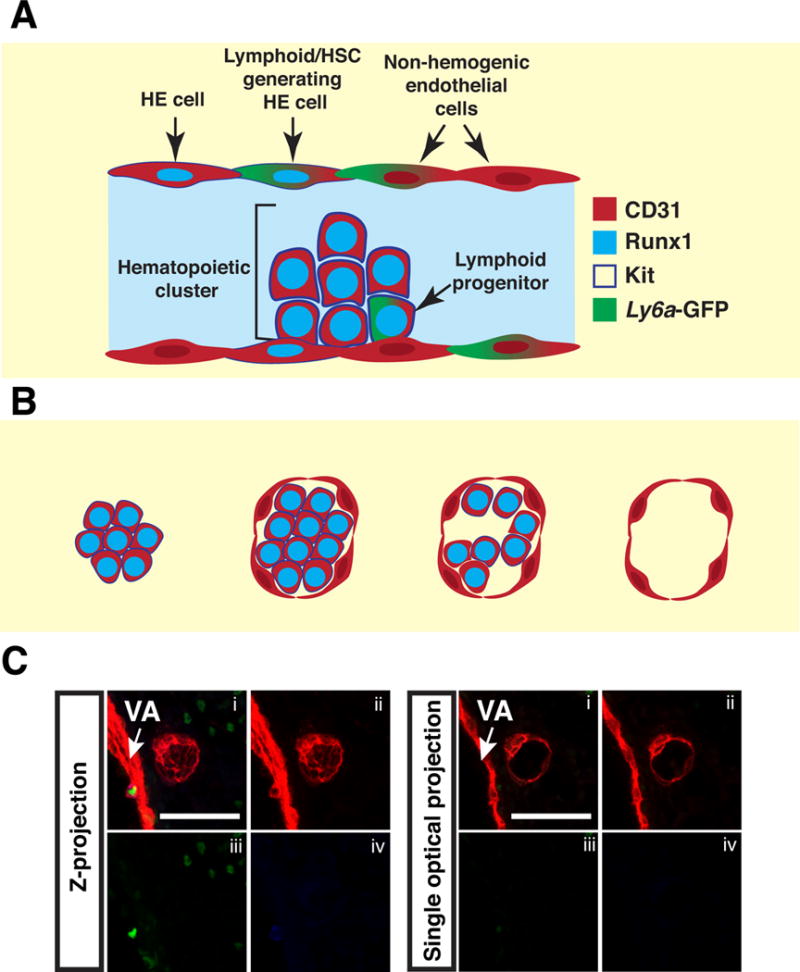
(A) Schematic illustrating the cell types identified by the combination of markers used in the analysis. (B) The four types of extravascular islands. Type I consists of tightly arranged hematopoietic cells with no endothelial component. Type II islands contain tightly arranged hematopoietic cells wrapped in a single layer of endothelial cells. Type III has loosely arranged hematopoietic cells wrapped in an endothelial layer. Type IV consists of a sphere of endothelial cells that are not associated with hematopoietic cells. (C) Z-projection of a type IV extravascular island that has been immunostained for CD31 (i,ii), Runx1 (i,iii) and Kit (i,iv). Panel on the right is a single optical projection illustrating the lack of Runx1+ Kit+ hematopoietic cells within a type IV extravascular island. Z-interval = 2μm and scale bar = 50μm.
Figure 2. Extravascular islands localize between the remodeling vitelline artery and umbilical artery at E10.5.
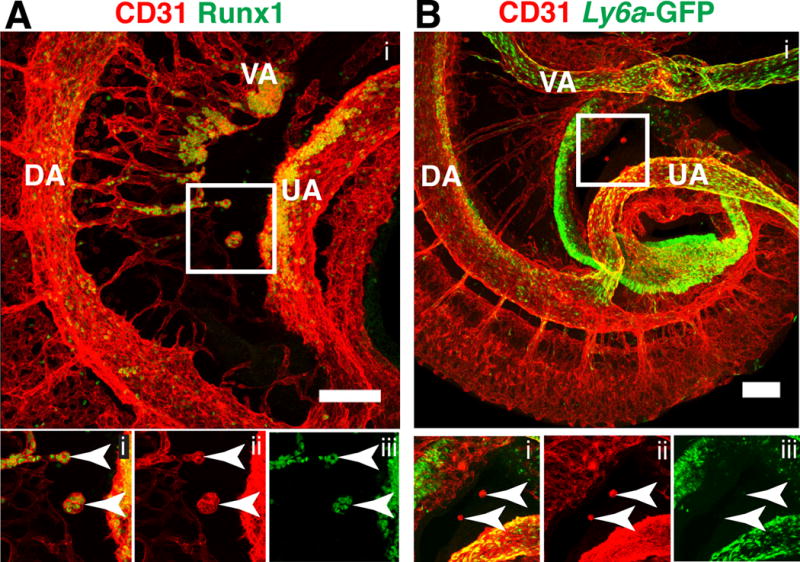
(A) Z-projection of an E10.5 embryo immunostained for CD31 (i, ii) and Runx1 (i, iii). The embryo is oriented with its cranial end on the top and dorsal edge on the left. Lower panels are split channel views of the boxed region. Arrowheads in bottom panels indicate extravascular islands. Z-interval = 1.5μm, scale bar = 100μm. (B) Z-projection of an E10.5 Tg(Ly6a-GFP) embryo immunostained for CD31 (i, ii) and GFP (i, iii). Lower panels are split channel views of the boxed region. Arrowheads indicate extravascular islands. Z-interval = 5μm, scale bar = 100μm. DA = dorsal aorta, VA = vitelline artery, UA = umbilical artery.
Confocal analysis of the umbilical artery of a 40 somite pair (sp) embryo reveals a large type II extravascular island consisting of 25–35 tightly arranged Runx1+ CD31+ Ly6a-GFP− hematopoietic cells (Fig. 3A, arrowhead) wrapped in a single layer of endothelial cells (Fig. 3B, arrows). The endothelial cells do not express Runx1 or Ly6a-GFP and are therefore unlikely to be hemogenic (Fig. 3B). Individual Z-slices through the extravascular island demonstrate that it is circumscribed by endothelium but independent of the vascular network (Fig. 3B and Movie 1). Currently, it is not possible to separate extravascular islands from intravascular clusters, so gauging the function and potential of these cells is difficult. However, the lack of Ly6a-GFP expression suggests that they do not contain lymphoid progenitors or HSCs as these populations are enriched in the Ly6a-GFP+ fraction of hematopoietic cluster cells (de Bruijn et al., 2002, Li et al., 2014).
Figure 3. Extravascular islands do not contain Runx1+ hemogenic endothelial cells.
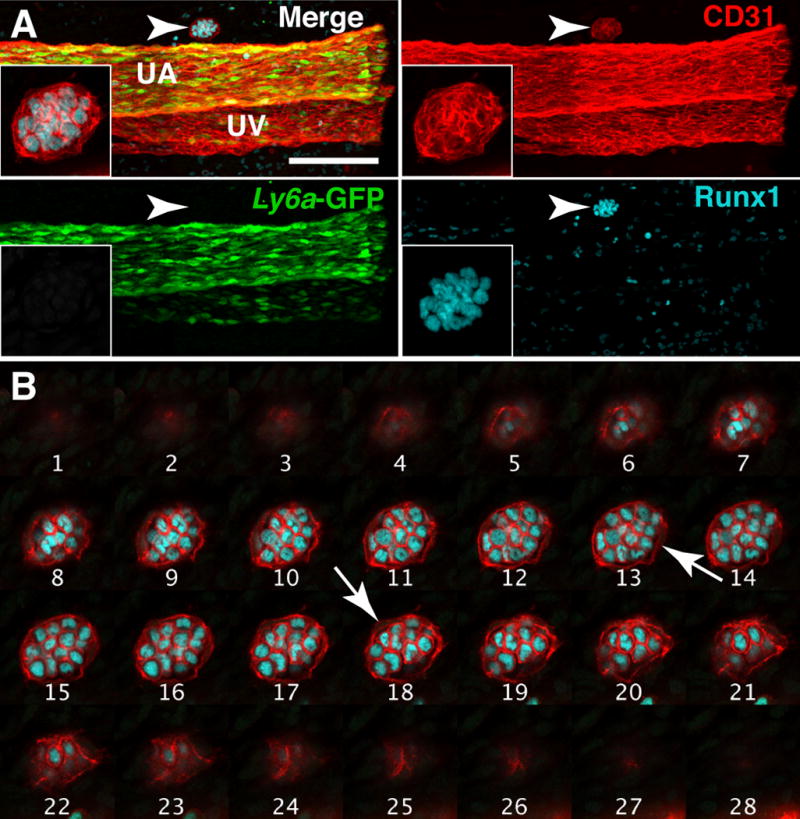
(A) Whole-mount Z-projection of the umbilical artery and vein of a 40sp Tg(Ly6a-GFP) embryo immunostained for CD31, Runx1 and GFP. Arrowhead indicates a Type II extravascular island on the cranial side of the umbilical artery. The extravascular island is located in between the embryo proper (which would be located to the left) and the chorion; inset is a magnified view of the extravascular island. Z-interval = 2μm, scale bar = 100μm; Z-interval of inset image = 1.17μm. UA = umbilical artery and UV = umbilical vein. (B) Montage of Z-slices through the extravascular island in (A). Each slice is 1.17μm apart. White arrows indicate endothelial cells.
We also found extravascular islands in extra-embryonic locations, at the distal ends of the umbilical and vitelline arteries near the chorion and yolk sac, respectively. Confocal analysis of a 36 sp yolk sac revealed a large type III extravascular island containing 75–100 Runx1+ cells located between the vitelline artery and the vascular plexus (Fig. 4A–B, arrowhead). Single optical projections through the extravascular island reveals that the hematopoietic cells are heterogenous; most Runx1+ cells express Kit but several near the periphery of the island lack Kit expression (Fig. 4C, arrows). Confocal analysis of the chorion from a 40sp Tg(Ly6a-GFP) embryo immunostained for CD31 and GFP demonstrates that type I extravascular islands also form near the distal end of the umbilical artery near the chorionic vascular plexus (Fig. 4D and E, arrowheads). Although we found no intravascular hematopoietic clusters in the chorion, we identified a type III extravascular island at the base of the umbilical artery where it connects to the placenta in an E11.5 (46sp) embryo (Fig. 4F, arrowhead). The type III extravascular island was heterogenous containing 32 CD31+ Runx1+ Kit+ cells and 29 CD31+ Runx1+ Kit− cells (Fig. 4F). Since extravascular islands are usually located near arteries that contain intravascular hematopoietic clusters, the type III extravascular island near the base of the umbilical artery may have migrated to the chorion from a more proximal location in the umbilical artery where clusters are abundant.
Figure 4. Extravascular islands in the extraembryonic yolk sac and placenta.
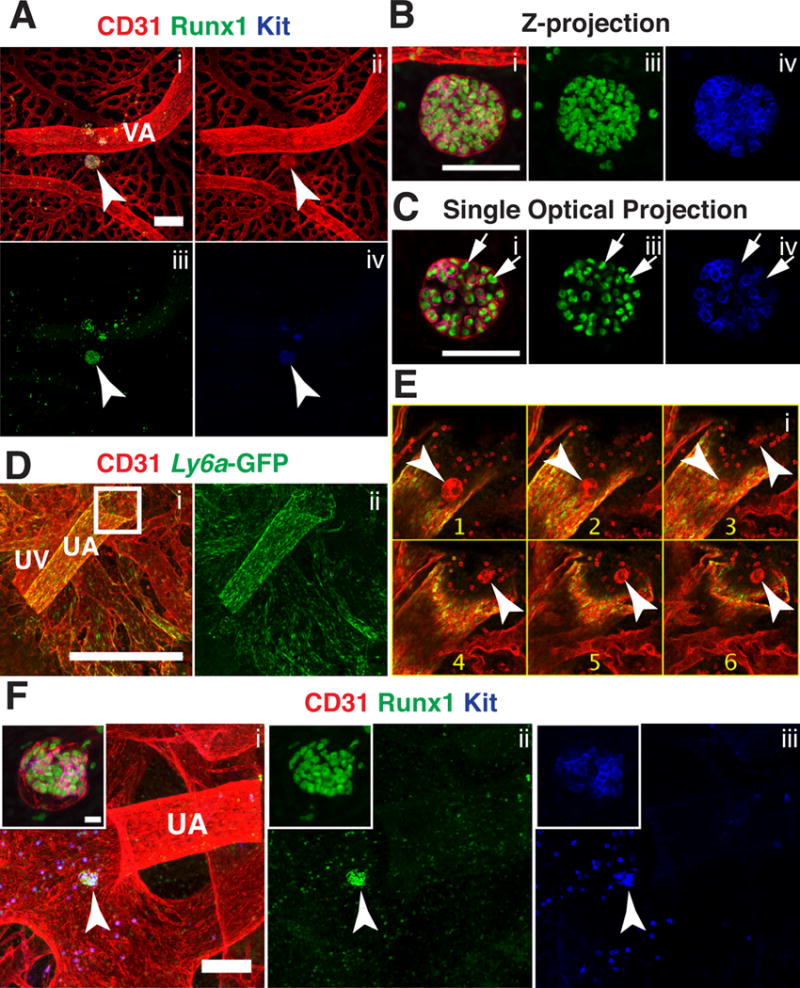
(A–C) Immunostaining for CD31 (i,ii) Runx1 (i,iii) and Kit (i,iv). (A) Confocal Z-projection of a 36sp yolk sac. Arrowhead indicates a large type III extravascular island near the vitelline artery (VA). Z-interval= 2μm and scale bar = 100μm. (B) Magnified view of the extravascular island in (A). Z-interval =2μm and scale bar = 50μm. (C) Single optical projection from Z-projection in (B), arrows indicate representative CD31+ Runx1+ Kit− cells within the island. (D–E) Immunostaining for CD31 (i) and Ly6a-GFP (i,ii). (D) Whole-mount confocal Z-projection of the umbilical artery, vein and chorionic vessels of a 40sp Tg(Ly6a-GFP) placenta. Z-interval = 5μm, scale bar = 500μm. (E) Montage of Z-slices from the boxed in region in (D). Z-slices are 5μm apart and arrowheads indicate type IV extravascular islands near the intersection of the umbilical artery and the chorion. (F) Z-projection of umbilical artery and chorionic vessels of a 46sp placenta immunostained for CD31 (i), Runx1 (i,ii) and Kit (i,iii). Arrowhead points to a type III extravascular island. Z-interval = 5μm and scale bar bar = 100μm. Inset is a magnified image of the type III extravascular island. Z-interval of the inset = 2μm and scale bar = 10μm.
Extravascular islands exit blood vessels via vascular remodeling and ballooning
Between E9.0 and E11.0 the vitelline artery (VA) undergoes an extensive remodeling process. At E9.0 the VA runs parallel to the paired dorsal aorta (DA), connecting to the DA at the caudal end of the embryo. By E11 the connection of the VA to the DA has moved in the rostral direction and is located at the level of the midgut. The rostral movement of the intersection between the VA and DA occurs via a tributary of smaller connecting vessels that are established then lost during the remodeling process. The tributary vessels are associated with a large concentration of CD31+ Runx1+ Kit+ hematopoietic clusters at E10.5 (Fig. 5A). As reported by Zovein et al. (Zovein et al., 2010) hematopoietic clusters in the small tributary vessels of the remodeling vitelline artery may become extravascular as the vessels surrounding them retract during the remodeling process. This idea is supported by the observation that CD31+ Runx1+ Kit+ hematopoietic clusters in these small vessels are often connected to the large diameter vessels via endothelial projections that do not appear to contain lumens, thus preventing the hematopoietic cluster cells from entering the circulation (Fig. 5B, arrows).
Figure 5. Hematopoietic clusters in the remodeling vitelline artery.
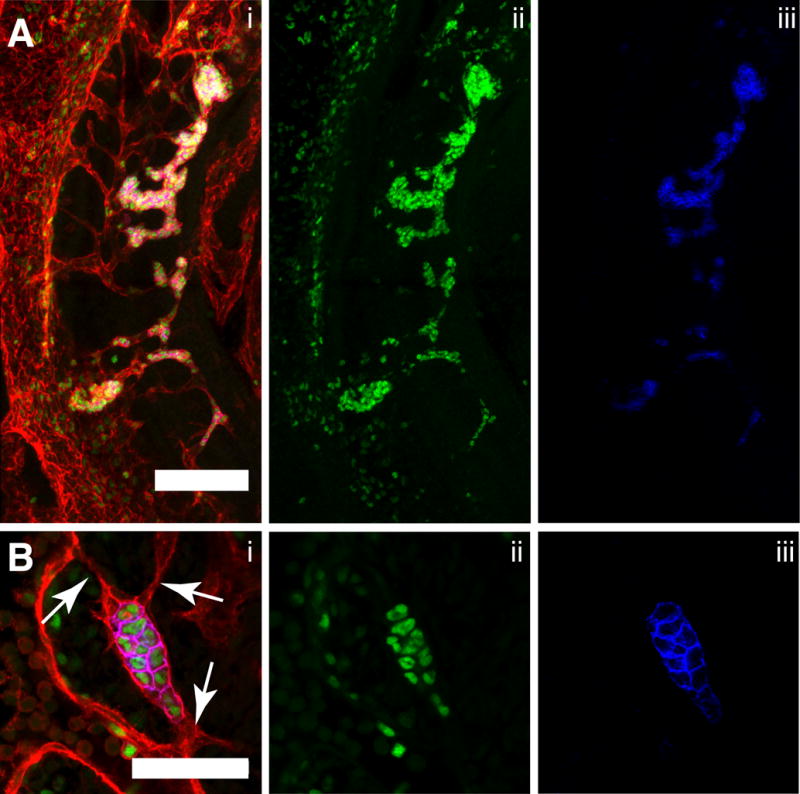
(A–B) Immunostaining for CD31 (i), Runx1 (i,ii) and Kit (i,iii). (A) Confocal Z-projection of the tributary vessels of the remodeling vitelline artery (VA) of a 34sp embryo. Z-interval = 5μm, scale bar = 100μm, DA = dorsal aorta. The embryo is oriented with its cranial end on the top and dorsal edge on the left. (B) Single optical projection of a hematopoietic cluster in the small tributary vessels of the vitelline artery of a 32sp embryo. Arrows indicate small endothelial projections that do not contain lumens. Scale bar = 50μm.
During lymphangiogenesis, lymphatic endothelial cells balloon out from the cardinal vein to create primitive lymphatic sacs (François et al., 2012). We observed a similar phenomenon in the umbilical and vitelline arteries as hematopoietic clusters extruded (“ballooned”) from the vessels, eventually pinching off to form extravascular islands. Single optical projections of a 34sp embryo revealed CD31+ Runx1− Kit− endothelial cells beneath a hematopoietic cluster egressing away from the lumen of the umbilical artery as the cluster is extruded (Fig. 6A, arrowhead). Ballooning also occurred in the vitelline artery, as illustrated in a confocal Z-projection of a 32 sp embryo (Fig. 6B,C). The balloon-shaped extrusion of CD31+ endothelial cells from the vitelline artery has entrapped part of a cluster, the rest of which remains intra-arterial (Fig. 6C and Movie 2). These images suggest that extravascular islands emerge from the vitelline artery both through ballooning and vascular remodeling. We analyzed the dorsal aortas of 14 E10.5 embryos between 32 and 39sp and 6 E11.5 embryos between 42 and 49sp and did not observe ballooning of endothelium beneath hematopoietic clusters in the dorsal aorta (Fig. 6A).
Figure 6. Extrusion of hematopoietic clusters from the umbilical and vitelline artery through ballooning.
(A) Single optical projection of hematopoietic clusters immunostained for CD31 (i,ii), Runx1 (i,iii) and Kit (i, iv) in the umbilical artery (top panels) and dorsal aorta (bottom panels) of 34–36sp embryos. Outline highlights endothelial layer beneath the hematopoietic clusters. Arrowhead points to extruding cluster. Scale bar = 50μm. (B) Z-projection of a 32sp embryo immunostained for CD31. Scale bar = 500μm. Z-interval = 11.04μm. DA = dorsal aorta, VA = vitelline artery and UA = umbilical artery. (C) Magnified view of boxed area in (B). Dotted line outlines cranial side of the vitelline artery to highlight the ballooning hematopoietic cluster (arrowhead). Z-interval = 2.34μm.
Since type I extravascular islands do not contain an endothelial component, their mechanism of vascular exit may be more akin to transendothelial migration, or similar to the EHT that occurs in zebrafish embryos which is oriented away from the vessel lumen. This demonstrates that there are at least three mechanisms by which extravascular islands form: 1) extrusion, in which endothelial cells balloon from the vessel trapping hematopoietic clusters as they pinch off from the vessel (Fig. 7A); 2) the retraction of vessels surrounding hematopoietic clusters due to vascular remodeling (Fig. 7B); and 3) transendothelial migration of type I hematopoietic cluster cells or EHT away from rather than into the vessel lumen.
Figure 7. Schematics illustrating the different mechanisms of the extravascular budding of clusters.
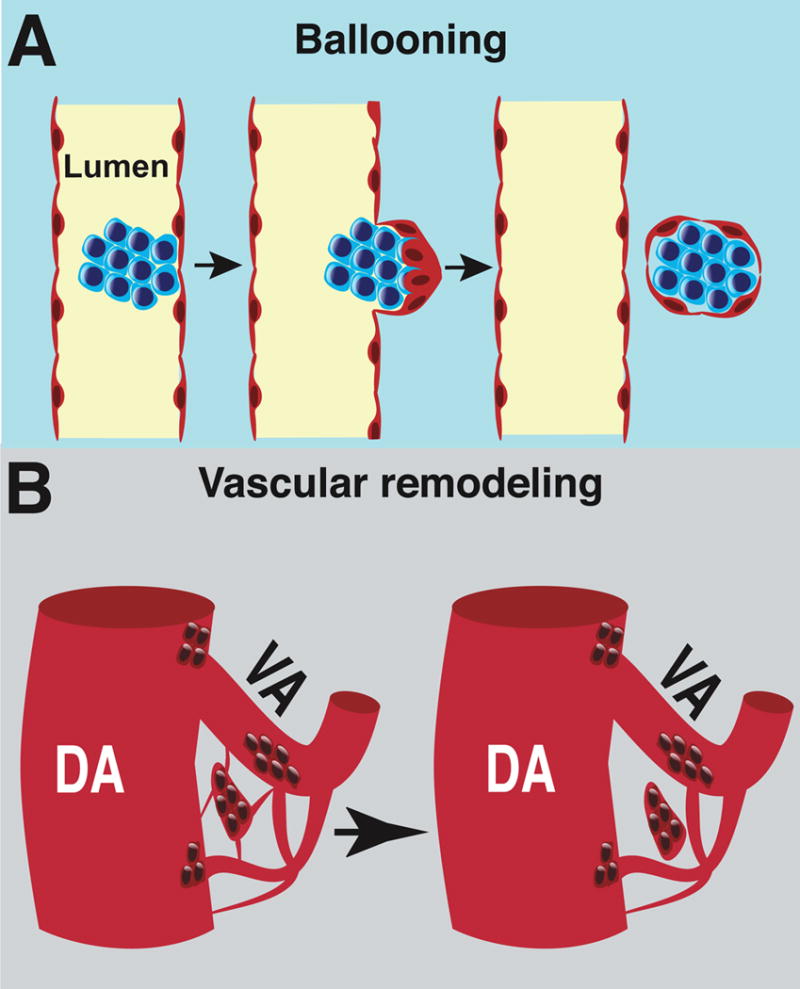
(A) Schematic illustrating ballooning of a hematopoietic cluster from an artery. (B) Vascular remodeling as a mechanism that promotes the formation of extravascular islands.
Extravascular islands contribute to vasculogenesis
An identical process to extravascular budding of hematopoietic clusters has previously been described in the heart. Between E11 and E14 ventricular endocardial cells near the interventricular sulci balloon outwards into the myocardium (Jankowska-Steifer et al., 2015, Red-Horse et al., 2010, Ratajska et al., 2009, Ratajska et al., 2006). These protrusions, called cardiac blood islands consist primarily of erythroblasts and megakaryocytes circumscribed by a single layer of endocardial cells (Jankowska-Steifer et al., 2015). Cardiac blood islands are thought to pinch off from the ventricular endocardium and fuse with the coronary vessels, contributing to the development of the coronary vasculature (Ratajska et al., 2009, Red-Horse et al., 2010). We also noted the apparent merging of extravascular islands with the vasculature in the embryo proper. A confocal Z-projection of the umbilical artery of a 32sp Tg(Ly6a-GFP) embryo shows extravascular islands on the cranial side of the umbilical artery. They included a spherical type II extravascular island consisting of CD31+ Runx1+ GFP− hematopoietic cells circumscribed by CD31+ Runx1− GFP− endothelial cells (Fig. 8A, white boxed region, 8B, and Movie 4), as well as several type IV extravascular islands that lack hematopoietic cells (Fig. 8A, yellow boxed region and C). Between 32 and 38 sp, large, elongated type II, III and IV extravascular islands are visible on the cranial side of the umbilical artery (Fig. 8D–F, Movie 5).
Figure 8. Extravascular islands contribute endothelial and hematopoietic cells to nearby vascular beds.
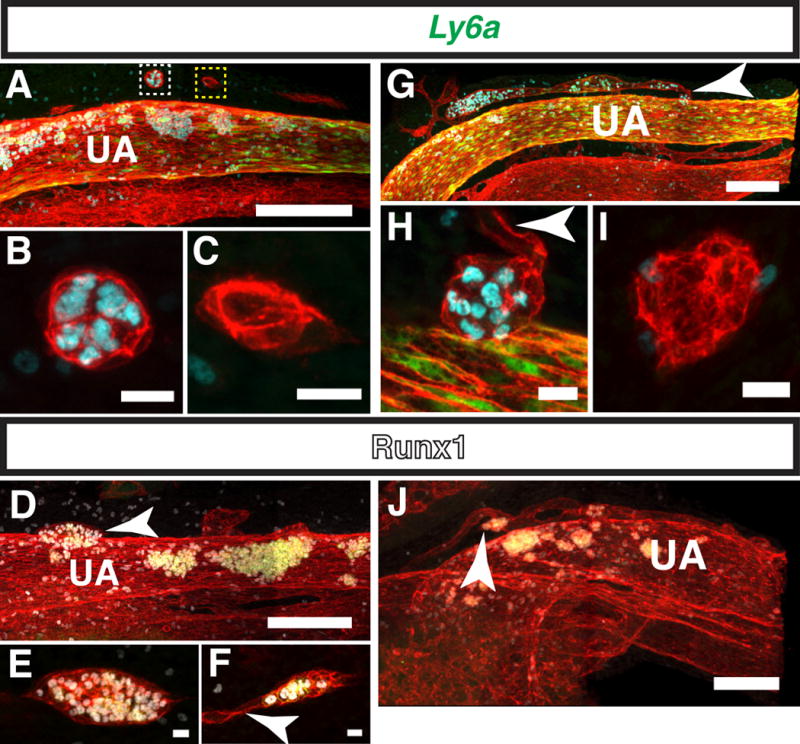
(A–C) Immunostaining for CD31, Runx1 and Ly6a-GFP. (A) Z-projection of the umbilical artery (UA) of a 32sp embryo. Z-interval = 2μm and scale bar = 100μm. White boxed region contains a type II, and yellow box a type IV extravascular island) (see movie 3 for 3D reconstruction) (B) Magnified image of a type II extravascular island on the cranial side of the UA in the white boxed region in (A). Scale bar = 10μm and Z-interval = 1.5μm. (C) Magnified image of an elongated type IV extravascular island in the yellow boxed region in (A). Scale bar = 10μm and Z-interval = 1.5μm. (D–F) Immunostaining for CD31, Runx1 and Kit. (D) Z-projection of the UA of 36sp embryo. Arrowhead points to a large elongated type III extravascular island. Scale bar = 100μm and Z-interval = 2μm (see movie 4 for 3D reconstruction). (E) Magnified image of a type III elongated extravascular island in (D). Scale bar = 10μm and Z-interval = 1.5μm. (F) Z-projection of an extravascular island near the UA. Arrowhead points to endothelial cells sprouting from one end of the extravascular island. Scale bar = 50μm and Z-interval = 1.5μm. (G–I) Immunostaining for CD31, Runx1 and Ly6a-GFP. (G) Z-projection of the UA of a 40sp embryo. Arrowhead points to a type II hematopoietic island that appears to have merged with a small diameter vessel running parallel to the UA. Z-interval = 2μm and scale bar = 100μm (see movie 5 for 3D reconstruction). (H) Magnified image of the extravascular island on the cranial side of the UA indicated by an arrowhead in (G). Arrowhead points to endothelial cells projecting from the extravascular island. Scale bar = 10μm and Z-interval = 1.5μm. (I) Magnified image of a type IV extravascular island behind the UA in (G) (not visible in panel G, see Movie 5). Scale bar = 10μm and Z-interval = 1.5μm. (J) Z-projection of the UA of a 32sp embryo immunostained for CD31, Runx1 and Kit. Arrowhead points to an extravascular island that is incorporated into a small diameter vessel running parallel to the UA. Scale bar = 100μm and Z-interval = 2μm. (See movie 6 for 3D reconstruction)
Some extravascular islands at this stage are connected to small diameter vessels running parallel to the umbilical artery, suggesting that they contributed to the formation of those vessels (Fig. 8F and H, arrowheads). More well-developed small diameter vessels running parallel to the umbilical vessels were also observed in 32sp to 40sp embryos (Fig. 8G and J). Confocal analysis of these small vessels demonstrates that they are often associated with a large concentration of Runx1+ hematopoietic cells, but do not contain Runx1+ CD31+ hemogenic endothelial cells (Fig. 8 G and J, Movies 5 and 6). As the majority of hematopoietic cells in circulation at this stage are CD31low Runx1− Kit− erythroid cells, the numerous CD31+ Runx1+ Ly6a-GFP+/− hematopoietic cells in the small vessels are most likely derived from extravascular islands. This is confirmed by 3D reconstruction of the umbilical artery of a 40sp Tg(Ly6a-GFP) embryo; the small vessel above the umbilical artery does not appear to be connected to the rest of the vasculature, indicating that the hematopoietic cells are unlikely to have arrived there via the circulation (Fig. 8G, Movie 6). A Z-projection of the umbilical artery of a 38sp embryo immunostained for CD31, Runx1 and Kit confirms the absence of Runx1+ hemogenic endothelial cells in the small parallel vessel located cranial to the umbilical artery (Fig. 8J). Interestingly, a cluster of CD31+ Runx1+ Kit+ hematopoietic cells connected to the small vessel above the umbilical artery has a diameter larger than that of the vessel (Fig. 8J, arrowhead). Therefore it is unlikely that this hematopoietic cluster was derived from the vessel, and more likely that it was an extravascular island that fused to the vessel. These observations suggest that extravascular islands derived from established large diameter vessels contribute to the development of nearby blood vessels.
Extravascular islands are unlikely to contribute to the lymphatic vasculature
Development of the lymphatic vasculature begins with expression of the transcription factor Sox18, and following that the lineage specifying transcription factor Prox1 in endothelial cells in the cardinal vein (Oliver and Srinivasan, 2010, Wigle and Oliver, 1999, Wigle et al., 2002, Hong et al., 2002, Petrova et al., 2002). The Prox1-expressing endothelial cells then balloon out to form primary lymphatic sacs (François et al., 2012). However, recently it was proposed that hemogenic endothelium in the major arteries also contributes to the lymphatic vasculature (Stanczuk et al., 2015).This hypothesis was based on lineage tracing experiments in which Cre recombinase driven from the Kit regulatory sequences (Kit is expressed at low levels on hemogenic endothelium (Marcelo et al., 2013)) was shown to mark cells in the lymphatic vasculature (Stanczuk et al., 2015). We reasoned that if hemogenic endothelium contributes to the formation of the lymphatic vasculature, that extravascular islands might be involved. To determine whether a subset of extravascular islands contained prospective lymphatic endothelium we determined whether they contained Prox1+ endothelial cells. Prox1 is expressed starting at E9.5 in endothelial cells in the cardinal vein, that at E10.5 begin delaminating from the vessel (Wigle and Oliver, 1999). At E10.5 (32–38sp) we observed Prox1+ (Kit−) lymphatic endothelial cells in the cardinal vein, as expected (Fig. 9A) (François et al., 2012, Wigle and Oliver, 1999, Yang et al., 2012, Hägerling et al., 2013). In the same embryos, we observed several extravascular islands including a small type I island near the vitelline artery consisting of 4–5 Kit+ CD31+ Prox1− hematopoietic cells with no endothelial component (Fig. 9B–C, arrowhead), and a type III island near the umbilical artery, which consisted of 12–15 loosely arranged CD31+ Kit+ Prox1− hematopoietic cells and CD31+ Kit− Prox1− endothelial cells (Fig. 9D–E, arrowhead). Also shown are two type II extravascular islands near the vitelline artery in the yolk sac of a 38 sp embryo (Fig. 9F–G, arrowheads). Neither the hematopoietic nor the endothelial components of the extravascular islands expressed Prox1. In total we identified 7 extravascular islands in 7 E10.5 embryos, and found none with Prox1+ cells, suggesting that extravascular islands do not contribute to the lymphatic vasculature.
Figure 9. Extravascular islands do not express the lymphatic endothelial marker Prox1.
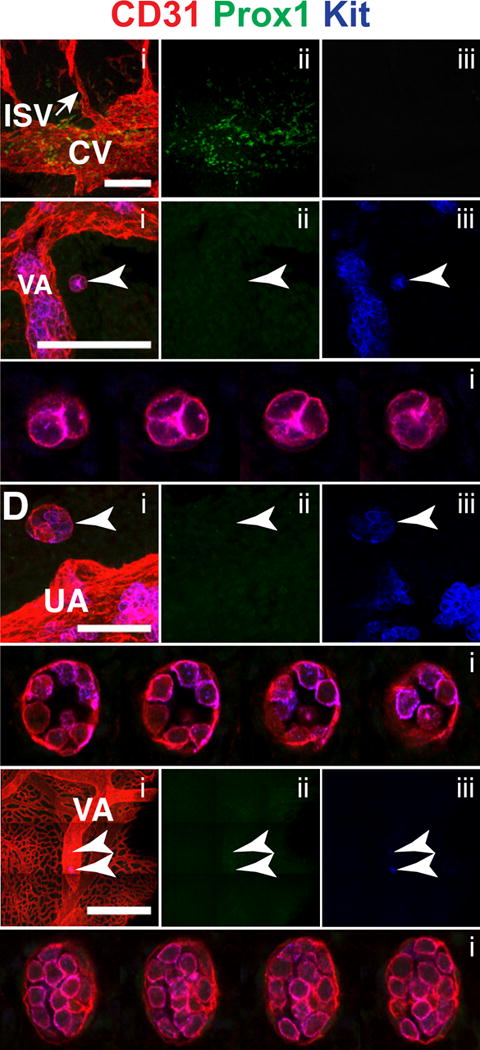
(A–G) Immunostaining for CD31 (i), Prox1 (i,ii) and Kit (i,iii). (A) Z-projection of the cardinal vein (CV) and intersomitic vessels (ISV) of a 32sp embryo. Scale bar = 100μm and Z-interval = 2μm. (B) Z-projection of the vitelline artery (VA) of a 32sp mouse embryo. The arrowhead indicates an extravascular island. Scale bar = 100μm and Z-interval = 2μm. (C) Montage of Z-slices of the type I extravascular island seen in (B). Z-slices are 2μm apart. (D) Z-projection of the umbilical artery of a 36 sp mouse. Arrowhead indicates a type III extravascular island. Scale bar = 50μm and Z-interval = 2μm. (E) Montage of Z-slices through the type III extravascular island seen in (D). Z-slices are 2μm apart. (F) Z-projection of the yolk sac of a 38sp embryo. Arrowheads indicate extravascular islands near the VA. Scale bar = 500μm and Z-interval = 2μm. (G) Montage of Z-slices of the type II extravascular islands seen in (F). Z-slices are 2μm apart.
Conclusion
Whole mount confocal microscopy revealed that extravascular islands are common in midgestation mouse embryos. The islands are released from the arteries via several mechanisms including extrusion (ballooning), the retraction of vessels during vascular remodeling, and transendothelial migration or EHT away from the vessel lumen. Although extravascular islands could simply be byproducts of vascular remodeling and EHT, we instead posit that they actively contribute to the formation of new vessels, similar to what has been described for the coronary vasculature (Red-Horse et al., 2010). Our data suggest that extravascular islands do not, however, contribute to the lymphatic vasculature, based on the absence of Prox1 expression. It is formally possible, though, that Prox1 is expressed after extravascular islands have fused with the lymphatic vessels.
Although the budding of hematopoietic cluster cells away from the arterial lumen was not believed to occur in mammalian embryos, our data suggest that this process, which is common in chick embryos, is also conserved in mammalian embryos, although not in the dorsal aorta. Our pictures are necessarily static. Nevertheless, we believe that they capture a dynamic aspect of vascular remodeling in the embryo.
Supplementary Material
Movie 1. 3-dimensional reconstruction of a type II extravascular island on the cranial side of the umbilical artery in a 40sp Tg(Ly6a-GFP) embryo. Immunostaining for CD31 (red), Runx1 (cyan) and GFP (green). Z-projection of this sample is shown in Figure 3.
Movie 2. Animation of a Z-stack of a 32 sp embryo immunostained for CD31 (red), showing an extruding (ballooning) extravascular island (arrow). The Z-projection of this sample is shown in Fig. 6C.
Movie 3. 3-dimensional reconstruction of extravascular islands on the cranial side of the umbilical artery in a 32sp Tg(Ly6a-GFP) embryo. Immunostaining for CD31 (red), Runx1 (grey) and GFP (green). A Z-projection of this sample shown in Figure 8A.
Movie 4. 3-dimensional reconstruction of an elongated type III extravascular island on the cranial side of the umbilical artery of a 36sp embryo. Immunostaining for CD31 (red), Runx1 (grey) and Kit (green). Z-projection of this sample is shown in Figure 8D.
Movie 5. 3-dimensional reconstruction of a small diameter vessel on the cranial side of the umbilical artery in a 40sp Tg(Ly6a-GFP) embryo. Immunostaining for CD31 (red), Runx1 (grey) and GFP (green). Z-projection of this sample is shown in Figure 8G.
Movie 6. 3-dimensional reconstruction of extravascular islands connected to a small diameter vessel (arrow) on the cranial side of the umbilical artery of a 36sp embryo. Immunostaining for CD31 (red), Runx1 (grey) and Kit (green). Z-projection of this sample is shown in Figure 8J.
Highlights.
Extravascular islands consist of hematopoietic and/or endothelial cells.
Extravascular islands are physically unconnected to the cardiovascular system.
Extravascular islands are observed in embryonic and extraembryonic tissues.
Extravascular islands emerge from the umbilical and vitelline arteries.
Extravascular islands emerge through ballooning and vascular remodeling.
Acknowledgments
We thank Andrea Stout and Jasmine Zhao at the UPenn Cell & Developmental Biology Microscopy Core for confocal microscopy assistance. This work was supported by NHLBI R01HL091724 (NAS), NHLBI U01HL100405 (NAS), NHLBI 1F31HL120615 (ADY) and NCI T32CA09140 (ADY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- BOISSET JC, VAN CAPPELLEN W, ANDRIEU-SOLER C, GALJART N, DZIERZAK E, ROBIN C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–20. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- CHEN MJ, LI Y, DE OBALDIA ME, YANG Q, YZAGUIRRE AD, YAMADA-INAGAWA T, VINK CS, BHANDOOLA A, DZIERZAK E, SPECK NA. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–52. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN MJ, YOKOMIZO T, ZEIGLER BM, DZIERZAK E, SPECK NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–91. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BRUIJN MF, MA X, ROBIN C, OTTERSBACH K, SANCHEZ MJ, DZIERZAK E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–83. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- FRAME JM, FEGAN KH, CONWAY SJ, MCGRATH KE, PALIS J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells. 2015 doi: 10.1002/stem.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANÇOIS M, SHORT K, SECKER GA, COMBES A, SCHWARZ Q, DAVIDSON TL, SMYTH I, HONG YK, HARVEY NL, KOOPMAN P. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol. 2012;364:89–98. doi: 10.1016/j.ydbio.2011.12.032. [DOI] [PubMed] [Google Scholar]
- GARCIA-PORRERO JA, GODIN IE, DIETERLEN-LIÈVRE F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol (Berl) 1995;192:425–35. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- GARCIA-PORRERO JA, MANAIA A, JIMENO J, LASKY LL, DIETERLEN-LIÈVRE F, GODIN IE. Antigenic profiles of endothelial and hemopoietic lineages in murine intraembryonic hemogenic sites. Dev Comp Immunol. 1998;22:303–19. doi: 10.1016/s0145-305x(98)00006-8. [DOI] [PubMed] [Google Scholar]
- HONG YK, HARVEY N, NOH YH, SCHACHT V, HIRAKAWA S, DETMAR M, OLIVER G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–7. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- HOOGENKAMP M, LICHTINGER M, KRYSINSKA H, LANCRIN C, CLARKE D, WILLIAMSON A, MAZZARELLA L, INGRAM R, JORGENSEN H, FISHER A, TENEN DG, KOUSKOFF V, LACAUD G, BONIFER C. Early chromatin unfolding by RUNX1: a molecular explanation for differential requirements during specification versus maintenance of the hematopoietic gene expression program. Blood. 2009;114:299–309. doi: 10.1182/blood-2008-11-191890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HÄGERLING R, POLLMANN C, ANDREAS M, SCHMIDT C, NURMI H, ADAMS RH, ALITALO K, ANDRESEN V, SCHULTE-MERKER S, KIEFER F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32:629–44. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFREDO T, GAUTIER R, BRAJEUL V, DIETERLEN-LIÈVRE F. Tracing the progeny of the aortic hemangioblast in the avian embryo. Dev Biol. 2000;224:204–14. doi: 10.1006/dbio.2000.9799. [DOI] [PubMed] [Google Scholar]
- JAFFREDO T, GAUTIER R, EICHMANN A, DIETERLEN-LIÈVRE F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–83. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- JANKOWSKA-STEIFER E, MADEJ M, NIDERLA-BIELIŃSKA J, RUMINSKI S, FLAHT-ZABOST A, CZARNOWSKA E, GULA G, RADOMSKA-LEŚNIEWSKA DM, RATAJSKA A. Vasculogenic and hematopoietic cellular progenitors are scattered within the prenatal mouse heart. Histochem Cell Biol. 2015;143:153–69. doi: 10.1007/s00418-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSA K, HERBOMEL P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–5. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- KISSA K, MURAYAMA E, ZAPATA A, CORTÉS A, PERRET E, MACHU C, HERBOMEL P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–56. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- LAM EY, HALL CJ, CROSIER PS, CROSIER KE, FLORES MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116:909–14. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- LANCRIN C, MAZAN M, STEFANSKA M, PATEL R, LICHTINGER M, COSTA G, VARGEL O, WILSON NK, MÖRÖY T, BONIFER C, GÖTTGENS B, KOUSKOFF V, LACAUD G. GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood. 2012;120:314–22. doi: 10.1182/blood-2011-10-386094. [DOI] [PubMed] [Google Scholar]
- LANCRIN C, SROCZYNSKA P, STEPHENSON C, ALLEN T, KOUSKOFF V, LACAUD G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–5. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y, ESAIN V, TENG L, XU J, KWAN W, FROST IM, YZAGUIRRE AD, CAI X, CORTES M, MAIJENBURG MW, TOBER J, DZIERZAK E, ORKIN SH, TAN K, NORTH TE, SPECK NA. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28:2597–612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z, LAN Y, HE W, CHEN D, WANG J, ZHOU F, WANG Y, SUN H, CHEN X, XU C, LI S, PANG Y, ZHANG G, YANG L, ZHU L, FAN M, SHANG A, JU Z, LUO L, DING Y, GUO W, YUAN W, YANG X, LIU B. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11:663–75. doi: 10.1016/j.stem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- LICHTINGER M, INGRAM R, HANNAH R, MÜLLER D, CLARKE D, ASSI SA, LIE-A-LING M, NOAILLES L, VIJAYABASKAR MS, WU M, TENEN DG, WESTHEAD DR, KOUSKOFF V, LACAUD G, GÖTTGENS B, BONIFER C. RUNX1 reshapes the epigenetic landscape at the onset of haematopoiesis. EMBO J. 2012;31:4318–33. doi: 10.1038/emboj.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA X, ROBIN C, OTTERSBACH K, DZIERZAK E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells. 2002;20:514–21. doi: 10.1634/stemcells.20-6-514. [DOI] [PubMed] [Google Scholar]
- MARCELO KL, SILLS TM, COSKUN S, VASAVADA H, SANGLIKAR S, GOLDIE LC, HIRSCHI KK. Hemogenic endothelial cell specification requires c-Kit, Notch signaling, and p27-mediated cell-cycle control. Dev Cell. 2013;27:504–15. doi: 10.1016/j.devcel.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER A. Histogenesis and morphogenesis of the thoracic duct in the chick; development of blood cells and their passage to the blood stream via the thoracic duct. The American Journal of Anatomy. 1913;15:131–197. [Google Scholar]
- MURAYAMA E, KISSA K, ZAPATA A, MORDELET E, BRIOLAT V, LIN HF, HANDIN RI, HERBOMEL P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–75. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- NAKANO H, LIU X, ARSHI A, NAKASHIMA Y, VAN HANDEL B, SASIDHARAN R, HARMON AW, SHIN JH, SCHWARTZ RJ, CONWAY SJ, HARVEY RP, PASHMFOROUSH M, MIKKOLA HK, NAKANO A. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun. 2013;4:1564. doi: 10.1038/ncomms2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH T, GU TL, STACY T, WANG Q, HOWARD L, BINDER M, MARIN-PADILLA M, SPECK NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–75. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- OLIVER G, SRINIVASAN RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development. 2010;137:363–72. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETROVA TV, MÄKINEN T, MÄKELÄ TP, SAARELA J, VIRTANEN I, FERRELL RE, FINEGOLD DN, KERJASCHKI D, YLÄ-HERTTUALA S, ALITALO K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–9. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATAJSKA A, CZARNOWSKA E, KOŁODZIŃSKA A, JABŁOŃSKA A, STACHURSKA E. New morphological aspects of blood islands formation in the embryonic mouse hearts. Histochem Cell Biol. 2009;131:297–311. doi: 10.1007/s00418-008-0542-4. [DOI] [PubMed] [Google Scholar]
- RATAJSKA A, CZARNOWSKA E, KOŁODZIŃSKA A, KLUZEK W, LEŚNIAK W. Vasculogenesis of the embryonic heart: origin of blood island-like structures. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:223–32. doi: 10.1002/ar.a.20311. [DOI] [PubMed] [Google Scholar]
- RED-HORSE K, UENO H, WEISSMAN IL, KRASNOW MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–53. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDELIN J, ARGANDA-CARRERAS I, FRISE E, KAYNIG V, LONGAIR M, PIETZSCH T, PREIBISCH S, RUEDEN C, SAALFELD S, SCHMID B, TINEVEZ JY, WHITE DJ, HARTENSTEIN V, ELICEIRI K, TOMANCAK P, CARDONA A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANCZUK L, MARTINEZ-CORRAL I, ULVMAR MH, ZHANG Y, LAVIÑA B, FRUTTIGER M, ADAMS RH, SAUR D, BETSHOLTZ C, ORTEGA S, ALITALO K, GRAUPERA M, MÄKINEN T. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- WIGLE JT, HARVEY N, DETMAR M, LAGUTINA I, GROSVELD G, GUNN MD, JACKSON DG, OLIVER G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGLE JT, OLIVER G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- YANG Y, GARCÍA-VERDUGO JM, SORIANO-NAVARRO M, SRINIVASAN RS, SCALLAN JP, SINGH MK, EPSTEIN JA, OLIVER G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–8. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOMIZO T, DZIERZAK E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–61. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOMIZO T, NG CE, OSATO M, DZIERZAK E. Three-dimensional imaging of whole midgestation murine embryos shows an intravascular localization for all hematopoietic clusters. Blood. 2011;117:6132–4. doi: 10.1182/blood-2011-02-334037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOMIZO T, OGAWA M, OSATO M, KANNO T, YOSHIDA H, FUJIMOTO T, FRASER S, NISHIKAWA S, OKADA H, SATAKE M, NODA T, ITO Y. Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes Cells. 2001;6:13–23. doi: 10.1046/j.1365-2443.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- YOKOMIZO T, YAMADA-INAGAWA T, YZAGUIRRE AD, CHEN MJ, SPECK NA, DZIERZAK E. Whole-mount three-dimensional imaging of internally localized immunostained cells within mouse embryos. Nature protocols. 2012;7:421–31. doi: 10.1038/nprot.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOVEIN AC, TURLO KA, PONEC RM, LYNCH MR, CHEN KC, HOFMANN JJ, COX TC, GASSON JC, IRUELA-ARISPE ML. Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood. 2010;116:3435–44. doi: 10.1182/blood-2010-04-279497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. 3-dimensional reconstruction of a type II extravascular island on the cranial side of the umbilical artery in a 40sp Tg(Ly6a-GFP) embryo. Immunostaining for CD31 (red), Runx1 (cyan) and GFP (green). Z-projection of this sample is shown in Figure 3.
Movie 2. Animation of a Z-stack of a 32 sp embryo immunostained for CD31 (red), showing an extruding (ballooning) extravascular island (arrow). The Z-projection of this sample is shown in Fig. 6C.
Movie 3. 3-dimensional reconstruction of extravascular islands on the cranial side of the umbilical artery in a 32sp Tg(Ly6a-GFP) embryo. Immunostaining for CD31 (red), Runx1 (grey) and GFP (green). A Z-projection of this sample shown in Figure 8A.
Movie 4. 3-dimensional reconstruction of an elongated type III extravascular island on the cranial side of the umbilical artery of a 36sp embryo. Immunostaining for CD31 (red), Runx1 (grey) and Kit (green). Z-projection of this sample is shown in Figure 8D.
Movie 5. 3-dimensional reconstruction of a small diameter vessel on the cranial side of the umbilical artery in a 40sp Tg(Ly6a-GFP) embryo. Immunostaining for CD31 (red), Runx1 (grey) and GFP (green). Z-projection of this sample is shown in Figure 8G.
Movie 6. 3-dimensional reconstruction of extravascular islands connected to a small diameter vessel (arrow) on the cranial side of the umbilical artery of a 36sp embryo. Immunostaining for CD31 (red), Runx1 (grey) and Kit (green). Z-projection of this sample is shown in Figure 8J.


