Abstract
Although osmotic minipumps are a reliable method for inducing nicotine dependence in rodents, continuous nicotine administration does not accurately model the intermittent pattern of nicotine intake in cigarette smokers. Our objectives, therefore, were to investigate whether intermittent nicotine delivery via osmotic minipumps could induce dependence in rats, and to compare the magnitude and duration of withdrawal following forced abstinence from intermittent nicotine to that induced by continuous nicotine administration. In order to administer nicotine intermittently, rats were surgically implanted with saline-filled osmotic minipumps attached to polyethylene tubing that contained hourly unit doses of nicotine alternating with mineral oil to mimic “injections”. Three doses of nicotine (1.2, 2.4, and 4.8 mg/kg/day) and saline were administered for 14 days using this method. In order to compare our intermittent delivery method with the more traditional continuous nicotine delivery, a second group of rats were implanted with minipumps attached to tubing that delivered continuous nicotine for 14 days. Rats were administered a 1.5 mg/kg subcutaneous (SC) mecamylamine challenge and observed for somatic signs of withdrawal on days 7, 14, 21, and 28 following minipump implantation. Fifteen somatic withdrawal signs were summed within a 50-minute observation period to obtain a composite Dependence Score. A generalized linear mixed-effects model revealed a significant Day × Dose × Method interaction. Amongst continuously-treated rats, only 4.8 mg/kg/d nicotine resulted in dependence scores significantly greater than those of controls at 14 days of exposure. In contrast, all intermittent nicotine groups showed significantly higher scores beginning at 7 days of exposure and persisting beyond 7 days of abstinence. In general, intermittent delivery produced a more robust withdrawal syndrome than continuous delivery, and did so at a lower dose threshold and with greater persistence after forced abstinence.
Keywords: nicotine, nicotine dependence, nicotine withdrawal, osmotic minipump, mecamylamine HCl
1. Introduction
Withdrawal from chronic nicotine usage in humans is generally characterized by irritability/agitation, aggression, anxiety, depression, increase in weight and appetite, restlessness, and craving for nicotine (Hughes et al 1991). Importantly, these symptoms are often reported to be the proximal cause of relapse to cigarette smoking in those trying to remain abstinent (USDHHS 1988). A better understanding of the neurobiological changes that occur as a consequence of nicotine dependence and withdrawal may aid in the development of better preventative and treatment intervention therapies.
Appropriate preclinical models of drug dependence allow for in-depth, mechanistic examination of susceptibility to and factors responsible for addiction, and for developing potential treatment therapies prior to human testing. Self-administration (Paterson and Markou 2004), daily intraperitoneal (IP) or subcutaneous (SC) nicotine injections (Clarke et al. 1983; Pandey et al. 2001), oral nicotine administration via drinking water (Flynn et al. 1989), and osmotic minipumps (Besheer and Bevins 2003; Malin et al. 1992) have commonly been employed to induce nicotine dependence in rodent models. The most frequently employed nicotine administration method is the osmotic minipump, which is implanted SC or IP and infuses nicotine continuously. Osmotic minipumps overcome many of the challenges associated with other methods, including lengthy training, costly equipment, or stress and conditioned drug effects induced by repeated drug injections.
While the minipump method obviates many of the limitations inherent to those described above, it too has a notable limitation- its continuous mode of drug delivery. One of the main drawbacks of using continuous nicotine infusion to induce dependence is that this delivery method does not closely mimic the intermittent pattern of nicotine intake typical of human smokers—i.e., smoking a cigarette for a few minutes every few hours throughout the day, with an extended period of withdrawal during sleep. This intermittent pattern of human nicotine administration is thought to allow nicotinic acetylcholine receptors (nAChRs), which undergo rapid upregulation but desensitization, to return to their full active state during the administration period (Marks et al 1993). This is arguably a very important aspect of nicotine tolerance and withdrawal development and maintenance (Dani 2001).
Because the aversiveness of nicotine withdrawal plays an important role in the maintenance of its use among human smokers, rodent models of nicotine withdrawal have become a widely-used means of assessing the degree of dependence among nicotine-treated animals. Malin et al. (1994) first demonstrated that an acute injection of the nAChR antagonist mecamylamine induces a nicotine withdrawal syndrome in rats receiving chronic nicotine via osmotic minipump, and others (e.g., Hildebrand et al., 1997) have confirmed this finding. The severity of withdrawal symptoms has also been found to correspond to the amount of prior nicotine exposure (Malin et al. 1992). To our knowledge, no study has yet examined the mecamylamine-precipitated withdrawal syndrome in rats receiving passive, intermittent nicotine for an extended period of time.
As such, the goal of the present study was to adapt and validate a method for inducing nicotine dependence in rats via automatic, repeated, intermittent injections of nicotine over a duration of 14 days using osmotic minipumps. Additionally, we sought to compare the dependence induced by this novel method to dependence induced by continuous administration via the same device. Nicotine dependence was assessed behaviorally, and quantified by summation of somatic behavioral signs of withdrawal following an acute challenge of mecamylamine.
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (Charles River Laboratory) weighing 300–325 g were single-housed under a 12-h light/dark cycle (0600 hours lights on). N=13–15 rats per group were administered intermittent nicotine or saline, and n=6 rats per group were administered continuous nicotine. Animals had ad libitum access to food and water and were handled for 1 week prior to experimentation. The Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program approved all procedures used in the study.
2.2 Drugs
(−)-Nicotine hydrogen tartrate and mecamylamine hydrochloride were purchased from Sigma Aldrich (St. Louis, MO, USA). Nicotine was dissolved in 0.9% sodium chloride (Hospira) to achieve the desired concentration, and pH was adjusted to 7.2 (±0.5) with sodium hydroxide (Sigma Aldrich).
2.3 Nicotine administration
We developed an automated method to non-continuously deliver discrete nicotine “injections” for 14 days using an osmotic minipump. Our goal was to deliver a single injection of nicotine every other hour, 24 hours/day for 2 weeks. We accomplished this by forming a long piece of PE-60 tubing (Instech Laboratories, Plymouth Meeting, PA, USA) into a coil using a method first described by Lynch et al. (1980) and prefilling the coil with nicotine solutions separated by mineral oil (Fig. 1A). Taking into account the inner diameter of the PE-60 tubing and the 2.5 μl/h flow rate of the osmotic minipump (model 2ML4; Alzet, Palo Alto, CA, USA), we calculated the total length of tubing needed for 14 days of infusions (187 cm) and the drug concentrations needed for each infusion period given the constant injection volume.
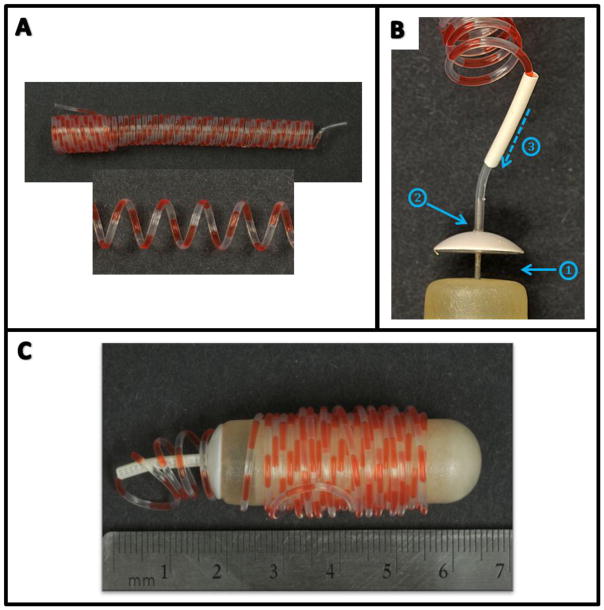
Figure 1. Design of the intermittent delivery device.
A: Coils measuring 190 cm in length were filled with alternating 2.5-μl infusions of nicotine solution and oil. B: Filled coils were attached to primed osmotic minipumps, and glue was used to attach the flow moderator cap to the pump (1) and the coil to the cap/flow moderator (2). A piece of heat-shrink tubing was then placed over the flow moderator (3) and sealed. C: The completed device measured less than 7 cm in length and fit comfortably in the IP cavity of a rat weighing ≥ 350 g.
Once the coils were formed, they were filled with nicotine (2.5 μl) and mineral oil (2.5 μl) in an alternating fashion using a Y-connector (Instech Laboratories) and two infusion pumps (Harvard Apparatus) to deliver precise volumes. In a second cohort of rats, coils were filled with only nicotine solution to allow for comparison of intermittent delivery with continuous delivery. The osmotic minipump, which contains a reservoir that holds enough solution for 28 days of delivery, was filled with saline and primed for 24 hours in 37° C saline for both method groups.
Prior to implantation for IP drug administration, the prefilled coil was attached to the flow moderator of the minipump (Fig. 1B). Because it was determined in vitro that the length of the filled coil produced backpressure sufficient to cause leakage of saline from the pump at the point of connection to the coil, Instant Krazy Glue (Elmer’s Products Inc., Columbus, OH, USA) was applied below the cap of the flow moderator to seal it to the top of the pump and to the end of the coil once it had been attached to the flow moderator. Additionally, a 1.5 cm-long piece of 1.6 mm diameter heat-shrink tubing (RadioShack Corp., Fort Worth, TX, USA) was placed at the end of the coil where it attached to the flow moderator in order to prevent the coil from cracking when animal movement created additional stress at the point of connection. A soldering station (Weller model WSD81, Germany) heated to 700° F was used to seal the heat-shrink tubing around the coil. The length of the coil was then wrapped around the minipump (Fig. 1C), and the device was implanted immediately. A pilot in vitro study confirmed that the flow rate of nicotine and oil through the coil was the same as the expected flow rate of solution from a minipump used without a catheter (2.5 μl/hr).
In order to compare the intermittent delivery method with continuous infusion of nicotine, we implanted an additional group of rats (n=6/group) with saline-filled minipumps attached to Lynch coils that were only filled with nicotine solution. These group sizes, though smaller than the intermittent groups, are comparable in size to those of other nicotine behavioral studies (e.g. Malin et al., 1994). We selected three nicotine doses from the literature reflecting a high (0.4 mg/kg every other hour or 0.2 mg/kg/hr continuously for a total of 4.8 mg/kg/day), moderate (0.2 mg/kg every other hour or 0.1 mg/kg/hr continuously for a total of 2.4 mg/kg/day; Benwell et al. 1995) and low (0.1 mg/kg/hr every other hour or 0.05 mg/kg/hr continuously for a total of 1.2 mg/kg/day; Malin et al. 1992) dose previously shown to induce dependence; doses are expressed as the free-base. Control animals were implanted with minipumps attached to coils filled with alternating saline and oil. Following the last day of testing, rats were sacrificed and the integrity of the tubing and pump were assessed.
2.4 Osmotic minipump implantation
Rats were anesthetized with 2–3% isoflurane in a 1 to 1 mixture of O2 and air. An incision was made in the abdomen large enough to fit the pump and tubing inside the peritoneal cavity. Gut sutures were used to close the muscular peritoneal layer and wound clips were used to close the skin incision. Surgical procedures were performed according to an aseptic protocol.
2.5 Somatic signs of nicotine withdrawal
An acute injection of mecamylamine HCl (1.5 mg/kg, SC) was administered at 7, 14, 21, and 28 days after pump implantation to precipitate nicotine withdrawal (note: nicotine was delivered only for the first 14 days of the 28 days of minipump capacity). Rats were observed for withdrawal signs in square Plexiglas chambers (35 × 35 × 40 cm). They were habituated to the chambers for 10 min/day 3 days prior to testing. On the day of testing, an additional 10-min habituation period was followed by a 60-min observation period: 10-min baseline before and 50 min precipitated withdrawal immediately following mecamylamine administration. The following somatic signs were tallied within 10-min intervals during the test (Malin et al. 1992): teeth chattering, chewing, gasping, writhing, head shakes, body shakes, tremors, blinks, yawns, seminal ejaculation, genital licks, hind foot scratches, escape attempts, and ptosis. Ptosis, if present, was counted only once per minute. Withdrawal behaviors were scored by two raters, who were blinded to drug condition. There was over 90% inter-rater reliability of the behavioral scoring between observers.
2.6 Statistics
Somatic signs of withdrawal were summed across the 50-min precipitated withdrawal period to yield a composite Dependence Score. A generalized linear mixed-effects model with a Poisson probability mass function and a log link function, an approach appropriate for count data, (O’Hara and Kotze, 2010; Stroup 2015) was used to model the behaviors. The model included fixed effects of nicotine dose, method of administration, and day, and their interaction, and random effects included by-rat intercepts and slopes across days. The analysis used glmer from the ‘lme4’ package within R, with posthoc tests utilizing the ‘phia’ package with the Holm-Bonferroni adjustment for multiple comparisons (R Core Team 2013; Bates et al. 2013; DeRosario-Martinez 2013). A p≤0.05 was considered significant throughout.
3. Results
Rats were treated for 14 days with 1.2, 2.4, or 4.8 mg/kg/d nicotine administered either continuously or intermittently via osmotic minipump. Analysis of precipitated withdrawal signs by generalized linear mixed-effects model revealed a significant Dose × Method × Day interaction [χ2 (9, N=75)=96.88, p<0.0001], including a significant main effect of Method [χ2(1, N=75)=13.15, p=0.0003] and significant Dose × Day [χ2(9, N=75)=190.56, p<0.0001] and Method × Day interactions [χ2(3, N=75)=67.51, p<0.0001]. There was no difference in baseline data between treatment groups or across time points (data not shown).
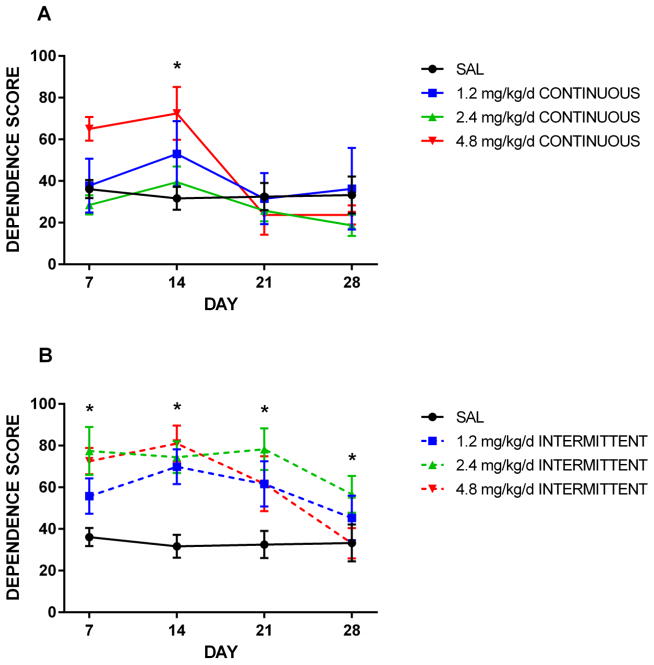
Amongst intermittently-treated animals, the degree of dependence across testing points varied by dose. All three nicotine doses resulted in a dependence score significantly greater than that seen amongst control animals at days 7, 14, and 21 (p≤0.03). There was a non-significant trend toward dose-dependence at day 14, at which point scores peaked in the 1.2 and 4.8 mg/kg/d groups (Figure 2). The 2.4 mg/kg/d dose also induced significantly higher dependence scores at day 28 (p<0.0001).
Figure 2. Effect of intermittent and continuous delivery dose on dependence score.
Rats were administered nicotine continuously (A) or intermittently in an hour on/hour off pattern (B) for 14 days via osmotic minipump. Mean counts of somatic withdrawal signs were summed across 50-min observation periods following a 1.5 mg/kg SC mecamylamine challenge. *All three intermittent nicotine doses resulted in significantly higher dependence scores at days 7, 14, and 21; 2.4 mg/kg/d also resulted in significantly higher scores at day 28. In contrast, only the 4.8 mg/kg/d dose resulted in significantly more withdrawal signs at day 14 in the continuously-treated groups. Error bars represent SEM.
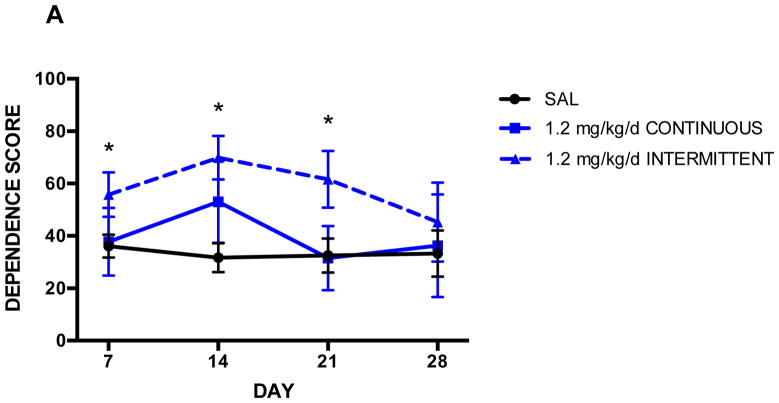
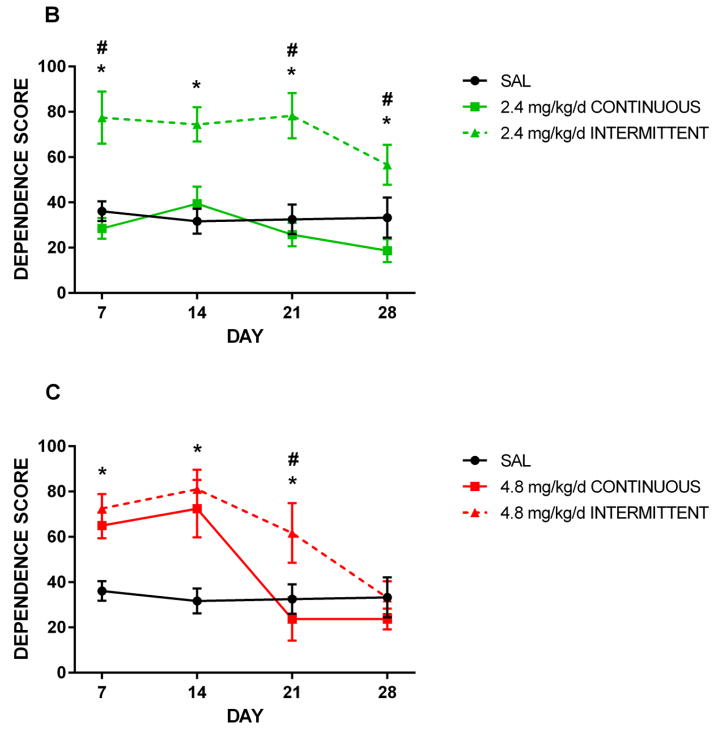
Figure 3 presents the effects of 1.2, 2.4, and 4.8 mg/kg/d nicotine administered either continuously or intermittently. The 4.8 mg/kg/d dose resulted in significantly elevated withdrawal signs relative to saline controls at 14 days of exposure (p≤0.003), regardless of administration method (Fig. 3C). Dependence scores between continuously- and intermittently-treated animals also did not significantly differ at Day 7; therefore, the lack of difference between saline controls and continuously-treated animals at this time point was likely due to the smaller ‘n’ in the continuous group. At Day 21, intermittently-treated animals still displayed elevated signs relative to controls (p=0.01), while continuously-treated animals were no longer behaviorally distinguishable from controls. Thus, this dose of nicotine administered intermittently appeared to induce heightened dependence relative to continuous nicotine, and spontaneous withdrawal also persisted longer in rats receiving intermittent nicotine.
Figure 3. Effect of administration method on dependence score.
Rats were treated for 14 days with 1.2 (A), 2.4 (B), or 4.8 (C) mg/kg/d nicotine administered either continuously or every other hour via osmotic minipump. Mean counts of somatic withdrawal signs were summed across 50-min observation periods following a 1.5 mg/kg SC mecamylamine challenge. Animals receiving 1.2 mg/kg/d nicotine intermittently scored significantly higher (p ≤ 0.03) than saline controls at days 7, 14, and 21 (A). Animals receiving 2.4 mg/kg/d nicotine intermittently scored significantly higher than saline controls at days 7, 14, 21, and 28, and significantly higher than continuously-treated animals at days 7, 21, and 28. Intermittently-treated animals receiving 4.8 mg/kg/d scored significantly higher (p ≤ 0.05) than saline controls at days 7, 14, and 21; additionally, they scored significantly higher than continuously-treated animals at day 21. Continuously-treated animals scored significantly higher than saline controls at day 14. * denotes intermittent groups that scored significantly higher than saline; # denotes intermittent groups that scored significantly higher than continuous groups. Error bars represent SEM.
In animals treated with 1.2 mg/kg/d, a similar behavioral pattern was observed across the four time points. Withdrawal signs following mecamylamine peaked at 14 days of nicotine exposure, and intermittently-treated animals showed significantly more signs than controls at days 7, 14, and 21 (p≤0.03). Continuously-treated animals appeared behaviorally identical to controls after 7 and 14 days of abstinence, while intermittently-treated animals experienced a more gradual decline in dependence signs over the 14-day abstinence period (Fig. 3A).
A moderate dose of 2.4 mg/kg/d was also tested, but for unknown reasons, continuous administration of this dose did not result in elevated withdrawal signs relative to controls at any time point; furthermore, scores amongst these animals were significantly lower than those of animals treated with the same dose in an intermittent fashion (p≤0.005) (Fig. 3B).
4. Discussion
Using an automated method to discretely deliver nicotine to rats over a relatively long period (14 days), we demonstrated a quantitative precipitated abstinence syndrome consistent with previous preclinical nicotine dependence models (Malin et al. 1992; Hildebrand et al. 1998). Behavioral measures of nicotine withdrawal, which include the 15 behaviors we scored, have been used and previously validated (e.g., Epping-Jordan et al. 1998; Harrison et al. 2001; Hildebrand et al. 1998). The delivery method described in this paper provides a novel approach to more closely approximating the intermittent smoking behavior in humans and produced a nicotine-dependent animal in the absence of experimenter interventions and with greater magnitude and more persistence than continuous minipump delivery. This method is less expensive and time-consuming than intravenous drug SA (or programmed passive administration).
In order to compare our intermittent delivery method to continuous delivery, we implanted a second group of rats with minipumps attached to nicotine-filled Lynch coils. This allowed us to compare patterns of delivery across the same daily dose rates and time points. Amongst continuously-treated subjects, only the 4.8 mg/kg/d dose of nicotine resulted in significantly elevated dependence scores relative to controls at 14 days of exposure. This is in contrast to the results seen in intermittently-treated rats, as all three doses resulted in dependence at day 7, 14, and 21 (Fig. 2). This suggests a lower dependence threshold when animals are given drug intermittently, although it should be noted that there are fewer animals in the continuous treatment groups which reduces the strength of statistical comparison between the two methods. It is also notable that dependence scores amongst intermittently-treated animals decrease at Day 28 relative to the previous testing points (Fig. 2). This is suggestive of a recovery from nicotine dependence induced by the 14 days of intermittent drug delivery.
There are two differences in our results that were surprising and warrant discussion. First, the moderate dose of 2.4 mg/kg/d nicotine, when administered continuously, did not induce dependence according to the behavioral assessment we used. This could be due to a methodological issue; however, we believe that is unlikely considering that animals from each group were run in different experimental groupings, and nicotine solutions were freshly made before each experiment. Additionally, considering that there were no outliers in the data for this group, it seems more likely that the lack of effect was a true negative result. Second, when the 2.4 mg/kg/d dose was administered intermittently, there were withdrawal symptoms that persisted until 14 days of abstinence, which was not the case with other doses. However, animals treated with this dose only showed more withdrawal signs than the control group, and did not differ from other nicotine doses. There was a decrease in withdrawal signs in all nicotine-treated groups at this time point. Therefore, it seems likely that the greater withdrawal signs in this group at 28 days might be due to chance.
Of note, saline-treated rats, which remained nicotine-naïve, showed some somatic signs following mecamylamine. Our dose of 1.5 mg/kg has been used in previous studies that employed a similar observational behavior assay (e.g. George et al., 2010), and was chosen to ensure that a withdrawal syndrome could be elicited in all nicotine treatment groups. Malin et al. (1994) previously determined that an acute dose of 5 mg/kg mecamylamine induces a withdrawal syndrome in nicotine-naïve rats that is comparable to that seen in rats receiving 9 mg/kg/d nicotine over 7 days, after they are administered 1 mg/kg mecamylamine. Thus, though our dose of 1.5 mg/kg is well below that which Malin et al. (1994) reported to induce a “quasi-nicotine abstinence syndrome”, the observance of some ‘withdrawal behaviors’ following 1.5 mg/kg mecamylamine in saline-treated animals is not surprising.
In addition to demonstrating a withdrawal response that is generally consistent with previous preclinical studies (e.g. Paterson and Markou 2004), our data suggest that nicotine somatic withdrawal signs (and presumably brain receptor alterations) persist beyond a 7 day abstinence period following only 14 days of prior intermittent drug exposure. This finding is also in agreement with previous studies that have examined withdrawal after a protracted period of abstinence. Paterson and Markou (2004) noted that following extended nicotine self-administration over a 31-day period, mecamylamine induced a withdrawal syndrome for up to 28 days of abstinence. In light of this finding, our observation that elevated withdrawal signs persisted for up to 14 days of abstinence is not surprising and might reflect persistent nAChR up-regulation following nicotine exposure (Paterson and Markou 2004). Though the exact mechanisms of this up-regulation are not known, α4β2 receptors have been shown to increase in response to chronic nicotine exposure (Colombo et al., 2013) The α3-α5-β4 nAChR subunit gene cluster, and function of the α5 subunit in particular, is implicated in nicotine dependence susceptibility (Colombo et al., 2013). Similarly, nicotine withdrawal symptoms in humans produce physiological, somatic, and affective changes (Hughes et al. 1994). Affective and behavioral effects include anxiety, craving, irritability, and restlessness. Withdrawal symptoms in humans begin within 6–12 hours after cessation, peak in 1–3 days, and on average last about 3–4 weeks (Gross and Stitzer, 1989; Hughes 1992). One notable difference in our method compared to self-administration is that it is non-contingent, and there may be differences in catecholamine levels in response to passive versus contingent nicotine (Donny et al., 2000). However, although our model does not incorporate the motivational aspects of nicotine dependence, due to its relative simplicity and consistency, it may be usefully applied to study the time course of withdrawal signs and receptor regulation in physiological nicotine dependence.
There are several examples in the literature that suggest very different dependent variable outcomes when considering continuous vs. discrete administration and highlight the importance of distinguishing between the two in experimental design. For example, tolerance to the behavioral effects of nicotine, such as locomotor sensitization is seen in rats after intermittent nicotine delivery (Stolerman et al. 1973; Clarke and Kumar 1983; Ksir et al. 1985) but not after continuous nicotine exposure (Ksir et al. 1987; Benwell et al. 1995). Rowell and Li (1997) have previously investigated the relationship between nicotine dose and pattern of administration by examining nAChR density in the striatum, cortex, and hippocampus in rats receiving 0.6–4.8 mg/kg/d nicotine passively via either continuous infusion or discrete, repeated infusions over 10 days. While continuous infusion resulted in significantly greater up-regulation of nAChRs relative to controls in all of these brain areas, 8 discrete infusions per day (resulting in the same daily dose) did not. Skjei and Markou (2003) have also previously identified that the dose and duration of nicotine exposure affect the severity of nicotine withdrawal. The results of this study support the notion of a “multi phase withdrawal”, wherein withdrawal from 4 to 16 days after minipump removal depended on both the dose and durations of nicotine infusions previously administered. In light of these findings, the significant behavioral differences we saw between continuously- and intermittently-treated animals are not surprising. Further studies will be necessary in order to characterize nicotinic receptors and assess plasma levels of nicotine during intermittent nicotine administration using the presently-described model.
Other recent studies (George et al., 2010; Gilpin et al., 2014) have employed nicotine vapor inhalation as a method for studying nicotine dependence in preclinical rodent models. This method better simulates the method of human nicotine intake than IP or SC administration methods. As yet, however, there is not a method for nicotine inhalation that simulates the transient on/off periods experienced by human cigarette smokers during waking hours.
There are methodological limitations to our study that warrant mentioning, notably animal weight gain over the course of the experiment. However, based on the starting weight of our animals, the additional weight gain over the 14 day administration period (25±5g) did not create a large difference in the daily dose/body weight concentration. Nevertheless, if consistency of drug delivery based on body weight is desirable, this can easily be accomplished by increasing the nicotine concentration during the catheter filling process prior to surgery. Other limitations include the relevance of and appropriateness of the chosen doses, the IP route of administration, which provides slower access to the brain than the IV or inhaled route, and the as yet unknown effects of the method on nicotinic receptor alterations. Additionally, we were unable to collect blood samples for analysis of plasma nicotine levels during these experiments. Doing so would have strengthened this study by allowing for the comparison of peak plasma levels between continuously and intermittently treated animals, although at the expense of additional handling and sampling stress. Although the intermittent pattern of nicotine delivery we used is more similar to human nicotine intake than continuous delivery, it did not account for the long period of abstinence during sleep. However, the intermittent delivery could easily be adapted to account for this extended period of abstinence—for instance, by interspersing the alternating nicotine and oil infusions with larger oil infusions that would interrupt regular nicotine infusions for 8 or more hours. One item of less concern with the method is the stability of nicotine solution within the rat over the 14-day injection period, as a previous study demonstrated that over 90% of the nicotine in osmotic pumps remains stable over a 14 day period (Benwell et al. 1995).
Although it is not known why humans do not take nicotine continuously (or more often than they do), and why for example, nicotine replacement therapy via continuous patch administration is only modestly effective in smoking cessation therapy, it seems reasonable to suggest that the discrete ad lib administration of nicotine produces an appropriate level of nicotinic modulation of dopaminergic (and other neurotransmission) firing to support the behavior (Brody et al. 2004; Le Foll et al. 2013). Our findings that automatic, intermittently administered nicotine induces dependence complement much of the preclinical literature and also expand the field by providing a new model that more closely approximates human self-administration of nicotine. This simple non-continuous infusion method is easily modifiable (e.g. to accomplish different dosage spacing regimens--including longer spacing during the sleep phase as in humans) and could provide a valuable tool to further dissect the neurobiological mechanisms underlying nicotine abuse.
Highlights.
Novel method to intermittently administer nicotine by osmotic minipump.
Nicotine dependence assessed as a function of dose and delivery method.
Intermittent nicotine caused more persistent dependence than continuous delivery.
Acknowledgments
This research was supported by a grant from the FDA Center for Tobacco Products and the Intramural Research Program of NIDA/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bates D, Maechler M, Bolker B, Walker S. Linear mixed-effects models using Eigen and S4. [Accessed 15 Dec 2013];Package ‘lme4’. R Package Version 0.1–5. 2013 Available via CRAN. http://cran.r-project.org/package=lme4.
- Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Brit J Pharmacol. 1995;114(2):454–460. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Bevins RA. Impact of nicotine withdrawal on novelty reward and related behaviors. Behav Neurosci. 2003;117(2):327–340. doi: 10.1037/0735-7044.117.2.327. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Brit J Pharmacol. 1983;78(2):329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo SF, Mazzo F, Pistillo F, Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochemical Pharmacology. 2013;86(8):1063–1073. doi: 10.1016/j.bcp.2013.06.023. http://doi.org/10.1016/j.bcp.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiat. 2001;49(3):166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- De Rosario-Martinez H, Fox J R Core Team. Post-hoc interaction analysis. [Accessed 15 Dec 2013];Package ‘phia’. R Package Version 0.1–5. 2013 Available via CRAN. http://cran.r-project.org/package=phia.
- Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF. Differential effects of response-contingent and response-independent nicotine in rats. European Journal of Pharmacology. 2000;402(3):231–240. doi: 10.1016/s0014-2999(00)00532-x. http://doi.org/10.1016/S0014-2999(00)00532-X. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Webster M, Ksir C. Chronic voluntary nicotine drinking enhances nicotine palatability in rats. Behav Neurosci. 1989;103(2):356–364. doi: 10.1037//0735-7044.103.2.356. [DOI] [PubMed] [Google Scholar]
- George O, Grieder TE, Cole M, Koob GF. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacology Biochemistry and Behavior. 2010;96(1):104–107. doi: 10.1016/j.pbb.2010.04.013. http://doi.org/10.1016/j.pbb.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapor inhalation escalates nicotine self-administration. Addiction Biology. 2014;19(4):587–592. doi: 10.1111/adb.12021. http://doi.org/10.1111/adb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Stitzer ML. Nicotine replacement: ten-week effects on tobacco withdrawal symptoms. Psychopharmacology. 1989;98(3):334–341. doi: 10.1007/BF00451684. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacol. 2001;25(1):55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH. Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology. 1997;129:348–356. doi: 10.1007/s002130050200. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779(1–2):214–225. doi: 10.1016/S0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, Shea P, Solomon LJ, Flynn BS. Smoking cessation among self-quitters. Health Psychol. 1992;11(5):331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiat. 1991;48(1):52–59. doi: 10.1001/archpsych.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89(11):1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan R, Hall DP, Jr, Kellar KJ. Exposure to nicotine enhances the behavioral stimulant effect of nicotine and increases binding of [3H]acetylcholine to nicotinic receptors. Neuropharmacology. 1985;24(6):527–531. doi: 10.1016/0028-3908(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan RL, Kellar KJ. Chronic nicotine and locomotor activity: influences of exposure dose and test dose. Psychopharmacology. 1987;92(1):25–29. doi: 10.1007/BF00215474. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Guranda M, Wilson AA, Houle S, Rusjan PM, Wing VC, Zawertailo L, Busto U, Selby P, Brody AL, George TP, Boileau I. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-(+)-PHNO PET study in humans. Neuropsychopharmacol. 2014;39:415–424. doi: 10.1038/npp.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HJ, Rivest RW, Wurtman CJ. Artificial induction of melatonin rhythms by programmed microinfusion. Neuroendocrinology. 1980;31(2):106–111. doi: 10.1159/000123059. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Be. 1992;43(3):779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology. 1994;115(1–2):180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266(3):1268–1276. [PubMed] [Google Scholar]
- O’Hara RB, Kotze DJ. Do not log-transform count data. Methods in Ecology and Evolution. 2010;1(2):118–122. http://doi.org/10.1111/j.2041-210X.2010.00021.x. [Google Scholar]
- Pandey SC, Roy A, Xu T, Mittal N. Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J Neurochem. 2001;77(3):943–952. doi: 10.1046/j.1471-4159.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology. 2004;173(1–2):64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- R Core Team. A language and environment for statistical computing. [Accessed 19 Dec 2013];R Foundation for Statistical Computing. 2013 http://web.mit.edu/r_v3.0.1/fullrefman.pdf.
- Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology. 2003;168(3):280–292. doi: 10.1007/s00213-003-1414-1. http://doi.org/10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30(4):329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Stroup WW. Rethinking the Analysis of Non-Normal Data in Plant and Soil Science. Agronomy Journal. 2015;107(2):811. http://doi.org/10.2134/agronj2013.0342. [Google Scholar]
- US Department of Health and Human Services. DHHS Publication (CDC) 88–8406. US Government Printing Office; Washington, DC: 1988. The Health Consequences of Smoking: Nicotine Addiction. A report by the Surgeon General. [Google Scholar]