Abstract
One potential setback to the use of gene therapy for the treatment of Sjögren’s syndrome is the presence of neutralizing antibodies (nAb) against adeno-associated virus (AAV) serotypes. In order to evaluate the efficacy of this treatment option, nAb titers were measured in both healthy individuals and Sjögren’s patients. Several serotypes with known transduction activity in mouse salivary glands were tested and only AAV5 showed a statistically significant change in the prevalence of nAbs between Sjögren’s and healthy participants. Both groups showed a higher rate of nAbs for AAV2 compared with most of the other serotypes tested, except for bovine AAV (BAAV). Although a similar rate of seropositivity was seen against BAAV and AAV2, the percentage of samples with high titer was significantly lower with BAAV. Furthermore, the majority of positive samples exhibited low nAb titers in the primary Sjögren’s syndrome (pSS) group for all serotypes except for AAV2. AAV5 was the only serotype that showed a statistically significant shift in the percentage of medium or high neutralizing titer. Based on these results, many serotypes are viable vectors in a gene therapy approach and pSS patients do not have a statistically significant higher rate of seropositivity or titer compared with healthy donors.
INTRODUCTION
Primary Sjögren’s syndrome (pSS) is an autoimmune disease generally characterized by an infiltration of T and B lymphocytes into glandular tissue such as salivary and lacrimal glands.1 Patients with Sjögren’s syndrome specifically experience xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes), while some also experience arthralgia (painful joints) and fatigue.2 Xerostomia patients are often at an increased risk of oral infection and caries, and have impairment in their ability to speak, swallow and taste.3 The exact etiology of the disease remains unknown; however, its pathogenesis is also characterized by an upregulation of interferon genes and antigen presentation/synthesis of immunomodulatory molecules by salivary epithelial duct cells.4,5 Currently, symptomatic treatment for the disease is largely ineffective and palliative in nature.6,7
Because of the limited nature of the conventional therapies, research has looked to gene therapy as a means for restoring normal cellular activity in the salivary gland, which is one of two primary sites of dysfunction. Gene therapy using recombinant viruses as gene transfer vectors in the salivary gland is currently being evaluated and was shown to be safe and possibly effective in treating radiation-induced xerostomia, an unrelated form of the disease.8 Because of their low immunogenic profile and ability to promote long-term gene transfer, vectors based on adeno-associated virus (AAV) are being actively pursued for gene transfer to the salivary gland.9 Currently, over 100 AAV capsid sequences have been cloned from a variety of sources and several serotypes have been reported to have transduction activity in salivary glands, including AAV2, AAV4, AAV5, bovine AAV (BAAV) and AAV12.10,11
Although effective in animal models at promoting long-term gene transfer, several experiments have demonstrated that circulating antibodies to AAV particles can neutralize them and prevent transduction. Lin et al. demonstrated that an inverse correlation exists between the pre-existing capsid neutralization titer and AAV-based HIV vaccine efficacy. Furthermore, patients with inflammatory bowel diseases show a significant difference in the prevalence of neutralizing antibodies (nAbs) for AAV serotypes between healthy donors (HD) and the patient population.12 In clinical trials, pre-existing antibodies to AAV2 are not reported to prevent gene transfer or expression in localized muscle-targeted gene therapy clinical trials for hemophilia B. However, a cell-mediated immune response to AAV2 vectors has been reported, which limited transgene expression when the vector dose was greater than 2 × 1012 particles per kg body weight and was delivered by portal vein injection.13,14
As a characteristic of Sjögren’s syndrome is elevated antibody levels, the focus of our study was to measure the level of neutralizing anti-AAV antibodies in pSS patients compared with HD and determine the likelihood that they would interfere with vector transduction in serotypes reported to have transduction activity in rodent salivary glands.
RESULTS
Subgroup analysis
Within our patient population, several characteristics were analyzed and are summarized in Supplementary Table 1. Ninety-five percent of pSS participants had focus scores greater than 2. Eighteen percent of pSS participants had decreased salivary flow and tested positive for auto-antibodies, but did not have ocular symptoms. 2.6% of pSS participants had decreased salivary flow, but did not test positive for auto-antibodies or have accompanying ocular symptoms. Seventy-five percent of male pSS participants had decreased salivary flow and ocular symptoms, and were positive for autoantibodies. Twenty-five percent of male pSS participants had decreased salivary flow and ocular symptoms, but were negative for autoantibodies. All participants that had focus scores less than 2 were female.
Prevalence of neutralizing antibodies to AAV
Serum samples were obtained from 39 pSS donors and 38 HD, and the samples were heat inactivated before testing in a virus neutralization assay as described in Materials and Methods. The highest prevalence of nAb was observed with AAV2 followed by BAAV in both HD and pSS groups (45% vs 62% and 39% vs 62%, respectively; Figure 1). As previously reported, a low prevalence of nAb was observed for serotype 5 (11% in HD).15 The lowest prevalence of nAb was observed for AAV12 in both HD and pSS groups (0 vs 8% respectively). Only AAV5 had a significant difference for the prevalence of AAV nAb of any serotype when compared between pSS and the HD groups (P<0.05).
Figure 1.
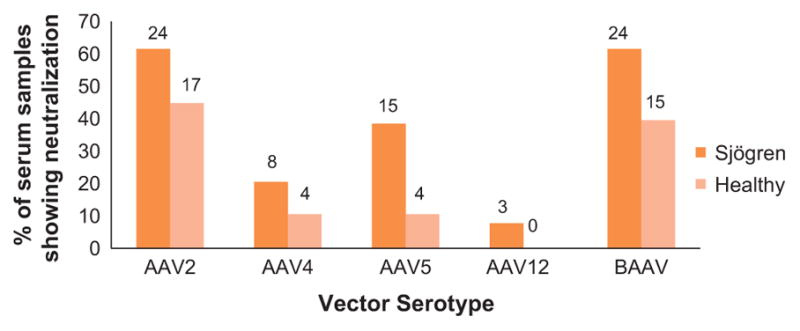
Percentage of serum samples showing neutralization activity in the pSS and healthy donor groups (HD). Fisher’s exact test was used to compare the prevalence difference between HD and pSS (N =38 and 39, respectively). Only AAV5 had a significant difference for the prevalence of AAV nAb of any serotype when compared between the pSS and HD groups (P<0.05).
Titer of neutralizing antibodies in the positive serum samples Neutralization activity is dependent upon the titer in the serum sample. For each positive serotype, the titers were classified as high, medium or low as described in the Materials and Methods section.
To assess the change in samples with an increase in titer from low to medium or high, a two-sample test of proportion was applied to the data. Only AAV5 showed a statistically significant change in distribution between the groups, with an increase in the medium and high in pSS compared with HD (P<0.05). Other serotypes showed some shift, but it was not statistically significant (Figure 2). Calculation of the relative risk ratio (RR), of developing a medium or high level of neutralizing antibodies in pSS vs HD, was >1.6 for all serotypes except AAV2. A A Spearman correlation analysis of the neutralizing titers between serotypes in either the HD or the pSS groups showed statistical significance for several of the serotypes (Supplementary Table 2). Both in the HD and the pSS groups, individuals that were positive for AAV2 were also likely to be positive for AAV4 or BAAV. AAV12 showed the least likelihood for correlation with other serotypes.
Figure 2.
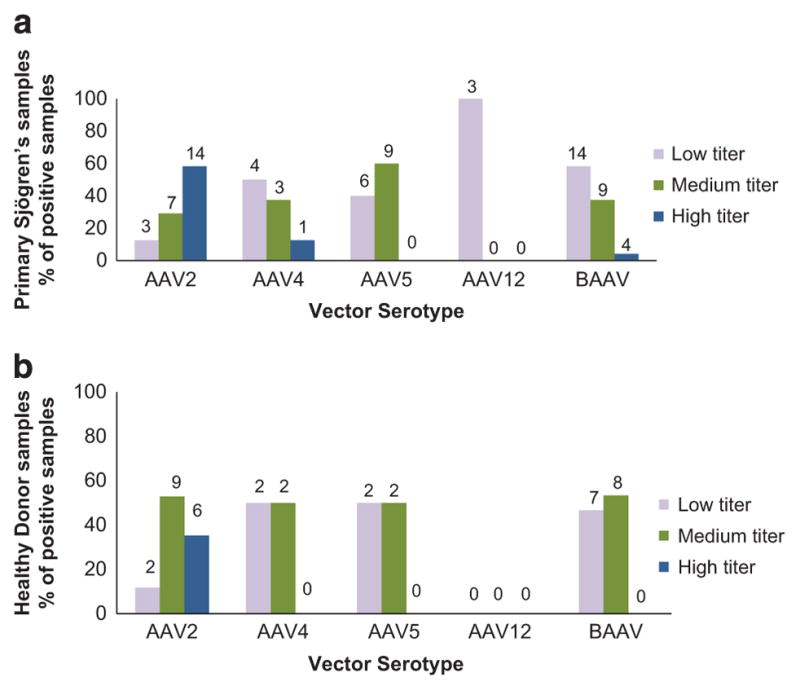
Titers of nAb in positive serum samples from the pSS and HD groups. Positive samples were divided into three groups (low, medium, and high) as described in Materials and Methods. A two-sample test of proportion was applied to the data. Only AAV5 showed a statistically significant change in distribution between the groups, with an increase in the medium and high in pSS compared with HD (P<0.05). Values above each column refer to the number of samples in that group. (a) Data from pSS and (b) data from HD. Values above each column represent the number of positive patients in that category.
Both AAV2 and BAAV had a similar prevalence of nAb in the two populations (Figure 1); however, for AAV2, the majority of the individuals with positive titers had high titer (58% vs 35% in pSS and HD, respectively), compared with BAAV where a few or none had high titer (4% vs 0% in pSS and HD, respectively; Figure 2). Overall the lowest neutralizing titers were seen with AAV12, which also had the lowest prevalence (7% vs 0% in pSS and HD, respectively) of nAb in either population, followed by AAV4 (21% vs 11% in pSS and HD, respectively). pSS patients showed a trend of having more individuals with medium and high titers than a low titer (= 1:25 dilution), but the increase was not statistically significant. Therefore, this change is likely to have minimal effect on vector transduction following systemic delivery.
Correlation between auto-antibody titers and neutralization titer to AAV serotypes
In addition to comparing neutralizing titers between pSS and HD groups, antinuclear antibodies and immunoglobulin G (IgG) values were correlated with each of the serotypes AAV2, AAV4, AAV5, AAV12, and BAAV within the pSS population using Pearson or Spearman rank correlation test. There was no correlation between antinuclear antibodies or IgG alone, and the neutralizing titer of the AAV serotypes tested, or between auto-antibodies (antinuclear antibodies and IgG; P>0.05).
DISCUSSION
Patients suffering from pSS often experience an increased risk of oral infections and caries along with an impaired ability to speak, swallow and taste. Because of these detrimental effects of pSS and the limited nature of existing treatment, a new long-term treatment, extending beyond simple symptomatic relief, has become necessary. Recent successes in the field of gene therapy have suggested this may be a viable solution provided the correct immunomodulatory or physiological basis for the loss of gland function can be identified. Due to the ability of the recombinant AAV (rAAV) to successfully target the salivary glands, their use has been the focus of future gene therapy options for pSS.16–18
This study focused on assessing the impact of the pre-existing immunity to the vector and if Sjögren’s patients had a higher prevalence of neutralizing activity to the vector, which could negatively impact this approach. Research on parvoviruses has shown they are well adapted to evade the neutralization in the presence of at least low levels of antibodies.19 The results for most serotypes demonstrate no significant difference in the prevalence of Abs between the study populations. As there is a general trend toward increased titers in the pSS population an increase in sample size could result in a statistically significant finding. For AAV5, the increase in prevelance of nAb in the pSS population is the result of an increase in the medium- and high-titer individuals within the Sjögren’s group and suggests that this serotype would be less effective in pSS patients compared with the others. Although the overall seropositivity for AAV5 is low compared with other serotypes like AAV2, pre-existing immunity to this vector could decrease transduction. Even with AAV2, which has the most robust data and has the highest number of positives in the high group, it is not significant.
The overall importance of pre-existing neutralizing antibodies on gene transfer to the salivary gland is not clear as the most common route of delivery is by retroductal cannulation to the gland, minimizing exposure to serum antibodies. In rodents, retroductal repeat administration within a month resulted in an anti-AAV2 immune response that interfered with transduction.20 In contrast, transduction via retroductal delivery of adenovirus vectors to the salivary glands of humans showed no relationship to pre-existing adenovirus serotype 5 neutralizing antibodies.8 A clinical trial using AAV2 to transfer aquaporin 1 to the salivary glands of radiation-induced xerostomia patients has been approved (NCT02446249) and results from this study may help to answer this question.
A statistically significant correlation exists between the positive population for some serotypes. This observation has been previously reported in other studies21,22 and suggests that despite many AAVs being serologically independent there is likely a correlation in seroconversion frequency for some serotypes. Further research would be required to determine the mechanism associated with this observation and if this is the result of coinfection with multiple serotypes, multiple subsequent infections, or an association with a common helper virus. Alternatively, the lack of non-specific individuals could arise from the rapid molecular evolution of multiple co-infecting parental viruses in an individual leading to a spectrum of related antigens as suggested by Calcedo et al.21
In vivo, vector neutralization can occur through a variety of mechanisms, including Fc-mediated phagocytosis, complement binding and activation, opsonization, and antibody-dependent cellular cytotoxicity of infected cells.23 Our study has focused on antibody-mediated neutralization, which likely is affecting vector cell surface binding and attachment. Most serotypes studied in this manuscript remain viable options in the treatment of Sjögren’s syndrome patients via gene therapy. Despite the higher titers found for rAAV2 within the pSS population, they remain statistically similar to healthy participants and AAV2 would likely be as effective a vector in either population. Serotypes 4, 5, and 12 show low nAb titers in both populations, therefore posing minimal chance of neutralization. Serotype BAAV showed a higher prevalence of nAb similar to that of 2; however, the majority of positive individuals had only low titers, which would have a minimal effect on transduction. Our results also indicate there is no correlation between auto-antibodies (antinuclear antibodies or IgG) and neutralizing titers for all serotypes.
The current treatment of Sjögren’s syndrome is limited to the relief of symptoms without addressing salivary dysfunction. Although Sjögren’s syndrome is considered a systemic disease, therapies targeting localized correction of the loss of salivary gland activity have been investigated, as patients with more severe sicca symptoms (loss of secretory function) report a significantly greater impact on many aspects of their daily life.24 Several molecules locally expressed in the salivary gland have recently shown promise in preclinical studies, including soluble CTLA4IgG fusion proteins and modulators of the interleukin-17 pathways.25,26 Clinical trials are evaluating systemic delivery of abatacept (sCTLA4:IgG) and Belimumab, a monoclonal antibody against BAFF. Both are approved for the treatment of adult auto-antibody-positive, systemic lupus erythematosus and arthritis, respectively. Although effective in these indications, both have significant side effects, including risk of infection that could limit their utility in treating Sjogren’s syndrome patients. Delivery of these local immunomodulators to the salivary gland via gene transfer would result in a higher local concentration within the gland and reach a comparable treatment effect, while minimizing some of the side effects associated with the drugs. Indeed, this concept has been supported with sCTLA4 expression in preclinical animal models.26 In addition, it would offer a more constant level of protein in circulation than is currently possible with injections.
MATERIALS AND METHODS
Cell culture
African green monkey kidney COS cells, obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA), were cultured. Cells were maintained at 37 °C in a 5% CO2 humidified atmosphere in Dulbecco’s Modified Eagle’s Medium (DMEM). DMEM was supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U of penicillin ml−l, and 0.1 mg of streptomycin ml −1 (Invitrogen, Carlsbad, CA, USA).
Samples
Serum samples from 38 HD (Group HD) and 39 pSS (Group pSS) were obtained from the NIH blood bank and the NIDCR Sjögren’s clinic, respectively (Table 1). All HD serum was from females. The study was approved by the Institutional Review Board of the National Institute of Dental and Craniofacial Research at the NIH (NCT00001390) and is registered at www.clinicaltrials.gov. All subjects signed a written consent document before enrollment. All serum samples were stored frozen and heat inactivated before testing in a virus neutralization assay in order to prevent complement-mediated cell lysis.
Table 1.
Demographics of the study subjects
| Healthy donors | Sjögren’s syndrome patients | |
|---|---|---|
| Population size (n) | 38 | 39 |
| Mean age (years) ±s.d. | 48.7 ±14.2 | 52.2 ±13.2 |
| Minimum age (years) | 17 | 24.5 |
| Maximum age (years) | 72 | 75.8 |
AAV vector production and characterization
AAV vectors, expressing a nuclear localized green fluorescent protein (GFP), were produced as described earlier.10 Briefly, 293T cells were transfected with pAAV2-NLS-GFP, the appropriate rep and cap genes for the serotype, along with the Ad helper plasmids 449B.27 Recombinant particles were purified by cesium chloride gradient centrifugation. DNase-resistant genome copy numbers of the vector stocks were determined by quantitative real-time PCR using the TaqMan system (Applied Biosystems) with probes specific to the cytomegalovirus promoter.
Neutralization assay
COS cells were seeded at a density of 7000 cells per well in a 96-well plate 1 day before inoculation. 2 × 107 recombinant particles were pre-incubated with serial dilutions of serum in medium for 1 h at room temperature. Cells were then inoculated with this mixture for 1 h at 37 °C and then washed with medium. Forty-eight hours after transduction, cells were analyzed for GFP expression by flow cytometry (BD FACSArray, Sparks, MD, USA). As a positive control, cells incubated with virus only were analyzed.
Samples were classified as either positive or negative for neutralizing activity. Serum samples were considered positive for neutralizing activity if transduction was inhibited by at least 50% at the 1:25 dilution. Of the positive samples, titer values were assigned to the sera based on the dilution that inhibited at least 50% transduction. The positive samples were then further categorized as having low, medium, or high neutralizing titer if greater than 50% transduction was achieved with the ≤1:25, 1:51–1:200 or >1:201 dilutions, respectively.
Statistical analysis
A Fisher’s exact test was used to compare the proportion of positive vs negative samples. Analysis of the proportion of the low to medium or high samples was carried out using a two-sample test of proportion. A sample size calculation for a generic AAV with a 50% difference in prevalence between HD and pSS and an alpha of 0.05, power of 0.8 would require a sample size of 15 in each group. For the AAV5 nAb with a prevalence of 11 or 38% for neutralizing antibodies when compared between pSS and the HD groups (P<0.05), the Fisher’s exact test with a 0.05 two-sided significance level has a 76% power to detect the difference between the pSS and HV when the sample sizes are 39 and 38, respectively. A Spearman correlation analysis was used to analyze the correlation between the nAbs of the two AAV serotypes in HD and pSS, respectively. The Fisher’s exact test was done by using SAS 9.2 (SAS Institute, Cary, NC, USA) and power analysis was done using nQuery Advisor 7 (Statistical Solutions Ltd, Boston, MA, USA). Spearman correlation was performed with GraphPad Prism statistical software (GraphPad Software Inc., Version 4.02, La Jolla, CA, USA). All statistical analyses were done using a P value ≤0.05 as statistically significant.
Supplementary Material
Acknowledgments
This work is supported by NIH and NIDCR intramural grants to JAC.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Fox RI. Sjogren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Hansen A, Lipsky PE, Dorner T. Immunopathogenesis of primary Sjogren’s syndrome: implications for disease management and therapy. Curr Opin Rheumatol. 2005;17:558–565. doi: 10.1097/01.bor.0000172801.56744.c3. [DOI] [PubMed] [Google Scholar]
- 3.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 4.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci USA. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsunawaki S, Nakamura S, Ohyama Y, Sasaki M, Ikebe-Hiroki A, Hiraki A, et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjogren’s syndrome. J Rheumatol. 2002;29:1884–1896. [PubMed] [Google Scholar]
- 6.Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E, et al. Inefficacy of infliximab in primary Sjogren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjogren’s Syndrome (TRIPSS) Arthritis Rheum. 2004;50:1270–1276. doi: 10.1002/art.20146. [DOI] [PubMed] [Google Scholar]
- 7.Steinfeld SD, Demols P, Appelboom T. Infliximab in primary Sjogren’s syndrome: one-year followup. Arthritis Rheum. 2002;46:3301–3303. doi: 10.1002/art.10674. [DOI] [PubMed] [Google Scholar]
- 8.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA. 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katano H, Kok MR, Cotrim AP, Yamano S, Schmidt M, Afione S, et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 2006;13:594–601. doi: 10.1038/sj.gt.3302691. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Katano H, Bossis I, Chiorini JA. Cloning and characterization of a bovine adeno-associated virus. J Virol. 2004;78:6509–6516. doi: 10.1128/JVI.78.12.6509-6516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H, et al. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis. 2011;17:2436–2442. doi: 10.1002/ibd.21673. [DOI] [PubMed] [Google Scholar]
- 13.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 14.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 15.Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodde BM, Mineshiba F, Wang J, Cotrim AP, Afione S, Tak PP, et al. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren’s syndrome. Ann Rheum Dis. 2006;65:195–200. doi: 10.1136/ard.2005.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vosters JL, Roescher N, Illei GG, Chiorini JA, Tak PP. TACI-Fc gene therapy improves autoimmune sialadenitis but not salivary gland function in non-obese diabetic mice. Oral Dis. 2012;18:365–374. doi: 10.1111/j.1601-0825.2011.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, et al. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol. 2005;185:363–372. doi: 10.1677/joe.1.06171. [DOI] [PubMed] [Google Scholar]
- 19.Nelson CD, Palermo LM, Hafenstein SL, Parrish CR. Different mechanisms of antibody-mediated neutralization of parvoviruses revealed using the Fab fragments of monoclonal antibodies. Virology. 2007;361:283–293. doi: 10.1016/j.virol.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kok MR, Voutetakis A, Yamano S, Wang J, Cotrim A, Katano H, et al. Immune responses following salivary gland administration of recombinant adeno-associated virus serotype 2 vectors. J Gene Med. 2005;7:432–441. doi: 10.1002/jgm.678. [DOI] [PubMed] [Google Scholar]
- 21.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 24.Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjogren’s Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. doi: 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjogren’s syndrome using adenovirus-mediated gene transfer. Lab Invest. 2011;91:54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin H, Nguyen CQ, Samuni Y, Uede T, Peck AB, Chiorini JA. Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6. NOD-Aec1Aec2 mouse model of Sjogren’s syndrome. Arthritis Res Ther. 2012;14:R40. doi: 10.1186/ar3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RH, Afione SA, Kotin RM. Transposase-mediated construction of an integrated adeno-associated virus type 5 helper plasmid. Biotechniques. 2002;33:204–206. 8, 10–1. doi: 10.2144/02331dd04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


