Abstract
Given the prevalence and societal impact of autism spectrum disorders (ASD), there is an urgent need to develop innovative preventative strategies and treatments to reduce the alarming number of cases and improve core symptoms for afflicted individuals. Translational efforts between clinical and preclinical research are needed to (i) identify and evaluate putative causes of ASD, (ii) determine the underlying neurobiological mechanisms, (iii) develop and test novel therapeutic approaches and (iv) ultimately translate basic research into safe and effective clinical practices. However, modeling a uniquely human brain disorder, such as ASD, will require sophisticated animal models that capitalize on unique advantages of diverse species including drosophila, zebra fish, mice, rats, and ultimately, species more closely related to humans, such as the nonhuman primate. Here we discuss the unique contributions of the rhesus monkey (Macaca mulatta) model to ongoing efforts to understand the neurobiology of the disorder, focusing on the convergence of brain and behavior outcome measures that parallel features of human ASD.
Keywords: animal model, autism spectrum disorder, rhesus monkey, preclinical
Integrating Clinical and Preclinical ASD Research Efforts
Autism spectrum disorder (ASD) is a collection of neurodevelopmental disorders characterized by early onset deficits in social behavior and communication, paired with repetitive behaviors and restricted interests. Current estimates indicate that ASD affects over 1% of children in the United States [1], yet there remains relatively little understanding of the underlying cause and few treatment options. Early behavioral interventions are effective for some, but not all, children with ASD [2, 3] and at present there are no pharmacological interventions targeting the core symptoms. There is a growing consensus that ASD is unlikely to have a single etiology and is actually a number of distinct brain disorders each caused by a complex interplay of genetic and environmental factors [4, 5]. This is perhaps not surprising given that individuals with ASD may exhibit a range of social and communication symptoms (mild to profound impairments), cognitive abilities (enhanced function to intellectual disability), and brain growth (both micro and macrocephaly) paired with a multitude of common comorbidities, which may include epilepsy, anxiety, gastrointestinal dysfunction, sleep disturbances, and abnormal sensory processing. The clinical heterogeneity of ASD has made it exceedingly difficult to determine how genetic and environmental risk factors interact to alter neurodevelopmental processes and ultimately produce ASD symptomatology. These challenges pose major roadblocks to developing novel therapeutic interventions and preventative strategies for ASD.
In spite of these challenges, there are some consistent neuroanatomical features emerging from research in ASD patient populations [6, 7]. A large body of neuroimaging work suggests brain regions that modulate social and emotional behavior, such as the frontal and temporal cortices as well as the amygdala, undergo an aberrant growth trajectory in children and adults with ASD [8–11]. However, neuroimaging studies lack the resolution to pinpoint the underlying neurobiology that contributes to these aberrant brain developmental trajectories. Postmortem brains from individuals diagnosed with ASD during life are essential to evaluate the neuropathology of the disorder [12] and have revealed consistent alterations in brain regions that play a role in social development (reviewed in [13]). However, due to the paucity of ASD postmortem brain tissue, relatively few cellular and molecular studies have been carried out in ASD as compared to other CNS disorders, such as schizophrenia or Alzheimer’s. This, in turn, has severely limited progress toward development of targeted therapeutic approaches and preventative strategies. Understanding the neurobiology of ASD will require increasingly coordinated research efforts between clinical and preclinical approaches. Research using animal models can be used to fill in the gaps in our knowledge by providing an experimental system to evaluate hypotheses that, for ethical and practical reasons, are impossible to test using human subjects [14, 15]. A unique strength of an animal model is the ability to integrate behavior, in vivo neuroimaging, and ultimately postmortem pathology in a single organism – an impossibility in human research. This allows animal models to play a key role in both “forward translation”, in which new basic science discoveries are developed into novel clinical therapies and in “reverse translation”, which allows for mechanistic exploration of clinical findings [16]. In this review we focus exclusively on nonhuman primate models of autism, emphasizing features of neuroanatomy and behavioral complexity that uniquely position the nonhuman primate model to bridge the gap between rodent models and ASD patient populations [17]. The reader is referred to a series of recent review articles describing other species used in ASD preclinical research [18–21].
Assessing Validity in Animal Models of ASD
Developing valid animal models has proven exceptionally challenging for complex brain disorders, including ASD, where the varied symptoms are difficult to model in any nonhuman species [22–26]. Although efforts to improve reproducibility in preclinical research may address some of these challenges [27–29], the fundamental issue of attempting to model a uniquely human disorder in a nonhuman species remains a paramount concern. Historically, the validity of animal models has been determined by: (i) Construct validity - etiological relevance of the model to human disease(s), (ii) Face validity - resemblance of outcome measures of the model to features of the human disease and (iii) Predictive validity - response of the model to therapeutic agents used to treat the human disease [30]. Although interpretation of these criteria vary [31], a basic goal for a valid animal model of ASD is to stem from an etiologically relevant experimental paradigm (high construct validity), produce an animal that exhibits species-specific changes in behavior related to core diagnostic features of ASD (high face validity) that may ultimately be used to develop and evaluate novel therapeutic interventions (high predictive validity).
Construct validity
Although much emphasis has been placed on evaluating the face validity of ASD animal models, it is equally important to begin with a model which stems from an etiologically relevant question. Early efforts to develop valid animal models of ASD were hindered by the lack of knowledge of the underlying cause(s) of ASD. Recent advances from clinical research now allows for animal models to experimentally evaluate putative genetic and environmental risk factors identified through ASD patient studies. It is, however, important to recognize that the vast majority of ASD animal models continue to focus on a single risk factor, though ASD likely results from a complex interplay of genetic and environmental factors. Single risk factor models are thus expected to produce a circumscribed series of brain and behavioral alterations, rather than the full symptomatology of ASD. This limitation is important to bear in mind when interpreting the validity of any single hit animal model of human disease.
Face validity
An animal model with high face validity will produce animals that exhibit symptoms that parallel features of the human disease. Given that ASD is a behaviorally defined disorder, the behavioral outcome of the animal model is the primary outcome measure of face validity. Individuals with ASD exhibit persistent deficits in social communication and social interaction across multiple contexts paired with restricted, repetitive patterns of behavior, interests, or activities [32]. In this review we will highlight the potential of the nonhuman primate to enhance the translational value of ASD animal models by utilizing a nonhuman primate battery of ASD-relevant behavioral tests developed over the past two decades [33, 34] and by integrating new technologies, such as non-invasive eye tracking. We also acknowledge that other innovative approaches are underway, such as the NIH led Research Domain Criteria (RDoC) initiative, that have tremendous potential to improve translation of basic and clinical neurodevelopmental disorder research [35]. The RDoC objective is to provide a novel framework for CNS disorder research that utilizes a dimensional classification based on genes, neural circuits and behavioral constructs rather than traditional DSM criteria [36]. We are, however, in the earliest stages of applying this approach to neurodevelopmental disorders and to our interpretation of preclinical models [37].
Predictive validity
Predictive validity addresses the specificity of the animal model to treatments that are effective in the human disease (i.e., treatments that ameliorate the human symptoms should also reverse pathological features in the animal model). As no drug treatment has been approved for the core symptoms of ASD, predictive validity cannot currently be determined in animal models of ASD. However, epidemiologically informed animal models that utilize behavioral assays with strong face validity will likely contribute to the development of pharmacological interventions targeting ASD symptomatology [38].
Unique Features of the Nonhuman Primate Model
The vast majority of ASD preclinical research is carried out in rodent models. In early 2017, a Pub Med search of “mouse model autism” yielded 915 articles and “rat model autism” yielded 294 articles, while a search of “nonhuman primate model autism” yielded only 15 articles. Mouse models have indeed laid the foundation for developing animal models of ASD and will undoubtedly continue to be an important species in ASD research, especially in models that incorporate genetic susceptibility. There are, however, limitations in relying on a single species to study complex human brain disorders, such as ASD. Renewed interest in developing rat ASD models may improve the translational utility of rodent models by utilizing animals with complex brains and display an enriched repertoire of social behavior [39, 40]. However, we suggest that a uniquely human disorder, such as ASD, may ultimately benefit from the use of animal species even more closely related to humans, such as the rhesus macaque (Macaca mulatta). Compared with rodents, which are separated from humans by more than 70 million years of evolution [41, 42], macaques diverged from human evolution closer to 25 million years ago and thus exhibit greater similarity to humans in genetics, neurobiology and, ultimately, behavior (Figure 1).
Figure 1.
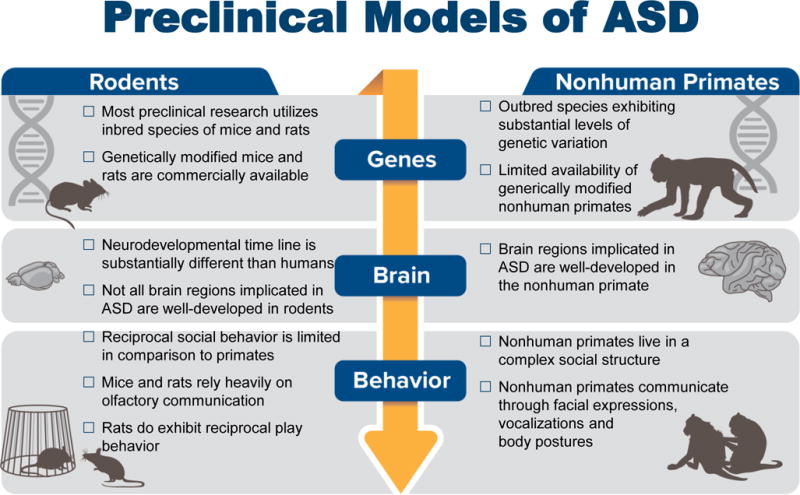
Comparison of ASD relevant considerations of genes, brain and behavior in rodent and nonhuman primate preclinical models.
The commonalities between rhesus monkeys and humans provide experimental platforms that can be used to evaluate etiologies, identify underlying neuropathology, and ultimately develop and test novel pharmacological interventions to alter brain and behavioral impairments not found in rodents [43]. Much like humans, rhesus macaques live in complex social groups and have evolved a sophisticated social communication system that includes a variety of facial expressions, body postures and vocalizations (Figure 2) [44]. Early social development in an infant macaque in many ways parallels that of human infants, albeit at an approximately four times faster maturational rate. Both human and nonhuman primate infants must rapidly learn to interpret and produce social interactions in order to successfully integrate into their social group where they will form lasting relationships with peers [45, 46]. Among macaque social signals, the use of facial expressions is one of the most salient features of macaque social behavior and the most similar to our own social communication [47–50]. Like humans, monkeys readily attend to social images and show a remarkably human-like pattern of visual attention that focuses heavily on the eyes and mouth [51]. Although not all human behaviors can be precisely assessed in a nonhuman primate (i.e., language, theory of mind), these animals are a closer approximation to humans in both neural and behavioral complexity and provide a valuable tool for bridging the gap between rodent models and ASD patient populations. Indeed, brain regions underlying complex social behaviors also show similar developmental and activity patterns in human and nonhuman primates [52] and are either not well-developed or nonexistent in rodents. For example, the frontal cortex has demonstrated the greatest phylogenetic growth of any brain region, which likely reflects the demands of living in more complex social environments that require increasing complexity of connections between brain regions [53]. There are cytoarchectonic regions that are identifiable in the human and nonhuman primate brain that are significantly less developed or absent in the rodents, including prefrontal regions that are highly implicated in social cognition [54–56]. In addition, the amygdala has nearly identical arrangement of nuclei cytoarchitecture, neurochemical distribution, connectivity and functional properties in the human and nonhuman primate [57, 58], but remarkably different nuclei distribution in the rodent brain [59]. Although the nonhuman primate model may provide a bridge from rodent models to human disease, the increased costs and ethical considerations constrain the use of nonhuman primates in research. Below we provide examples of how the nonhuman primate can be selectively used to improve translation between ASD preclinical and clinical research efforts.
Figure 2.

Rhesus monkeys live in large social groups and communicate with a variety of facial expressions, vocalizations and body postures. (K. West/California National Primate Research Center).
Maximizing Translational Utility of the Nonhuman Primate ASD Model
In this review, we focus specifically on the social domain of ASD symptomatology, which is inherently complex and requires an ethologically valid approach to assess in animal models [60]. Social impairments vary greatly among individuals with ASD [61] and is currently defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) by early onset “persistent deficits in social communication and social interaction across multiple contexts” that may manifest as impairments in (i) social-emotional reciprocity, ranging from abnormal social approach and failure to initiate or respond to social interactions, (ii) nonverbal communicative behaviors used for social interaction, ranging from poorly integrated verbal and nonverbal communication to a total lack of facial expressions and nonverbal communication and (iii) deficits in developing, maintaining, and understanding relationships, including difficulties adjusting behavior to suit various social contexts and/or an absence of interest in peers [32]. There are obvious limitations in our ability to model these complex behaviors in a non-human species, and results from preclinical models need to be interpreted cautiously [62].
A variety of paradigms have been developed to measure social interactions in rodent ASD models, ranging from simple automated procedures to labor intensive quantification of reciprocal social interactions [63, 64]. For many mouse models of ASD, simple, automated assessments of social interest, such as the three-chambered social approach test is commonly used as a first line screening assay for autism like phenotypes [65]. In mice, for example, the species-typical response to an unfamiliar conspecific is to approach and investigate; decreased time spent investigating a stimulus animal is operationally defined as diminished sociability [66–68]. The three-chamber social approach assay does not, however, allow animals to engage in species-typical reciprocal social behavior, and may not be sensitive to more subtle alterations in social behavior that may be detected in rats, nonhuman primates, and other species that engage in reciprocal social behaviors [69]. Although standardized behavioral phenotyping tools developed for rodents have led to more coordinated preclinical research efforts, there remains a need to develop additional behavioral tests that capture the full spectrum of social deficits relevant to ASD [70]. The rich social repertoire of the rhesus monkey provides an opportunity to address the fundamental issue of how to develop behavioral assays with high relevance to the diagnostic symptoms of ASD.
The diagnosis of ASD usually occurs in two stages, often beginning with concerns from parents and/or ASD-specific screening that has been incorporated into well-child visits. Children identified through early screening may then require a comprehensive evaluation carried out by a developmental pediatrician, child psychologist, or psychiatrist. These commonly include the Autism Diagnostic Observation Schedule (ADOS), which is a semi-structured, standardized assessment that consists of carefully planned social interactions and communication and opportunities to elicit spontaneous behaviors within specific contexts [71]. During the ADOS, a trained examiner presents the child with various prompts (i.e., response to name, requests for toys etc.) and then rates items on a 4-point scale that are summed into two algorithms: Social Affect and Restricted and Repetitive Behaviors. The ADOS has a strong predicative validity [72] and, along with the semi-structured parent interview Autism Diagnostic Interview Revised (ADI-R), are considered the gold standard for diagnosing ASD [73]. Because the ADOS requires a well-trained examiner to engage the child using a series of carefully scripted prompts and the ADI-R requires an extensive interview to provide the developmental history, it is not feasible to develop comparable screening tools for a nonhuman species. However, caregiver-questionnaires commonly used in ASD research, such as the Social Responsiveness Scale (SRS) [74], may be more amenable for adaptation in a nonhuman species. The SRS provides a quantitative measure of behavioral variability that can be used to identify individuals who do not reach diagnostic criteria for ASD, but nonetheless display atypical social behaviors in comparison to the general population [75]. The 65-item human SRS has been adapted for use in chimpanzees by modifying items, such as language, which cannot be ascertained in nonhuman primates [76]. Chimpanzee colony caretakers have demonstrated strong interrater reliability on the resulting 36-item chimpanzee version and are able to accurately detect individual variation in chimpanzee social behavior, suggesting that the SRS may be a useful tool for measuring social responsiveness in both humans and chimpanzees. Subsequent efforts to adapt the chimpanzee version of the SRS for use in rhesus monkeys have yielded mixed results [77]. Highly trained caretakers using the monkey version of the SRS were able to identify a subset of macaques displaying atypical patterns of social responsiveness related to social avoidance, social anxiety/inflexibility, lack of social confidence, and social awkwardness. However, only 4 of 36 items on the monkey SRS were considered highly reliable, suggesting that additional work will be needed to address concerns of low intra-item reliability. Nonetheless, the potential of adapting ASD screening tools for use in nonhuman primates would provide a useful tool for measuring behavioral variability and improve translational relevance of preclinical ASD models.
Given that ASD is a behavioral disorder defined by impairments in early social development, one of the most direct ways to assess the validity of a putative animal model of ASD is to quantify the emergence of species-typical social interactions. Over the past two decades, our research team has worked closely with primatologists and child psychologists to develop a battery of behavioral tests that can be used to quantify ASD-relevant changes in social development in the rhesus monkey (for review, [78]). Although it is beyond the scope of the current review to provide a detailed description of approaches to measuring social behavior in nonhuman primates, here we highlight ASD-relevant behavioral phenotyping tools that have been developed specifically for use in nonhuman primates by our laboratory and others (Table 1). Many of the nonhuman primate behavioral outcome measures we have developed have been specifically designed to bridge the gap between rodent models and ASD patient populations [81]. Deficits in social play, for example, are a prominent feature of ASD that can be measured in preclinical animal models by measuring the frequency and duration of species-typical play behaviors [79, 80]. Our recommendations for an ASD-relevant behavioral battery include: (i) provide the offspring with a social rearing environment, ideally consisting of age-matched peers and adults to facilitate species typical social development, (ii) screen for delays in motor, reflex or sensory development that may interfere with social development, (iii) utilize testing paradigms that allow the animals to engage in complex, reciprocal social interactions with both familiar and unfamiliar conspecifics, (iv) utilize a comprehensive behavioral ethogram and focal sampling methods to obtain a detailed assessment of social interactions and (v) collect longitudinal behavioral data to map the trajectory of social development over time.
Table 1.
ASD diagnostic criteria for social communication/interaction domains and examples of relevant rhesus monkey behavioral assays highlighted in this review
| ASD Social Communication and Social Interaction Domains | Rhesus Monkey ASD-Relevant Behavioral Phenotyping |
|---|---|
ASD deficits in social-emotional reciprocity:
|
Rhesus monkey reciprocal social interaction:
|
ASD deficits in nonverbal communicative behaviors used for social interaction:
|
Rhesus monkey social communication:
|
ASD deficits in developing, maintaining, and understanding relationships:
|
Rhesus monkey peer social networks:
|
Note that the references listed above are not the only tools available for quantifying macaque social behavior. These studies, have, however, been previously utilized in studies of infant and juvenile macaque monkeys and provide the reader with a valuable catalog of ASD-relevant behavioral assays.
In addition to behavioral observations of the animal model, it is essential to integrate other ASD-relevant outcome measures, such as eye-tracking, in vivo neuroimaging, and ultimately postmortem neuropathology, to provide insight into the underlying neural mechanisms. Direct behavioral observations can be supplemented with less subjective measurements, such as non-invasive eye tracking that allow investigators to measure social attention in young humans and nonhuman primates [82]. Eye-tracking outcome measures are highly relevant to ASD, as converging evidence from numerous experimental paradigms indicate that individuals with ASD demonstrate diminished attention to social stimuli across development [83]. Adaptation of human eye-tracking paradigms for use in nonhuman primates provides an opportunity to evaluate social attention [84–88], and better identify neural circuitry underlying social processing [89]. The use of noninvasive eye-tracking in the nonhuman primate model will provide a powerful tool to evaluate novel preventative and therapeutic interventions in future studies and can improve translation from animal models to clinical populations [90].
Although we are at the earliest stages of understanding the neural underpinnings of ASD social deficits, efforts are underway to identify robust biomarkers that may lead to earlier identification, improved treatment efficacy and discovery of targeted treatments. Due to similarities in brain structure and function, nonhuman primates are particularly well-suited for adapting neuroanatomical methods commonly used in ASD clinical populations, such neuroimaging and postmortem brain pathology, to map structural alterations in brain development. Over the past two decades, structural magnetic resonance imaging (MRI) studies have provided insight into the neurobiological basis of ASD in vivo [91]. Structural imaging approaches are readily available for nonhuman primate models, which often have the additional benefit of collecting longitudinal data from individuals at multiple time points throughout development [92, 93]. Including longitudinal neuroimaging as an outcome measure, as described in the models below, can improve the translational utility of the nonhuman primate model and provide insight into underlying neurobiological and genomic mechanisms that can later be explored through extensive evaluation of postmortem tissue duplicating studies carried out in human brains (reviewed in, [94]). In fact, key “social brain” regions implicated in ASD, such as the amygdala, anterior cingulate, prefrontal and temporal cortices, are remarkably similar in the human brain and nonhuman primate brain and undergo similar developmental trajectories [95, 96] (Box 1). Recent efforts to integrate neuroscience tools, such as electroencephalography (EEG), into preclinical models will enable researchers to assess circuit integrity in ASD populations as well as animal models [97].
Box 1. Regions of the brain most commonly implicated in social processing for both humans and nonhuman primates. Although the macaque brain is only approximately one tenth the size of the human brain, the regions of the “social brain” are well developed. Many of these regions are also implicated in ASD neuropathology, thus illustrating the translational utility of the nonhuman primate model.
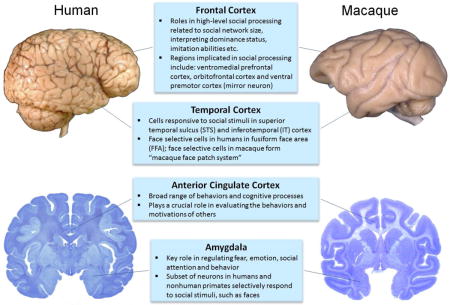
Suggested Readings:
Apps MA, Rushworth MF, Chang SW. The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron. 2016;90(4):692-707. doi: 10.1016/j.neuron.2016.04.018. PubMed PMID: 27196973; PubMed Central PMCID: PMCPMC4885021.
Bauman MD, Bliss-Moreau E, Machado C J & Amaral DG (2011). The Neurobiology of Primate Social Behavior. In: J. Decety and J. T. Cacioppo (eds.), Handbook of Social Neuroscience. New York. Oxford University Press.
Chang L, Tsao DY. The Code for Facial Identity in the Primate Brain. Cell. 2017;169(6):1013-28 e14. doi: 10.1016/j.cell.2017.05.011. PubMed PMID: 28575666.
Ferrari PF. The neuroscience of social relations. A comparative-based approach to empathy and to the capacity of evaluating others’ action value. Behaviour. 2014;151(2-3):297-313. doi: 10.1163/1568539X-00003152. PubMed PMID: 25258451; PubMed Central PMCID: PMCPMC4172363.
Rushworth MF, Mars RB, Sallet J. Are there specialized circuits for social cognition and are they unique to humans? Curr Opin Neurobiol. 2013;23(3):436-42. doi: 10.1016/j.conb.2012.11.013. PubMed PMID: 23290767.
Rutishauser U, Mamelak AN, Adolphs R. The primate amygdala in social perception - insights from electrophysiological recordings and stimulation. Trends Neurosci. 2015;38(5):295-306. doi: 10.1016/j.tins.2015.03.001. PubMed PMID: 25847686; PubMed Central PMCID: PMCPMC4417366.
Tremblay S, Sharika KM, Platt ML. Social Decision-Making and the Brain: A Comparative Perspective. Trends Cogn Sci. 2017;21(4):265-76. doi: 10.1016/j.tics.2017.01.007. PubMed PMID: 28214131.
Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci U S A. 2008;105(49):19514-9. doi: 10.1073/pnas.0809662105. PubMed PMID: 19033466; PubMed Central PMCID: PMCPMC2614792.
Overview of Nonhuman Primate Models of ASD
Below we provide a brief history of nonhuman primate models of ASD, then shift our focus to recent models that evaluate a specific etiology and/or treatment, highlighting the unique potential of the nonhuman primate model to integrate behavior and brain outcome measures. The reader is referred to excellent reviews on contributions of nonhuman primates to our basic understanding of the neural basis of social behavior [98, 99], including new approaches which integrate functional MRI, electrophysiology and anatomical tracer injections to redefine the neural basis of social processing [100]. Although these basic research studies undoubtedly contribute to the field of ASD research, we have elected to focus the review on more direct efforts to model ASD in nonhuman primates (Table 2), including: (i) Experimentally induced brain pathology models, (ii) Naturally occurring behavior based models, (iii) Epidemiologically informed models, (iv) Genetic models, and (v) Treatment models.
Table 2.
Rhesus Monkey Models Relevant to ASD
| Approach (References) | Brain and Behavioral Outcomes | Construct Validity | Face Validity | Predictive Validity |
|---|---|---|---|---|
| Experimentally-Induced Brain Pathology Models | ||||
| Neonatal amygdala lesion model (Bachevalier et al., 1994; Bauman et al., 2004a, 2004b; Bliss-Moreau et al., 2011, 2013; Raper et al., 2013, 2014a, 2014b) | Monkeys with complete bilateral amygdala damage demonstrate subtle impairments in social development and pronounced deficits in fear processing | low | low | N/A |
| Naturally Occurring Behavior-Based Models | ||||
| Endogenous variability in social behavior (Sclafani et al., 2016) | Juvenile monkeys identified as lowsocial show early impairments in recognizing familiar vs. novel faces and in the species-typical gaze aversion | low | moderate | N/A |
| ASD-Relevant Etiology Models | ||||
| Vaccine exposure (Hewitson et al., 2010; Curtis et al., 2015; Gadad et al., 2015) | No evidence of ASD relevant brain and behavior pathology | low | low | N/A |
| Prenatal exposure to maternal autoantibodies (Martin et al., 2008; Bauman et al., 2013) | Treated monkeys exhibit repetitive behaviors, social impairments and increased total brain volume that parallels features of ASD | high | high | N/A |
| Prenatal exposure to viral or bacterial infections (Willette et al., 2011; Short et al., 2010) | Monkeys born to dams treated with influenza or LPS late in gestation exhibit changes in behavior and/or brain development | moderate | moderate | N/A |
| Prenatal exposure to maternal immune activation (Bauman et al., 2014; Machado et al., 2015; Weir et al., 2015; Rose et al., 2016) | Monkeys born to dams treated with the viral mimic polyIC demonstrate inappropriate social interactions, fail to attend to salient social cues and brain pathology relevant to ASD | moderate | high | N/A |
| Genetic Models | ||||
| Transgenic MECP2 model (Liu et al., 2016) | Monkeys over expressing MECP2 demonstrate increased repetitive behaviors and decreased social interactions | moderate | moderate | N/A |
| ASD-Treatment Models | ||||
| Oxytocin (OT) treatment models (Chang et al., 2012; Ebitz et al., 2013;Parr et al., 2013, 2014, 2016; Dal Monte et al., 2014; Landman et al., 2014; Liu et a., 2015; Putnam et al., 2016;Simpson et al., 2017) | OT administration associated with pro-social outcomes in some, but not all, OT treatment models | moderate | moderate | moderate |
Experimentally Induced Brain Pathology Models
Early attempts to develop nonhuman primate models of ASD utilized a neural-systems approach to evaluate the role of specific brain structures, such as the amygdala, which had been implicated in ASD pathophysiology. The “amygdala theory of autism” that emerged in the early 1990s was based on reports of postmortem amygdala neuropathology in individuals with ASD [101] paired with emerging functional imaging studies that also implicated the amygdala in ASD pathophysiology [102]. Bachevalier and colleagues developed a nonhuman primate model to evaluate the role of the amygdala in social development utilizing a lesion based approach that had successfully contributed to our understanding of the role of the medial temporal lobe structure in memory. Early studies by Bachevalier described impairments in social development in peer-reared rhesus monkeys that sustained bilateral damage to the amygdala and surrounding cortex early in development [103]. The observation that animals with neonatal amygdala damage initiated less social contact and more social withdrawal than controls was interpreted as being highly relevant to ASD, though concerns were raised over the lack of specificity of the lesions and the restricted rearing environment which is known to impact social development. Subsequent studies of rhesus monkeys with more selective amygdala lesions that were raised in an enriched social environment failed to replicate these early social deficits [104–106], though the animals did demonstrate abnormal fear responses [107, 108] and subtle differences in socioemotional processes later in life [109–113]. More recent amygdala lesion studies by Bachevalier and colleagues have focused on refined changes in emotional reactivity [114, 115], including alterations in the hypothalamic-pituitary-adrenal (HPA) axis [116] following neonatal amygdala damage. Collectively, these studies suggest that the primate amygdala most likely plays a protracted role in socioemotional behaviors throughout development, but offers little evidence that complete damage to the amygdala early in development yields changes in behavior specifically relevant to ASD core social impairment. Although there is compelling evidence for structural and functional amygdala abnormalities in individuals with ASD [117–121], complete amygdala lesions in an animal model clearly do not represent findings in the clinical population. Moreover, amygdala pathology is not specific to ASD and appears to be a common feature of most all neurodevelopmental and neuropsychiatric disorders [122]. Although amygdala lesion research has provided valuable insight into the role of the amygdala in socioemotional development, the low construct and face validity of the neonatal amygdala lesion model would argue against this approach as a valid animal model of ASD.
Naturally Occurring Behavior Based Models
To the best of our knowledge, ASD is a uniquely human disorder that does not naturally occur in other nonhuman species [123]. Nonetheless, substantial effort has been placed on identifying animal models that exhibit ASD-relevant behaviors through selective breeding practices or through quantification of endogenous variability in species-typical social behavior. We suggest that caution should be utilized when selecting an animal model based solely on behavioral outcomes (i.e., models that demonstrate high face validity), rather than ASD-relevant etiology (i.e., demonstrate high construct validity). Inbred mouse strains, such as the BTBR T+tf/J (BTBR), exhibit impairments in social interaction and repetitive behaviors interpreted as being highly reminiscent of ASD symptoms [124] and have been used to test ASD-relevant social interventions [125–127]. However, the pronounced neural deficits present in the BTBR strain that are not present in ASD, such as congenital corpus callosum agenesis, have raised questions about the construct validity of this model [128, 129].
While there are no nonhuman primate models comparable to the BTBR mouse, there is renewed interest in capitalizing on naturally occurring variability in social behavior as a pre-clinical ASD research tool. Capitanio and colleagues have found that like humans, rhesus monkeys, show marked and stable individual differences in the tendency to interact socially with conspecifics [130, 131]. Adult monkeys classified as “low-sociable” are less interested in social interactions and differ from animals categorized as “high-sociable” in response to social stimuli [132]. Recent interest in using the low-social monkeys as an ASD model for the social deficit seen in ASD suggests that the trait of low sociability appears to result from a complex interplay of genes, brain, and environment that manifest as impaired social information processing very early in development [133]. Although the early developmental time frame of the low social model is relevant to ASD, it is not clear at this point if the low social monkeys reflect impaired social functioning or simply a continuum of sociability that is within the range of species-typical social behavior. It is plausible that low sociability in monkeys may be more relevant to other human conditions such as behavioral inhibition [134] and loneliness [135], rather than the profound social impairments that are characteristic of ASD. Nonetheless, naturally occurring variability in nonhuman primate social behavior could prove to be a useful testbed for identification of potential pro-social pharmacological interventions and/or understanding the neurobiological underpinnings of social behavior [136]. For example, previous studies have demonstrated that endogenous variability in cerebrospinal fluid oxytocin levels in juvenile and sub-adult macaques are positively associated with affiliative social behaviors [137].
Epidemiologically Informed Models
The previous examples of nonhuman primate ASD models illustrate the challenges in developing valid models for a uniquely human disorder when the cause(s) are unknown. However, recent progress in identifying putative causes of ASD has led to increasingly sophisticated preclinical research efforts. Here we highlight examples to demonstrate how nonhuman primate models have been used to directly evaluate risk factors initially identified through studies in patient populations, including: (i) Vaccine exposure and (ii) Prenatal immune challenge.
i. Vaccine Models
In the 1990s, concerns were raised regarding associations between the rising rate of ASD and the use of vaccines that contain thimerosal and/or the measles mumps rubella (MMR) vaccines. It is important to clearly state that human epidemiology studies from the past 20 years have provided no evidence linking vaccines with an increased risk of ASD [138]. A series of studies in nonhuman primates were initiated to empirically test the theory that vaccines cause alterations in brain and behavioral development in a species closely related to humans. The initial nonhuman primate vaccine models utilized small cohorts that yielded inconsistent and sometimes controversial findings [139, 140]. Subsequent studies with a large sample size, however, found no consistent evidence of neurodevelopmental deficits or aberrant behavior in vaccine exposed animals [141, 142]. Male nursery-reared nonhuman primates that received either thimerosal containing vaccines recommended in the 1990s or the 2008 expanded pediatric vaccine schedule did not exhibit consistent differences from unvaccinated controls during a number of neurobehavioral tests, including the acquisition of neonatal reflexes, the development of object permanence, the formation of discrimination learning strategies, and assessments of social development [141]. Moreover, there was no evidence of neuropathology in the cerebellum, hippocampus or amygdala of the vaccine treated monkeys [142]. This study also included behavioral data from two additional treatment groups: (i) animals that received the thimerosal containing vaccines, but no MMR vaccines and (ii) animals that received the MMR vaccines, but not the thimerosal containing vaccines, allowing investigators to differentiate between thimerosal and MMR exposure groups. Daily observations of the animals in their peer-socialization groups between 12-18 months of age revealed subtle differences in exploratory behavior, but not social interactions, between unvaccinated and vaccinated groups. However, there were no significant differences in any behavior measured between the control and experimental groups after 6 months of social living (at ~18 months of age). Collectively, the nonhuman primate models of vaccine exposure lend further support to epidemiological research that has found no link between vaccine and ASD-related brain and behavior pathologies.
ii. Prenatal Immune Challenge Models
Recent evidence suggests that the prenatal environment, and in particular, the maternal fetal immune environment, may be a promising area of ASD etiology research [143–145]. The focus on the prenatal environment is due in part to our growing understanding of the overlap between the developing nervous system and the immune environment. We now know that immune signaling molecules play a key role in all stages of fetal brain development [146, 147]. Experiences that alter the maternal-fetal immune environment may disrupt the finely orchestrated events of neural development, thereby increasing the risk of offspring central nervous system (CNS) disorders [148–150]. The nonhuman primate may prove particularly relevant to evaluate prenatal risk factors of ASD given the similarities in gestational and neurodevelopmental timeline, immune systems, and placental physiology. Below we describe two prenatal immune-based risk factors implicated in ASD: (i) maternal autoantibodies that target fetal brain tissue and (ii) prenatal exposure to immune challenges that activate the maternal immune system.
During mid-late gestation, IgG isotype antibodies from the mother are transported across the placenta in order to equip the immunologically naïve fetus with protection [151]. However, in addition to immunoprotective antibodies, autoantibodies that react to fetal ‘self’-proteins can also cross the placenta resulting in a potential neonatal autoimmune disease. IgG antibodies targeting fetal brain proteins have been reported in a subset of mothers who have given birth to a child with ASD (for reviews, [144, 152]. Specific combinations of these anti-brain antibodies targeting proteins at 37 and 73 kDa have only been found in mothers who have a child with ASD and not in mothers of typically developing children. The recently identified protein targets of these antibodies play critical roles in neural development, supporting the hypothesis that prenatal exposure to anti-brain autoantibodies could disrupt the trajectory of brain development and ultimately lead to one form of ASD [153]. As maternal autoantibodies become increasingly implicated in ASD, it has become imperative to utilize animal models to evaluate causative effects between prenatal exposure to anti-brain antibodies and ASD related brain and behavior pathology [154, 155]. A pilot study of rhesus monkeys prenatally exposed to nonspecific ASD-associated maternal antibodies produced offspring that exhibited high levels of motor stereotypies [156]. A subsequent nonhuman primate model that utilized ASD-specific maternal antibodies found only mothers of human children with ASD yielded offspring with social impairments, including increased protectiveness from their mothers, inappropriate social approach with a novel animal and deficits in reciprocal social interactions with familiar peers [157] (Figure 3). Male monkeys exposed to these ASD-specific autoantibodies also demonstrate enlarged brain volumes that parallel features of children exposed to the same maternal antibodies in utero [158]. Similar to children with ASD, differences in brain volume in male monkeys exposed to ASD-specific autoantibodies are predominantly accounted for by increases in frontal lobe volume. While the convergence of aberrant brain growth in both the clinical populations and the nonhuman primate model is compelling (Figure 3), the underlying neuronal mechanisms responsible for aberrant primate neurodevelopment following maternal brain-reactive antibody exposure remain unknown. Because there are no known human postmortem brain tissue samples from an individual prenatally exposed to ASD-specific maternal autoantibodies, the nonhuman primate model may provide the only opportunity to evaluate neurobiological mechanisms in a closely related species.
Figure 3.
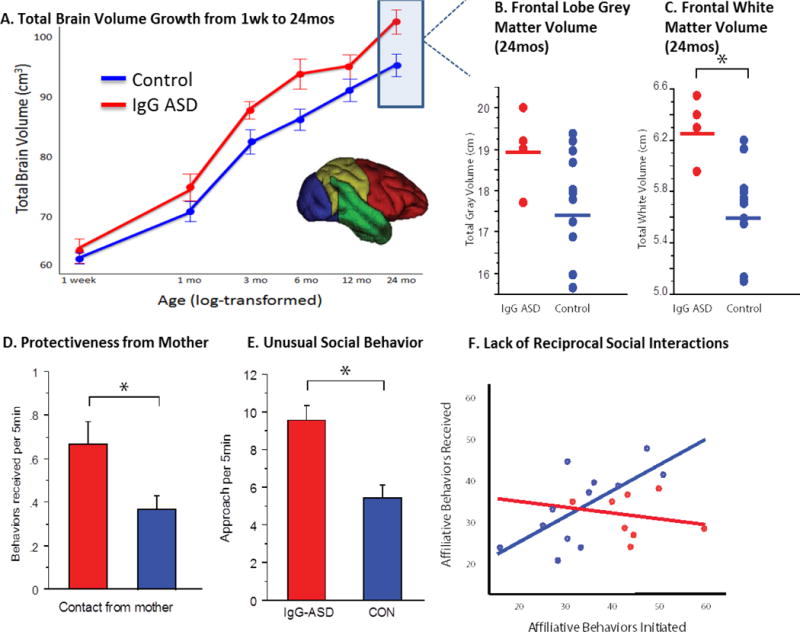
Aberrant brain (A–C) and behavioral (D–F) development in nonhuman primate offspring prenatally exposed to autism associated maternal antibodies (IgG-ASD) compared to control offspring. Adapted from (157), Bauman et al., Transl Psychiatry, 2013. 3: p. e278.
Other prenatal challenges, such as maternal infection during pregnancy, have also been associated with an increased risk of neurodevelopmental disorders, including ASD [159–161]. Although initial evidence was based primarily on case reports of ASD following prenatal exposure to infectious agents, such as rubella or cytolomegalovirus [160], recent large-scale epidemiology studies [162–165] paired with quantification of inflammatory markers obtained from archived maternal samples [166–169] lends further support to the link between maternal infection and increased ASD risk. Clearly not all women exposed to infections during pregnancy go on to have a child with altered neurodevelopment, suggesting that genetic susceptibility, gestational timing, and intensity of the prenatal challenge are critical factors to consider. Animal models are playing a key role in determining the mechanisms by which prenatal infection may alter fetal brain development. Initial nonhuman primate studies by Coe and colleagues evaluated the effects of prenatal exposure to viral or bacterial infections on subsequent brain and behavioral development of the offspring [170, 171]. Rhesus monkeys born to dams exposed to low dose endotoxemia in the third trimester exhibited subtle alterations in behavior and failed to show species-typical attenuation of startle responses on tests of prepulse inhibition [170]. At 1 year of age, the exposed offspring exhibited a global increase in white matter volume paired with region specific alterations in gray matter compared to controls. In a similar study, rhesus monkeys born to dams infected with influenza in the third trimester did not differ from controls on measures of behavioral development, but did exhibit global reductions of cortical gray matter and reduced white matter in the parietal lobe [171]. While these studies were not specifically designed as animal models of ASD, their findings demonstrate the power of pairing sophisticated behavioral assessments with structural MRI to systematically evaluate the effects of a prenatal insult on both brain and behavior development.
The diversity of infections associated with alterations in neurodevelopment suggests that the maternal immune response may be the critical link between prenatal exposure to infection and subsequent alterations in fetal brain development. Our research team has focused on the maternal immune response, rather than a specific pathogen, as the key link between prenatal exposure to infection and altered fetal brain development. The emerging maternal immune activation (MIA) hypothesis has been directly tested in animal models by artificially activating the immune system of pregnant rodents with the viral mimic, polyinosinic:polycytidylic acid (polyIC), a toll-like receptor-3 (TLR3) agonist that stimulates an inflammatory response in the absence of a specific pathogen. Rodent pups born to dams treated with poly IC at mid-gestation demonstrate behavioral abnormalities, neuropathology, and altered gene expression relevant to both ASD and SZ (reviewed in, [172–175]). Our laboratory adapted this approach to develop the first MIA nonhuman primate model by using a modified form of polyIC to stimulate the primate maternal immune response. Pregnant rhesus monkeys injected with poly IC at the end of either the first or second trimester produce offspring with abnormal motor stereotypies and repetitive behaviors [176]. While both first and second trimester MIA offspring produced fewer affiliative vocalizations than controls, only the first trimester MIA offspring showed signs of atypical social interactions with unfamiliar peers. Given that neurodevelopmental disorders, including ASD, are characterized by changes in social cognition and emotion, as well as altered visual attention devoted to facial expressions, we then initiated a series of noninvasive eye-tracking studies to provide further insight into the nature of the social impairments observed in the MIA offspring. As juveniles, male rhesus monkeys born to first trimester MIA treated dams differed from controls on several measures of social attention, particularly when viewing macaque faces depicting the “fear grimace” facial expression [177]. Compared to controls, these MIA offspring had a longer latency before fixating on the eyes, fewer fixations directed at the eyes, and spent less total time fixating on the eyes of the fear grimace images. The lack of attention to the eye region exhibited by treated animals represents a significant departure from social processing in normal macaques and typically developing humans [178] and parallels findings of both ASD and SZ clinical populations [179].
The relatively high construct and face validity of the nonhuman primate MIA model suggests that this model may provide the opportunity to evaluate underlying neuropathology associated with prenatal immune challenge, which may in turn provide insight into neural circuits that are vulnerable in ASD. Our preliminary evaluation of postmortem tissue also revealed differences in dendritic morphology in DLPFC of MIA-exposed offspring [180]. Compared to controls, apical dendrites of MIA-treated offspring are smaller in diameter and exhibit a greater number of oblique dendrites. These data provide the first evidence that prenatal exposure to MIA alters dendritic morphology in a nonhuman primate MIA model, which may have implications for revealing the underlying neuropathology of neurodevelopmental disorders related to maternal infection. MIA exposed offspring also demonstrate long lasting changes in immune function that may be relevant to ASD, including evidence of elevated plasma cytokine concentrations and increased functional immune responses following stimulation [181]. Collectively, the changes in brain, behavior and immune function observed in the nonhuman primate MIA model lends support to the hypothesis that prenatal infection acts as a “neurodevelopmental disease primer” that is possibly relevant to a number of neurodevelopmental disorders [182], including ASD [183].
Genetic Manipulation Models
Family and twin studies suggest that ASD is a highly heritable disorder, though understanding genetic contributions to ASD has proven to be exceedingly complex [184]. In spite of the large number of transgenic mouse models targeting ASD-relevant genes [185], there remains little understanding of how specific genetic mutations lead to a cascade of molecular abnormalities, alterations in neural circuitry and ultimately changes in behavioral development. Recent advances in genetic modification tools may also open the door to explore, for the first time, genetic risk factors in a species more closely related to humans [186–188]. Preliminary efforts have focused on the autism-related MECP2 gene [189]. Many ASD symptoms are present in humans who either have an extra copy of this gene (MECP2-duplication syndrome) or who have mutations in this gene (Rett syndrome) [190]. Genetically engineered long-tailed macaques (Macaca fascicularis) expressing between one and seven extra copies of MECP2, display aberrant behaviors that may be relevant to ASD [191]. Compared to controls, transgenic monkeys exhibit increased frequency of repetitive circular locomotion, increased anxiety, reduced social interaction with peers, and weak evidence of cognitive impairments. Although the monkeys do not mimic the entire spectrum of MECP2-duplication symptoms, including seizures and severe cognitive deficits, the nonhuman primate model has the potential to improve the translational utility of ASD-relevant mouse models. For example, future efforts utilizing neuroimaging and postmortem histological studies could identify regions of the brain impacted by MECP2 over-expression and CRISPR gene-editing technology could be employed to knock out extra copies. Moreover, the ability to experimentally manipulate genetics in a nonhuman primate model could expand upon promising gene x environment studies underway in nonhuman primate models [192–194].
ASD Treatment Models
The prevalence and societal impact of ASD creates an urgent need for innovative treatments that will improve the core social deficits in children with ASD. At present, drug treatments target only peripheral symptoms such as aggression, anxiety, and depression, and not the core social impairments present in every child with ASD [195, 196]. Efficacious pro-social pharmacological treatments delivered early in childhood in parallel with behavioral interventions could play a critical role in correcting the social development trajectory, greatly improve the quality of life for individuals with autism, and reduce the emotional and financial burden on families and society.
Here we highlight one of the most promising areas for ASD treatment research – the oxytocin (OT) system [197, 198] - to demonstrate the utility of the nonhuman primate model for evaluating ASD-relevant pharmacological interventions. OT is a neuropeptide that enhances social attachment and motivation in rodent models [199] and may improve social functioning in some individuals with ASD [200]. Although nasal OT therapy has previously been promoted as a “safe and promising” therapy for ASD [201], we know very little about the efficacy or mechanism of OT treatment in humans [202, 203]. For example, it is not clear if OT has a universal pro-social influence on human behavior, or an indirect effect by reducing overall anxiety [204, 205]. Moreover, it is not known if the effects of intranasal OT in humans are mediated by activity of central OT receptors or OT receptors in peripheral tissue. This is due, in part, to our incomplete understanding of the primate OT receptor system [206]. There is a clear need for pre-clinical, systematic evaluation of the long term safety, efficacy, and mechanism of OT in a species more closely related to humans.
Initial studies carried out in adult nonhuman primates demonstrated that inhaled OT in rhesus monkeys penetrates the CNS [207] and increases pro-social behaviors in a reward allocation test [208]. Subsequent studies in adult monkeys have reported that intranasal OT enhances socially reinforced learning [209], selectively attenuates rhesus monkeys’ attention to negative facial expressions [210], decreases social vigilance [211], enhances gaze following responses [212], increases fixations to the eye region relative to the mouth [213], and alters attention to emotional distractors [214]. Together, these studies support the hypothesis that OT promotes social exploration both by amplifying social motivation and by attenuating social vigilance in the rhesus monkey [215, 216]. Given that ASD is a developmental disorder, studies in younger animals are especially important. Nursery reared macaques treated with intranasal OT show an increase in facial gesturing at human care givers [217]. Interestingly, early indices of imitative skills predicted OT associated increases in affiliative behaviors, suggesting that infants who demonstrate a high propensity for social interactions early in life may be more sensitive to OT manipulation. A subsequent study from this same group reports that female nursery-reared monkeys are more socially skilled at baseline compared to males and that oxytocin improved working memory and gaze following, but only for males [217]. Although these initial pro-social results are intriguing, many questions remain regarding the long term effects of OT exposure. For example, chronic administration of OT to infant macaques increases the time spent viewing videos of dynamic facial expression, but also selectively reduces the attention to the eye region of neutral faces in a dose dependent manner [218]. The authors speculate that the mechanism of the non-prosocial outcome may be that repeated administration of OT down regulates OT receptors in regions of the brain that regulate social attention.
There is a clear need for additional studies focusing on the neural mechanisms that mediate the effects of OT in primates. In the rhesus monkey brain, OT receptors are most robustly expressed in regions involved with visual processing, including the nucleus basalis of Meynert, the superficial gray layer of the superior colliculus, the pedunculopontine tegmental nucleus, the trapezoid body, and the ventromedial hypothalamus [219]. A recent functional imaging study in macaques revealed that OT reduces responses to both fearful and aggressive faces and reduces functional coupling between the amygdala and areas in the occipital and inferior temporal cortex selectively in response to fearful and aggressive faces [220]. Although a high density of OT receptors has not been found in these regions of interest, activity in regions, such as the amygdala, may be modulated by projections from areas that do contain OT receptors, including the nucleus basalis of Meynert [221, 222]. The homologies between monkeys and humans in neural circuits mediating social behavior may provide a valuable test bed for the continued evaluation of OT and future pro-social pharmacological manipulations [223].
Future Directions
Evidence from clinical studies suggests that ASD is not a single disease, but rather a number of conditions with distinct etiology and pathophysiology that ultimately lead to similar behavioral impairments. As our understanding of ASD improves, so does our ability to develop increasingly sophisticated preclinical models to evaluate potential etiologies, identify underlying pathophysiology, and ultimately to develop novel therapeutic and preventative strategies. Although rodent models will continue to be the logical starting point for ASD preclinical research efforts, we suggest that it may ultimately be necessary to assess validity of an ASD model in a species that is more closely related to humans, such as the rhesus macaque. Thus, we would advocate for more coordinated research efforts between laboratories that utilize rodent and nonhuman primate models to advance mechanistic understanding and ultimately evaluate the efficacy of biological treatments for core impairments in ASD.
Highlights.
Advanced animal models are needed to study complex brain disorders, such as ASD
Rhesus monkeys demonstrate sophisticated social processing capabilities
Brain regions implicated in social processing are well developed in rhesus monkeys
This combination makes the rhesus monkey an invaluable preclinical tool for ASD
Acknowledgments
We dedicate this review to the memory of our collaborator, Dr. Paul Patterson, whose research continues to inspire the next generation of scientists committed to improving the lives of individuals with neurodevelopmental disorders. We also thank the members of the Bauman and Schumann Laboratories for ongoing discussion about the topics covered in this review. M.D.B. is supported by grants from the National Institute of Mental Health (NIMH; P50MH106438), the National Institute of Child Health and Human Development (NICHD; R21HD080498, U54HD079125), and the UC Davis Behavioral Health Center of Excellence. C.M.S is supported by grants from the NIMH (R21MH108909, R01MH097236). The authors wish to thank Katherine Kim and Allison Lau for assistance in preparing the manuscript, Steve Dana and Kathy West for assistance with figures and Dr. John Capitanio for feedback on a previous draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 2.Bacon EC, et al. Measuring outcome in an early intervention program for toddlers with autism spectrum disorder: use of a curriculum-based assessment. Autism Res Treat. 2014;2014:964704. doi: 10.1155/2014/964704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37(1):8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vorstman JA, et al. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017 doi: 10.1038/nrg.2017.4. [DOI] [PubMed] [Google Scholar]
- 5.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. doi: 10.1186/s13229-017-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–47. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donovan AP, Basson MA. The neuroanatomy of autism - a developmental perspective. J Anat. 2017;230(1):4–15. doi: 10.1111/joa.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazlett HC, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumann CM, et al. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66(10):942–9. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumann CM, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30(12):4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumann CM, Nordahl CW. Bridging the gap between MRI and postmortem research in autism. Brain Res. 2011;1380:175–86. doi: 10.1016/j.brainres.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JA, et al. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 2015;10:111–44. doi: 10.1146/annurev-pathol-012414-040405. [DOI] [PubMed] [Google Scholar]
- 14.Ecker C, Spooren W, Murphy DG. Translational approaches to the biology of Autism: false dawn or a new era? Mol Psychiatry. 2012 doi: 10.1038/mp.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauman MD, Schumann CM. Is ‘bench-to-bedside’ realistic for autism? An integrative neuroscience approach. Neuropsychiatry (London) 2013;3(2):159–168. doi: 10.2217/npy.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.t Hart BA. Reverse translation of failed treatments can help improving the validity of preclinical animal models. Eur J Pharmacol. 2015;759:14–8. doi: 10.1016/j.ejphar.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Watson KK, Platt ML. Of mice and monkeys: using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. J Neurodev Disord. 2012;4(1):21. doi: 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homberg JR, et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci Biobehav Rev. 2016;65:292–312. doi: 10.1016/j.neubiorev.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Kazdoba TM, Leach PT, Crawley JN. Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 2016;15(1):7–26. doi: 10.1111/gbb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazdoba TM, et al. Translational Mouse Models of Autism: Advancing Toward Pharmacological Therapeutics. Curr Top Behav Neurosci. 2016;28:1–52. doi: 10.1007/7854_2015_5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kas MJ, et al. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future perspectives. Psychopharmacology (Berl) 2014;231(6):1125–46. doi: 10.1007/s00213-013-3268-5. [DOI] [PubMed] [Google Scholar]
- 22.Kaffman A, Krystal JH. New Frontiers in Animal Research of Psychiatric Illness. Methods Mol Biol. 2012;829:3–30. doi: 10.1007/978-1-61779-458-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braff L, Braff DL. The neuropsychiatric translational revolution: still very early and still very challenging. JAMA Psychiatry. 2013;70(8):777–9. doi: 10.1001/jamapsychiatry.2013.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson PH. Modeling autistic features in animals. Pediatr Res. 2011;69(5 Pt 2):34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tordjman S, et al. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet. 2007;37(1):61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- 27.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612–3. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis SC, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–91. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilkenny C, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83(1):1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 31.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1(1):9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th. Arlington, Viginia: American Psychiatric Association; [Google Scholar]
- 33.Bauman MD, Amaral DG. Nonhuman primate models for autism spectrum disorders. In: Buxbaum JD, Hof PR, editors. The Neuroscience of Autism Spectrum Disorders. Elsevier; 2013. pp. 379–390. [Google Scholar]
- 34.Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2(5):295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 35.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76(5):350–3. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosgrove VE, Kelsoe JR, Suppes T. Toward a Valid Animal Model of Bipolar Disorder: How the Research Domain Criteria Help Bridge the Clinical-Basic Science Divide. Biol Psychiatry. 2016;79(1):62–70. doi: 10.1016/j.biopsych.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Ecker C, Spooren W, Murphy D. Developing new pharmacotherapies for autism. J Intern Med. 2013;274(4):308–20. doi: 10.1111/joim.12113. [DOI] [PubMed] [Google Scholar]
- 39.Vanderschuren LJ, Trezza V. What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr Top Behav Neurosci. 2014;16:189–212. doi: 10.1007/7854_2013_268. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton SM, et al. Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci. 2014;128(2):103–9. doi: 10.1037/a0035988. [DOI] [PubMed] [Google Scholar]
- 41.Gibbs RA, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428(6982):493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392(6679):917–20. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 43.Ruhela RK, Prakash A, Medhi B. An urgent need for experimental animal model of autism in drug development. Ann Neurosci. 2015;22(1):44–9. doi: 10.5214/ans.0972.7531.220210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang SW, et al. Neuroethology of primate social behavior. Proc Natl Acad Sci U S A. 2013;110(Suppl 2):10387–94. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein TA, Capitanio JP. Longitudinal stability of friendships in rhesus monkeys (Macaca mulatta): individual- and relationship-level effects. J Comp Psychol. 2012;126(1):97–108. doi: 10.1037/a0025607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein TA, et al. Early involvement in friendships predicts later plasma concentrations of oxytocin and vasopressin in juvenile rhesus macaques (Macaca mulatta) Front Behav Neurosci. 2014;8:295. doi: 10.3389/fnbeh.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15(6):543–8. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 48.Bower S, Suomi SJ, Paukner A. Evidence for kinship information contained in the rhesus macaque (Macaca mulatta) face. J Comp Psychol. 2012;126(3):318–23. doi: 10.1037/a0025081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrari PF, et al. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr Biol. 2009;19(20):1768–72. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrari PF, et al. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machado CJ, et al. Social and nonsocial content differentially modulates visual attention and autonomic arousal in Rhesus macaques. PLoS One. 2011;6(10):e26598. doi: 10.1371/journal.pone.0026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt ML, Seyfarth RM, Cheney DL. Adaptations for social cognition in the primate brain. Philos Trans R Soc Lond B Biol Sci. 2016;371(1687) doi: 10.1098/rstb.2015.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semendeferi K, et al. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5(3):272–6. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- 54.Preuss T. Do rats have a prefrontal cortex? The Rose-Woolsey-Akert reconsidered. Journal of Cognitive Neuroscience. 1995;(7):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Bicks LK, et al. Prefrontal Cortex and Social Cognition in Mouse and Man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80(3):633–47. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schumann CM, Vargas M, Lee A. Synopsis of primate amygdala neuroanatomy. In: Amaral DG, Adolphs R, editors. Living without an amygdala. Guilford Press; 2016. [Google Scholar]
- 58.Rutishauser U, Mamelak AN, Adolphs R. The primate amygdala in social perception - insights from electrophysiological recordings and stimulation. Trends Neurosci. 2015;38(5):295–306. doi: 10.1016/j.tins.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chareyron LJ, et al. Stereological analysis of the rat and monkey amygdala. J Comp Neurol. 2011;519(16):3218–39. doi: 10.1002/cne.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters SM, Pothuizen HH, Spruijt BM. Ethological concepts enhance the translational value of animal models. Eur J Pharmacol. 2015;759:42–50. doi: 10.1016/j.ejphar.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 61.Lord C, Bishop SL. Recent advances in autism research as reflected in DSM-5 criteria for autism spectrum disorder. Annu Rev Clin Psychol. 2015;11:53–70. doi: 10.1146/annurev-clinpsy-032814-112745. [DOI] [PubMed] [Google Scholar]
- 62.Servadio M, Vanderschuren LJ, Trezza V. Modeling autism-relevant behavioral phenotypes in rats and mice: Do ‘autistic’ rodents exist? Behav Pharmacol. 2015;26(6):522–40. doi: 10.1097/FBP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 63.Silverman JL, et al. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasciuto E, et al. Autism Spectrum Disorders: Translating human deficits into mouse behavior. Neurobiol Learn Mem. 2015;124:71–87. doi: 10.1016/j.nlm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0826s56. Chapter 8: p. Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang M, et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012;107(5):649–62. doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silverman JL, et al. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015;40(9):2228–39. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silverman JL, et al. AMPAKINE enhancement of social interaction in the BTBR mouse model of autism. Neuropharmacology. 2013;64:268–82. doi: 10.1016/j.neuropharm.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ku KM, et al. Behavioral Phenotyping of Juvenile Long-Evans and Sprague-Dawley Rats: Implications for Preclinical Models of Autism Spectrum Disorders. PLoS One. 2016;11(6):e0158150. doi: 10.1371/journal.pone.0158150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bishop SL, Lahvis GP. The autism diagnosis in translation: shared affect in children and mouse models of ASD. Autism Res. 2011;4(5):317–35. doi: 10.1002/aur.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lord C, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–23. [PubMed] [Google Scholar]
- 72.Gotham K, et al. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37(4):613–27. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 73.de Bildt A, et al. Interrelationship between Autism Diagnostic Observation Schedule-Generic (ADOS-G), Autism Diagnostic Interview-Revised (ADI-R), and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) classification in children and adolescents with mental retardation. J Autism Dev Disord. 2004;34(2):129–37. doi: 10.1023/b:jadd.0000022604.22374.5f. [DOI] [PubMed] [Google Scholar]
- 74.Constantino JN. Social Responsiveness Scale Second Edition (SRS-2) Los Angeles: Western Psychological Services; 2012. [Google Scholar]
- 75.Constantino JN, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163(2):294–6. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 76.Marrus N, et al. Initial description of a quantitative, cross-species (chimpanzee-human) social responsiveness measure. J Am Acad Child Adolesc Psychiatry. 2011;50(5):508–18. doi: 10.1016/j.jaac.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feczko EJ, et al. The Macaque Social Responsiveness Scale (mSRS): A Rapid Screening Tool for Assessing Variability in the Social Responsiveness of Rhesus Monkeys (Macaca mulatta) PLoS One. 2016;11(1):e0145956. doi: 10.1371/journal.pone.0145956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bauman MD, Amaral DG. Nonhuman primate models of autism. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism Spectrum Disorders. Oxford University Press; 2011. pp. 963–980. [Google Scholar]
- 79.Jordan R. Social play and autistic spectrum disorders: a perspective on theory, implications and educational approaches. Autism. 2003;7(4):347–60. doi: 10.1177/1362361303007004002. [DOI] [PubMed] [Google Scholar]
- 80.Young RL, Brewer N, Pattison C. Parental identification of early behavioural abnormalities in children with autistic disorder. Autism. 2003;7(2):125–43. doi: 10.1177/1362361303007002002. [DOI] [PubMed] [Google Scholar]
- 81.Bauman MD, Crawley J, Berman RF. Autism: Animal Models. 2010 [Google Scholar]
- 82.Machado CJ, Nelson EE. Eye-tracking with nonhuman primates is now more accessible than ever before. Am J Primatol. 2011;73(6):562–9. doi: 10.1002/ajp.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chita-Tegmark M. Social attention in ASD: A review and meta-analysis of eye-tracking studies. Res Dev Disabil. 2016;48:79–93. doi: 10.1016/j.ridd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 84.Paukner A, et al. Neonatal imitation predicts how infants engage with faces. Dev Sci. 2014;17(6):833–40. doi: 10.1111/desc.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simpson EA, et al. Face Detection and the Development of Own-Species Bias in Infant Macaques. Child Dev. 2017;88(1):103–113. doi: 10.1111/cdev.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simpson EA, et al. Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Sci Rep. 2016;6:20233. doi: 10.1038/srep20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simpson EA, Suomi SJ, Paukner A. Evolutionary relevance and experience contribute to face discrimination in infant macaques (Macaca mulatta) J Cogn Dev. 2016;17(2):285–299. doi: 10.1080/15248372.2015.1048863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paukner A, et al. Sensitivity to first-order relations of facial elements in infant rhesus macaques. Infant Child Dev. 2013;22(3):320–330. doi: 10.1002/icd.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang B, et al. Amygdala volume predicts patterns of eye fixation in rhesus monkeys. Behav Brain Res. 2012;229(2):433–7. doi: 10.1016/j.bbr.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Senju A, Johnson MH. Atypical eye contact in autism: models, mechanisms and development. Neurosci Biobehav Rev. 2009;33(8):1204–14. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Ecker C. The neuroanatomy of autism spectrum disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism. 2017;21(1):18–28. doi: 10.1177/1362361315627136. [DOI] [PubMed] [Google Scholar]
- 92.Scott JA, et al. Longitudinal analysis of the developing rhesus monkey brain using magnetic resonance imaging: birth to adulthood. Brain Struct Funct. 2016;221(5):2847–71. doi: 10.1007/s00429-015-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Young JT, et al. The UNC-Wisconsin Rhesus Macaque Neurodevelopment Database: A Structural MRI and DTI Database of Early Postnatal Development. Front Neurosci. 2017;11:29. doi: 10.3389/fnins.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schumann CM, Noctor S, Amaral DG. Postmortem neuropathology of autism. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism Spectrum Disorders. Oxford University Press; 2011. [Google Scholar]
- 95.Bauman MD, et al. The neurobiology of primate social behavior. In: Decety J, Cacioppo JT, editors. Handbook of Social Neuroscience. Oxford University Press; New York: 2011. [Google Scholar]
- 96.Bauman MD, Amaral DG. Neurodevelopment of social cognition. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge: 2008. [Google Scholar]
- 97.Modi ME, Sahin M. Translational use of event-related potentials to assess circuit integrity in ASD. Nat Rev Neurol. 2017;13(3):160–170. doi: 10.1038/nrneurol.2017.15. [DOI] [PubMed] [Google Scholar]
- 98.Tremblay S, Sharika KM, Platt ML. Social Decision-Making and the Brain: A Comparative Perspective. Trends Cogn Sci. 2017;21(4):265–276. doi: 10.1016/j.tics.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Platt ML, Seyfarth RM, Cheney DL. Adaptations for social cognition in the primate brain. Philos Trans R Soc Lond B Biol Sci. 2016;371(1687):20150096. doi: 10.1098/rstb.2015.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grimaldi P, Saleem KS, Tsao D. Anatomical Connections of the Functionally Defined “Face Patches” in the Macaque Monkey. Neuron. 2016;90(6):1325–42. doi: 10.1016/j.neuron.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bauman ML. Microscopic neuroanatomic abnormalities in autism. Pediatrics. 1991;87(5 Pt 2):791–6. [PubMed] [Google Scholar]
- 102.Baron-Cohen S, et al. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 103.Bachevalier J. Medial Temporal Lope Structures and Autism - a Review of Clinical and Experimental Findings. Neuropsychologia. 1994;32(6):627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 104.Bauman MD, et al. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24(3):711–21. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bauman MD, et al. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16(8):1388–411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 106.Prather MD, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106(4):653–8. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 107.Bliss-Moreau E, et al. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Dev Psychobiol. 2010;52(5):487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bliss-Moreau E, et al. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience. 2011;178:123–32. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci. 2011;125(6):848–58. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bliss-Moreau E, et al. The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci. 2013;25(12):2124–40. doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bliss-Moreau E, et al. The effects of neonatal amygdala or hippocampus lesions on adult social behavior. Behav Brain Res. 2017;322(Pt A):123–137. doi: 10.1016/j.bbr.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moadab G, Bliss-Moreau E, Amaral DG. Adult social behavior with familiar partners following neonatal amygdala or hippocampus damage. Behav Neurosci. 2015;129(3):339–50. doi: 10.1037/bne0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moadab G, et al. Early amygdala or hippocampus damage influences adolescent female social behavior during group formation. Behav Neurosci. 2017;131(1):68–82. doi: 10.1037/bne0000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raper J, et al. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Horm Behav. 2013;63(4):646–58. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raper J, et al. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Dev Psychobiol. 2014;56(8):1711–22. doi: 10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raper J, et al. Neonatal amygdala lesions lead to increased activity of brain CRF systems and hypothalamic-pituitary-adrenal axis of juvenile rhesus monkeys. J Neurosci. 2014;34(34):11452–60. doi: 10.1523/JNEUROSCI.0269-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dalton KM, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26(29):7674–9. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schumann CM, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nordahl CW, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69(1):53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen MD, et al. Functional Connectivity of the Amygdala Is Disrupted in Preschool-Aged Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(9):817–24. doi: 10.1016/j.jaac.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49(4):745–59. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31(2):109–30. doi: 10.1017/S0140525X08003543. discussion 130–178. [DOI] [PubMed] [Google Scholar]
- 124.McFarlane HG, et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 125.Bales KL, et al. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burket JA, et al. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res Bull. 2011;86(3–4):152–8. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Silverman JL, et al. Influence of stimulant-induced hyperactivity on social approach in the BTBR mouse model of autism. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971(1):47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- 129.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(8):1663–77. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47(4):299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 131.Capitanio JP, Widaman KF. Confirmatory factor analysis of personality structure in adult male rhesus monkeys (Macaca mulatta) Am J Primatol. 2005;65(3):289–94. doi: 10.1002/ajp.20116. [DOI] [PubMed] [Google Scholar]
- 132.Capitanio JP. Sociability and responses to video playbacks in adult male rhesus monkeys (Macaca mulatta) Primates. 2002;43(3):169–77. doi: 10.1007/BF02629645. [DOI] [PubMed] [Google Scholar]
- 133.Sclafani V, et al. Early Predictors of Impaired Social Functioning in Male Rhesus Macaques (Macaca mulatta) PLoS One. 2016;11(10):e0165401. doi: 10.1371/journal.pone.0165401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chun K, Capitanio JP. Developmental consequences of behavioral inhibition: a model in rhesus monkeys (Macaca mulatta) Dev Sci. 2016;19(6):1035–1048. doi: 10.1111/desc.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Capitanio JP, et al. A behavioral taxonomy of loneliness in humans and rhesus monkeys (Macaca mulatta) PLoS One. 2014;9(10):e110307. doi: 10.1371/journal.pone.0110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Capitanio JP. Naturally Occurring Nonhuman Primate Models of Psychosocial Processes. ILAR J. 2017:1–9. doi: 10.1093/ilar/ilx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Winslow JT, et al. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–8. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 138.Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32(29):3623–9. doi: 10.1016/j.vaccine.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 139.Hewitson L, et al. Delayed acquisition of neonatal reflexes in newborn primates receiving a thimerosal-containing hepatitis B vaccine: influence of gestational age and birth weight. J Toxicol Environ Health A. 2010;73(19):1298–313. doi: 10.1080/15287394.2010.484709. [DOI] [PubMed] [Google Scholar]
- 140.Hewitson L, et al. Influence of pediatric vaccines on amygdala growth and opioid ligand binding in rhesus macaque infants: A pilot study. Acta Neurobiol Exp (Wars) 2010;70(2):147–64. doi: 10.55782/ane-2010-1787. [DOI] [PubMed] [Google Scholar]
- 141.Curtis B, et al. Examination of the safety of pediatric vaccine schedules in a non-human primate model: assessments of neurodevelopment, learning, and social behavior. Environ Health Perspect. 2015;123(6):579–89. doi: 10.1289/ehp.1408257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gadad BS, et al. Administration of thimerosal-containing vaccines to infant rhesus macaques does not result in autism-like behavior or neuropathology. Proc Natl Acad Sci U S A. 2015;112(40):12498–503. doi: 10.1073/pnas.1500968112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16(8):469–86. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fox-Edmiston E, Van de Water J. Maternal Anti-Fetal Brain IgG Autoantibodies and Autism Spectrum Disorder: Current Knowledge and its Implications for Potential Therapeutics. CNS drugs. 2015;29(9):715–724. doi: 10.1007/s40263-015-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 147.Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353(6301):772–7. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Knuesel I, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643–60. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 150.Meltzer A, Van de Water J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology. 2017;42(1):284–298. doi: 10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuo TT, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30(6):777–89. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69(6):693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Braunschweig D, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013;3:e277. doi: 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Camacho J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. 2014;266:46–51. doi: 10.1016/j.bbr.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martinez-Cerdeno V, et al. Prenatal Exposure to Autism-Specific Maternal Autoantibodies Alters Proliferation of Cortical Neural Precursor Cells, Enlarges Brain, and Increases Neuronal Size in Adult Animals. Cereb Cortex. 2016;26(1):374–83. doi: 10.1093/cercor/bhu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martin LA, et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22(6):806–16. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bauman MD, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. 2013;3:e278. doi: 10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nordahl CW, et al. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun. 2013;30:61–5. doi: 10.1016/j.bbi.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69(5 Pt 2):26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72(10):1272–6. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Atladottir HO, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 163.Atladottir HO, et al. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447–54. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zerbo O, et al. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43(1):25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zerbo O, et al. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2015;45(12):4015–25. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goines PE, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jones KL, et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. 2017;22(2):273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Abdallah MW, et al. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain, behavior, and immunity. 2012;26(1):170–176. doi: 10.1016/j.bbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 169.Abdallah MW, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. The World Journal of Biological Psychiatry. 2013;14(7):528–538. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- 170.Willette AA, et al. Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res. 2011;219(1):108–15. doi: 10.1016/j.bbr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Short SJ, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67(10):965–73. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain, behavior, and immunity. 2010;24(6):881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 173.Meyer U, Feldon J. To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62(3):1308–21. doi: 10.1016/j.neuropharm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 174.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 175.Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33(7):1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 176.Bauman MD, et al. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75(4):332–41. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Machado CJ, et al. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol Psychiatry. 2015;77(9):823–32. doi: 10.1016/j.biopsych.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leonard TK, et al. How macaques view familiarity and gaze in conspecific faces. Behav Neurosci. 2012;126(6):781–91. doi: 10.1037/a0030348. [DOI] [PubMed] [Google Scholar]
- 179.King BH, Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 180.Weir RK, et al. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rose DR, et al. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75(4):307–15. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 183.Careaga M, Murai T, Bauman MD. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol Psychiatry. 2017;81(5):391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.de la Torre-Ubieta L, et al. Advancing the understanding of autism disease mechanisms through genetics. Nature Medicine. 2016;22(4):345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hulbert SW, Jiang YH. Monogenic mouse models of autism spectrum disorders: Common mechanisms and missing links. Neuroscience. 2016;321:3–23. doi: 10.1016/j.neuroscience.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qiu Z, Li X. Non-human Primate Models for Brain Disorders - Towards Genetic Manipulations via Innovative Technology. Neurosci Bull. 2017 doi: 10.1007/s12264-017-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jennings CG, et al. Opportunities and challenges in modeling human brain disorders in transgenic primates. Nat Neurosci. 2016;19(9):1123–30. doi: 10.1038/nn.4362. [DOI] [PubMed] [Google Scholar]
- 188.Chen Y, Niu Y, Ji W. Genome editing in nonhuman primates: approach to generating human disease models. J Intern Med. 2016;280(3):246–51. doi: 10.1111/joim.12469. [DOI] [PubMed] [Google Scholar]
- 189.Liu H, et al. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell. 2014;14(3):323–8. doi: 10.1016/j.stem.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ramocki MB, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66(6):771–82. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu Z, et al. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature. 2016;530(7588):98–102. doi: 10.1038/nature16533. [DOI] [PubMed] [Google Scholar]
- 192.Kinnally EL, et al. Effects of early experience and genotype on serotonin transporter regulation in infant rhesus macaques. Genes Brain Behav. 2008;7(4):481–6. doi: 10.1111/j.1601-183X.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- 193.Kinnally EL, et al. Serotonin pathway gene-gene and gene-environment interactions influence behavioral stress response in infant rhesus macaques. Dev Psychopathol. 2010;22(1):35–44. doi: 10.1017/S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kinnally EL, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010;9(6):575–82. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues Clin Neurosci. 2012;14(3):263–79. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Doyle CA, McDougle CJ. Pharmacotherapy to control behavioral symptoms in children with autism. Expert Opin Pharmacother. 2012;13(11):1615–29. doi: 10.1517/14656566.2012.674110. [DOI] [PubMed] [Google Scholar]
- 197.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61(3):340–50. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Green JJ, Hollander E. Autism and oxytocin: new developments in translational approaches to therapeutics. Neurotherapeutics. 2010;7(3):250–7. doi: 10.1016/j.nurt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154(6):726–35. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 200.Guastella AJ, I, Hickie B. Oxytocin Treatment, Circuitry, and Autism: A Critical Review of the Literature Placing Oxytocin Into the Autism Context. Biol Psychiatry. 2016;79(3):234–42. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 201.Tachibana M, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23(2):123–7. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- 202.Meyer-Lindenberg A, et al. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 203.Bethlehem RA, et al. Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 204.Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61(3):392–9. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Evans SL, et al. Intranasal oxytocin effects on social cognition: a critique. Brain Res. 2014;1580:69–77. doi: 10.1016/j.brainres.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Horm Behav. 2012;61(3):313–9. doi: 10.1016/j.yhbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 207.Dal Monte O, et al. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One. 2014;9(8):e103677. doi: 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chang SW, et al. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci U S A. 2012;109(3):959–64. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Parr LA. Intranasal oxytocin enhances socially-reinforced learning in rhesus monkeys. Frontiers in Behavioral Neuroscience. 2014;8(278) [Google Scholar]
- 210.Parr LA, et al. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38(9):1748–56. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proc Natl Acad Sci U S A. 2013;110(28):11630–5. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Putnam PT, et al. Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys. Psychoneuroendocrinology. 2016;72:47–53. doi: 10.1016/j.psyneuen.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dal Monte O, et al. Oxytocin enhances attention to the eye region in rhesus monkeys. Front Neurosci. 2014;8:41. doi: 10.3389/fnins.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Landman R, et al. Effect of distracting faces on visual selective attention in the monkey. Proc Natl Acad Sci U S A. 2014;111(50):18037–42. doi: 10.1073/pnas.1420167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chang SW, Platt ML. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Res. 2013 doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ebitz RB, Platt ML. An evolutionary perspective on the behavioral consequences of exogenous oxytocin application. Front Behav Neurosci. 2013;7:225. doi: 10.3389/fnbeh.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Simpson EA, et al. Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques. Psychopharmacology (Berl) 2017;234(3):497–506. doi: 10.1007/s00213-016-4480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Parr LA, et al. Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology. 2016;74:149–157. doi: 10.1016/j.psyneuen.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Freeman SM, et al. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–41. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu N, et al. Oxytocin modulates fMRI responses to facial expression in macaques. Proc Natl Acad Sci U S A. 2015;112(24):E3123–30. doi: 10.1073/pnas.1508097112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mesulam MM, et al. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214(2):170–97. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 222.Jones EG, et al. Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. J Comp Neurol. 1976;167(4):385–419. doi: 10.1002/cne.901670402. [DOI] [PubMed] [Google Scholar]
- 223.Golub MS, Hogrefe CE, Bulleri AM. Peer social interaction is facilitated in juvenile rhesus monkeys treated with fluoxetine. Neuropharmacology. 2016;105:553–60. doi: 10.1016/j.neuropharm.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


