Abstract
Purpose of review
The Assessment of Spondyloarthritis International Society (ASAS) axial spondyloarthritis (axSpA) classification criteria marked a major step forward in SpA research, distinguishing axial from peripheral disease, and allowing earlier identification through MRI. This facilitated all aspects of research including epidemiology, therapeutics and patient outcomes.
Recent findings
The ASAS axSpA classification criteria have been applied broadly in research, and were validated in a recent meta-analysis of international studies. Concerns arose due to clinical differences between the clinical and imaging arms, which imply different risk for radiographic progression, and perform differently in validation studies. Low specificity of the MRI finding of sacroiliac joint bone marrow edema may lead to misclassification in populations with low axSpA prevalence. We suggest methodology to improve upon the criteria, including rigorous assessment of potential candidate criteria sets, discrete choice experiments to allow consideration of feature weights, and validation. Separately, assessment of structural and inflammatory MRI abnormalities should be performed to refine the MRI definition of sacroiliitis.
Summary
The debate regarding the validation and modification of the ASAS axSpA classification criteria should lead to international efforts to build upon the gains made by these criteria, to further refine the axSpA population definitions for research and ultimately improve patient outcomes.
Keywords: Spondyloarthritis, Classification, Outcomes research
Introduction
Spondyloarthritis (SpA) is a family of related diseases affecting the axial and peripheral skeleton. Ankylosing spondylitis (AS), the prototypic form of SpA, is commonly diagnosed in the presence of definitive sacroiliitis on conventional x-rays. The modified New York Classification Criteria (mNYCC) for AS, which rely on radiographic changes and have been commonly used in research, were developed in 1984. The radiographic changes of sacroiliitis, however, are estimated to take 6 to 10 years to develop following symptom onset.[1] Moreover, while the degree of “radiographic sacroiliitis” as seen on the plain x-rays is essential to classify a patient according to mNYCC, the reliability of this grading is poor, and training makes little impact on improving the reproducibility.[2] In the decades since the mNYCC development, with increasing availability and utilization of magnetic resonance imaging (MRI), it was recognized that MRI features of sacroiliitis preceded radiographic changes, allowing earlier identification of patients who would go on to develop AS. In 2003, the FDA approval of tumor necrosis factor inhibitors (TNFi) for the treatment of AS indicated a paradigm shift in the management of AS. Around the same time, the dramatic efficacy of TNFi and other biologic therapies in treating rheumatoid arthritis (RA) had led to efforts to treat the disease early and aggressively. Rheumatologists wondered if treating AS early would improve patient outcomes, but diagnosing AS early and reliably was the main hurdle.
Development of the ASAS Classification Criteria
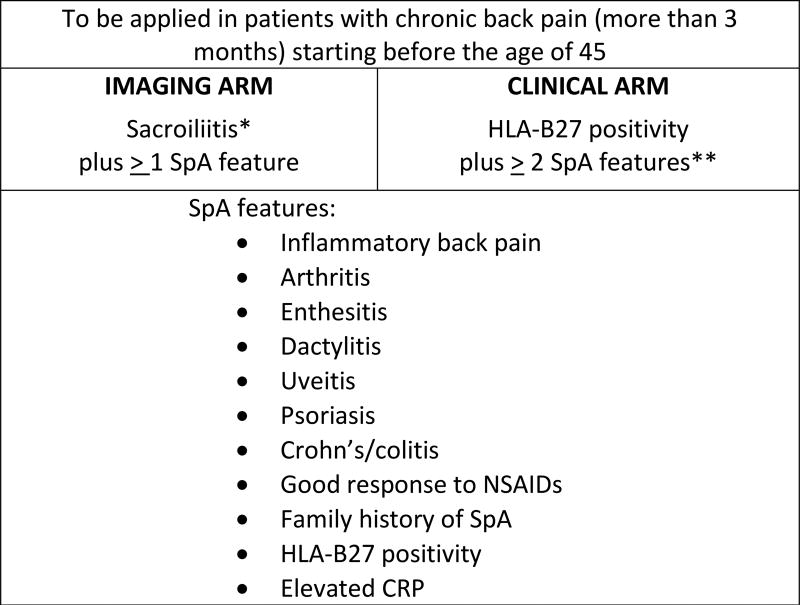
In 2009, the Assessment of SpondyloArthritis International Society (ASAS) published new criteria for classification of axial spondyloarthritis (axSpA). [3, 4] These criteria were developed beginning with expert review of paper cases of chronic back pain of unknown origin, whose detailed clinical information was duplicated both with and without information of SI joint MRI results (inflammation present versus absent) for a total of 142 cases. Candidate criteria sets were developed using clinical reasoning and considering combinations of any, up to 2, or up to 3 extra-spinal SpA manifestations. Two criteria sets were selected for further testing by their maximal sensitivity and specificity. ASAS members then contributed chronic back pain patients (onset <45 years) to a cohort study comprising 649 patients. In 40% of this sample, the candidate sets were further developed using various definitions of inflammatory back pain, and subsequently validated in the remaining 60% sample (Figure 1).
Figure 1.
ASAS classification criteria for axial spondyloarthritis
*Sacroiliitis may be definite radiographically according to modified NY criteria, or by active inflammation on SI joint MRI (clear presence of bone marrow edema or osteitis). **Persons may satisfy the clinical arm if imaging results are unknown. References: [3, 4]
In addition to the goal of classifying persons with axSpA who had imaging findings of sacroiliitis, either on x-ray or MRI, the 2009 ASAS criteria also allow classification of those with solely clinical features of SpA. Thus the ASAS axSpA criteria may be fulfilled by patients with chronic back pain (≥3 months) with onset prior to age 45 years who have either: (A) X-ray changes meeting the mNYCC or MRI findings of active sacroiliitis and one other clinical feature of SpA (“imaging arm”) or (B) HLA-B27 positivity in combination with at least 2 other clinical features of (“clinical arm”; Figure 1). In 2011, ASAS also published criteria for peripheral SpA, which aided in differentiating axSpA as a distinct entity from peripheral SpA for further research.[5]
The positive impact of the ASAS Classification Criteria
The ASAS axSpA classification criteria have been a huge step forward in our understanding of the spectrum of SpA, and have been widely adopted by the international rheumatology community.[6] The notion of dividing ‘spondyloarthritis’ in to axSpA and peripheral SpA as distinct phenotypes, and then dividing axSpA further in to ankylosing spondylitis(AS) and non-radiographic axSpA (nr-axSpA) has revolutionized our thinking of these conditions. The introduction of two new concepts, axSpA and nr-axSpA, has allowed identification of patients early in the course of their disease, before they can be classified as AS. The ability to identify axSpA patients who lack radiographic changes (nr-axSpA) has led to a clearer understanding of the natural history and risk factors for axSpA, and greater appreciation for the burden of axSpA worldwide.[7] Additionally, the use of MRI to identify axSpA patients earlier in the disease course, prior to development of radiographic changes, has led to identification of more homogeneous populations for inclusion in research studies. These developments have led to advanced research into the possibility of prevention of disease progression, particularly through the development and testing of new medications in early stages of the disease.
Performance of the ASAS axSpA Criteria
To assess performance of the axSpA criteria, ASAS previously established an international 25-center cohort study, which included 649 patients seeking evaluation for back pain with onset <45 years. Axial SpA was diagnosed in 60% of these patients. Estimates of the performance of these criteria vary according to the specific population assessed. When compared to the older ESSG and Amor criteria at the time of the original study, sensitivity of the ASAS axSpA criteria were similar, and specificity was greater (84% vs 65–78%).[3] Subsequently, a long term follow-up study assessed the predictive validity of the axSpA criteria by reassessing the diagnosis of patients in the original ASAS axSpA criteria development cohort. Mean follow up was 4.4 years (range 1.9–6.8) for the 394 participants from the original cohort, and the gold standard against which ASAS axSpA criteria were compared was the rheumatologist’s diagnosis (not necessarily the same clinician). The imaging and clinical arms of the axSpA criteria were considered both separately and in combination with resulting positive predictive values ranging from 86–96%.[8]
The ProSpA study assessed the performance of the ASAS criteria in a population of US adults age 18 or older with chronic back pain with onset prior to age 45. [9] With direct application of the ASAS criteria, 47% were classified as axSpA. The overall specificity of the ASAS criteria was 79%, somewhat lower than reported in more “selected” referral populations, which was thought to be related to a relatively lower prevalence of males and HLA-B27 positive individuals in the ProSpA study than in previous studies.
A recent meta-analysis assessed the performance of the ASAS axSpA classification criteria in 7 observational cohorts comprising nearly 5000 patients, including the US ProSpA study.[10] Individual studies reported overall specificities ranging from 62% to 95%, with the pooled specificity of 87%. Specificities for the imaging arm (+/− clinical arm) ranged from 74% to 99% and for the clinical arm (+/− imaging arm) ranged from 71% to 97% for the individual studies. The pooled specificity was maintained across groups that fulfilled the imaging or clinical arms, irrespective of fulfillment of the other arm (range: 87–97%). Taken together, studies on the performance of the ASAS axial SpA criteria demonstrate overall good specificity, which is critical to identification of the intended population for study.
Concerns regarding the ASAS axSpA classification criteria
Despite the good performance of these criteria as noted above, there is variability in how the criteria perform across different populations – especially in unselected chronic low back pain populations where the pre-test probability of axSpA is low – and within other subgroups.
In October 2013, the FDA rejected the application by two TNFi (certolizumab and adalimumab) for the treatment of nr-axSpA. Amongst many other reasons, the FDA’s concern about the specificity of the ASAS axSpA classification criteria when erroneously used for diagnostic purposes. While four TNFi have been approved in the European Union to treat nr-axSpA, none have garnered FDA’s approval in the US. These issues continue to be debated, and we highlight the concerns raised about these criteria below, related to subgroups identified by the criteria, the issues around specificity of the MRI sacroiliitis definition, and the lack of weights given to SpA features.
Lack of homogeneity between the clinical and imaging arms of the ASAS criteria
Studies indicate that important differences exist in the clinical characteristics of patients fulfilling the clinical and imaging arms, and their prognosis regarding disease progression. The clinical arm, which spans the spectrum of those who duly fulfill the imaging requirements all the way to those with normal imaging, has reduced specificity relative to the imaging arm (83% versus 97%, respectively).[11] Studies showed that subjects fulfilling the clinical arm were less likely to be male (42% versus 59%, p<.05) and had lower mean C-reactive protein (CRP; 5.2 versus 11.6, p<.05), two factors associated with lower radiographic progression to AS.[12, 13] HLA-B27 positivity is 100% in the clinical arm (by definition), and lower in the imaging arm.[12, 13] The proportion of subjects with nr-axSpA fulfilling each of the arms is not consistently reported, but would be important to consider in future work.
Clinicians’ reliance on MRI results and reduced MRI specificity in typical chronic back pain populations
MRI is the imaging modality of choice for axSpA, and has allowed identification of early disease where x-ray changes are minimal or absent. Both structural and inflammatory abnormalities may be present in/around the SI joints in axSpA. The current ASAS definition of MRI sacroiliitis is a single bone marrow edema lesion in the SI joint on 2 consecutive slices, or more than one bone marrow edema lesions on a single slice. [14, 15] However, concern exists regarding this definition, as degenerative changes in the SI joints can also lead to similar bone marrow edema lesions, and they can be found in normal healthy individuals too.[16] The clinicians’ reliance on MRI results influenced the ASAS criteria development process, in that expert diagnosis changed in 21% of paper cases when MRI results were added.[3] This influence of MRI results was also demonstrated in the ProSpA study, in which the majority of patients diagnosed with axSpA fulfilled the imaging arm, while those not diagnosed with axSpA more commonly fulfilled the clinical arm.[9] In addition, the reported high specificity of the imaging arm (97%) warrants closer consideration in populations different than the ASAS cohort, which had an axSpA prevalence of 60%.[4] The expected estimate for axSpA prevalence among unselected young adults with chronic back pain would be 5%,[17] and in this setting the misclassification of patients as axSpA would outweigh the correct classification.[18] Indeed this is suggested by a study in young adults with chronic low back pain onset prior to age 45, in which MRI “sacroiliitis” was present in 21%, but expected to be much less common.[19] Taken together, these findings illustrate a need to re-evaluate the role of MRI features in classification of axSpA, and the definition of “positive MRI”.
Inability to weigh clinical features
While the ASAS criteria require at least 1 or 2 clinical features of SpA (the imaging and clinical arms, respectively), each of the clinical features carries the same weight in the current ASAS criteria. For example, an HLA-B27 positive patient with inflammatory back pain and elevated CRP has a positive likelihood ratio (LR+) product of 70, while a HLA-B27 positive patient with sacroiliitis on MRI and acute uveitis has an LR+ product of 1314. Current criteria do not distinguish the relatively greater risk of SpA conferred by some features.[20] Additionally, the ASAS criteria do not have a mechanism to address correlation between features (eg- HLA-B27 positivity and positive family history of SpA). Classification criteria for gout and systemic sclerosis have both used methodology to incorporate weighting of disease features, which could be used in classifying axSpA, as outlined below.
Potential methodology for improving the specificity of the axSpA classification criteria
Various processes can be used for improving the specificity of the existing criteria. Here we describe a method similar to those used in development of classification criteria for rheumatoid arthritis (RA), gout and systemic sclerosis.[21–25]
Item generation and reduction
A list of candidate items that differentiate axSpA from mimicking conditions could be generated from literature review, and patient/expert opinion. The resulting extensive list of candidate items would be reduced through a Delphi exercise and/or nominal group technique and then be tested for their discriminatory capacity for axSpA in a sample from existing North American as well as registries from the rest of the world and cohorts that contain both axSpA and mimicking conditions.
Consensus process to identify factors that impact the probability of axial SpA
Rheumatologists with a special interest in axSpA would be asked to contribute paper cases of patients for whom axSpA was in the differential diagnosis. An international axSpA expert panel would review the results of the item generation/reduction process, then rank-order each case from the lowest to highest probability of having axSpA. The item generation/ reduction process and paper cases will form the basis for in-depth discussion to identify key features that positively or negatively impact the probability of axSpA and to develop potential classification criteria sets.
Item weighting
Item weighting may be performed through discrete-choice experiments, a process that has been used in development of RA, systemic sclerosis and gout classification criteria.[21–25] Paper cases from SpA experts internationally would be purposively sampled to reflect the spectrum of probability that each case could be classified as having axSpA (i.e. - early versus established, and radiographic versus non-radiographic disease). Standard software would be used to develop paired case scenarios that differ in two attributes. The case pairs would be presented to an international panel of axSpA experts, who will select the case from the pair that he/she believes has a higher probability of being classified as having axSpA. Through iterative pairwise choices, the software will assign relative weights to each case attribute.
Defining a threshold for classifying axSpA
The expert panel would consider candidate criteria sets (some incorporating item weighting) relative to the gold-standard axSpA definition of expert opinion. For each criteria set, a cutoff score would be calculated to maximize the sum of specificity and sensitivity. The expert panel will then participate in a threshold identification exercise, which will assess experts’ willingness to enroll paper cases into a study (e.g. a phase 3 trial for a new biologic agent for axSpA with unclear efficacy and safety).
Validation of the final criteria
Using data from existing axSpA registries and cohorts (remainder of the split-sample reserved for this purpose), the sensitivity and specificity of the revised axSpA classification criteria will be assessed. In subsequent studies, performance of the revised criteria will be compared with that of the original ASAS criteria, as well as the ESSG and Amor criteria, and assessed in other populations such as unspecified chronic back pain.
MRI features in axSpA
To further establish the role of specific MRI features that differentiate inflammatory arthritis involving the SI joints from mechanical or other causes of back pain, MRIs will need to be compared among patients presenting with back pain, and whose diagnosis is subsequently established though other clinical/laboratory assessments. This may be conducted in existing cohorts, if such imaging is available, or a new cohort may be assembled in order to address this question.
Conclusion
The 2009 ASAS SpA classification criteria marked an important step forward in our understanding of the spectrum of SpA, and our ability to define the clinical spectrum of peripheral and axSpA for studies of novel therapies and patient outcomes. These criteria have succeeded in changing our vocabulary, has expanded our ability to treat axial spondyloarthritis patients early and have been instrumental in garnering European regulatory authority approval for four TNFi to treat nr-axSpA. Nonetheless, opportunities exist to improve these criteria; to reconcile the heterogeneity that exists in populations classified by the clinical and imaging arms, to determine weighting of axSpA features, and to define and refine definitions of MRI-associated sacroiliitis. Herein, we have outlined the arguments on both side of the debate, and also one of the many possible methodologies to improve specificity of the classification criteria. ASAS classification criteria have been a major breakthrough in the field of spondyloarthritis with important implications for research and patient care. A healthy debate about the pros and cons of the existing criteria leading to further modifications can only improve patient care.
Key Points.
-
-
The Assessment of Spondyloarthritis International Society (ASAS) 2009 axial spondyloarthritis (axSpA) classification criteria have been a breakthrough in the rheumatology community, distinguishing axial from peripheral disease SpA, and allowing earlier identification of axSpA though use of MRI.
-
-
Following the broad implementation of the ASAS axSpA criteria, concerns have arisen regarding important clinical differences and specificities between the clinical and the imaging arms of the axSpA criteria, which have prognostic implications for radiographic progression
-
-
The specificity of the ”positive MRI definition” considering bone marrow edema alone without structural changes is another cause for concern in populations where axSpA prevalence is lower than that in the original ASAS cohort, and in which misclassification would be more common.
-
-
Opportunities to improve upon the ASAS axSpA criteria include reconciling heterogeneity between the clinical and imaging arms, to incorporating weighting of axSpA features, and refining the MRI definition of sacroiliitis.
Acknowledgments
None
Financial support and sponsorship: MD is supported by the National Institutes of Health AR069127
Footnotes
Conflicts of interest: AD: Past-Chair, Spondyloarthritis Research & Treatment Network (SPARTAN); MD: None
References
- 1.Poddubnyy D, M R. Early spondyloarthritis. Rheum Dis Clin North Am. 2012;38:387–403. doi: 10.1016/j.rdc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 2.van Tubergen A, Heuft-Dorenbosch L, Schulpen G, et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis. 2003;62(6):519–25. doi: 10.1136/ard.62.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68(6):770–6. doi: 10.1136/ard.2009.108217. [DOI] [PubMed] [Google Scholar]
- 4.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 5.Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 6.Deodhar A, Reveille JD, van den Bosch F, et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration's comments and concerns. Arthritis Rheum. 2014;66(10):2649–56. doi: 10.1002/art.38776. [DOI] [PubMed] [Google Scholar]
- 7.van Tubergen A. The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol. 2015;11(2):110–8. doi: 10.1038/nrrheum.2014.181. [DOI] [PubMed] [Google Scholar]
- 8.Sepriano A, Landewe R, van der Heijde D, et al. Predictive validity of the ASAS classification criteria for axial and peripheral spondyloarthritis after follow-up in the ASAS cohort: a final analysis. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208730. [DOI] [PubMed] [Google Scholar]
- 9.Deodhar A, Mease PJ, Reveille JD, et al. Frequency of Axial Spondyloarthritis Diagnosis Among Patients Seen by US rheumatologists for Evaluation of Chronic Back Pain. Arth & Rheum. 2016;68(7):1669–76. doi: 10.1002/art.39612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Sepriano A, Rubio R, Ramiro S, et al. Performance of the ASAS classification criteria for axial and peripheral spondyloarthritis: a systematic literature review and meta-analysis. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210747. Published Online First(08 Feb 2017). * This meta-analysis assessed the performance of the ASAS axSpA classification criteria in 7 observational cohorts comprising nearly 5000 patients, reporting pooled specificities for the clinical and imaging arms separately and combined ranging from 87 to 97%. [DOI] [PubMed] [Google Scholar]
- 11.Deodhar A. Sacroiliac joint MRI in the diagnosis of axial SpA: "A tiny bit of white on two consecutive slices" may be objective, but not specific. Arthritis Rheumatol. 2015 doi: 10.1002/art.39549. [DOI] [PubMed] [Google Scholar]
- 12.Molto A, Paternotte S, van der Heijde D, et al. Evaluation of the validity of the different arms of the ASAS set of criteria for axial spondyloarthritis and description of the different imaging abnormalities suggestive of spondyloarthritis: data from the DESIR cohort. Ann Rheum Dis. 2015;74(4):746–51. doi: 10.1136/annrheumdis-2013-204262. [DOI] [PubMed] [Google Scholar]
- 13.Akkoc N, Khan MA. ASAS classification criteria for axial spondyloarthritis: a look at the unfilled part of the glass. Clin Exp Rheumatol. 2014;32(6 Suppl 87):S-14–5. [PubMed] [Google Scholar]
- 14.Lambert RG, Bakker PA, van der Heijde D, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208642. [DOI] [PubMed] [Google Scholar]
- 15.Rudwaleit M, Jurik AG, Hermann KG, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68(10):1520–7. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 16.Weber U, Lambert RG, Pedersen SJ, et al. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res(Hoboken) 2010;62(12):1763–71. doi: 10.1002/acr.20312. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Weinstein JN. Low back pain. New England Journal of Medicine. 2011;344(5):363–70. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- *18.van der Linden S, Khan MA. Can we currently and confidently assess the true burden of illness due to non-radiographic axial spondyloarthritis? Clin Exp Rheumatol. 2016;34(6):963–5. * An editorial highlighting some concerns regarding heterogeneity introduced by the multi-arm design of the ASAS axSpA classification criteria, and concerns regarding insufficient specificity leading to misclassification in populations with low or moderate axSpA prevalence. [PubMed] [Google Scholar]
- 19.Arnbak B, Grethe Jurik A, Hørslev-Petersen K, et al. Associations Between Spondyloarthritis Features and Magnetic Resonance Imaging Findings: A Cross-Sectional Analysis of 1,020 Patients With Persistent Low Back Pain. Arthritis Rheum. 2016;68(4):892–900. doi: 10.1002/art.39551. [DOI] [PubMed] [Google Scholar]
- 20.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;52(4):1000–8. doi: 10.1002/art.20990. [DOI] [PubMed] [Google Scholar]
- 21.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SR, Naden RP, Fransen J, et al. Multicriteria decision analysis methods with 1000Minds for developing systemic sclerosis classification criteria. J Clin Epidemiol. 2014;67(6):706–14. doi: 10.1016/j.jclinepi.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67(10):2557–68. doi: 10.1002/art.39254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neogi T, Aletaha D, Silman AJ, et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum. 2010;62(9):2582–91. doi: 10.1002/art.27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]