Abstract
Purpose of review
Extracellular vesicles (EV) carry bioactive molecules that can be transferred between cells and tissues. This purpose of this review is to describe how EV regulate functions of cells in cartilage and other joint tissues. The potential application of EV in the treatment of osteoarthritis (OA) and as biomarkers will also be discussed.
Recent findings
EV are found in synovial fluid, in articular cartilage and in the supernatants of synoviocytes and chondrocytes. EV in cartilage have been proposed to be involved in cross talk between cells in joint tissues and to affect extracellular matrix turnover and inflammation. EV from arthritic joints can promote abnormal gene expression and changes in cartilage extracellular matrix, including abnormal mineralization. Promising results were obtained in the therapeutic application of mesenchymal stem cell derived EV for cartilage repair and experimental OA.
Summary
EV have emerged as vehicles for the exchange of bioactive signalling molecules within cartilage and between joint tissues to promote joint homeostasis and arthritis pathogenesis. As the molecular content of EV can be customized, they offer utility in therapeutic applications.
Keywords: extracellular vesicles, exosomes, cell-cell communication, chondrocytes, synoviocytes, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) represents the most common musculoskeletal disorder 1. It is a complex and multifaceted disease, characterized by the degradation of articular cartilage, subchondral bone remodeling, joint inflammation and changes in meniscus and ligaments 2. Various risk factors for OA have been identified and these include aging, joint injury, excessive chronic mechanical stress, genetic factors, and metabolic disorders 3. Although several pathogenesis pathways have been characterized 4, current knowledge is incomplete and has not led to effective approaches for prevention or treatment. These limitations can be overcome by advances in the understanding of molecular mechanisms that are involved in the maintenance of joint tissues which involves communication of cells within the different joint tissues. Cells are able to communicate with neighboring or distant cells through cytokines and hormones. In addition, extracellular vesicles (EV) that are released from cells have attracted attention as novel a mechanism of cell-cell communication. EV transfer bioactive molecules to recipient cells to modulate their activity 5. In this review, we focus on the role of EV in cell-cell communication within and among joint tissues during homeostasis and OA pathogenesis and address the potential therapeutic application of EV.
EXTRACELLULAR VESICLES
Extracellular vesicles (EV) had previously been regarded as inert cellular debris, that was generated as a consequence of cell damage or the result of dynamic plasma membrane turnover. However, recently cell-cell communication via EV has become the center of attention in research of diseases and tissue repair. EV contain bioactive molecules, including proteins, mRNAs, microRNAs, lipids, and DNA 6. EV have been classified depending on size and biogenesis pathway. Exosomes are small EV (30–150 nm in diameter) that are generated in multivesicular endosome (MVE)/multivesicular bodies (MVB) and are released when these compartments fuse with the plasma membrane. Microvesicles/microparticles (MVs) (50–1000 nm in diameter) are released by budding from the surface of the plasma membrane 7. Exosome biogenesis is a very tightly regulated process governed by multiple signaling molecules, and begins with receptor activation that is unique to each cell type 8. Detailed and conclusive characterization of the various types of EV has not yet been accomplished. The International Society for Extracellular Vesicles provided a minimal set of biochemical, biophysical and functional standards 9. Size alone can not distinguish exosomes from MVs 10. Furthermore, some proteins previously used as exosome markers, such as MHC class II or class I molecules, heat-shock proteins, flotillins, or actin, are present in all types of EV, and thus cannot be considered as either exosome or even EV-specific markers 11. New specific markers of medium and large size EV (e.g., actinins), of endosome-derived exosomes (co-expressing three tetraspanins CD9/CD63/CD81 and including TSG101 and syntenin-1), and of non-endosomal EV (some ITGs) have been proposed 7, 11. There remains a continuing need to better understand the molecular mechanisms of the biogenesis and release of EV and to discover better markers for the various types. The released EV have surface receptors/ligands of their cell source and have potential to interact with specific target cells 6. EV directly stimulate target cells by receptor-mediated interactions or transfer of the enclosed bioactive molecules 8.
EV FROM CARTILAGE AND CHONDROCYTES
EV have long been known to be present in the pericellular matrix of articular cartilage and growth plate cartilage 12–17. Various terms have been used to describe them, including matrix vesicles (MaV), articular cartilage-derived extracellular vesicles (ACEV) or apoptotic bodies but there is no conclusive distinction (Table 1). Originally, MaV were described in growth plate as derived from budding or disintegrating cells that are associated with hydroxyapatite deposition. Alkaline phosphatase activity is abundant in MaV and is used as a marker for their identification 13. MaV also contain pyrophosphate-generating nucleoside triphosphate pyrophosphohydrolase (NTPPH) activities. Matrix vesicles can be isolated from collagenase-digested articular cartilage and separated from chondrocytes by differential centrifugation and used for functional, biochemical, and ultrastructural studies 18. Isolated MaV can incorporate calcium, hydrolyze ATP or other nucleotide triphosphates, and facilitate precipitation of hydroxylapatite 19. Although MaV from different sources are heterogeneous 20, they are similar with respect to the capacity to mineralize matrix 12, 17. ACEV have been shown to have a physiological function in endochondral bone development and pathologic role in calcium crystal deposition in articular cartilage 21. The majority of the proteome was shared by EV isolated from normal and OA cartilage, but immunoglobulins and complement components were present only in OA ACEV which also contained lower levels of matrix proteoglycans 22. Importantly, the ACEV proteome shares fewer similarities with exosomal proteomes. The heterotrimeric G proteins, HSP70 and 90 and members of the tetraspanin family such as CD9, CD63 and CD81 that are particularly characteristic of exosomes were not seen in ACEV 22. CD9, CD63 and CD81 were previously considered to be specific markers for exosomes; however, in recent proteomics comparison, these proteins were observed in all EV including MV and apoptotic bodies 11, 23.
Table 1.
Characteristics of Articular Cartilage-derived Extracellular Vesicle (ACEV).
| Exosome | Matrix vesicles/Microvesicle | Apoptotic body | |
|---|---|---|---|
| Origin | Endocytic pathway Autophagic pathway |
Budding off/fusion from the Plasma membrane Autophagic pathway |
Plasma membrane in apoptotic cell |
| Size | 30–150 nm | 100 – 1000 nm | 100 nm < |
| Marker | CD9, CD63, CD81, Flotillin-1, Alix, Tsg101, LC3 etc. No specific markers |
||
| Content | mRNA, non-coding-RNA (microRNA etc), protein, lipid, DNA | ||
| Isolation method | Differential centrifugation/Density gradient centrifugation Commercial kit | ||
RNAs are also packaged in EV and are transferable genetic material from tissue to tissue and from human to human 5. RNAs are protected from degradation by the lipid membrane of the EV. Coding and non-coding small RNAs in EV were in proportions that differed from parent cells with an enrichment of specific miRNAs suggesting that miRNAs are selectively packaged into EV. For example, small RNAs such as miRNAs were enriched in EV isolated from cultures of costochondral growth zone chondrocytes, while large RNAs such as 18S and 28S rRNA were not detected 24. EV from normal articular cartilage contain full length mRNAs for factor XIIIA, type II transglutaminase, collagen II, aggrecan, ANKH and GAPDH. When transferred to chondrocytes, ACEV-derived RNA was internalized. This was associated with changes in the expression of alkaline phosphatase and osteopontin 25.
The mechanisms of ACEV formation include apoptosis which is increased in OA-affected cartilage 26. Chondrocyte-derived apoptotic bodies contain alkaline phosphatase and NTP pyrophosphohydrolase activities, and can precipitate calcium 27. A role of apoptosis in generating this type of ACEV has been demonstrated in experiments where apoptosis was induced by the nitric oxide donor sodium nitroprusside or antibody to the Fas antigen 27. EV accumulate around apoptotic cells (Figure 1). The levels of pyrophosphate produced by apoptotic bodies were increased by pretreatment of the chondrocytes with transforming growth factor-β and decreased by IL-1β 27. It has also been suggested that ACEV from primary articular chondrocytes can be generated through the autophagy pathway 28. In normal but not in OA chondrocytes, rapamaycin, which induces autophagy by inhibiting mTOR signaling, increased the release of ACEV that contained LC3, a marker of autophagosomes 28. Release of ACEV was inhibited by gene knock down of caspase-3, suggesting an involvement of apoptosis-related mechanisms 28. Thus, ACEV include various types of EV that differ in mechanism of generation and apparently in molecular content.
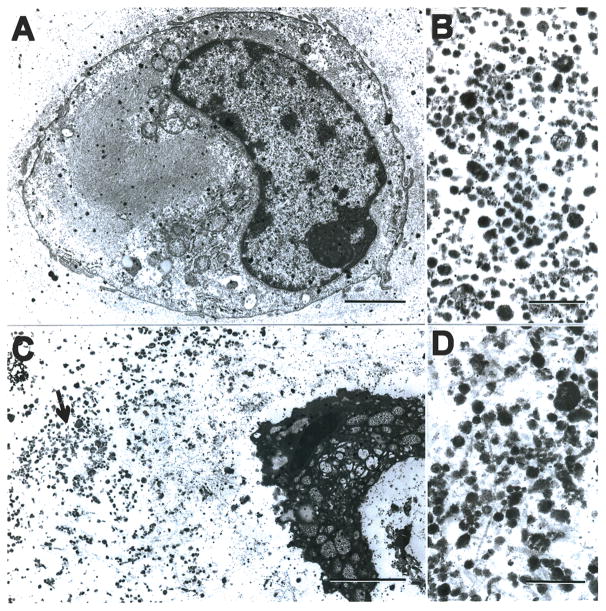
Figure 1. Electron microscopy of apoptotic chondrocytes, apoptotic bodies and matrix vesicles.
(A) Electron micrograph of a chondrocyte from normal articular cartilage. Bar represents 2 μM.
(B) Isolated matrix vesicles from normal cartilage. Bar represents 0.5 μM.
(C) Electron micrograph of an apoptotic chondrocyte in cartilage treated with the NO donor SNP. The area indicated by the arrow is shown at higher magnification in (D). Bar represents 2 μM.
(D) High magnification view of the area indicated by the arrow in (C). Bar represents 0.5 μM.
More detailed information about calcium crystal deposition by ACEV is presented in a recent review 21. A database of MaV proteins also provide comprehensive information on protein components of mineralization-related MaV 29.
EV IN COMMUNICATION AMONG JOINT TISSUES
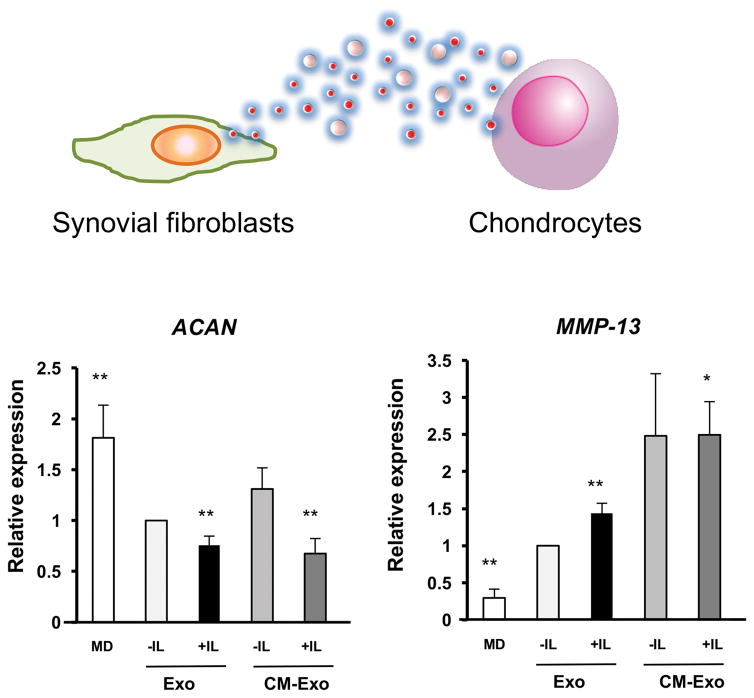
The concept that EV can mediate communication among cells from different joint tissues has thus far only been tested in a limited number of examples. Exosomes from IL-1β stimulated synoviocytes significantly up-regulated MMP-13 and ADAMTS-5 expression in articular chondrocytes, and down-regulated COL2A1 and ACAN compared with synoviocyte derived exosomes 30 (Figure 2). Migration and tube formation activity were significantly higher in human umbilical vein endothelial cells treated with the exosomes from IL-1β stimulated synoviocytes, which also induced significantly more proteoglycan release from cartilage explants. Inflammatory cytokines, IL-6, MMP-3 and VEGF in exosomes were only detectable at low level. IL-1β, TNFα MMP-9 and MMP-13 were not detectable in exosomes. NanoString analysis showed that levels of 50 miRNAs were differentially expressed in exosomes from IL-1β stimulated synoviocytes compared to non-stimulated cells 30.
Figure 2. The effect of EV from IL-1β stimulated synovial fibroblasts on normal articular chondrocytes.
Articular chondrocytes were treated with fresh-DMEM, exosomes from non-stimulated synovial fibroblasts (SFB) or exosomes from IL-1β stimulated SFB. The expression of OA-related genes was analyzed by real-time PCR. MMP-13 was significantly up-regulated, and ACAN was significantly down-regulated by exosomes from IL-1β stimulated SFB. **P < 0.01 versus Exo -IL. MD; fresh-DMEM with 10% FBS, Exo -IL; exosomes from non-stimulated SFB, Exo + IL; exosomes from IL-1β stimulated SFB.
EV IN SYNOVIAL FLUID AS POTENTIAL OA BIOMARKERS
EV are abundant in synovial fluid, and can be derived from resident cells in joint tissues and from leukocytes that infiltrate arthritis-affected joints. Synovial fluid EV modulate the release of chemokines and cytokines in synoviocytes 31, 32. The expression patterns of miRNAs in synovial fluid of OA were similar to miRNAs secreted by synovial tissues 33. A recent microarray analysis of miRNAs in synovial fluid exosomes showed that in samples from female OA patients miR-16-2-3p was up-regulated and miR-26a-5p, miR-146a-5p and miR-6821-5p were down-regulated. In synovial fluid from male OA patients miR-6878-3p was down-regulated and miR-210-5p was up-regulated. Thus, synovial fluid exosomal miRNA content is altered in patients with OA and these changes are gender specific 34. This is the first study to analyze exosomal molecules as biomarkers in OA. Future studies need to address the possibility of detecting joint-derived EV in blood and of identifying the cellular origin of EV. This has potential to detect tissue specific changes as biomarkers for OA.
EV IN THERAPEUTIC APPLICATIONS
Mesenchymal stem cells (MSC) have been used successfully in tissue engineering approaches to treat cartilage lesions and OA in animal 35 and human studies 36, 37. These beneficial functions of MSC are at least in part mediated by paracrine effects of cytokines and growth factors that decreased inflammation, enhanced progenitor cell proliferation, improved tissue repair. In a mouse model of myocardial ischemia/reperfusion injury it was first demonstrated that the protective paracrine effect was mediated by secreting exosomes 38.
Since then additional studies have demonstrated that EV derived mesenchymal stem/stromal cells (MSC) have therapeutic effects 39–41. We reported that MSC-derived EV promote skeletal muscle repair and bone fracture healing in mouse models through acceleration of biological function such as angiogenesis and cell differentiation 32, 42.
Exosomes can be used in therapeutic approaches, either from specific cell types such as MSC or from cells that are transfected with genes that have therapeutic potential to enrich for RNA levels for the gene of interest. Exosomes derived from synovial membrane MSC promoted chondrocyte proliferation and migration but inhibited their secretion of extracellular matrix (ECM). Wnt5a and Wnt5b carried by exosomes activated the alternative Wnt signaling pathway and enhanced proliferation and migration of chondrocytes but significantly decreasing ECM secretion. We previously reported that miRNAs, in particular miRNA-140, one of the most abundant miRNAs in chondrocytes are important regulators of cartilage homeostasis 43, 44. Exosomes were prepared from cells that were transduced with lentiviral miR-140-5p enhanced the proliferation and migration of articular chondrocytes and reduced OA severity in a rat model 45. Human embryonic MSC exosomes promoted cartilage regeneration in a rat osteochondral defect model 46. In that study, MSC exosomes accelerated neotissue filling and enhanced matrix synthesis of type II collagen and sulphated glycosaminoglycans. By the end of 12 weeks, exosome-treated rats displayed complete restoration of cartilage and subchondral bone.
Exosomes from conditioned culture media of embryonic stem cell derived MSC (ESC-MSC) maintained the chondrocyte phenotype by increasing collagen type II synthesis and decreasing ADAMTS5 expression in the presence of IL-1β. Intra-articular injection of ESC-MSC alleviated cartilage destruction and matrix degradation in the DMM model. Immunocytochemistry revealed colocalization of the exosomes and collagen type II-positive chondrocytes. Subsequent intra-articular injection of exosomes derived from ESC-MSC successfully impeded cartilage destruction in the DMM model. The exosomes from ESC-MSC exert a beneficial therapeutic effect on OA by balancing the synthesis and degradation of chondrocyte ECM, which in turn provides a new target for OA drug and drug-delivery system development 47. This study demonstrated the utility of MSC exosomes as a ready-to-use and ‘cell-free’ therapeutic alternative to cell-based MSC therapy.
FUTURE PERSPECTIVES
The role of EV in joint homeostasis and OA pathogenesis is of great interest and further research on this topic has potential implications for the discovery of novel biomarkers and therapeutic approaches. Currently this field is at an early stage and the topic that seems most advanced is the use of EV as therapeutic vehicles. Key questions that need to be investigated are: regulation of the types, amounts and compositions of EV that are generated and released by cells; stability of EV in the various joint tissue environments and transport of EV through dense ECM structures such as in cartilage; mechanisms of recognition and internalization by cells; the role of EV in joint homeostasis and pathogenesis. Markers of EV in synovial fluid or blood that allow tracking their cellular origin and thus profiling the status of these cells.
CONCLUSION
EV are present in articular cartilage and synovial fluid and represent a heterogeneous mixture that varies in regard to mechanism of generation and molecular content. Synovial Fluid EV are potential new OA biomarkers. Composition of EV from OA cartilage appears to be altered and may contribute to abnormal mineral and crystal deposition. EV are released from synovial fibroblasts and affect gene expression in chondrocytes. The pattern of mRNAs and miRNAs in EV can be altered by stimulation or gene transduction of cells and thus be designed to specifically change the function of target cells for therapeutic use.
KEY POINTS.
EV are released from chondrocytes through various mechanisms, including autophagy and apoptosis.
EV from chondrocytes can play a role in abnormal articular cartilage mineralization.
EV from mesenchymal stem cells can be enriched for certain bioactive molecules, such as miRNAs and used for tissue repair and in the treatment of OA.
Acknowledgments
Financial support and sponsorship
This work was supported by NIH grant AG 007996 to ML and MEXT/JPS KAKENHI fund for the Promotion of Joint International Research 15KK0308 to SM.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Hoy DG, Smith E, Cross M, Sanchez-Riera L, Blyth FM, Buchbinder R, et al. Reflecting on the global burden of musculoskeletal conditions: lessons learnt from the global burden of disease 2010 study and the next steps forward. Ann Rheum Dis. 2015;74:4–7. doi: 10.1136/annrheumdis-2014-205393. [DOI] [PubMed] [Google Scholar]
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–64. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol. 2015;22:51–63. doi: 10.1016/j.coph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 9.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Journal of extracellular vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E1433–42. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derfus BA, Rachow JW, Mandel NS, Boskey AL, Buday M, Kushnaryov VM, et al. Articular cartilage vesicles generate calcium pyrophosphate dihydrate-like crystals in vitro. Arthritis Rheum. 1992;35:231–40. doi: 10.1002/art.1780350218. [DOI] [PubMed] [Google Scholar]
- 13.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali SY, Griffiths S. Formation of calcium phosphate crystals in normal and osteoarthritic cartilage. Ann Rheum Dis. 1983;42(Suppl 1):45–8. doi: 10.1136/ard.42.suppl_1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein H, Bab IA, Sela J. The occurrence of hydroxyapatite crystals in extracellular matrix vesicles after surgical manipulation of the rabbit knee joint. Cell Tissue Res. 1981;214:449–54. doi: 10.1007/BF00233487. [DOI] [PubMed] [Google Scholar]
- 16.Einhorn TA, Gordon SL, Siegel SA, Hummel CF, Avitable MJ, Carty RP. Matrix vesicle enzymes in human osteoarthritis. J Orthop Res. 1985;3:160–9. doi: 10.1002/jor.1100030205. [DOI] [PubMed] [Google Scholar]
- 17.Derfus BA, Kurtin SM, Camacho NP, Kurup I, Ryan LM. Comparison of matrix vesicles derived from normal and osteoarthritic human articular cartilage. Connect Tissue Res. 1996;35:337–42. doi: 10.3109/03008209609029209. [DOI] [PubMed] [Google Scholar]
- 18.Wuthier RE, Chin JE, Hale JE, Register TC, Hale LV, Ishikawa Y. Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J Biol Chem. 1985;260:15972–9. [PubMed] [Google Scholar]
- 19.Ali SY. Analysis of matrix vesicles and their role in the calcification of epiphyseal cartilage. Fed Proc. 1976;35:135–42. [PubMed] [Google Scholar]
- 20.Mitchell NS, Shepard NL. Electron microscopic evaluation of the occurrence of matrix vesicles in cartilage. Anat Rec. 1990;227:397–404. doi: 10.1002/ar.1092270403. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Rosenthal AK. Articular cartilage vesicles and calcium crystal deposition diseases. Curr Opin Rheumatol. 2016;28:127–32. doi: 10.1097/BOR.0000000000000244. This is a comprehensive review of the role of extracellular vesicles in abnormal calcification and other OA-related changes in articular cartilage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenthal AK, Gohr CM, Ninomiya J, Wakim BT. Proteomic analysis of articular cartilage extracellular vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011;63:401–11. doi: 10.1002/art.30120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. Journal of extracellular vesicles. 2013:2. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Lin Z, Rodriguez NE, Zhao J, Ramey AN, Hyzy SL, Boyan BD, et al. Selective enrichment of microRNAs in extracellular matrix vesicles produced by growth plate chondrocytes. Bone. 2016;88:47–55. doi: 10.1016/j.bone.2016.03.018. This study supports the concept that miRNAs are selectively enriched in extracellular vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitton E, Gohr CM, McNally MT, Rosenthal AK. Articular cartilage vesicles contain RNA. Biochemical and biophysical research communications. 2009;388:533–8. doi: 10.1016/j.bbrc.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–8. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto S, Ochs RL, Rosen F, Quach J, McCabe G, Solan J, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3094–9. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Grewal R, Ninomiya J, Coyne CB, et al. Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. J Biol Chem. 2015;290:13028–38. doi: 10.1074/jbc.M114.630558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y, Xu Q, Luan J, Hu S, Pan J, Han J, et al. MVsCarta: A protein database of matrix vesicles to aid understanding of biomineralization. Bioscience trends. 2015;9:190–2. doi: 10.5582/bst.2015.01061. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis research & therapy. 2014;16:R163. doi: 10.1186/ar4679. This is the first study to demonstrate that extracellular vesicles mediate communication among cells from different joint tissues and that the effects of extracellular vesicles depend on theactivation state of the cells releasing them. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berckmans RJ, Nieuwland R, Tak PP, Boing AN, Romijn FP, Kraan MC, et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002;46:2857–66. doi: 10.1002/art.10587. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, et al. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem cells translational medicine. 2016;5:1620–30. doi: 10.5966/sctm.2015-0285. This study supports the concept that extracellular vesicles can be used in therapeutic approaches for connective tissue healing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murata K, Yoshitomi H, Tanida S, Ishikawa M, Nishitani K, Ito H, et al. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis research & therapy. 2010;12:R86. doi: 10.1186/ar3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪▪.Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Scientific reports. 2017;7:2029. doi: 10.1038/s41598-017-01905-y. This is the first report that synovial fluid exosomal miRNA content is altered in patients with OA, supporting the potential for extracellular vesicles as a new source of OA biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem Cells. 2007;25:2964–71. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 36.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees With Cartilage Defects. Clin Orthop Relat Res. 2015;473:2316–26. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017;25:2494–501. doi: 10.1007/s00167-016-3984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem cell reviews. 2015;11:150–60. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 40.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23:812–23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851–58. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS letters. 2015;589:1257–65. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 43.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nature reviews Rheumatology. 2012;8:543–52. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–85. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪▪.Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95. doi: 10.7150/thno.17133. This is an outstanding study demonstrating that intraarticular injection of exosomes that are enriched in miR-140 can be used to reduce the severity of experimental osteoarthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135–40. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]