Abstract
Nonalcoholic fatty liver disease (NAFLD) is a major public health problem afflicting approximately 1 billion individuals worldwide. Liver biopsy is considered the gold standard to assess severity of liver disease in patients with NAFLD. However, it is invasive, has high inter-observer variability, and is associated with adverse effects, including pain, infection, and rarely death. It is also impractical due to the large number of individuals who have NAFLD. Therefore, there is a strong need for the non-invasive assessment of disease severity in NAFLD. Over the last 2 decades, tremendous advances have been made in non-invasive imaging assessment of NAFLD. In this review, we will discuss the available evidence to quantify liver fat as well as liver fibrosis non-invasively using imaging modalities. We would also discuss the limitations of current modalities to detect the progressive form for NAFLD termed as nonalcoholic steatohepatitis (NASH). Finally we will discuss emerging comparative efficacy of various imaging based elastographic modalities and their diagnostic test characteristics to detect advanced fibrosis or cirrhosis.
Keywords: MRE, VCTE, SWE, ARFI, liver fibrosis, liver fat, nonalcoholic steatohepatitis, NAFLD, cirrhosis, bridging fibrosis, biomarker, CAP, CUS, QUS
Introduction
When a clinician sees a patient with NAFLD in a clinic setting. There are three broad clinical questions that emerge: 1) Does my patient have NAFLD, 2) Does my patient has NASH, and 3) Does my patient has any fibrosis or advanced fibrosis. These are the current unmet needs in the field and an area of intense investigation to search for an ideal biomarker or a clinical prediction rule for the assessment of disease severity in NAFLD. Tremendous advances have been made over the last decade to develop and refine the role of imaging based biomarkers to fulfill this unmet need in the field. In this section of this review, we will discuss the role of imaging-based biomarkers for addressing above-mentioned key clinical questions in assessing disease severity in NAFLD.
NAFLD is defined as presence of hepatic steatosis on either imaging or liver histology with at least 5% of hepatocytes showing steatosis in individuals who consume little or no alcohol and do not have any other secondary cause of hepatic steatosis such as excessive alcohol use, steatogenic drugs, viral hepatitis among others1. It can be broadly sub-divided in to two forms: Nonalcoholic fatty liver (NAFL); a non-progressive form of NAFLD, with limited or no risk of progression to cirrhosis and dying from liver disease, and nonalcoholic steatohepatitis (NASH), the progressive form of NAFLD, with substantial risk of progression to cirrhosis and dying from liver disease2. Emerging data from recent studies have shown that presence of fibrosis especially advanced fibrosis (stage 3: defined as bridging fibrosis, or stage 4: defined as cirrhosis) portends a worse prognosis and significantly increases the risk of death from liver disease3–5.
Liver biopsy, the current clinical gold standard to diagnose NASH and stage of fibrosis in NAFLD 6, is invasive and costly, it may be complicated by morbidity and even death 7, and so is not practical for screening the millions of asymptomatic, at-risk individuals or for monitoring changes in fibrosis stage over time 8, 9. Therefore, accurate, objective, quantitative, reproducible, precise biomarkers are needed to detect presence of hepatic steatosis, NASH, stage of fibrosis and presence of advanced fibrosis to risk stratify patients who need to be referred and treated by hepatologists1, 10.
As a clinician our goal is to reduce the risk of progression to cirrhosis and death from liver disease. We need diagnostic tools to help us identify the sub-group of patients who may need interventions. The imaging tests that are available or under development may be assessing different properties of the tissue including diffusion, texture, amount of fat, number of bonds in the fat, liver stiffness among others. The most common tissue property that has emerged as a surrogate for estimating liver fibrosis is liver stiffness or elasticity quantified by the various elastographic modalities. These emerging imaging-based biomarkers also provide an objective rather than subjective and a quantitative assessment of liver fat or fibrosis11. We will divide this review into two broad categories: Does my patient have NAFLD? And then answer, Does my patient have progressive form of liver disease? Provide data on NASH, presence of fibrosis. Finally we will discuss emerging methodologies that may have a role in future.
Assessment of liver fat by imaging
Role of Magnetic Resonance Spectroscopy (MRS) and Imaging for liver fat quantification
The gold standard for quantification of triglyceride (liver fat) content in the liver is magnetic resonance spectroscopy12. Therefore, when used in an appropriate clinical context MRS can yield an accurate diagnosis of NAFLD when hepatic steatosis is present as defined by a liver fat content of 5% or higher, and also help quantify the liver fat content beyond the presence of hepatic steatosis13. MRS noninvasively measures proton signals as a function of their resonance frequency. The signal intensity at frequencies corresponding to water or fat can be quantified, and the fat-signal fraction can be calculated. When performed properly, MRS is sensitive to even trace amounts of liver fat, and MRS is widely accepted as the most accurate non-invasive method to quantify liver fat14–16.
Limitations of MRS
MRS has important limitations that preclude its widespread clinical and research implementations17. MRS requires special expertise, is time consuming to perform, requires additional equipment and is not readily available clinically. It is restricted in spatial coverage. Sampling error is difficult to avoid which is problematic for longitudinal monitoring. Imaging based methods that evaluate the entire liver and that are simple to perform and analyze would be preferable17.
MRI-Proton Density Fat Fraction (MRI-PDFF)
Therefore, MRI based methods have been developed to estimate the liver fat content without using spectroscopy. The benefits of imaging based methods to quantify fat is that one can get an image of the liver so it is much easier to know the exact region of interest where fat was measured and this is particularly useful when monitoring patients longitudinal especially in the setting of a trial18–20. The reason for knowing the location of the region of interest for fat quantification is that liver fat is heterogenous and is not uniformly distributed and can vary from one segment to another. PDFF is a fundamental property of tissue and represents the ratio of MR-visible triglyceride protons to the sum of triglyceride and water protons21. The PDFF corrects for T1 decay and R2* and hence provides an accurate estimate of liver fat content as it corrects for inflammation, edema and iron content, respectively. It utilizes spectrally corrected, T1-independent, T2*-corrected magnitude and/or complex-based spoiled gradient echo pulse sequences and are available on both 1.5 and 3T scanners across a variety of scanner types22.
Comparison between ultrasound, CT and CAP for liver fat quantification
Conventional ultrasound lacks the sensitivity for detection of liver fat content at 5% reference23, 24. Due in part to the limitations of other noninvasive tests, conventional ultrasonography (CUS) remains the most commonly used imaging modality to diagnose and grade hepatic steatosis. CUS is widely available, safe, well tolerated, and relatively inexpensive, and it can be performed on scanners of any manufacturer. In this procedure, radiologists review conventional brightness B-mode sonographic images of the liver and surrounding structures and assess numerous sonographic features known to be affected by fat content such as the “echogenicity” of the liver relative to adjacent right kidney and the obscuration of liver structures. From these qualitative features, the presence and degree of hepatic steatosis are inferred 8, 24–27. The degree of steatosis may be reported using subjective terms (“mild, moderate, severe”) or more formally using ordinal CUS scores 15, 24, 27–30 designed to match the ordinal scores used by pathologists for grading hepatic steatosis on histology. Fundamental limitations of CUS are that individual sonographic features are interpreted qualitatively and these features are affected by numerous factors other than the presence and degree of hepatic steatosis, including patient (obesity, coexistent renal disease) and acquisition (scanner, transducer, operator, and instrument settings) factors. Consequently, CUS is limited in accuracy, repeatability, and reproducibility for both diagnosis and grading of hepatic steatosis 27, 31–33, particularly in obese individuals 8, 34, 35 who are at risk for NAFLD. Computed tomography (CT) is rarely used to look for NAFLD. It is typically an incidental finding on an abdominal CT performed for another indication. Density of liver to spleen ratio is typically used to detect hepatic steatosis on CT scan. Due to exposure to ionizing radiation, CT scans are not favored for assessment of hepatic steatosis.
Recent studies have shown that quantitative ultrasound may be superior than conventional ultrasound to detect hepatic steatosis36.
Controlled attenuation parameter (CAP) is a tool that may be helpful in the assessment of liver fat. CAP is available on newer fibroscan machines as an adjunct to liver stiffness measurement by Vibration Controlled Transient Elastography (VCTE). Recent studies have shown that CAP may be utilized to detect hepatic steatosis but does not provide a reliable quantitative estimate of liver fat content37. Karlas et al conducted a meta-analysis, including data from 19 of the 21 available studies of CAP performance.38 This study included 2,735 patients with various causes for liver disease of which 537 (19.6%) had NAFLD. The AUROC for the presence of hepatic steatosis on liver biopsy by CAP was 0.823. Wong and colleagues have provided much needed guidance regarding the reliability criteria for its utility in practice37. Data also suggest that IQR would serve as a key measure of reliability of CAP reading for the detection of hepatic steatosis37. Caussy and colleagues have recently provided that the optimal cut-point of CAP for the detection of 5% liver fat on MRI-PDFF is 288 db/sec. The criteria for the reliability of CAP has been further refined and it appears that a 30db/sec variability in CAP is optimal threshold for a reliable reading on CAP for liver fat assessment. CAP values may attenuate the accuracy of liver stiffness values especially in those with higher CAP in the setting of low fibrosis score (stage 0–2)39. Furthermore, emerging data suggest that XL-probe may slightly overestimate the CAP reading relative to M-probe but head to head studies comparing XL-probe versus M-probe within the same patient are needed to evaluate the CAP values, and the exact cut-point for a 5% liver fat content cut point40.
CAP versus MRI-PDFF
Two studies have performed head to head comparison between CAP and MRI-PDFF with contemporaneous liver biopsy assessment41, 42. Both studies have consistently demonstrated that MRI-PDFF is better than CAP for the each dichotomous category for grade of steatosis. Table 1. provides data on various liver fat assessment imaging modalities and their characteristics and caveats associated with their use in clinical practice and research.
Table 1.
Comparison between commonly used modalities for liver fat quantification
| Modality | Cost | Accuracy | Point of care | Quantitative | Caveats |
|---|---|---|---|---|---|
| Conventional ultrasound | + | ++ | Yes | No | May fail in obesity and in iron overload and cirrhosis |
| CAP | + | ++ | Yes | Yes, but not linear in higher liver fat content | Affected by type of probe and fibrosis |
| CT | ++ | ++ | No | Semi-quantitative | Ionizing radiation |
| MRI-PDFF | ++ | +++ | No | Yes | Not suitable for screening |
CAP: controlled attenuation parameter, CT: computed tomography, MRI-PDFF: magnetic resonance imaging proton density fat fraction
Novel ultrasound based tests for liver fat assessment
Back Scatter Coefficient (BSC) and Attenuation Parameters (AP) are emerging as two quantitative biomarkers for liver fat quantification. Both BSC and AP appear to provide high diagnostic accuracy for detection of hepatic steatosis36. However, future multicenter trials are needed to determine a reproducible protocol and apply these QUS parameters to routine ultrasound imaging modalities.
Assessment of NASH and fibrosis by Imaging
Most of the imaging modalities perform poorly in the detection of NASH and may not be reliable for the detection of NASH by themselves. However, significant progress has been made for assessing the role of liver stiffness as a quantitative imaging based biomarkers for detection and quantification of liver fibrosis in patients with NAFLD. We would refer the reader to recent reviews and guidelines written on this topic (Ajmera et al. Seminars in Liver Disease in press)1, 10.
Role of VCTE
VCTE (fibroscan, Echosens, Paris) is a point of care elastographic method that provides estimation of liver stiffness measurement (LSM). Liver stiffness measurement has been surrogate to provide an estimate of liver fibrosis stage. Details regarding the VCTE procedure and shear wave propagation have been well-described in previous reviews. There are two probes that are now available: M-probe and XL-probe in which the wave propagation are examined at a fixed depth of 25–65 mm in the case of M-probe and 35–75 mm in the case of XL-probe. A LSM is provided in KiloPascals (Kpa)39, 42. A notable limitation of VCTE is the high failure rates in obese patients 43, 44, which limits reliable measurement of liver stiffness by VCTE in a significant proportion of NAFLD patients. Therefore, an XL-probe was designed to gain a better estimation of LSM in morbidly obese individuals as M-probe may fail in such cases as liver is deeper than 25 mm from the skin surface45. XL-probe has a lower shear wave frequency, with increased amplitude and a deeper focal length that has shown to reduce the failure rate for estimating LSM and may provide more reliable estimates in the setting of morbid obesity 46, 47.
VCTE has been well-studied in the setting of viral hepatitis and well-designed studies using large cohorts with paired assessment of VCTE with biopsy have yielded robust quality criteria for it’s application. The VCTE quality criteria are similar across various etiologies of liver disease. VCTE quality criteria includes a minimum of 10 measurements are made to obtain the median valid liver stiffness measurements in kilopascals (kPa) and the interquartile range (IQR), an interquartile range (IQR)-to-median LSM ratio of ≤0.3. LSM, and a success rate of ≥ 60% to obtain the 10 measurements8, 39. Siddiqui and colleagues conducted a prospective study including 393 patients with NAFLD who underwent VCTE within one year of liver biopsy assessment as a sub-study of the NASH CRN study cohort. In this multicenter study, the diagnostic accuracy of VCTE using cross-validated AUROC for differentiating fibrosis stage 0 from stages1–4 was: 0.74 (95% CI 0.68–0.79); fibrosis stages 0–2 from stages 3–4 was: 0.83 (95%: 0.79, 0.87); and fibrosis stages 0–3 from stage 4 was: 0.93 (95%: 0.90, 0.97), respectively (personal communication). As expected the diagnostic accuracy of VCTE in this multicenter study is lower than as previously reported by Tapper et al in their seminal, single center study among patients with biopsy-proven NAFLD from the United States48. The optimal cut-point to be utilized for population screening or referral to a hepatology clinic or perform a liver biopsy still remains to be determined to inform practice guidelines1.
Role of ARFI and SWE
Acoustic Resonance Forced Impulse Imaging (ARFI) (Antares or Acuson S2000, Siemens Medical Solutions, Mountain view, CA) elastography is integrated into a conventional ultrasound device and provides an estimate of liver stiffness in shear wave speed (m/sec). SWE (ElastQ; Philips, Andover, USA. Logiq E9; GE Healthcare, Wauwatosa, USA. Aixplorer; Supersonic Imagine, Aix-en-Provence, France) is an FDA approved technique that adapts ultrasound imaging to produce LSM. Similar to ARFI, the SWE operator must define a large, vessel-free region of interest themselves in ultrasonic B-mode imaging using curved abdominal probe during breath hold, and take a series of measurements (typically seven to eleven), and then a median of those values is obtained. There are limited quality criteria for the clinical application of ARFI or SWE, and further studies are needed to standardize the protocols for obtaining ARFI and SWE readings.
In general, SWE and ARFI provide a better and more reliable estimate of liver fibrosis than VCTE. However, the data and experience in the use of these devices in NAFLD is currently limited. Future studies are needed to define their role and help establish quality criteria so the ARFI and SWE readings can be standardized.
Palmeri and colleagues examined 172 patients with NAFLD and found that the diagnostic accuracy of ARFI for the detection of advanced fibrosis was 0.9049. More recently, Liu et a conducted a meta-analysis to examine the role of ARFI in detection of advanced fibrosis in NAFLD and found it to be of moderate accuracy:50.
Head to head comparison between MRE and VCTE, VCTE and ARFI and SWE, and MRE and ARFI
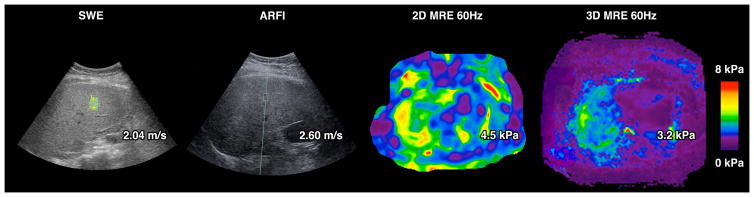
Table 2. provides data on various elastographic modalities and their characteristics and caveats associated with their use in clinical practice. Figure 1. Provides a contemporaneous imaging assessment of a patient with stage 3 fibrosis who underwent an ARFI, SWE, 2D and 3D MRE. There are emerging data on head to head comparison between various elastographic methods with liver biopsy assessment as the reference standard. Imajo and colleagues examined 142 patients with biopsy-proven NAFLD residing in Japan (Average BMI 28.1 Kg/m2) who underwent VCTE using M-probe as well as a contemporaneous MRE41. They found that MRE was better than VCTE for the detection of any fibrosis. In a more recent study, Park and colleagues examined 104 patients with biopsy-proven NAFLD residing in Southern California (Average BMI 30.4 Kg/m2 ) who underwent VCTE using both M and XL-probe as well as a contemporaneous MRE42. Both the studies noted that MRE was better than VCTE for the detection of early stages of fibrosis. In a study including morbidly obese individuals, Chen and colleagues demonstrated that MRE is better than VCTE (M and XL probe) for the detection of stage 3–4 fibrosis (AUROC 0.97 vs 0.87, p = 0.046)51. To put these three studies in context, it is notable that the average BMI is lower in Imajo et al than Park et al than Chen et al suggesting that as the BMI of the cohort increases MRE is likely to outperform VCTE especially if the BMI ≥ 35 Kg/m2 even for the presence of advanced fibrosis. These three single center studies are helpful but individual patient meta-analyses are needed to further assess the comparative efficacy of MRE versus VCTE.
Table 2.
Comparison between elastographic modalities
| Modality | Cost | Accuracy | Point of care | Quality Criteria | Caveats |
|---|---|---|---|---|---|
| VCTE | + | ++ | Yes | Standardized | Increased variability in morbid obesity and cirrhosis |
| ARFI/SWE | + | ++ | Can be | QIBA* is working on it | Increased variability in morbid obesity and cirrhosis |
| MRE | ++ | +++ | No | QIBA* is working on it | Excellent accuracy in obesity and cirrhosis May fail in the setting of Iron overload |
VCTE: vibration controlled transient elastography, ARFI: Acoustic radiation forced impulse imaging, SWE: shear-wave elastography, MRE: magnetic resonance elastography
Figure 1.
A patient with stage 3 fibrosis and NASH underwent a SWE (using GE) with a reading of 2.04 m/s, an ARFI (Siemens ultrasound) with a reading of 2.60 m/s, a 2D MRE at 60 Hz (GE 3 Tesla) with a reading 4.5 K pa, and a 3D MRE at 60 hz (GE 3 Tesla) with a reading of 3.2 Kpa. These readings are suggestive of advanced fibrosis across all four modalities.
A small number of studies with >100 patients have compared VCTE with ARFI or SWE. Cassinoto et al.52 showed that the findings for the assessment for the presence of advanced fibrosis were comparable (AUROC for VCTE was 0.86, AUROC for ARFI was 0.84 and AUROC for SWE was 0.89). In this study, the authors noted that in the subgroup of patients with BMI < 30 kg/m2, ARFI may be associated with more reliable results for the assessment of liver fibrosis stage.
Cui and colleagues examined 125 patients with biopsy-proven NAFLD residing in Southern California who underwent ARFI as well as a contemporaneous MRE53. They found that MRE was better than ARFI for the detection of any fibrosis especially among obese patients with NAFLD.
Other imaging parameters of interests including 3D MRE, multiparametric MRI, and multiscan
Recent studies have shown that 3D MRE may be better than 2D MRE. 3D MRE is a more advanced version of the technology that can image shear-wave fields in 3 dimensions of the entire liver rather than a smaller area of interest in the case of 2D MRE. Loomba and colleagues showed that the AUROC for diagnosing advanced fibrosis was 0.981 for 3D MRE at 40 Hz versus 0.921 for 2D MRE at 60 Hz (standard shear-wave frequency available clinically)54. 3D MRE has been utilized in the setting of treatment response assessment in NASH but it requires significant expertise and is not ready for routine clinical practice18. However, 3D MRE derived liver stiffness assessments provide proof-of-concept data that we can further refine the available modalities for the assessment of fibrosis in NAFLD. Other emerging modalities of interest include corrected T1 decay using the multiscan platform (Perspectum Diagnostics Inc)55. Pavilides and colleagues conducted a study including 71 patients with biopsy-proven NAFLD who underwent a contemporaneous MRI assessment using multiscan platform and VCTE. They reported that mulitscan was able to diagnose NASH with an AUROC of 0.856. These data are encouraging and remain to be validated in larger validation studies that are underway. In addition, there are emerging data on multiparametric MRI/MRE that may have utility in the assessment of inflammation and ballooning beyond liver stiffness57. A combined assessment using multiparametric MRI/MRE including damping ratio derived from 3D MRE, and liver stiffness by MRE, and MRI-PDFF may be combined to inform detection of NASH and early stages of fibrosis (personal communication Richard Ehman). These data require multicenter validation, and further studies are needed before clinical application and implementation.
Longitudinal assessment of changes in liver fat and fibrosis assessment
MRI-PDFF is now increasingly utilized especially in the setting of clinical trials to assess longitudinal changes in liver fat content. Emerging data suggest that a 30% relative decline in liver fat content corresponds to a 2-point reduction in the NAFLD activity score. Hence, a 30% relative reduction may be the threshold for classifying a clinically meaningful reduction in liver fat in the setting of a clinical trial58. However, further studies are needed to validate these findings.
MRE is also now been utilized for longitudinal assessment of changes in liver fibrosis especially in the setting of clinical trials along with other elastographic methods including VCTE. A recent study has shown that approximately 15–19% relative reduction in MRE derived liver stiffness corresponds to a 5% reduction in body weight (usually the lower threshold associated with improvement in liver histology in NASH)59. QIBA document under review also suggests that a 19% reduction in MRE is not likely to due to chance or error and would suggest a significant decrease in MRE. However, exact clinical significance of a change in liver stiffness measurement still remains to be defined. This is an intense area of research currently. Longitudinal studies are underway to address these queries.
Utilization of these modalities in clinical practice
Assessment of NAFLD
MRI-PDFF may be utilized as the gold standard to either detect or quantify liver fat content. However, it is expensive and may not be routinely available. Therefore, it should be reserved in the setting of clinical trials or epidemiologic studies. It may be used in clinical practice when other tests fail or there is a critical need to quantify liver fat content e.g. living liver donor assessment. Conventional ultrasound is not sensitive but when it is positive it has a high specificity for detection of moderate to severe hepatic steatosis. CUS is neither sensitive nor specific for liver fat quantification. CAP may be used to detect hepatic steatosis if the IQR is less than 30 db/m as the readings are likely to be highly reliable60. A threshold of 288 db/sec of CAP reading has a 80% accuracy for the presence of hepatic steatosis. CAP lacks the ability to quantify liver fat content on a dynamic range especially when the liver fat content ranges between 15% and 50%42. Further research is needed to optimize CAP to achieve higher degree of correlation with MRI-PDFF.
Assessment of Advanced fibrosis
All of the imaging tests currently utilized have a high negative predictive value for excluding advanced fibrosis. A strategy that may be considered in clinical practice is to utilize a clinical prediction rule such as a FIB-4 to exclude advanced fibrosis, and then perform an elastography-based method to further assess for advanced fibrosis in those who are either in the grey-zone or have a high likelihood of advanced fibrosis. Individuals with a BMI less than 35 kg/m2 may undergo any elastography based test depending upon the availability and the cost to the institutions/payers. As the diagnostic accuracy of ultrasound based modalities is significantly lower in the setting of morbid obesity (BMI ≥ 35 kg/m2)61, an MRE may be utilized if it is available and is not cost-prohibitive. Overall, MRE provides the highest diagnostic accuracy among all imaging modalities for the assessment of fibrosis in NAFLD. However, it is not practical to be used in routine clinical care across the millions of individuals who are at risk for advanced fibrosis. VCTE offers real-time results and is performed as a point-of-care test. This approach adds value to the clinic visit as it allows for in-clinic counselling and a decision to pursue a liver biopsy can be made expediously. Similar to VCTE, SWE or ARFI may also be utilized for risk stratification of NAFLD patients based upon their availability and local expertise and familiarity with their cut-points for the presence of advanced fibrosis for shear wave speed or stiffness, respectively. Furthermore, e a discussion with the local hepatologist and/or radiologist should be performed to decide on the preferred techniques, requirements for operator experience, and pre-specified criteria for reporting to ensure correct interpretation of results. SWE or ARFI are currently not used as a point-of-care test but they have the potential to be developed into a point-of-care test in future.
Future research directions
Imaging assessment of NAFLD is an area of intense research and clinical significance. Following are the key unmet needs in the field.
Diagnosis
Prospective studies are needed to develop an optimal algorithm for step-wise utilization of multiple elastographic methods along with clinical prediction rules and/or serum-based biomarkers to develop a cost-effective strategy to screen for not only stage 3 and stage 4 patients but also those with stage 2 or higher fibrosis so we can detect patients who should be enrolled in registration trials for the treatment of NASH related fibrosis without needing a liver biopsy. Clinicians also need guidance on which modalities are likely to provide a better estimate of fibrosis stage based upon patient-related factors such as morbid obesity or ascites or severe iron overload. Finally, a strategy that involves initial test to screening out individuals who are at a low risk of any fibrosis followed by a second more specific test to rule in for the presence of stage 2 or higher fibrosis.
Context of use
We need to established cut-points for each modality depending upon the context of use. The cut-points for excluding any fibrosis at the level of population will be very different than those when the same test is being utilized in the NAFLD clinic in a tertiary care center. Better understanding of the context of use is critical to develop evidence-based practice guidelines for screening for NAFLD related fibrosis.
Quality criteria and cost-effectiveness
The field needs further studies to provide key quality criteria for the application of SWE and ARFI and other ultrasound based modalities similar to what has been established for VCTE. Once criteria for an optimal examination is established we will need comparative cost-effectiveness studies to examine the role of these modalities in routine clinical practice.
Optimal cut-points for each modality in NAFLD
The clinicians would benefit from guidelines derived from evidence generated from individual patient meta-analyses for an optimal cut-point for detection of specific stages of fibrosis in NAFLD, and their clinical utility.
Association with long-term clinical outcomes in NAFLD
Prospective cohort studies are needed to examine the longitudinal association between baseline imaging based modalities or biomarkers and their longitudinal change over-time and incident development of cirrhosis, risk of hepatic decompensation, need for liver transplant and liver-related mortality.
Clinically meaningful improvement in NAFLD and fibrosis
Future studies are needed regarding the amount of decrease in liver stiffness by VCTE, SWE, ARFI and MRE (and other imaging based biomarkers) that is associated with 1-stage improvement in liver fibrosis in NAFLD or reversal of NASH or cirrhosis or risk of hepatic decompensation. These data are needed to replace liver histology with non-invasive imaging assessment as the primary endpoint in Phase 3 trials.
Concluding remarks: Is the liver biopsy still needed?
Liver biopsy assessment remains the gold standard as the optimal strategy for screening for advanced fibrosis remains to be established. The exact cut-off for each modality and their optimal utility in the appropriate context of use remains to be studied and then clearly articulated in future practice guidelines. Whether the patient has NAFLD can be accurately determined using MRI-PDFF without subjecting the patient for a liver biopsy assessment. However, we are not able to differentiate whether the patient has NAFL versus NASH especially in early stages of fibrosis or in the setting of no fibrosis. Presence of any fibrosis can now be detected using MRE alone with an accuracy of approximately 80%. This needs to be further improved and the current status of all imaging modalities is suboptimal for routine clinical assessment. Therefore, a liver biopsy is still needed to answer this question. Whether a patient has advanced fibrosis can be reliably answered using an MRE. Although MRE can provide a diagnostic accuracy ranging between 0.92–0.95 for the detection of advanced fibrosis it is impractical to suggest using it for the routine non-invasive detection of advanced fibrosis at this time. VCTE, SWE and ARFI provide good discrimination for detection of advanced fibrosis and cirrhosis in non-obese but have lower accuracy due to higher variability or lesser reliability especially among obese individuals.
The field needs a step-wise strategy that is developed on a solid foundation of evidence-based medicine rather than cut-points derived from retrospective studies. Cost-effectiveness studies are needed to determine the optimal strategy for the assessment of liver fibrosis in patients with NAFLD. We believe that a step-wise approach may be studied and then utilized if proven to improve quality of life years saved by using a clinical prediction rule such as a FIB-4 followed by an ultrasound based method depending upon availability including VCTE, ARFI or SWE, and then consider MRE in those who either fail the ultrasound-based methods or have risk factors such as morbid obesity (BMI ≥ 35 Kg/m2) to receive an MRE assessment. Before clinical implementation of such an approach, prospective studies should be performed so that the decisions are well-informed and we do not harm the patients by neglecting those who would test negative but still have advanced fibrosis or bring in individuals who do not have any fibrosis for a more detailed assessment or subject them to a liver biopsy examination when they may not need it. Multicenter, collaborative, prospective studies with a built-in-strategy of sequential testing versus standard of care are needed to further inform clinical practice.
Acknowledgments
I would like to thank Drs. Veeral Ajmera and Cyrielle Caussy for their thoughtful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017 doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. e1–9. doi: 10.1016/j.cgh.2014.04.014. quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: Systematic Review and Meta-analysis. Hepatology. 2017 doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–97. e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 7.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49:1017–44. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 8.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666–75. doi: 10.1038/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver. Electronic address eee. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 11.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeder SB, Cruite I, Hamilton G, et al. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–49. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong VW, Wong GL, Yeung DK, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62:182–9. doi: 10.1016/j.jhep.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Tang A, Tan J, Sun M, et al. Nonalcoholic Fatty Liver Disease: MR Imaging of Liver Proton Density Fat Fraction to Assess Hepatic Steatosis. Radiology. 2013 doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwenzer NF, Springer F, Schraml C, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–45. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 17.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–50. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–32. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369–76. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoo T, Serai SD, Pirasteh A, et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology. 2017:170550. doi: 10.1148/radiol.2017170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton MS, Heba ER, Hooker CA, et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-assigned Steatosis Grades of Liver Biopsies from Adults with Nonalcoholic Steatohepatitis. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paige JS, Bernstein GS, Heba E, et al. A Pilot Comparative Study of Quantitative Ultrasound, Conventional Ultrasound, and MRI for Predicting Histology-Determined Steatosis Grade in Adult Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2017;208:W168–W177. doi: 10.2214/AJR.16.16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–90. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamer OW, Aguirre DA, Casola G, et al. Imaging features of perivascular fatty infiltration of the liver: initial observations. Radiology. 2005;237:159–69. doi: 10.1148/radiol.2371041580. [DOI] [PubMed] [Google Scholar]
- 26.Hamer OW, Aguirre DA, Casola G, et al. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637–53. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 27.van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159–68. doi: 10.1148/radiol.10091790. [DOI] [PubMed] [Google Scholar]
- 28.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 30.Ballestri S, Lonardo A, Romagnoli D, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32:1242–52. doi: 10.1111/j.1478-3231.2012.02804.x. [DOI] [PubMed] [Google Scholar]
- 31.Strauss S, Gavish E, Gottlieb P, et al. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320–3. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 32.Fishbein M, Castro F, Cheruku S, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619–25. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007–19. doi: 10.1016/j.jhep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14:635–7. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 35.de Moura Almeida A, Cotrim HP, Barbosa DB, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14:1415–8. doi: 10.3748/wjg.14.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SC, Heba E, Wolfson T, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337–1345. e6. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong VW, Petta S, Hiriart JB, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67:577–584. doi: 10.1016/j.jhep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Karlas T, Petroff D, Sasso M, et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. Journal of Hepatology. 2016 doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Petta S, Wong VW, Camma C, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145–1155. doi: 10.1002/hep.28843. [DOI] [PubMed] [Google Scholar]
- 40.Chan WK, Nik Mustapha NR, Wong GL, et al. Controlled attenuation parameter using the FibroScan(R) XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5:76–85. doi: 10.1177/2050640616646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637. e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 42.Park CC, Nguyen P, Hernandez C, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598–607. e2. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Ledinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–31. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Foucher J, Castera L, Bernard PH, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411–2. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Vuppalanchi R, Siddiqui MS, Van Natta ML, et al. Performance Characteristics of Vibration- Controlled Transient Elastography for Evaluation of Non-Alcoholic Fatty Liver Disease. Hepatology. 2017 doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Ledinghen V, Wong VW, Vergniol J, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R) J Hepatol. 2012;56:833–9. doi: 10.1016/j.jhep.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–71. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 48.Tapper EB, Challies T, Nasser I, et al. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients With Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2016;111:677–84. doi: 10.1038/ajg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmeri ML, Wang MH, Rouze NC, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666–672. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H, Fu J, Hong R, et al. Acoustic Radiation Force Impulse Elastography for the Non-Invasive Evaluation of Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review & Meta-Analysis. PLoS One. 2015;10:e0127782. doi: 10.1371/journal.pone.0127782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Yin M, Talwalkar JA, et al. Diagnostic Performance of MR Elastography and Vibrationcontrolled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology. 2017;283:418–428. doi: 10.1148/radiol.2016160685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassinotto C, Boursier J, de Ledinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016 doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 53.Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453–61. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loomba R, Cui J, Wolfson T, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavlides M, Banerjee R, Sellwood J, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308–15. doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavlides M, Banerjee R, Tunnicliffe EM, et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017;37:1065–1073. doi: 10.1111/liv.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology. 2017;284:694–705. doi: 10.1148/radiol.2017160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9:692–701. doi: 10.1177/1756283X16656735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel NS, Hooker J, Gonzalez M, et al. Weight Loss Decreases Magnetic Resonance Elastography Estimated Liver Stiffness in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15:463–464. doi: 10.1016/j.cgh.2016.09.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caussy C, Alquiraish MH, Nguyen P, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2017 doi: 10.1002/hep.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caussy C, Chen J, Alquiraish MH, et al. Association Between Obesity and Discordance in Fibrosis Stage Determination by Magnetic Resonance vs Transient Elastography in Patients with Nonalcoholic Liver Disease. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]