Abstract
Angiogenesis is a critical component during wound healing, and the process is sensitive to mechanical stimuli. Current in vitro culture environments used to investigate three-dimensional microvascular growth often lack dimensional stability and the ability to withstand compression. We investigated the ability of decorin, a proteoglycan known to modulate collagen fibrillogenesis, incorporated into a collagen hydrogel to increase construct dimensional stability while maintaining vascular growth. Decorin did not affect microvascular growth parameters, while increasing the compressive modulus of collagen gels and significantly reducing the contraction of 3% collagen gels after 16 days in culture.
Introduction
Vascular growth and remodeling are processes that are highly sensitive to mechanical cues; however, much of the existing research in this field has focused on luminal mechanics related to fluid flow (i.e. fluid shear and cyclic stretch) rather than abluminal stimulation of the vessel network itself [1]. Abluminal forces are particularly relevant to load-bearing tissues such as bone, and it is well established that angiogenesis and osteogenesis are intimately linked in both development and healing [2, 3]. Additionally, previous work in vivo showed that functional loading has a potent time-dependent influence on vascular growth during segmental bone defect healing; early loading impaired vessel growth, whereas delayed loading enhanced vascular growth [4]. Well-controlled in vitro studies are needed to better understand the mechanical cues associated with vascular growth and inhibition.
Microvascular fragments (MVF) are multicellular segments of vasculature that can sprout and form networks in vitro [5]. In combination with an appropriate 3D substrate, MVFs allow for interactions between multiple cell types and between cells and their matrix, thereby better representing the complex in vivo processes of angiogenesis than models utilizing single cells or 2D substrates. Previous work utilizing MVF constructs has shown that they are sensitive to chemical cues, extracellular matrix (ECM) mechanical properties, and tensile forces [6–8]; however, they have yet to be studied under compressive forces. MVF are typically cultured in three dimensions within type I collagen gels, which contract significantly over time in culture [5, 8]. Gel contraction prevents longitudinal analysis of cultures, which is a serious limitation for studies aiming to investigate time-dependent effects. Thus, to study the role of a compressive mechanical environment on MVF growth over time in vitro, there is a need for a material substrate that both supports MVF growth and has greater dimensional stability than a low percentage collagen gel.
Although increasing the percentage of collagen within the gel increases structural stability, MVF growth is hindered at high collagen densities [8]. Crosslinking methods exist to chemically link the collagen fibrils rather than relying on physical interactions; however, unreacted crosslinking reagents can be cytotoxic and are therefore not amenable to cell encapsulation prior to crosslinking [9]. While synthetic materials offer advantages of tunability and batch-to-batch consistency, both scaffold development and MVF culture would require optimization, whereas MVF growth in collagen is quite well characterized. Thus, this research focused on techniques to modify the mechanical properties of collagen hydrogel constructs.
Decorin (DCN) is a small leucine-rich proteoglycan (SLRP) expressed in connective tissue that modulates collagen fibrillogenesis and alters the mechanical properties of collagen hydrogels [10–14]. A previous in vitro study demonstrated that addition of DCN during collagen fibrillogenesis led to a denser fibril network with a higher tensile modulus [14]. Our objective here was to investigate the ability of DCN-supplemented collagen hydrogels to 1) support microvascular growth, 2) maintain dimensional stability throughout at least 14 days in culture, and 3) withstand physiologic compressive loads.
Methods
Decorin Purification
Methods for decorin purification were based on published protocols [14, 15]. Bovine knee ligament samples were frozen in liquid nitrogen and pulverized. The powder was suspended in an extraction solution [15] at 4 °C overnight before centrifugation at 20,000 g for 2 hours. The resulting supernatant was then sequentially filtered through a 1.1 μm glass fiber filter and a 0.45 μm regenerated cellulose filter. The filtrate was subjected to anion exchange chromatography over a POROS PI column at 4 °C. GAG-containing fractions were identified by dimethylmethylene blue (DMB) assay, pooled, concentrated via ultrafiltration, and purified by gel chromatography with a sepharose CL-4B column. GAG-containing fractions were pooled as above, and DCN-containing fractions were identified by SDS-PAGE [11] before ultrafiltration and buffer exchange. Hydrophobic chromatography was performed on the DCN fractions using an octyl-sepharose column at 25 °C. DCN-containing fractions were monitored and concentrated as above before buffer exchange into phosphate buffered saline (PBS) using PD-10 desalting columns. The solution was sterilized by filtration. DCN purity was confirmed with SDS-Page [11], and concentration was determined by DMB assay.
Gel Formation and Characterization
Collagen gels were created from rat-tail type I collagen (Corning Life Sciences, Corning, NY). Collagen solutions, 3 mg/mL or 6 mg/mL with or without 50 μg/mL DCN, were buffered with 1× Dulbecco’s modified eagle medium (DMEM) and mixed on ice. Gels were formed at 37 °C unless otherwise stated.
For turbidity measurements, which assess collagen fibrillogenesis kinetics, 100 μL of ice cold collagen solution was pipetted into a 96-well plate (n=5–6/group). Absorbance readings at 405 nm were made every minute for 60 minutes at room temperature [14]. Acellular gels were imaged using second harmonic generation (SHG) for qualitative comparison of collagen fibrils using a Zeiss LSM 710 confocal microscope (800 nm excitation).
To evaluate gel mechanical properties, acellular gels were tested in unconfined compression using a Bose ELF 3100 system with a 25 g load cell (Bose, Framingham, MA; Transducer Techniques, Temecula, CA). Gels were formed in a custom mold (8 mm diameter × 5.7 mm height) and incubated in PBS at 37 °C overnight prior to testing; gels were submerged in PBS in an acrylic chamber during testing at room temperature. Gels were preconditioned with 10 cycles of a triangle waveform varying from 0 to 10% strain at 0.05 Hz; testing was then performed using a monotonic ramp to 30% strain at 0.02 mm/s. Gels were held at 30% strain for 150 s to allow stress relaxation. Peak stress was determined by averaging the stress during the last one second of the strain ramp. The equilibrium modulus was calculated after stress relaxation by averaging the last ten seconds of the 150 s hold time at 30% strain. The linear modulus was defined as the slope of a linear regression fit to the linear region (10%-30%) of the applied strain ramp; all linear fits had a correlation coefficient ≥ 0.9.
Microvascular Fragment Culture and Analysis
MVF were isolated as previously described [16]. Briefly, adipose tissue was harvested from epididymal fat pads of Lewis rats, minced, and partially digested with a collagenase solution. Selective filtration was then used to remove tissue larger than 500 μm and single cells smaller than 20 μm. MVF were suspended at 20,000 fragments/mL of gel and cultured in rhVEGF-supplemented serum-free media [7].
Quantification of vessel growth was done on day 9 of culture. MVF-containing gels were fixed with 4% paraformaldehyde, and rhodamine labeled Griffonia simplicifolia (GS-1) lectin (Vector Laboratories, Burlingame, CA) was used to stain vessel structures for quantification. GS1-lectin-stained MVF networks were imaged using a Zeiss LSM 700 confocal microscope. Five randomly selected fields were imaged at 10× to a depth of 402 μm for each gel, and 7–8 gels were imaged per group. Maximum intensity z-projections were created for each stack, thresholded, and skeletonized in ImageJ to determine branch number and total length. Gel contraction was determined by quantification of gel area (ImageJ) from photographs (Gel Doc XR, Bio-Rad, Hercules, CA) of well plates over time in culture.
Statistics
All experiments were performed with n≥5, and results are presented as mean ± SEM. All analyses were performed as a two-way ANOVA with α level of 0.05 and Bonferonni post hoc tests.
Results
The addition of DCN to both 3% and 6% collagen solutions led to a lower ultimate turbidity (p<0.001 for 3%, p<0.01 for 6%). DCN did not affect the lag time, the initial phase of fibrillogenesis during which nucleation sites form and turbidity is low, in 3% collagen. However, in 6% collagen, DCN shortened the lag time (p<0.05), allowing for an earlier onset of fibrillogenesis. The rate of fibrillogenesis as evidenced by the slope of the turbidity curve growth phase was not significantly affected by DCN in either group (Fig. 1). There were overall effects of collagen percentage on both ultimate turbidity (p<0.0001) and rate of fibrillogenesis (p<0.0001), with higher collagen density increasing both parameters. Qualitatively, in both 3% and 6% gels, the addition of DCN during polymerization of the collagen gels appeared to cause a more spatially uniform but diffuse organization of collagen fibrils with fewer short, wide bundles. Both 6% gels appeared denser than either 3% gel (Fig. 2).
Figure 1.
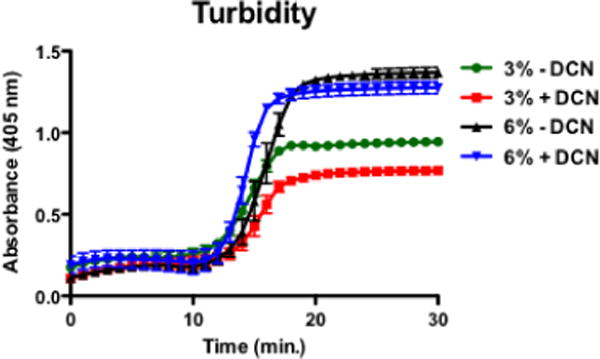
Turbidity curves for 3% and 6% collagen ± 50 μg/mL DCN (n=5–6/group).
Figure 2.
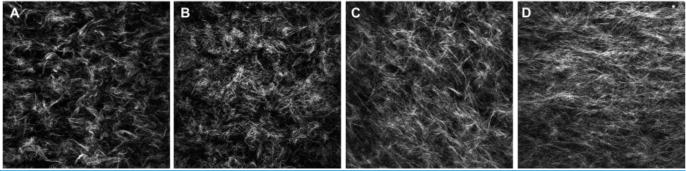
Representative single-plane 60× SHG images of A) 3% collagen without DCN, B) 3% + DCN, C) 6% − DCN, and D) 6% + DCN.
Both collagen density (p<0.0001) and DCN (p<0.0001) increased the peak stress experienced at 30% strain. There was also a significant, ordinal interaction effect (p=0.0004); the effect of DCN was greater in 6% than in 3% collagen (Fig. 3A). The equilibrium modulus was significantly higher in 6% vs. 3% collagen (p<0.001) but was not affected by DCN (p=0.125) (Fig. 3B). The linear modulus was also increased by increasing collagen density (p<0.0001), and DCN increased the linear modulus of 6% gels (p<0.01). The linear modulus of 3% gels was not affected by DCN, and there was no significant interaction (Fig. 3C).
Figure 3.
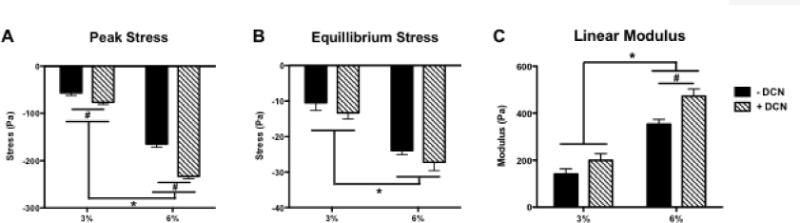
Gel compression testing results showing A) peak stress experienced at 30% load, B) equilibrium stress level under maintained 30% strain, and C) linear modulus (n=5/group). * indicates significant differences between 3% and 6% gels (p<0.0001). # indicates significant differences between ± DCN (p<0.01 for 6%; p<0.05 for 3%).
MVF cultured in 3% collagen gel resulted in significantly greater total network length (p<0.0001) and number of branches (p<0.0001) than those cultured in 6% gel. There was no significant effect of DCN on MVF growth, and there was no significant interaction. Qualitative observations of the cultures showed that the 3% gels had more multidirectional branch-points (arrows, Fig. 4) while 6% gels promoted bidirectional growth from the two fragment ends.
Figure 4.
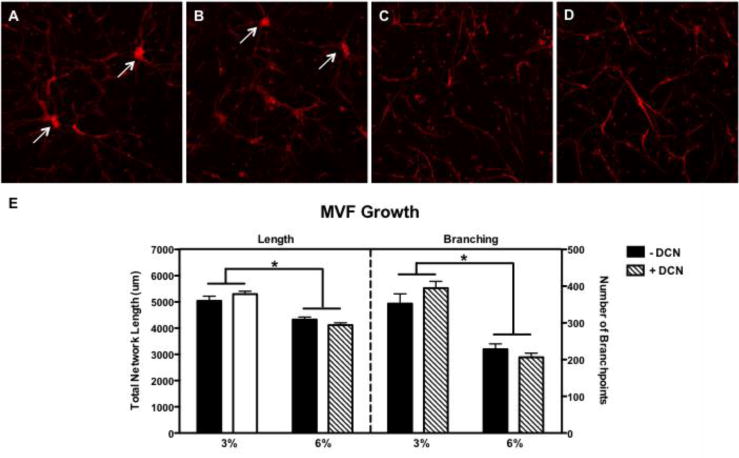
Representative 10× maximum intensity z-projections of MVF growth at day 9 in A) 3% collagen without DCN, B) 3% + DCN, C) 6% − DCN, and D) 6% + DCN and quantification (n=5 frames/7–8 gels/group) of E) total network length number of branch-points. * indicates significant differences between 3% and 6% gels (p<0.0001).
At day 16 of MVF culture, 3% collagen gels with no decorin had contracted to an average of 48% of their original (day 1) area, while 3% collagen gels with decorin and both 6% gels retained about 95% of their initial area. Both increased collagen density (p<0.0001) and DCN (p<0.0001) significantly attenuated gel contraction. A significant interaction effect was also revealed, and post hoc tests showed that contraction of only 3% collagen gel was significantly reduced by the presence of DCN (Fig. 5).
Figure 5.
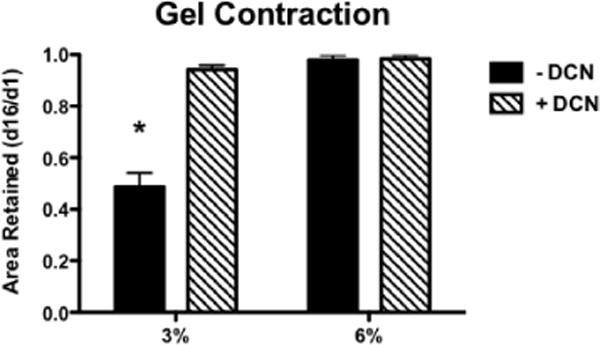
Average fractional area retained by MVF-seeded gels over 16 days in culture (n=6/group). * indicates significant difference from all other groups (p<0.001).
Discussion
The vasculature is an integral component of musculoskeletal regeneration, both providing nutrient transport and participating in VEGF-BMP-2 signaling interplay [17, 18]. Changes in the mechanical environment of bone injury repair tissue have been associated with vascular changes in vivo [4]. However, it has been challenging to directly examine the relationship between the compressive loads of a large bone defect and vascular regulation. Accordingly, the goal of this work was to investigate the ability of DCN-supplemented collagen scaffold to maintain dimensional stability while supporting microvascular growth in vitro. The addition of DCN markedly increased the stability of 3% collagen gel through day 16 in culture. Further, although DCN increased the modulus of both 3% and 6% collagen gels, DCN-supplemented constructs supported robust MVF growth.
The addition of DCN significantly reduced contraction of 3% collagen. Although 6% collagen was not impacted by the addition of DCN, the 6% gels do not undergo appreciable contraction during culture, and thus it is logical that their mechanical properties were less affected by DCN. The reduction in contraction of the 3% gel by DCN was dramatic despite a relatively modest increase in compressive properties, potentially suggesting a biological effect of DCN rather than a purely mechanical phenomenon. Endothelial cells cultured on a DCN substrate have shown reduced focal adhesion formation [19], and DCN has shown reduced collagen contraction by fibroblasts through TGF-ß inhibition [20, 21]. In contrast, DCN has also been shown to increase cell-mediated collagen contraction [22]; however, that study utilized highly contractile smooth muscle cells, and they may respond differently to DCN than other vascular cell populations. Despite potential biological effects of DCN, MVF growth was not significantly different as a result of DCN supplementation.
Collagen is known to have a higher tensile than compressive modulus [13, 23]. Since DCN affects collagen properties via affecting fibrillogenesis, the tensile properties of resultant fibers may better show the effect of DCN. Thus, while a primary objective of this work was to establish whether collagen with DCN could withstand compression, the tensile properties of the gels may have been affected to a greater degree than the compressive properties. Additionally, forces exerted by cells during sprouting angiogenesis that ultimately cause the gels to contract are primarily tensile rather than compressive, perhaps suggesting that gel contraction was mitigated by an increased tensile modulus [24].
While increased substrate stiffness tends to decrease microvascular growth [8], using DCN to increase the modulus of the vascularized collagen gels did not have this effect. DCN is known to play a complex role in angiogenesis, with studies suggesting both pro- and anti-angiogenic effects depending on the context [19, 25–27]. Many of these studies focus on the expression of DCN by endothelial cells rather than on its function as an ECM component [27]; however, when endothelial cells were cultured on a two-dimensional DCN substrate, tube-like structure formation was inhibited, suggesting an anti-angiogenic role [19]. While tube-like structure formation is a critical step in de novo angiogenesis, DCN may not affect the subsequent steps such as sprouting and elongation that are more relevant to the microvascular fragment model presented in this study. Also, the use of pure DCN in isolation from other ECM moieties may have magnified effects of DCN. DCN is also known to interact with growth factors such as transforming growth factor ß (TGF-ß) and has been shown to upregulate vascular endothelial growth factor (VEGF) [28, 29]. In the system presented here, DCN may bind, sequester, and better present growth factors [30], thereby potentially promoting angiogenesis and effectively balancing the inhibitory effect of increased substrate stiffness.
Finally, the 50 μg/mL DCN dose used in this study was chosen based on previous research utilizing DCN in 2% collagen; while higher concentrations of DCN further affected fibrillogenesis, the tensile modulus plateaued at 50 μg/mL [14]. In this study, we used 3% collagen to compare microvascular growth to results from previous studies using this in vitro model for angiogenesis [5–7, 16], and it is possible that a higher dose of DCN would produce a greater effect in higher percentage collagen. As the concentration of collagen monomer is increased, collagen-collagen interactions may dominate over any effects between collagen and DCN and effectively dampen the response to DCN. Both 3% and 6% collagen showed a reduction of final turbidity in the presence of DCN, consistent with previous results in 2% collagen. However, the rate of fibrillogenesis, evidenced by the slope of the growth phase, was not affected by DCN in either 3% of 6% collagen, and the lag time was not affected in 3% collagen and was in fact reduced in 6% collagen (Fig. 1), contrasting previous results [14]. Taken together, these data suggest that the 50 μg/mL dose of DCN does have differing effects on different densities of collagen. Despite the variances in turbidity curves, SHG images of the gels qualitatively showed collagen organization differences, which were also noted in 2% collagen [14], and the compressive modulus was increased for both densities.
Conclusions
By quantifying collagen hydrogel construct mechanical properties and vascular growth as a function of collagen density and presence of DCN, this study has demonstrated a method to improve dimensional stability of collagen gels without compromising vascular growth. While it is unclear whether the reduction of gel contraction is primarily due to biological or mechanical effects of DCN, both compressive modulus and fibril structures were altered. Taken together, these data suggest that decorin-supplemented collagen constructs are an attractive candidate for in vitro model studies on the effects of compressive mechanical loading on three-dimensional microvascular growth.
Acknowledgments
Financial support from NIH #R01AR069297 and NIH #R01HL131856 is greatly acknowledged.
References
- 1.Chien S. Effects of disturbed flow on endothelial cells. Annals of Biomedical Engineering. 2008;36(4):554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipowska J, et al. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017 doi: 10.1007/s10456-017-9541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafage-Proust MH, et al. Assessment of bone vascularization and its role in bone remodeling. Bonekey Rep. 2015;4:662. doi: 10.1038/bonekey.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerckel JD, et al. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci U S A. 2011;108(37):E674–80. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoying JB, Boswell CA, Williams SK. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev Biol Anim. 1996;32(7):409–19. doi: 10.1007/BF02723003. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan L, et al. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc Res. 2008;78(2):324–32. doi: 10.1093/cvr/cvn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgar LT, et al. Mechanical interaction of angiogenic microvessels with the extracellular matrix. J Biomech Eng. 2014;136(2):021001. doi: 10.1115/1.4026471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar LT, et al. Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS One. 2014;9(1):e85178. doi: 10.1371/journal.pone.0085178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang-Lee LL, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res. 1990;24(9):1185–201. doi: 10.1002/jbm.820240905. [DOI] [PubMed] [Google Scholar]
- 10.Vogel KG, Trotter JA. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987;7(2):105–14. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 11.Danielson KG, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136(3):729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson PS, et al. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127(1):181–5. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 13.Pins GD, et al. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73(4):2164–72. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese SP, Underwood CJ, Weiss JA. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 2013;32(7–8):414–23. doi: 10.1016/j.matbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HU, et al. Characterization of the dermatan sulfate proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage and skin isolated by octyl-sepharose chromatography. J Biol Chem. 1989;264(5):2876–84. [PubMed] [Google Scholar]
- 16.Krishnan L, et al. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol. 2007;293(6):H3650–8. doi: 10.1152/ajpheart.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouletreau PJ, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109(7):2384–97. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, et al. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143(2):199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- 19.Davies Cde L, et al. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62(1):26–42. doi: 10.1006/mvre.2001.2311. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, et al. Recombinant Human Decorin Inhibits TGF-b1 Induced Contraction of Collagen Lattice by Keloid Fibroblasts. Wounds. 2009;21(2):47–56. [PubMed] [Google Scholar]
- 21.Zhang Z, et al. Recombinant human decorin inhibits TGF-beta1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35(4):527–37. doi: 10.1016/j.burns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Paderi JE, et al. Collagen-binding peptidoglycans: a biomimetic approach to modulate collagen fibrillogenesis for tissue engineering applications. Tissue Eng Part A. 2009;15(10):2991–9. doi: 10.1089/ten.tea.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamant J, et al. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180(1060):293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- 24.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109(1):317–30. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schonherr E, et al. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res. 2004;41(6):499–508. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- 26.Grant DS, et al. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21(31):4765–77. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 27.Jarvelainen HT, et al. Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res. 1992;203(2):395–401. doi: 10.1016/0014-4827(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 28.Santra M, et al. Ectopic decorin expression up-regulates VEGF expression in mouse cerebral endothelial cells via activation of the transcription factors Sp1, HIF1alpha, and Stat3. J Neurochem. 2008;105(2):324–37. doi: 10.1111/j.1471-4159.2007.05134.x. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrand A, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–34. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferdous Z, et al. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282(49):35887–98. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]


