Abstract
Functional Near-Infrared Spectroscopy (fNIRS) maps human brain function by measuring and imaging local changes in hemoglobin concentrations in the brain that arise from the modulation of cerebral blood flow and oxygen metabolism by neural activity. Since its advent over 20 years ago, researchers have exploited and continuously advanced the ability of near infrared light to penetrate through the scalp and skull in order to non-invasively monitor changes in cerebral hemoglobin concentrations that reflect brain activity. We review recent advances in signal processing and hardware that significantly improve the capabilities of fNIRS by reducing the impact of confounding signals to improve statistical robustness of the brain signals and by enhancing the density, spatial coverage, and wearability of measuring devices respectively. We then summarize the application areas that are experiencing rapid growth as fNIRS begins to enable routine functional brain imaging.
Introduction
Functional Near-Infrared Spectroscopy (fNIRS) is a non-invasive, non-ionizing method for measuring and imaging the functional hemodynamic response to brain activity. Near-infrared light can propagate several centimeters through the scalp and skull, and spectroscopically interrogate the concentrations of oxygenated (HbO), deoxygenated (HbR), and total (HbT) hemoglobin within the brain. By shining near-infrared light on the scalp and placing a detector a few centimeters away, changes in the amount of diffuse light reaching the detector provide a measure of changes in cerebral hemoglobin concentrations. Since its first implementation over 20 years ago [1][2][3], fNIRS has proven to be an effective tool to study normal brain function and its alteration in disease [4]. Similar to EEG, fNIRS’ safety, low-cost, portability, and high temporal resolution give it the potential for widespread implementation. An important difference is that while EEG measures the fast, electrical responses associated with neuronal activity, fNIRS relies on neurovascular coupling and measures the hemodynamic response just as is done with fMRI [5]. fNIRS is particularly suited for populations and studies for which other imaging modalities are limited (e.g. fMRI), including infants and children, procedures involving mobility and inter-activity, and clinical environments. As recently reviewed in a NeuroImage special issue [4], dominant application areas include behavioral and cognitive neurodevelopment [6], perception and cognition, psychiatric conditions [7], and neurological applications including epilepsy [8], stroke and brain injury [9]. Figure 1 illustrates the exponential growth of fNIRS applications over the past 25 years and what we believe are major milestones in the field. Beyond the traditional continuous-wave approach that we discuss extensively in this review, the figure also highlights in red those technological innovations that are not yet routine but likely to advance the field and promote new applications in the future. Notably, time-domain (TD) NIRS [10] enables “null-distance” depth resolution, and diffuse correlation spectroscopy (DCS) is a NIRS cousin technique sensitive to the motion of red blood cells that provides an index of blood flow with enhanced brain sensitivity compared with NIRS [11][12].
Figure 1. Volume of fNIRS publications per year.
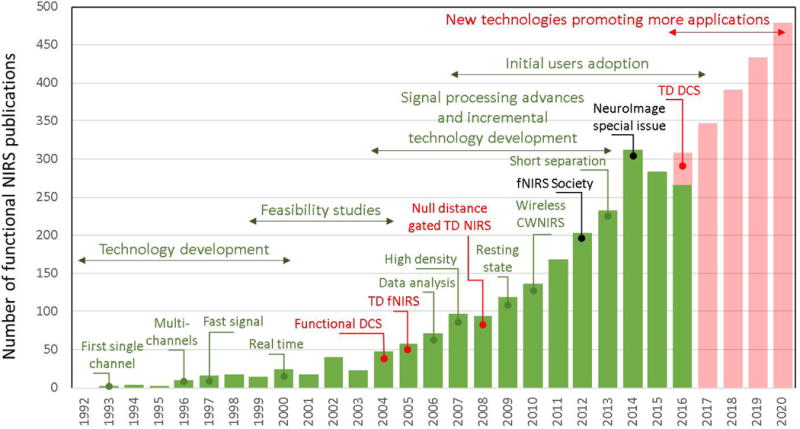
The graph illustrates the exponential growth of the technology applications since its first implementation in 1993 [1][2][3], and highlights what we believe are the major contributions to the field over the past 25 years. The establishment of the fNIRS society and the corresponding NeuroImage special issue [4] mark an increase in publications by users (in black). While the vast majority of current fNIRS applications rely on the continuous-wave (CW) modality, we marked in red some important technological innovations that go beyond this traditional CWNIRS approach: Time-Domain NIRS (TD-NIRS) [10] and Diffuse Correlation spectroscopy (DCS) [11][12]. We believe these new technologies will in turn promote the development of new applications in the future, as we illustrate in our predictions for the growth of the field in red bars.
This Current Opinion article briefly summarizes the main advances in the fNIRS field from the last 5 years and provides our perspective on what challenges will be overcome and what application areas will grow in the coming years. We start by describing recent advances in probe development, and in signal processing for improving brain sensitivity, spatial resolution, and for minimizing interference by systemic physiology and motion artifacts. We then review the continued growth in application to brain development, cognition and psychiatry, as well as emerging applications for brain-computer interfacing and hyperscanning.
Hardware advances
High-density fNIRS
A major limitation of fNIRS is its poor spatial resolution, both in depth and laterally. For typical sparse arrays of optodes, the lateral resolution is on the order of the source-detector separation (a few centimeters), and there is essentially no depth resolution. One approach to improve resolution is to increase the density of optodes on the scalp, with channels at various source-detector separations providing overlapping sensitivity volumes. Tomographic algorithms then allow reconstruction of 3-dimensional images of brain activation [13]. This approach is generally referred to as Diffuse Optical Tomography (DOT).
Zeff first introduced the term “high-density” (HD) DOT to describe their implementation of an array of 52 optodes over the visual cortex [14], demonstrating separation of extra-cerebral from cortical signals, and high-resolution eccentricity and angular mapping of the visual cortex. That system was later expanded to 188 optodes (1200 channels) (Figure 2.a–b) and showed excellent agreement between fNIRS and fMRI mapping of functional activation and resting state networks over different cortical regions [13] (Figure 2.c). Habermehl used a HD probe with 30 optodes concentrated over the primary somatosensory cortex to spatially resolve the activation responses from stimulation of two different fingers [15]. These HD approaches have demonstrated accurate mapping of brain function with fNIRS, pushing its spatial resolution close to that of fMRI [16] for cortical regions close to the skull.
Figure 2. Probe advances. a-b-c) High-density probe.
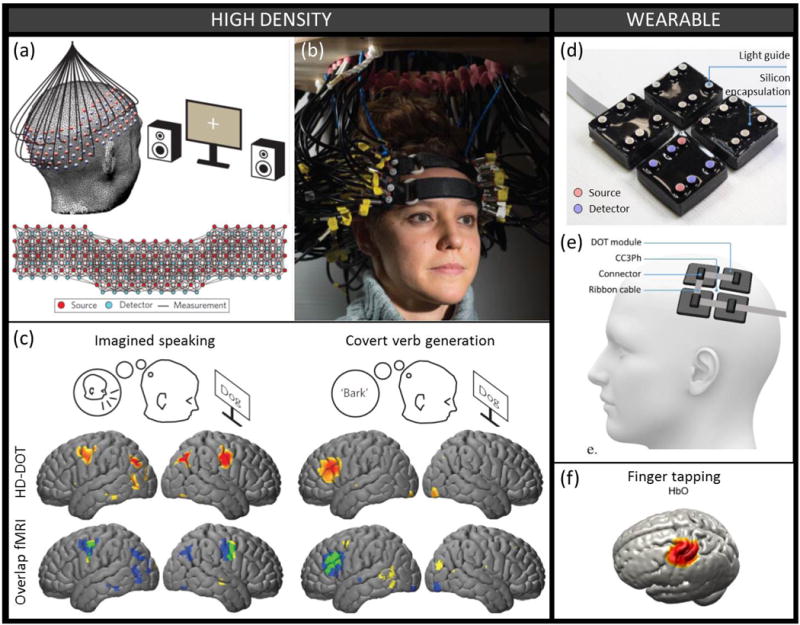
(a) Schematic and (b) photograph of a high-density probe with 96 sources and 92 detectors yielding over 1200 overlapping measurement channels. (c) The HD-DOT measurements, combined with anatomical light propagation modeling and reconstruction, enable mapping with high resolution on the cortical surface of the hemodynamic response to different cognitive brain tasks. The HD-DOT spatial response (blue) and the independently measured fMRI BOLD response (yellow) show very good spatial overlap (green). Figure panels a, b and c were modified with permission from [13] (d-e-f) Wearable probe. (d) Photograph of a modular wearable device consisting of 4 independent DOT modules each constructed from 30 × 30 mm printed circuit board, 4 photodiodes and 2 dual-wavelength sources. (e) Schematic representation of the wearable device positioned on the scalp over the primary somatomotor cortices (f) Group-average mapping of the hemodynamic response to a finger tapping task using the wearable probe presented above and image reconstruction based on a subject-registered atlas. Figure panels d, e and f were modified with permission from [19].
High density systems however remain rare as they suffer from their own challenges. A compromise needs to be made between the field of view and the sampling density, so that typically only a specific cortical region can be imaged [15][17]. The HD system of Eggebrecht [13] does not compromise on either, but its portability is significantly reduced because of the weight of the fibers that need to be supported in a rigid fixture (Figure 2b). It is unlikely that fiber-based HD imaging will translate to broad applications as the more costly and bulky probes greatly undermine the fNIRS benefits of portability and flexibility. Instead, efforts focusing on developing wearable, fiberless systems have strived recently for higher spatial sampling density [18]. For instance, a recent modular DOT system offers the promise of combining high-density measurements with wearability [19] (Figure 2.d–f), and we envision that similar systems will be developed in the upcoming years to reconcile the needs of portability and spatial mapping accuracy.
Wearable fNIRS
Wearable fNIRS is the next advance that will transform the technology, enabling studies of brain activity associated with natural behaviors in ecologically valid settings and dramatically reducing the cost of fNIRS systems [18]. Taking full advantage of the portability of fNIRS will dramatically increase the spectrum of applications. It will allow studies of normal and pathological brain function in more natural environments (social interactions, psychiatric diseases, stroke recovery), and more efficient monitoring of patients with brain injury or neurodegenerative disease (stroke, Alzheimer’s, concussion) and of normal and abnormal brain development (autism, language development). In addition, the lower cost of wearable devices and their flexibility will result in higher accessibility to the technology, compared to expensive conventional brain imaging technologies.
Dispensing with optical fibers and placing light emitters and receivers directly on the head, wearable devices offer lower weight and higher flexibility than traditional NIRS systems. The majority of wearable devices take advantage of light-emitting diodes (LEDs) and photodiodes [19] [20]. Early portable systems used electrical cables, more prone to RF interference, to transfer analog signals to a controller module [21][22], typically held in a backpack. More recent wearable systems implement digital conversion directly in the optode [19] [20]. These systems can be extremely light, but generally afford only low dynamic range on the detected signal, and restricted measurements to fixed source-detector separations and to forehead only. Choi developed a dedicated integrated circuit (IC) for multi-channel, high-dynamic range detection [23]. In 2016, seeing a need for probes that can be adjusted to users’ specific needs, two research groups proposed modular designs for scalable fNIRS probes [19][20]. Lühmann shared their design online for easy replication [20]. Chitnis showed very promising in vivo results (Figure 2.d–f) with good signal-to-noise ratio at larger than 5-cm source-detector separations on hairy regions of the head [19]. Given this impressive performance achieved with inexpensive photodiodes and LEDs, and continued miniaturization of digital converters and FPGAs, we anticipate significant design advances in wearable fNIRS systems over the coming five years that will have a profound impact on the growth and utility of fNIRS applications. There are no technical challenges to realizing these advances other than optimizing the circuit designs based on available components to achieve highly multiplexed measurements of brain activity. These wearable and low-cost systems will greatly aid with the adoption of high density fNIRS measurements that are presently constrained by the bulk of fiber optics which would now be replaced by much smaller and lighter electrical cables.
Signal Processing Advances
Statistics
Similar to the analysis of other neuroimaging modalities, specifically functional MRI, the contrast in fNIRS is based on changes in the level of oxy- and deoxy-hemoglobin between two or more task or rest conditions. During a typical functional brain study, a participant performs repeated trials of a specific task(s). A statistical model is used to detect differences in the level of hemoglobin between a pair of specific tasks or between task and baseline. The most common statistical model used is a linear regression model, where measured hemoglobin changes are modeled as linear combinations of regressors derived from the timing of the stimulus events, and the evoked hemodynamic response is solved by deconvolution or using a canonical model. As reviewed by Huppert [24], there is continued debate about the optimal model to maximize sensitivity of the fNIRS analysis. This is a particular problem in infant and pediatric studies where the shape of the appropriate canonical model is even less certain and evolving with age.
There are several important distinctions between fNIRS and fMRI analyses [24] in terms of noise structure and types of artifacts. Of particular concern is that the “noise” (i.e. the un-modelled signal) is generally correlated and not normally-distributed as it arises from systemic physiology and motion artifacts. Pre-coloring or pre-whitening general linear models are used to correct these problems [25][26], however approaches commonly used in fMRI-based GLM analysis do not necessarily directly translate to fNIRS [24]. In particular, the most appropriate model for physiological noise in fNIRS is still being debated with considerations being given to band-pass filtering [27], auto-regressive filters [26], adaptive or moving-averaging filters [28], and regression techniques including the use of polynomials [25], wavelets [29], or externally measured physiological time-courses such as finger-tip pulse-oximetry [27]. As these methods continue to be proposed, there is an increasing need for rigorous comparisons of the tradeoffs in the sensitivity and specificity of these methods. We discuss further some of the promising approaches to address the contamination by systemic physiology and motion artifacts in the following sections.
Short-separation regression of interfering physiological signals
As the majority of the optical signal comes from the scalp and not the brain, any hemodynamic fluctuations in the scalp interferes with accurate estimation of the stimulus-evoked responses in the brain and correction methods should be implemented to improve estimation accuracy [30]. Methods that use standard averaged long-distance fNIRS signals to derive an estimate of the global physiological interference to use as a regressor, run the risk of removing the actual brain response as this does not provide a true independent measure of global physiology. Methods that use auxiliary measurements such as blood pressure, respiration, and heart rate variability, on the other hand, cannot resolve spatial heterogeneity in the scalp hemodynamic fluctuations [31]. Moreover, different tasks result in different global physiological changes which can either mask the brain response or create false positives [32] [33]. For low-density probe designs, a short-separation channel that measures only scalp hemodynamics has been shown to be a robust solution to filtering the contaminating signals [33]. However, most current fNIRS systems and probe assemblies do not permit the inclusion of short-separation channels. Because of the robustness of this method, we anticipate that commercial instrumentation will be modified to permit short-separation measurements and that such measurements will become standard in the next five years.
Motion Artifacts
As fNIRS application area broadens to different age and disease groups, motion artifacts in the fNIRS signal is becoming an important challenge [34]. The fNIRS signal is susceptible to motion artifacts due to the subject movements that cause uncoupling of the optodes and the scalp, resulting in either high-frequency spikes or baseline shifts. These artifacts are commonly detected and removed or corrected before further processing. While smoothing methods such as wavelet filtering [35] are excellent in removing the sharp spikes, the baseline shifts in the signal will remain after this type of filtering. Methods such as spline interpolation [36] or targeted principal component analysis (tPCA) [37] are better at correcting baseline shifts, but they, on the other hand, leave some residual after correcting high-frequency spikes. There is clearly an opportunity to use hybrid methods that take advantage of different types of correction algorithms. For instance, a hybrid method can be implemented that first corrects for the baseline shifts and then uses a smoothing method to correct for the remaining spikes. Moreover, more objective detection methods that rely only on the natural physiological variations in the signal to set a threshold for motion artifact such as heart beat would be ideal. It is expected that motion correction algorithms will mature and become routine over the next five years.
Anatomical Guidance
fNIRS provides a measure of hemodynamic changes and does not provide structural images that can be used to guide interpretation of the signals. The simplest way to obtain structural guidance to permit, for instance, comparison of results across subjects and across studies, is to localize the fNIRS measurement channels with respect to the 10–20 reference points that were originally defined for electroencephalography. The relation between these 10–20 locations and the underlying cortical structure and the standard Montreal Neurological Institute (MNI) stereotactic coordinates was originally worked out in the context of fNIRS by Jurcak [38] and further developed as recently reviewed in [39]. These tools make it possible to determine the MNI coordinates of fNIRS measurements to facilitate not only comparisons across fNIRS studies, but also to compare fNIRS results with fMRI and PET studies that routinely use MNI coordinates for reporting the locations of brain activation.
An alternative approach is to reconstruct an image of brain activation from the fNIRS data using a head model as a spatial prior [40][13] (Figure 2c and 2f). This spatial prior has the dual advantage of improving the image reconstruction, and of providing a functional image on the brain structure, thus localizing activations to known anatomical regions. Importantly, these images permit comparison across subjects as the images are in a consistent brain space. Software analysis packages are becoming standardized [40], making these analyses available to the broader community. What is needed in the coming years is extension of the head models from “adults” to children and infants [41], as well as elderly adults.
Applications
Brain Development
fNIRS is ideal for neuroimaging in infants and children (Figure 3a,b), as it does not require subjects to be still, asleep or sedated, but instead allows them to interact freely with their environment. Also, because of the thinner scalp and skull compared to adults, the fNIRS cerebral sensitivity is great in infants. These advantages have led to the adoption of fNIRS for a vast range of studies of both typical and atypical neurodevelopment ([42][43][44]), including the development of object and face processing, number processing, language acquisition, social communication, and neuromotor development. Studies of atypical functional development have focused mainly on attention deficit hyperactivity disorder and autism spectrum disorder. Most studies have demonstrated discrimination between groups, but individual risk assessment and diagnosis remain challenging, even more so than in adults because established standards in children at different ages are still lacking.
Figure 3. Applications of fNIRS to various neuroscience studies.
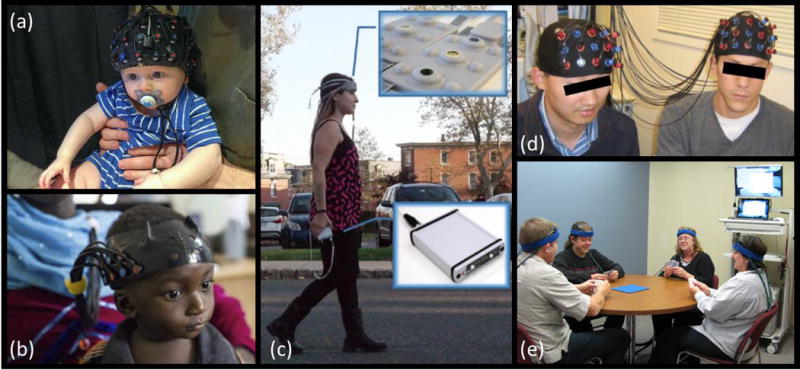
(a) Example of fNIRS cap on the head of a 7-month old infant sitting on his parent’s lap. Photograph courtesy of Dr. Katherine Perdue, Boston Children’s Hospital. (b) fNIRS headgear on a 13-month-old infant. Modified with permission from [42], photo credit to the Bill and Melinda Gates Foundation. (c) Battery operated and wireless unit allows untethered outdoor measurement during mobility studies. Modified with permission from [49]. (d) Hyperscanning fNIRS experiment simultaneously measuring brain activity in two people while they play a computer-based cooperation game side by side. Modified with permission from [59]. (e) An example of experimental setup for fNIRS hyperscanning of 4 volunteers playing a card game. Photograph courtesy of Arthur DiMartino, TechEn, Inc.
These studies foreshadow the crucial role fNIRS will play to deepen our understanding of the developing brain. But practical challenges specific to infants and children exist, including limited time to place and localize the probe, and the prevalence of motion artifacts. These issues are being addressed by the development of improved infant-specific probes. Optimal approaches and parameters for motion artifact identification and correction cannot be directly exported from adult to infant studies, and need to be established empirically in this population.
Another challenge resides in the data interpretation. For instance, brain atlases developed for adults cannot be simply scaled down to infants’ heads, as the brain undergoes structural changes early in life. Fortunately, reference brain atlas for infants and children at different ages are starting to appear [41]. Finally, neurovascular coupling is still developing in the young brain, and conflicting observations in infants have been reported with either hemodynamic responses resembling those observed in adults, or instead displaying a negative response [45]. fNIRS signals in infants need to be interpreted carefully, as they may not directly reflect neuronal activity, but instead the development of neurometabolic and neurovascular systems [45].
Application to the Cognitive and Psychological Sciences
fNIRS is proving to be an invaluable tool in the cognitive and psychological sciences because of the ease with which neural activity can be measured in natural settings with portable and low-cost systems (Figure 3c). Measurements of the prefrontal cortex are particularly easy because of reduced interference from hair, but studies are and should measure from more brain regions. Recent reviews have addressed the application to emotional processing [46], mobility and aging [47], clinical psychology [48], psychiatry [7], integration with neuromodulation [49], and brain computer interfaces [50]. fNIRS complements fMRI because it permits rapid studies of larger numbers of subjects and more frequent longitudinal measurements of particular relevance for learning and treatment studies. We anticipate that ongoing development of highly capable wearable fNIRS systems will greatly expand the repertoire of cognitive and psychological studies.
Brain Computer Interface
Brain computer interface (BCI) is a growing field of research that aims to facilitate human being’s communication and interaction with the environment by directly measuring and self-regulating the neuronal and/or hemodynamic activity in the brain [50]. While EEG is the most studied non-invasive BCI [51], we see more and more promising studies with fNIRS. In a recent work, Chaudhary and colleagues have succeeded to reach above chance level accuracy to a yes/no paradigm with patients who are in a complete locked-in state [52]. Another interesting study attempted to control a robot through mere motor imagery [53] comparable to what has been done with EEG [54]. This type of research can have a high impact on prosthetics such as controlling a robot-arm to perform daily functions, controlling a wheelchair or the environment without the need of a chip implant. Another future application would be to use fNIRS-BCI in personalized augmented reality applications. These can span from subjective preferences during daily life to personalized medicine. EEG and fNIRS can potentially complement each other through combining EEG’s ability to directly capture neural activity with millisecond time scale, fNIRS’s better spatial localization and its ability to measure slow and integrated hemodynamic changes which are more representative of the brain states. We expect such hybrid methods [55] [20][56] to improve classification accuracy.
Hyperscanning
Studying the social brain ideally involves imaging socially interacting people in a naturalistic environment. Hyperscanning is a technique that allows this type of research by measuring brain activity simultaneously from two or more people during real-time interactions [57] (Figure 3d,e). So far, various imaging modalities such as fMRI, MEG, EEG and fNIRS have been used in hyperscanning studies. Among these, fNIRS and EEG are the most suitable modalities as they provide the naturalistic environment that social interactions require, and EEG-fNIRS combination during hyperscanning can give invaluable insights into the nature of social interactions. With wearable fNIRS systems, we expect to see more studies with hyperscanning especially in these research areas: the study of diseases strongly linked with problems in social interactions as autism or depression; interactions such as student-teacher, parent-child or patient-clinician; the brain correlates of changes in social interactions during development; and differences due to gender or certain traits.
Social interactions typically involve both neuronal and systemic physiological processes [58]. Moreover, the type of signal processing methods used to analyze fNIRS hyperscanning data such as correlation, coherence [59], Granger causality or transfer entropy [60] are more sensitive to superficial contamination in fNIRS data. It will be essential to filter superficial contamination with, for instance, the short separation regression approach described above.
Conclusions and Future Prospects
The fNIRS field has grown exponentially since its first demonstration of measuring human brain activity in 1993 (Fig. 1). The field reached a tipping point with over 200 papers published in 2012 [4] and the formal establishment of the Society for Functional Near Infrared Spectroscopy in 2014 (http://fnirs.org). The Society plays a critical role in strengthening the advancement, adoption and application of fNIRS. This is accomplished by bringing together technology developers and users at a biennial conference to discuss best practices and problems in need of solutions as well as solutions in need of problems. Importantly, the Society supports the continuation of discussions between conferences via social media and committees that greatly facilitate the establishment and adoption of fNIRS standards. We anticipate that because of these efforts building a strong fNIRS community, the next five years will see the establishment of fNIRS as a routine functional brain imaging method with explosive growth in the impact on our understanding of how the human brain functions.
Highlights.
fNIRS allows functional human brain imaging in natural environments.
Wearable fiberless probes with increased density accelerate its widespread adoption.
Signal processing advances diminish physiological interference.
Continued growth in application to brain development, cognition and psychiatry
Emerging applications for brain-computer interfacing and hyperscanning
Acknowledgments
This work was supported by the National Institutes of Health P41-EB015896, R01-GM104986, and R24-NS104096.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Villringer A, Planck J, Hock C, Schleinkofer L, Dirnagl U. Near infrared spectroscopy (NIRS): A new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci Lett. 1993;154:101–104. doi: 10.1016/0304-3940(93)90181-j. [DOI] [PubMed] [Google Scholar]
- 2.Hoshi Y, Tamura M. Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man. Neurosci Lett. 1993;150:5–8. doi: 10.1016/0304-3940(93)90094-2. [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Kamei A, Takashima S, Ozaki T. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. J Cereb Blood Flow Metab. 1993;13:516–520. doi: 10.1038/jcbfm.1993.66. [DOI] [PubMed] [Google Scholar]
- 4**.Boas DA, Elwell CE, Ferrari M, Taga G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. Neuroimage. 2014;85:1–5. doi: 10.1016/j.neuroimage.2013.11.033. This paper gives an introduction to a special issue on fNIRS containing 58 papers, providing a broad snapshot of the status of the field. [DOI] [PubMed] [Google Scholar]
- 5.Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics. 2010;2:8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristia A, Dupoux E, Hakuno Y, Lloyd-Fox S, Schuetze M, Kivits J, Bergvelt T, van Gelder M, Filippin L, Charron S, et al. An Online Database of Infant Functional Near InfraRed Spectroscopy Studies: A Community-Augmented Systematic Review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlis A-C, Schneider S, Dresler T, Fallgatter AJ. Application of functional near-infrared spectroscopy in psychiatry. Neuroimage. 2014;85:478–488. doi: 10.1016/j.neuroimage.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen DK, Tremblay J, Pouliot P, Vannasing P, Florea O, Carmant L, Lepore F, Sawan M, Lesage F, Lassonde M. Non-invasive continuous EEG-fNIRS recording of temporal lobe seizures. Epilepsy Res. 2012;99:112–126. doi: 10.1016/j.eplepsyres.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Obrig H. NIRS in clinical neurology - a “promising” tool? Neuroimage. 2014;85:535–546. doi: 10.1016/j.neuroimage.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Pifferi A, Contini D, Mora AD, Farina A, Spinelli L, Torricelli A. New frontiers in time-domain diffuse optics, a review. J Biomed Opt. 2016;21:91310. doi: 10.1117/1.JBO.21.9.091310. [DOI] [PubMed] [Google Scholar]
- 11.Durduran T, Yodh AG. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage. 2014;85:5163. doi: 10.1016/j.neuroimage.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutin J, Zimmerman B, Tyulmankov D, Tamborini D, Wu KC, Selb J, Gulinatti A, Rech I, Tosi A, Boas DA, et al. Time-domain diffuse correlation spectroscopy. Optica. 2016;3:1006. doi: 10.1364/OPTICA.3.001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Eggebrecht AT, Ferradal SL, Robichaux-Viehoever A, Hassanpour MS, Dehghani H, Snyder AZ, Hershey T, Culver JP. Mapping distributed brain function and networks with diffuse optical tomography. Nat Photonics. 2014;8:448–454. doi: 10.1038/nphoton.2014.107. This paper demonstates the ability of fNIRS to provide high spatial resolution over a wide-field of view to measure distributed brain functions comparable to what canbe measured with fMRI, and then demonstrates applications in patients with deep brain stimulators that cannot be measured with fMRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeff BW, White BR, Dehghani H, Schlaggar BL, Culver JP. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proc Natl Acad Sci. 2007;104:12169–12174. doi: 10.1073/pnas.0611266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habermehl C, Holtze S, Steinbrink J, Koch SP, Obrig H, Mehnert J, Schmitz CH. Somatosensory activation of two fingers can be discriminated with ultrahigh-density diffuse optical tomography. Neuroimage. 2012;59:3201–3211. doi: 10.1016/j.neuroimage.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggebrecht AT, White BR, Ferradal SL, Chen C, Zhan Y, Snyder AZ, Dehghani H, Culver JP. A quantitative spatial comparison of high-density diffuse optical tomography and fMRI cortical mapping. Neuroimage. 2012;61:1120–1128. doi: 10.1016/j.neuroimage.2012.01.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph DK, Huppert TJ, Franceschini MA. Diffuse optical tomography system to image brain activation with improved spatial resolution and validation with functional magnetic resonance imaging. Appl Opt. 2006;45:8142–51. doi: 10.1364/ao.45.008142. [DOI] [PubMed] [Google Scholar]
- 18**.Zhao H, Cooper R. A review of recent progress towards a fibre-less, whole-scalp diffuse optical tomography system. Neurophotonics. 2017 doi: 10.1117/1.NPh.5.1.011012. A very recent review of the rapid advances in wearable fNIRS systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitnis D, Cooper RJ, Dempsey L, Powell S, Quaggia S, Highton D, Elwell C, Hebden JC, Everdell NL. Functional imaging of the human brain using a modular, fibre-less, high-density diffuse optical tomography system. Biomed Opt Express. 2016;7:4275. doi: 10.1364/BOE.7.004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Luhmann A, Wabnitz H, Sander T, Muller KR. M3BA: A mobile, modular, multimodal biosignal acquisition architecture for miniaturized EEG-NIRS based hybrid BCI and monitoring. IEEE Trans Biomed Eng. 2016 doi: 10.1109/TBME.2016.2594127. [DOI] [PubMed] [Google Scholar]
- 21.Everdell NL, Airantzis D, Kolvya C, Suzuki T, Elwell CE. A portable wireless near-infrared spatially resolved spectroscopy system for use on brain and muscle. Med Eng Phys. 2013;35:1692–1697. doi: 10.1016/j.medengphy.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Kiguchi M, Atsumori H, Fukasaku I, Kumagai Y, Funane T, Maki A, Kasai Y, Ninomiya A. Note: Wearable near-infrared spectroscopy imager for haired region. Rev Sci Instrum. 2012;83 doi: 10.1063/1.4704456. [DOI] [PubMed] [Google Scholar]
- 23.Choi JK, Kim JM, Hwang G, Yang J, Choi MG, Bae HM. A time-divided spread-spectrum code based 15pW-detectable multi-channel fNIRS IC for portable functional brain imaging. Digest of Technical Papers - IEEE International Solid-State Circuits Conference. 2015:196–197. [Google Scholar]
- 24**.Huppert TJ. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics. 2016;3:10401. doi: 10.1117/1.NPh.3.1.010401. This is an important commentary of how to properly analyze fNIRS data that should guide the establishment of standard fNIRS analysis procedures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. Neuroimage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 26.Barker JW, Aarabi A, Huppert TJ, Suzuki M, Miyai I, Ono T, Kubota K, Cooper RJ, Selb J, Gagnon L, et al. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Inst Stat Math 22 A M Dale Hum Brain Mapp. 2013;4:35–54. doi: 10.1364/BOE.4.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009;48:280–298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamran MA, Hong K-S. Linear parameter-varying model and adaptive filtering technique for detecting neuronal activities: an fNIRS study. J Neural Eng. 2013;10:56002. doi: 10.1088/1741-2560/10/5/056002. [DOI] [PubMed] [Google Scholar]
- 29.Jang KE, Tak S, Jung J, Jang J, Jeong Y, Ye JC. Wavelet minimum description length detrending for near-infrared spectroscopy. J Biomed Opt. 2009;14:34004. doi: 10.1117/1.3127204. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell M, Scholkmann F, Wolf U, Wolf M, Elwell C, Tachtsidis I. Modelling confounding effects from extracerebral contamination and systemic factors on functional near-infrared spectroscopy. Neuroimage. 2016;143:91–105. doi: 10.1016/j.neuroimage.2016.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagnon L, Cooper RJ, Yücel MA, Perdue KL, Greve DN, Boas DA. Short separation channel location impacts the performance of short channel regression in NIRS. Neuroimage. 2012;59:2518–2528. doi: 10.1016/j.neuroimage.2011.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yucel MA, Selb J, Aasted CM, Petkov MP, Becerra L, Borsook D, Boas DA. Short separation regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses. Neurophotonics. 2015;2:35005. doi: 10.1117/1.NPh.2.3.035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tachtsidis I, Scholkmann F. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics. 2016;3:31405. doi: 10.1117/1.NPh.3.3.031405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Brigadoi S, Ceccherini L, Cutini S, Scarpa F, Scatturin P, Selb J, Gagnon L, Boas DA, Cooper RJ. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. This paper shows the improvements that can result in the interpretation of fNIRS data corropted by motion artifacts during cognitive tasks. Importantly, this paper provides guidance on how to select the optimal motion correction algorithm for a given data set. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molavi B, Dumont GA. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol Meas. 2012;33:259–70. doi: 10.1088/0967-3334/33/2/259. [DOI] [PubMed] [Google Scholar]
- 36.Scholkmann F, Spichtig S, Muehlemann T, Wolf M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol Meas. 2010;31:649–662. doi: 10.1088/0967-3334/31/5/004. [DOI] [PubMed] [Google Scholar]
- 37.Yucel MA, Selb J, Cooper RJ, Boas DA. Targeted principle component analysis: A new motion artifact correction approach for near-infrared spectroscopy. J Innov Opt Health Sci. 2014;7:1–8. doi: 10.1142/S1793545813500661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34:1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 39**.Tsuzuki D, Dan I. Spatial registration for functional near-infrared spectroscopy: From channel position on the scalp to cortical location in individual and group analyses. Neuroimage. 2014;85:92–103. doi: 10.1016/j.neuroimage.2013.07.025. A recent review on the spatial registration of fNIRS data with anatomical landmarks to permit comparison with the breadth of neuroimaging data from fMRI, PET, MEG, and EEG, as well as other fNIRS studies. [DOI] [PubMed] [Google Scholar]
- 40*.Aasted CM, Yücel MA, Cooper RJ, Dubb J, Tsuzuki D, Becerra L, Petkov MP, Borsook D, Dan I, Boas DA. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics. 2015;2:20801. doi: 10.1117/1.NPh.2.2.020801. This paper is a tutorial on how to use a freely available software package to perform spatial registration of fNIRS data to improve anatomical interpretation of the functional measurements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brigadoi S, Aljabar P, Kuklisova-Murgasova M, Arridge SR, Cooper RJ. A 4D neonatal head model for diffuse optical imaging of pre-term to term infants. Neuroimage. 2014;100:385–394. doi: 10.1016/j.neuroimage.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd-Fox S, Begus K, Halliday D, Pirazzoli L, Blasi A, Papademetriou M, Darboe MK, Prentice AM, Johnson MH, Moore SE, et al. Cortical specialisation to social stimuli from the first days to the second year of life: A rural Gambian cohort. Dev Cogn Neurosci. 2017;25:92–104. doi: 10.1016/j.dcn.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderwert RE, Nelson CA. The use of near-infrared spectroscopy in the study of typical and atypical development. Neuroimage. 2014;85:264–271. doi: 10.1016/j.neuroimage.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Lee CW, Cooper RJ, Austin T. Diffuse optical tomography to investigate the newborn brain. Pediatr Res. 2017;82:376–386. doi: 10.1038/pr.2017.107. A recent comprehensive review of the application of fNIRS imaging to the newborn human brain. [DOI] [PubMed] [Google Scholar]
- 45.Kozberg MG, Chen BR, DeLeo SE, Bouchard MB, Hillman EMC. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc Natl Acad Sci U S A. 2013;110:4380–5. doi: 10.1073/pnas.1212785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendall RCA, Eachus P, Thompson C. A Brief Review of Research Using Near-Infrared Spectroscopy to Measure Activation of the Prefrontal Cortex during Emotional Processing: The Importance of Experimental Design. Front Hum Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: A targeted review. Journals Gerontol - Ser A Biol Sci Med Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adorni R, Gatti A, Brugnera A, Sakatani K, Compare A. Could fNIRS promote neuroscience approach in clinical psychology? Front Psychol. 2016;7:1–4. doi: 10.3389/fpsyg.2016.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKendrick R, Parasuraman R, Ayaz H. Wearable functional near infrared spectroscopy (fNIRS) and transcranial direct current stimulation (tDCS): expanding vistas for neurocognitive augmentation. Front Syst Neurosci. 2015;9:27. doi: 10.3389/fnsys.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naseer N, Hong K-S. fNIRS-based brain-computer interfaces: a review. Front Hum Neurosci. 2015;9:1–15. doi: 10.3389/fnhum.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He B, Gao S, Yuan H, Wolpaw JR. Brain–Computer Interfaces. Neural Engineering. 2013:87–151. [Google Scholar]
- 52.Chaudhary U, Xia B, Silvoni S, Cohen LG, Birbaumer N. Brain–Computer Interface–Based Communication in the Completely Locked-In State. PLoS Biol. 2017;15:1–25. doi: 10.1371/journal.pbio.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Batula AM, Kim YE, Ayaz H. Virtual and Actual Humanoid Robot Control with Four-Class Motor-Imagery Based Optical Brain Computer Interface. (Submitted) 2017. 2017 doi: 10.1155/2017/1463512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng J, Zhang S, Bekyo A, Olsoe J, Baxter B, He B. Noninvasive Electroencephalogram Based Control of a Robotic Arm for Reach and Grasp Tasks. Sci Rep. 2016;6:38565. doi: 10.1038/srep38565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan MJ, Hong MJ, Hong K-SS. Decoding of four movement directions using hybrid NIRS-EEG brain-computer interface. Front Hum Neurosci. 2014;8:244. doi: 10.3389/fnhum.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koo B, Lee HG, Nam Y, Kang H, Koh CS, Shin HC, Choi S. A hybrid NIRS-EEG system for self-paced brain computer interface with online motor imagery. J Neurosci Methods. 2015;244:26–32. doi: 10.1016/j.jneumeth.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Babiloni F, Astolfi L. Social neuroscience and hyperscanning techniques: Past, present and future. Neurosci Biobehav Rev. 2014;44:76–93. doi: 10.1016/j.neubiorev.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholkmann F, Holper L, Wolf U, Wolf M. A new methodical approach in neuroscience: assessing inter-personal brain coupling using functional near-infrared imaging (fNIRI) hyperscanning. Front Hum Neurosci. 2013;7:813. doi: 10.3389/fnhum.2013.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui X, Bryant DM, Reiss AL. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage. 2012;59:2430–2437. doi: 10.1016/j.neuroimage.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Y, Cheng X, Zhang Z, Li X, Hu Y. Cooperation in lovers: An fNIRS-based hyperscanning study. Hum Brain Mapp. 2016;841:831–841. doi: 10.1002/hbm.23421. [DOI] [PMC free article] [PubMed] [Google Scholar]


