SUMMARY
We recently identified the multipass transmembrane protein SERINC5 as an antiviral protein that can potently inhibit HIV-1 infectivity and is counteracted by HIV-1 Nef. We now report that the anti-HIV-1 activity, but not the sensitivity to Nef, is conserved among vertebrate SERINC5 proteins. However, a Nef-resistant SERINC5 became Nef sensitive when its intracellular loop 4 (ICL4) was replaced by that of Nef-sensitive human SERINC5. Conversely, human SERINC5 became resistant to Nef when its ICL4 was replaced by that of a Nef-resistant SERINC5. In general, ICL4 regions from SERINCs that exhibited resistance to a given Nef conferred resistance to the same Nef when transferred to a sensitive SERINC, and vice versa. Our results establish that human SERINC5 can be modified to restrict HIV-1 infectivity even in the presence of Nef.
In Brief
Human SERINC5 potently inhibits HIV-1 infectivity but is counteracted by HIV-1 Nef. Dai et al. show that an intracellular loop region of SERINC5 plays an important role in its sensitivity to Nef and that human SERINC5 can be modified to inhibit HIV-1 even in the presence of Nef.
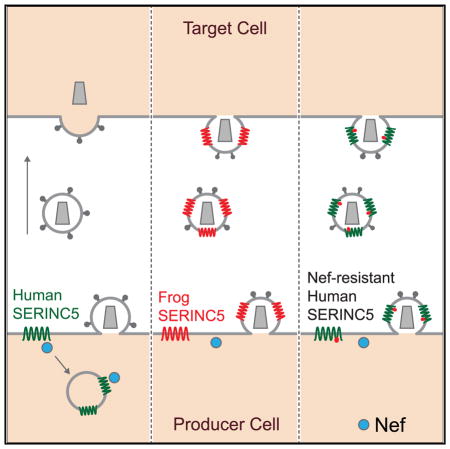
INTRODUCTION
Nef is an accessory protein of HIV-1 and other primate immunodeficiency viruses that is crucial for efficient virus replication in infected individuals and for virus pathogenicity (Deacon et al., 1995; Kestler et al., 1991). Although Nef is not an essential viral gene, it robustly enhances virus spreading in cultures of quiescent primary CD4+ T lymphocytes (Miller et al., 1994; Spina et al., 1994). Nef hijacks the clathrin-associated protein complex 2 (AP-2) adaptor to induce the internalization of the viral receptor CD4 (Aiken et al., 1994; Chaudhuri et al., 2007; Garcia and Miller, 1991). In addition, Nef downregulates numerous other cell surface proteins (Haller et al., 2014), including certain major histocompatibility class I molecules, which is thought to protect infected cells from cytotoxic T cells (Collins et al., 1998; Schwartz et al., 1996).
Nef also enhances the specific infectivity of progeny virions (Aiken and Trono, 1995; Chowers et al., 1994; Miller et al., 1995). This function of Nef requires its presence during virus production, but Nef has no detectable effect on the encapsidation of the genomic viral RNA, the incorporation of Env, or the morphology or stability of the mature core (Aiken and Trono, 1995; Forshey and Aiken, 2003; Schwartz et al., 1995). Nevertheless, Nef increases the efficiency of reverse transcription in target cells (Aiken and Trono, 1995; Chowers et al., 1995; Schwartz et al., 1995). The effects of Nef on HIV-1 infectivity are determined by variable HIV-1 Env regions (Usami and Göttlinger, 2013) and are dependent on clathrin-mediated endocytosis (Pizzato et al., 2007; Usami et al., 2014).
We and others have identified the multipass transmembrane proteins SERINC3 and SERINC5 as antiviral proteins that are incorporated into Nef-deficient HIV-1 virions and counteracted by Nef (Matheson et al., 2015; Rosa et al., 2015; Usami et al., 2015). SERINC5 in particular can dramatically inhibit the infectivity of Nef-deficient HIV-1 (Matheson et al., 2015; Rosa et al., 2015; Usami et al., 2015). Although primary HIV-1 Env proteins often confer significant resistance against SERINC5, virion-associated SERINC5 sensitizes even resistant Envs to some neutralizing antibodies (Beitari et al., 2017).
HIV-1 Nef removes SERINC3 and SERINC5 from the cell surface (Matheson et al., 2015; Rosa et al., 2015; Usami et al., 2015) and prevents their incorporation into progeny virions (Rosa et al., 2015; Usami et al., 2015). In contrast, Nef increased the cell surface expression of SERINC1 (Matheson et al., 2015), indicating that SERINC3 and SERINC5 are selectively downregulated. Furthermore, the effects of HIV-1 Nef proteins on SERINC3 and SERINC5 are strain dependent. For instance, the Nef proteins of the primary HIV-1 isolates 97ZA012 and 93BR020 strongly inhibit the incorporation of SERINC3 and SERINC5 into virions; in contrast, the Nef protein of the T cell line-adapted strain SF2 is only active against SERINC5 (Usami et al., 2015). Thus, Nef proteins can discriminate among SERINC family members. However, the determinants that govern sensitivity to Nef remain unknown.
We show that an intracellular loop of human SERINC5 confers sensitivity to widely divergent Nefs in the context of a Nef-resistant vertebrate SERINC5 and confers sensitivity to NefSF2 in the context of human SERINC3, which is normally resistant to this particular Nef. Human SERINC5 acquired resistance against Nefs from different HIV-1 clades when this intracellular loop was replaced by the corresponding region from a Nef-resistant SERINC5. Altogether, our results identify a major determinant of Nef responsiveness and establish that human SERINC5 can be made resistant to Nef-induced downregulation.
RESULTS
Anti-HIV-1 Activity, but Not Sensitivity to Nef, Is Conserved among Vertebrate SERINC5 Orthologs
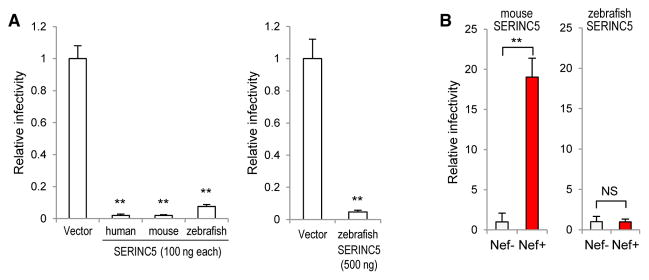
As little as 100 ng of a relatively weak (pBJ5-based) SERINC5 expression plasmid is sufficient to potently inhibit the single-cycle infectivity of Nef− HIV-1 virions (Usami et al., 2015). Under the same conditions, mouse SERINC5 reduced the specific infectivity of Nef− HIV-1 virions produced in 293T cells to a similar extent (40- to 50-fold) as human SERINC5 (Figure 1A). Zebrafish (Danio rerio) SERINC5, which is only 61% identical to human SERINC5, reduced progeny virus infectivity 13- or 21-fold when 100 or 500 ng of expression vector were used, respectively (Figure 1A).
Figure 1. Anti-HIV-1 Activity, but Not Responsiveness to Nef, Is Conserved among SERINC5 Orthologs.
(A) Human, mouse, and zebrafish SERINC5 proteins share the ability to inhibit the specific infectivity of HIV-1 progeny virions.
(B) NefSF2 expressed in trans in virus producer cells counteracts the effect of exogenous mouse, but not zebrafish, SERINC5 on Nef− HIV-1 progeny virion infectivity.
Bar graphs represent the mean + SD from 3 biological replicates.
Next, we examined the sensitivities of mouse and zebrafish SERINC5 to NefSF2, which selectively inhibits the incorporation of human SERINC5 into HIV-1 virions (Usami et al., 2015). Although the inhibitory effect of mouse SERINC5 on Nef− HIV-1 produced in 293T cells was clearly counteracted by NefSF2 expressed in trans, the effect of zebrafish SERINC5 was unaffected by NefSF2 (Figure 1B). We infer that widely divergent SERINC5 proteins share the ability of human SERINC5 to inhibit HIV-1 infectivity, but not necessarily its sensitivity to Nef.
Transfer of Nef Sensitivity to a Nef-Resistant SERINC5
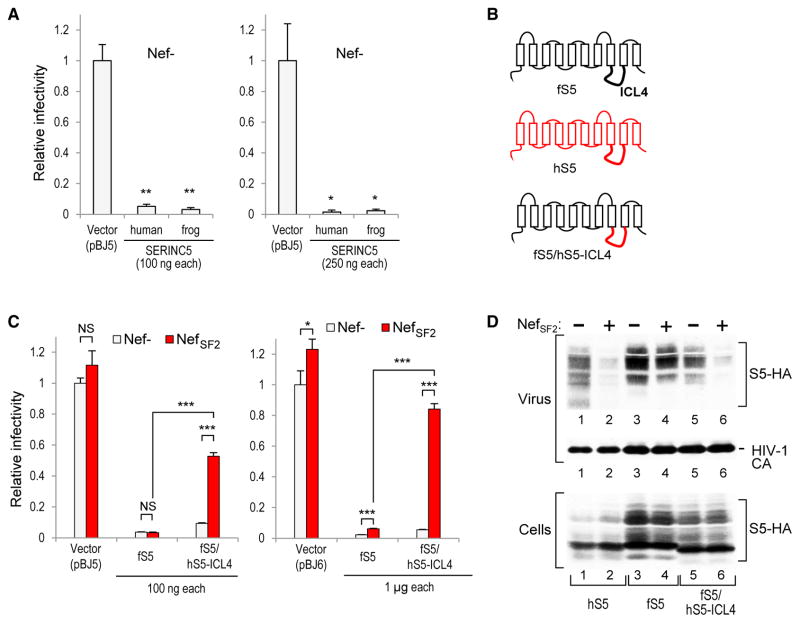
Because zebrafish SERINC5 was somewhat less active against Nef− HIV-1 than human SERINC5, we also tested the SERINC5 protein of the Western clawed frog (Xenopus tropicalis). Frog SERINC5 expressed from pBJ5 inhibited the single-cycle infectivity of Nef−HIV-1 progeny virions with a potency comparable to that of human SERINC5 (Figure 2A). However, whereas human SERINC5 is efficiently counteracted by NefSF2 (Usami et al., 2015), the inhibitory effect of frog SERINC5 expressed from pBJ5 on Nef− HIV-1 was unaffected by NefSF2 (Figure 2C). Even when expressed from pBJ6, which lacks an SV40 origin required for episomal amplification, as well as SV40 enhancer sequences (Rosa et al., 2015), frog SERINC5 reduced the specific infectivity of Nef− HIV-1 more than 40-fold, and this inhibitory effect largely persisted in the presence of NefSF2 expressed in trans (Figure 2C). In the absence of exogenous SERINC5, the effect of NefSF2 on the infectivity of Nef− HIV-1 virions was quite modest in these experiments (Figure 2C), perhaps because 293T cells express only low levels of endogenous SERINC5 (Usami et al., 2015).
Figure 2. Transfer of Human SERINC5 ICL4 Is Sufficient to Confer Nef Responsiveness to Frog SERINC5.
(A) Frog SERINC5 is as potent as human SERINC5 in inhibiting the single-round infectivity of Nef− HIV-1 progeny virions.
(B) Schematic illustration of the parental SERINC5 proteins and of the chimera examined.
(C) Effect of frog SERINC5 on Nef− HIV-1 progeny virion infectivity is largely resistant to NefSF2 but becomes sensitive upon replacement of its ICL4 by that of human SERINC5. SERINCs were expressed from pBJ5 or from the very weak pBJ6 expression plasmid.
(D) Western blots showing the effects of NefSF2 on the incorporation of human, frog, and chimeric SERINC5 into Nef− HIV-1 virions.
Bar graphs represent the mean + SD from 3 biological replicates.
See also Figures S1, S2, and S4.
We subsequently used frog SERINC5 as a recipient of human SERINC5 sequences to identify determinants that confer sensitivity to NefSF2. The transmembrane hidden Markov model (TMHMM) membrane protein topology prediction method (Krogh et al., 2001) predicts that SERINC5 has 10 transmembrane helices, and our previous findings indicate that the loop connecting helices 7 and 8 is surface exposed (Usami et al., 2015). This in turn suggested that the loop connecting helices 8 and 9 represents the fourth and longest intracellular loop (Figure 2B). To examine the role of this region, we replaced intracellular loop 4 (ICL4) of frog SERINC5 (fS5) with that of human SERINC5 (hS5) (Figure 2B). The resulting fS5/hS5-ICL4 chimera largely retained the ability of frog SERINC5 to inhibit the specific infectivity of Nef−HIV-1 progeny virions but lost much of its resistance to NefSF2 expressed in trans (Figure 2C). Thus, the transfer of the ICL4 of human SERINC5 was sufficient to confer substantial sensitivity to NefSF2.
As previously reported (Usami et al., 2015), the incorporation of hemagglutinin (HA)-tagged human SERINC5 into progeny virions was strongly inhibited by NefSF2 (Figure 2D, lanes 1 and 2). In contrast, the incorporation of frog SERINC5 was only slightly affected by NefSF2 (Figure 2D, lanes 3 and 4). Finally, the effect of NefSF2 on the incorporation of the fS5/hS5-ICL4 chimera resembled its effect on human SERINC5 (Figure 2D, lanes 5 and 6). We conclude that the transfer of the ICL4 was sufficient to allow NefSF2 to significantly inhibit both the anti-HIV-1 effect and the virion incorporation of frog SERINC5.
The ICL4 of Human SERINC5 Confers Sensitivity to Widely Divergent Nefs
Unlike NefSF2 (clade B), which selectively inhibits the incorporation of human SERINC5 into HIV-1 virions, Nef97ZA012 (clade C) also inhibits the incorporation of human SERINC3 (Usami et al., 2015). Nevertheless, the inhibitory effect of frog SERINC5 on HIV-1 infectivity was essentially unaffected by Nef97ZA012 (Figure S1A), as was the incorporation of frog SERINC5 into HIV-1 virions (Figure S1B). In contrast, Nef97ZA012 enhanced the specific infectivity of Nef− HIV-1 produced in the presence of the fS5/hS5-ICL4 chimera about 13-fold (Figure S1A) and strongly inhibited the incorporation of the chimeric protein (Figure S1B). Thus, the ICL4 of human SERINC5 conferred sensitivity to HIV-1 Nefs from different subtypes.
We also observed that the LL164,165AA AP-2 binding site mutant of NefLAI, which does not counteract SERINC5 (Rosa et al., 2015), was more abundant in HIV-1 virions produced in the presence of the fS5/hS5-ICL4 chimera than of frog SERINC5 (Figure S1C). Because frog SERINC5 and the fS5/hS5-ICL4 chimera were incorporated about equally in the absence of a functional Nef (Figure S1B), this finding provides indirect evidence for a physical interaction between Nef and the ICL4 of human SERINC5.
Because the ability to counteract SERINC5 is conserved among all primate lentiviral lineages (Heigele et al., 2016), we additionally examined the effects of simian immunodeficiency virus (SIV) Nefs. SIVmac239 Nef increased the specific infectivity of Nef− HIV-1 produced in the presence of frog SERINC5 or of the fS5/hS5-ICL4 chimera 17- or 27-fold, respectively (Figure S1D). Thus, SIVmac239 Nef counteracted even unmodified frog SERINC5. In contrast, unmodified frog SERINC5 was largely resistant to the Nef proteins of SIVagm155 and SIVagm677, and the transfer of the ICL4 of human SERINC5 substantially increased its sensitivity to these Nefs (Figures S1E and S1F). Overall, these findings demonstrate that the ICL4 of human SERINC5 can confer sensitivity to Nefs from widely divergent primate lentiviruses.
Transfer of Sensitivity against NefSF2 to SERINC3
Unlike other HIV-1 Nefs, NefSF2 has no effect on the incorporation of SERINC3 into HIV-1 virions (Usami et al., 2015). To examine whether the ICL4 of SERINC5 confers sensitivity to NefSF2 in the context of human SERINC3 (hS3), we generated the hS3/hS5-ICL4 chimera, which harbors the ICL4 of human SERINC5 in a human SERINC3 background (Figure S2A). Although the incorporation of authentic human SERINC3 was unaffected by NefSF2, as previously reported (Usami et al., 2015), the incorporation of the chimera was strongly inhibited (Figure S2B), confirming the role of the ICL4 in determining Nef sensitivity.
Transfer of Nef Resistance to Human SERINC5
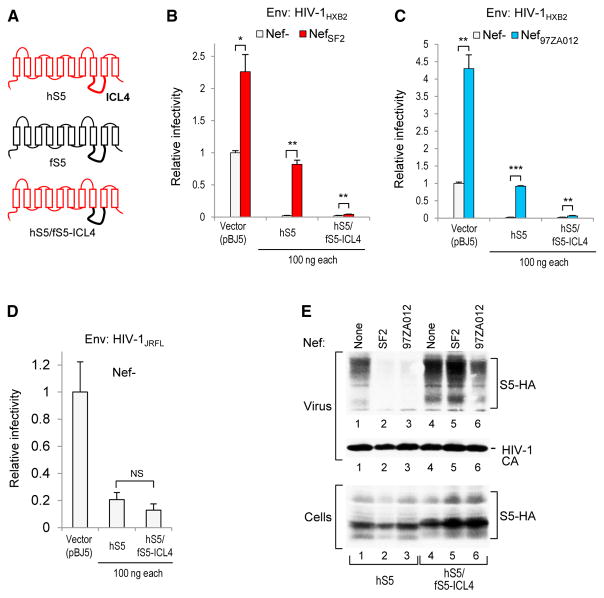
The results described earlier showed that the fS5/hS5-ICL4 chimera gained sensitivity to various Nefs. We also made the reciprocal hS5/fS5-ICL4 chimera (Figure 3A), which inhibited the infectivity of Nef− HIV-1 virions carrying EnvHXB2 as potently as authentic human SERINC5 (Figures 3B and 3C). Although the infectivity of Nef− HIV-1 virions carrying the relatively SERINC5-resistant EnvJRFL was inhibited to a lesser extent as expected (Rosa et al., 2015), the hS5/fS5-ICL4 chimera again was at least as potent as human SERINC5 (Figure 3D), confirming that it retained full anti-HIV activity. Crucially, whereas NefSF2 increased the infectivity of progeny virions carrying EnvHXB2 about 37-fold in the presence of exogenous human SERINC5, only a 2-fold increase was observed in the presence of the hS5/fS5-ICL4 chimera (Figure 3B). Furthermore, similar results were obtained with Nef97ZA012 (Figure 3C). Consistent with these observations, the incorporation of the hS5/fS5-ICL4 chimera into HIV-1 virions was not inhibited by NefSF2 and was only moderately reduced by Nef97ZA012 (Figure 3E). As expected, both Nef proteins strongly inhibited the incorporation of authentic human SERINC5 (Figure 3E). We conclude that the hS5/fS5-ICL4 chimera exhibits marked resistance to HIV-1 Nef proteins.
Figure 3. ICL4 of Frog SERINC5 Confers Resistance to Nef.
(A) Schematic illustration of the parental SERINC5 proteins and of the chimera examined.
(B) Human SERINC5 bearing the ICL4 of frog SERINC5 remains fully capable of inhibiting the infectivity of Nef− HIV-1 virions but exhibits strong resistance to a clade B HIV-1 Nef.
(C) SERINC5 chimera also exhibits resistance to a clade C HIV-1 Nef.
(D) SERINC5 chimera inhibits the infectivity of Nef− HIV-1 virions carrying a relatively resistant HIV-1 Env at least as well as the parental human SERINC5.
(E) Western blots showing the effects of clade B and clade C HIV-1 Nefs on the incorporation of human SERINC5 and of the SERINC5 chimera into Nef− HIV-1 virions.
Bar graphs represent the mean + SD from 3 biological replicates.
See also Figure S4.
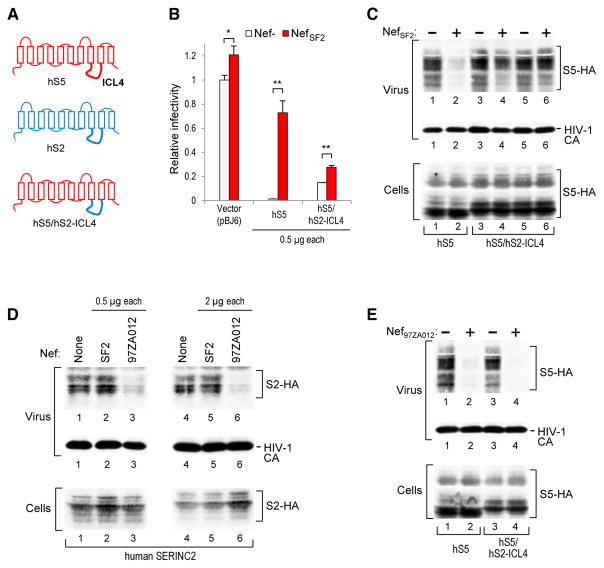
We also replaced the ICL4 of human SERINC5 with that of human SERINC2 (hS2), which exhibits no sequence similarity in this region. The resulting hS5/hS2-ICL4 chimera (Figure 4A) inhibited the specific infectivity of Nef− HIV-1 virions, albeit less potently than authentic human SERINC5 (Figure 4B). In this experiment, NefSF2 increased virion infectivity more than 50-fold in the presence of exogenous human SERINC5 but less than 2-fold in the presence of the chimera. Furthermore, the incorporation of the hS5/hS2-ICL4 chimera into HIV-1 virions was essentially resistant to NefSF2 (Figure 4C). In addition, flow cytometry revealed that the surface expression of SERINC5(iHA), which contains an internal HA tag within an extracellular loop, was not compromised when its ICL4 was replaced with that of SERINC2; it also revealed that the ICL4 swap conferred resistance to downregulation by NefSF2 (Figure S3). Altogether, these results establish that the ICL4 swap markedly reduced the sensitivity of human SERINC5 to NefSF2.
Figure 4. ICL4 of Human SERINC2 Confers Resistance to NefSF2, but Not Nef97ZA012.
(A) Schematic illustration of the parental human SERINC proteins and of the chimera examined.
(B) Inhibitory activity of human SERINC5 bearing the ICL4 of human SERINC2 on Nef− HIV-1 progeny virion infectivity is largely resistant to NefSF2. Bar graphs represent the mean + SD from 3 biological replicates.
(C) Western blots showing that the incorporation of the SERINC chimera into Nef− HIV-1 virions is largely unaffected by NefSF2 expressed in trans. The incorporation of the chimera was analyzed in duplicate to document reproducibility.
(D) Western blots showing that the incorporation of human SERINC2 into Nef− HIV-1 virions is unaffected by NefSF2 but inhibited by Nef97ZA012.
(E) Western blots showing that Nef97ZA012 inhibits the incorporation of human SERINC5 bearing the ICL4 of human SERINC2 into Nef− HIV-1 virions.
See also Figures S3 and S4.
During the course of these experiments, we observed that the incorporation of human SERINC2 into Nef− HIV-1 virions, although unaffected by NefSF2, is inhibited by Nef97ZA012 (Figure 4D). Similarly, the incorporation of the hS5/hS2-ICL4 chimera, while unaffected by NefSF2, was clearly inhibited by Nef97ZA012 (Figure 4E). Because the hS5/fS5-ICL4 chimera, which differs only in the ICL4, was resistant to Nef97ZA012 (Figure 3C), these findings raise the possibility that Nef97ZA012 recognizes a determinant within the ICL4 of SERINC2. In addition, these findings suggest that only ICL4 regions derived from SERINCs that exhibit resistance to a given Nef confer resistance to the same Nef when transferred to a sensitive SERINC.
Point Mutations in the ICL4 of Human SERINC5 Confer Resistance to Nef
Although Nef typically targets dileucine- or tyrosine-based sorting motifs, such motifs are not evident within the ICL4 of human or mouse SERINC5 (Figure S4A). To examine which region of the ICL4 determines sensitivity to Nef, we generated versions of Nef-resistant frog SERINC5 that have relatively poorly conserved segments replaced by the corresponding human SERINC5 sequences (Figure S4A). We observed that the transfer of ICL4 residues 9–26, but not of ICL4 residues 26–41, of human SERINC5 significantly increased the sensitivity of frog SERINC5 to NefSF2 (Figure S4B). Within the 9–26 segment, a leucine residue (L350 in human SERINC5) is present in Nef-sensitive, but not in Nef-resistant, SERINC5 proteins (Figure S4A). Point mutations that targeted this residue (L350A) or another small hydrophobic residue nearby (I353A) reduced the sensitivity of human SERINC5 to NefSF2 about 8- or 3-fold, respectively (Figure S4C). Together, these mutations (L350A/I353A) reduced the sensitivity of human SERINC5 more than 12-fold, and an 8-fold reduction was observed when L350 and I353 were replaced by the corresponding residues in frog SERINC5 (L350T/I353M) (Figure S4C). We also observed that the L350A and L350A/I353A mutations rendered the incorporation of human SERINC5 into HIV-1 virions largely resistant to NefSF2 (Figure S4D). These findings confirm the crucial role of the ICL4 in Nef sensitivity.
DISCUSSION
This study shows that widely divergent SERINC5 proteins from different vertebrate species share the ability to markedly inhibit the infectivity of Nef− HIV-1 virions. However, sensitivity to Nef is not conserved and can be transferred to a Nef-resistant SERINC5 by exchanging the ICL4 region. In the same manner, human SERINC3 can be made sensitive to a particular Nef, supporting the crucial role of the ICL4 region in Nef sensitivity. Conversely, our results show that the anti-HIV-1 activity of human SERINC5 can be made to persist even in the presence of Nef, which might provide a novel strategy to combat HIV-1. However, the in vivo significance of the effect of Nef on SERINC5 remains to be established.
Nearly the entire ICL4 region is predicted to be flexible by the PROFbval program (Schlessinger et al., 2006). The apparent flexibility of the ICL4 could conceivably facilitate interactions with widely divergent Nef proteins through a mechanism involving conformational selection (Boehr et al., 2009). Our data show that the ICL4 of human SERINC5 can confer sensitivity to Nefs from different primate lentiviral lineages.
The conservation of the anti-HIV activity among vertebrate SERINC5 proteins indicates that this activity is closely related to the biological function of SERINC5, which remains to be elucidated. SERINCs have been named for their reported function as carrier proteins that facilitate the synthesis of serine-derived lipids (Inuzuka et al., 2005). This activity suggested a model in which SERINCs inhibit HIV-1 infectivity by modifying the lipid content of progeny virions. However, a study argues strongly against this hypothesis by showing that SERINC5 does not alter the lipid composition of HIV-1 progeny virions or the exposure of phosphatidylserine on their surface (Trautz et al., 2017).
Usami et al. (2015) and Rosa et al. (2015) have presented evidence indicating that Nef counteracts SERINC5 by preventing its incorporation into HIV-1 virions. More recent work confirmed that Nef excludes SERINC5 from virions but suggested that Nef additionally inactivates the antiviral activity of any SERINC5 that becomes virion-associated even in the presence of Nef as a consequence of overexpression (Trautz et al., 2016). In the present study, the ability of Nef proteins to reduce the incorporation of native or chimeric versions of frog or human SERINC5 correlated well with their ability to enhance HIV-1 infectivity, which supports the notion that virion exclusion is the primary mechanism by which Nef counteracts SERINC5.
The ability of Nef to counteract human SERINC5 depends on the AP-2 clathrin adaptor complex (Rosa et al., 2015), whose cargo-binding AP2M1 and AP2S1 subunits in particular are more than 95% identical in human, frog, and zebrafish. Nef also hijacks clathrin adaptors to downregulate CD4 and major histocompatibility complex class I (MHC class I) molecules, and in these cases, Nef:AP complexes recognize linear epitopes within the cytosolic domains of CD4 and MHC class I molecules (Jia et al., 2012; Ren et al., 2014). Our results indicate that certain small hydrophobic residues within the ICL4 of human SERINC5 are critical for its sensitivity to Nef, and it is tempting to speculate that the region containing these residues is also recognized by Nef:AP complexes.
EXPERIMENTAL PROCEDURES
Analysis of Virus Infectivity
Pseudovirions capable of a single round of replication were produced by transfecting 293T cells using a calcium phosphate precipitation method. The cells were co-transfected with HXB/Env−/Nef− (1 μg in the experiments shown in Figures 1A, 1B, and 2A; 2 μg in all other experiments), a pSVIIIenv-based plasmid expressing EnvHXB2 or, where indicated, EnvJRFL (0.1 μg in the experiments shown in Figures 1A, 1B, 2A, and 3D; 0.4 μg in all other experiments), and the indicated amounts of pBJ5- or pBJ6-based plasmids expressing wild-type or chimeric SERINC5 proteins, or with equimolar amounts of empty vector. In addition, equal amounts of the nef-deficient pNefFS or of a plasmid expressing an HIV-1 or SIV Nef were co-transfected where indicated (2 μg in the experiments shown in Figure 1B and in the left panel of Figure 2C; 0.5 μg in all other experiments). Supernatants containing progeny virions were harvested 1 day post-transfection, clarified by low-speed centrifugation, filtered through 0.45-μm pore filters, and then used immediately to infect TZM-bl indicator cells in triplicate in 6-well plates. Aliquots of the virus stocks were frozen for HIV-1 capsid (p24) antigen quantitation by a standard ELISA. Three days post-infection, the indicator cells were lysed, and β-galactosidase activity induced as a consequence of infection was measured using a kit (E2000; Promega). Values were normalized for the amount of p24 antigen present in the supernatants used for infection.
Analysis of SERINC Incorporation
293T cells were co-transfected with 1 μg HXB/Env−/Nef−, pBJ5-based expression plasmids for C-terminally HA-tagged SERINCs or SERINC chimeras (0.1 μg, unless indicated otherwise), and the nef-deficient control plasmid pNefFS or a plasmid expressing an HIV-1 Nef (0.5 μg in the experiments shown in Figures 3E, S1B, S2B, and S4D; 2 μg in the experiments shown in Figures 2D, 4C, and 4E). Virions released into the medium were pelleted through 20% sucrose cushions, and virus- and cell-associated proteins were detected by western blotting as described (Accola et al., 2000). Because SERINC proteins are highly prone to aggregation (Usami et al., 2015), samples used for the detection of SERINCs were not boiled before loading. The antibodies used were 183-H12-5C against HIV-1 CA, and HA.11 (BioLegend) against the HA epitope.
Statistical Analysis
Statistical analysis was performed using a two-tailed unpaired t test with Welch’s correction in case of unequal variance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, NS, not significant (p > 0.05).
Supplementary Material
Highlights.
Anti-HIV-1 activity is a conserved property of widely divergent SERINC5 proteins
Sensitivity to HIV-1 Nef is not conserved
Nef sensitivity can be transferred by exchanging an intracellular loop region
Human SERINC5 can be modified to restrict HIV-1 even in the presence of Nef
Acknowledgments
We thank M. Pizzato for pBJ6-SERINC5 and the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 for monoclonal antibody 183-H12-5C and for TZM-bl cells. This work was supported by NIAID/NIH grant R01AI127263.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.12.082.
AUTHOR CONTRIBUTIONS
W.D., Y.U., and Y.W. performed the experiments. W.D. and H.G. designed the experiments and analyzed the data. H.G. wrote the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Accola MA, Strack B, Göttlinger HG. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol. 2000;74:5395–5402. doi: 10.1128/jvi.74.12.5395-5402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Beitari S, Ding S, Pan Q, Finzi A, Liang C. Effect of HIV-1 Env on SERINC5 antagonism. J Virol. 2017;91:e02214–e02216. doi: 10.1128/JVI.02214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol. 2009;5:789–796. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Lindwasser OW, Smith WJ, Hurley JH, Bonifacino JS. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol. 2007;81:3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, Guatelli JC. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers MY, Pandori MW, Spina CA, Richman DD, Guatelli JC. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- Forshey BM, Aiken C. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J Virol. 2003;77:4409–4414. doi: 10.1128/JVI.77.7.4409-4414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Haller C, Müller B, Fritz JV, Lamas-Murua M, Stolp B, Pujol FM, Keppler OT, Fackler OT. HIV-1 Nef and Vpu are functionally redundant broad-spectrum modulators of cell surface receptors, including tetraspanins. J Virol. 2014;88:14241–14257. doi: 10.1128/JVI.02333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigele A, Kmiec D, Regensburger K, Langer S, Peiffer L, Stürzel CM, Sauter D, Peeters M, Pizzato M, Learn GH, et al. The potency of Nef-mediated SERINC5 antagonism correlates with the prevalence of primate lentiviruses in the wild. Cell Host Microbe. 2016;20:381–391. doi: 10.1016/j.chom.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka M, Hayakawa M, Ingi T. Serinc, an activity-regulated protein family, incorporates serine into membrane lipid synthesis. J Biol Chem. 2005;280:35776–35783. doi: 10.1074/jbc.M505712200. [DOI] [PubMed] [Google Scholar]
- Jia X, Singh R, Homann S, Yang H, Guatelli J, Xiong Y. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol. 2012;19:701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Matheson NJ, Sumner J, Wals K, Rapiteanu R, Weekes MP, Vigan R, Weinelt J, Schindler M, Antrobus R, Costa AS, et al. Cell surface proteomic map of HIV infection reveals antagonism of amino acid metabolism by Vpu and Nef. Cell Host Microbe. 2015;18:409–423. doi: 10.1016/j.chom.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Warmerdam MT, Page KA, Feinberg MB, Greene WC. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J Virol. 1995;69:579–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzato M, Helander A, Popova E, Calistri A, Zamborlini A, Palù G, Göttlinger HG. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc Natl Acad Sci USA. 2007;104:6812–6817. doi: 10.1073/pnas.0607622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Park SY, Bonifacino JS, Hurley JH. How HIV-1 Nef hijacks the AP-2 clathrin adaptor to downregulate CD4. eLife. 2014;3:e01754. doi: 10.7554/eLife.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A, Chande A, Ziglio S, De Sanctis V, Bertorelli R, Goh SL, McCauley SM, Nowosielska A, Antonarakis SE, Luban J, et al. HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature. 2015;526:212–217. doi: 10.1038/nature15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger A, Yachdav G, Rost B. PROFbval: predict flexible and rigid residues in proteins. Bioinformatics. 2006;22:891–893. doi: 10.1093/bioinformatics/btl032. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Maréchal V, Danos O, Heard JM. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Spina CA, Kwoh TJ, Chowers MY, Guatelli JC, Richman DD. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautz B, Pierini V, Wombacher R, Stolp B, Chase AJ, Pizzato M, Fackler OT. The antagonism of HIV-1 Nef to SERINC5 particle infectivity restriction involves the counteraction of virion-associated pools of the restriction factor. J Virol. 2016;90:10915–10927. doi: 10.1128/JVI.01246-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautz B, Wiedemann H, Lüchtenborg C, Pierini V, Kranich J, Glass B, Kräusslich HG, Brocker T, Pizzato M, Ruggieri A, et al. The host-cell restriction factor SERINC5 restricts HIV-1 infectivity without altering the lipid composition and organization of viral particles. J Biol Chem. 2017;292:13702–13713. doi: 10.1074/jbc.M117.797332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami Y, Göttlinger H. HIV-1 Nef responsiveness is determined by Env variable regions involved in trimer association and correlates with neutralization sensitivity. Cell Rep. 2013;5:802–812. doi: 10.1016/j.celrep.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami Y, Popov S, Göttlinger HG. The Nef-like effect of murine leukemia virus glycosylated gag on HIV-1 infectivity is mediated by its cytoplasmic domain and depends on the AP-2 adaptor complex. J Virol. 2014;88:3443–3454. doi: 10.1128/JVI.01933-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami Y, Wu Y, Göttlinger HG. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature. 2015;526:218–223. doi: 10.1038/nature15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.