 Synthesis and antimicrobial evaluation of C-5 functionalized bis-isatins against pathogenic microorganisms.
Synthesis and antimicrobial evaluation of C-5 functionalized bis-isatins against pathogenic microorganisms.
Abstract
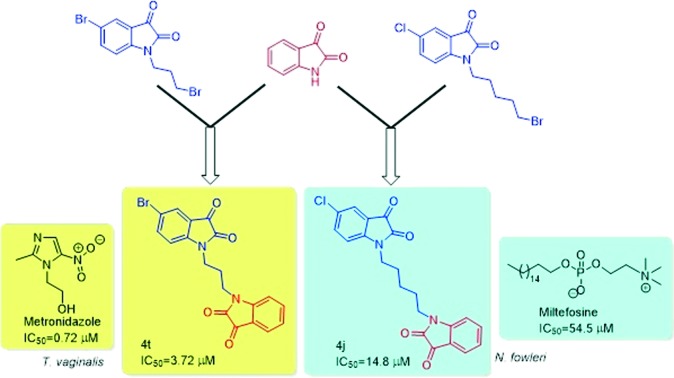
A series of N-alkyl-tethered C-5 functionalized bis-isatins were synthesized and evaluated for their antimicrobial activity against pathogenic microorganisms. The preliminary evaluation studies revealed compound 4t, with an optimal combination of a bromo substituent at the C-5 position of the isatin ring and a propyl chain linker, being the most active among the synthesized series exhibiting an IC50 value of 3.72 μM against Trichomonas vaginalis while 4j exhibited an IC50 value of 14.8 μM against Naegleria fowleri, more effective than the standard drug miltefosine. Compound 3f with an octyl spacer length was the most potent among the series against Giardia lamblia with an IC50 of 18.4 μM while 3d exhibited an IC50 of 23 μM against Entamoeba histolytica. This library was also screened against the fungal pathogen Aspergillus parasiticus. A number of the compounds demonstrated potency against this fungus, illustrating a possible broad-spectrum activity. Furthermore, an evaluation of these synthesized compounds against a panel of normal flora bacteria revealed them to be non-cytotoxic, demonstrating the selectivity of these compounds. This observation, in combination with previous studies that found isatin to be non-toxic to humans, presents a new possible scaffold for drug discovery against these important protozoal pathogens of humans and animals.
Introduction
Protozoal pathogens of humans and animals that colonize mucosal surfaces cause some of the most prevalent diseases. Among these mucosal pathogens are Trichomonas vaginalis (a sexually transmitted pathogen), Giardia lamblia and Entamoeba histolytica (both food- and waterborne pathogens), and Naegleria fowleri (a free-living amoeba that becomes parasitic once established in human tissue and is deadly in most cases). Sexually transmitted infections (STIs) are still considered a major health issue. Among these diseases, trichomoniasis is one of the most common non-viral sexually transmitted diseases and is caused by the protozoal pathogen Trichomonas vaginalis. Over 276.4 million cases of trichomoniasis are reported worldwide each year,1,2 with more than 8 million cases in North America alone.3T. vaginalis is more prevalent in females than males,4–6 however, the reason for this ambiguity is poorly understood. Trichomoniasis is associated with diverse reproductive health outcomes including cervical neoplasia, cervical carcinoma, post-hysterectomy infection, tubal infertility, preterm rupture of membranes, pelvic inflammatory disease, and birth complications including preterm birth, low birth-weight infants and neonatal infection.7–9 T. vaginalis has also been reported to act as a co-factor in the transmission of human immunodeficiency virus (HIV) leading to a two-fold increase in the risk of transmitting HIV.10–14
Giardia lamblia and Entamoeba histolytica are two common food- and waterborne protozoal parasites, which are responsible for ∼280 and ∼50 million diarrhoeal infections annually, respectively.15 Water or food contaminated with cysts or direct person-to-person contact is responsible for the transmission of these parasites.16 Symptomatically, giardiasis results in watery diarrhoea, abdominal discomfort, pain and cramps while chronic diseases may lead to malabsorption and decelerated physical growth among children.17 Amoebiasis, on the other hand, may result in abdominal pain, diarrhoea, and severe colitis with tissue death and perforation. Since contaminated food and water are the major sources for the transmission of these parasites, the global burden of these diseases is shouldered mainly by developing nations with poor sanitation.18 Metronidazole, together with other nitroimidazoles, such as tinidazole and ornidazole, is the mainstay for the treatment of these parasitic infections. The introduction of nitroimidazoles for the treatment of parasitic infections heralded a new era for the treatment of parasitic infections.19,20 However, the recent discovery of side effects including genotoxicity, gastric mucus irritation and development of clinical resistance including the growth of trophozoites of E. histolytica at therapeutically relevant levels of metronidazole demonstrated the need for the development of unique scaffolds against these parasitic diseases.21
Isatin and its functionalized derivatives have gained the interest of medicinal chemists.22 Currently, an isatin-based triple angiokinase inhibitor BIBF1120 II, reported by the pharmaceutical company Boehringer, is in phase III clinical trials for non-small cell lung cancer.23 Furthermore, isatin-based sunitinib III (Sutent) is a multikinase inhibitor targeting VEGFR-1, VEGFR-2, PDGFRb and c-Kit. Sunitinib was approved by the FDA for the treatment of gastrointestinal stromal tumors (GIST) and advanced renal cell carcinoma (RCC).24–26 Different combinations of bis-isatins have been reported to have significant biological activities. For example, indigo and indirubin have potential inhibitory action against CDK-2 while meisoindigo was used in the treatment of chronic myelogenous leukemia. 7,7′-Azaindirubin has been shown to possess inhibitory activity against casein kinase 2.27–29
Recently, we described the synthesis of 1H-1,2,3-triazole-tethered β-lactam–isatin and β-amino alcohol-based β-lactam–isatin conjugates along with their preliminary in vitro evaluation studies against T. vaginalis.30,31 The synthesized conjugates proved to be potent against T. vaginalis exhibiting IC50 values below 10 μM along with minimal cytotoxicity to cultured human cells. The methodology was further extended towards synthesis of N-propargylated-isatin Mannich adducts along with their in vitro evaluation against T. vaginalis and T. foetus with the most potent compound exhibiting an IC50 value of 11.3 μM against T. foetus.32,33 As a continuation of our interest in the synthesis of biologically relevant scaffolds34 and a logical follow-up of our recent study on uracil–isatin conjugates as potential anti-trichomonad agents,34a the present manuscript describes the synthesis of N-alkyl chain-tethered C-5 functionalized bis-isatins and their in vitro evaluation against a range of parasitic organisms.
Results and discussion
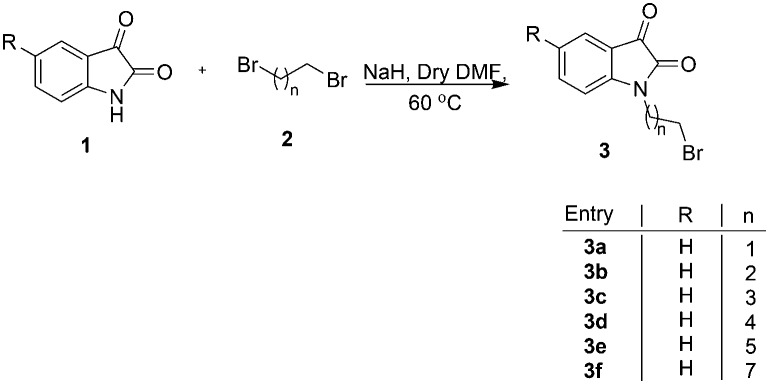
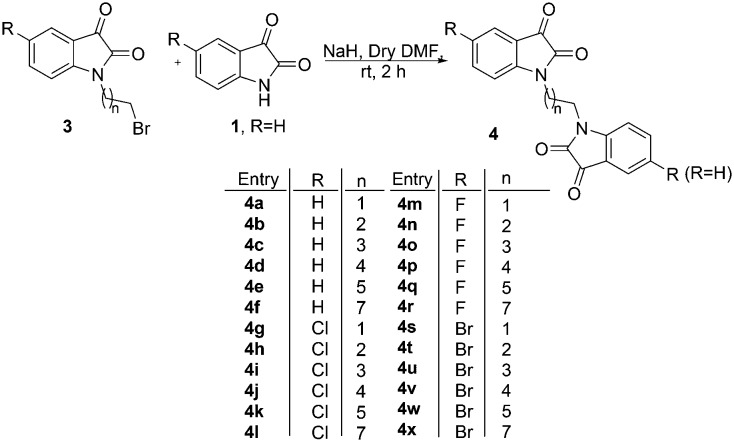
The precursor, viz. N-alkylbromoisatins 3, was prepared by an initial base-promoted reaction of C-5 substituted isatins 1 with dibromoalkanes 2 (Scheme 1).35 The target compound 4 was synthesized via a hydride-promoted reaction of appropriate precursors 1 and 3 in dry DMF as elucidated in Scheme 2. The purification of the reaction mixture, after usual work-up, via column chromatography resulted in the isolation of desired scaffolds, the structures to which were assigned on the basis of spectral data and analytical evidence.
Scheme 1. Synthesis of N-alkylbromoisatin.
Scheme 2. Synthesis of N-alkyl chain-tethered C-5 functionalized bis-isatin.
Compound 4f, for example, characterized as 1-[8-(2,3-dioxo-2,3-dihydro-indol-1-yl)octyl]-1H-indole-2,3-dione, was analysed as C24H24N2O4 and showed a molecular ion peak at m/z = 404.1743 (M+) in its mass spectrum. The salient features of its 1H NMR spectrum included a singlet at δ 1.37, a triplet at 1.71 (J = 7.0 Hz) and another triplet at 3.73 (J = 7.0 Hz) corresponding to the methylene protons of the alkyl chain along with the required number of aromatic protons. The appearance of characteristic absorptions at δ 158.2 and 183.6 corresponding to isatin ring carbonyls (C O) along with the requisite number of carbons in the 13C NMR spectrum further substantiated the assigned structure.
The synthesized compounds were evaluated for their inhibitory effects against trichomoniasis, giardiasis and amoebiasis. For anti-trichomonal activities, the synthesized compounds were evaluated against the G3 strain of T. vaginalis tested at 50 μM (Table 1). The anti-trichomonad activities of the tested compounds showed dependence upon the nature of the substituent at the C-5 position of the isatin ring as well as the alkyl chain length, introduced as a spacer. Bis-isatins showed better activity profiles compared to N-alkylbromoisatins 3a–3f. In the case of unsubstituted isatins (R H), the compounds having odd numbered alkyl chain length have been shown to possess better activity profiles compared to the compounds having even numbered alkyl chain length except 4a. The presence of an electron-withdrawing chloro substituent at the C-5 position of the isatin ring improved the activity, while a decrease in activity was observed at longer alkyl chain length as evident for conjugate 4l. Further, the introduction of fluoro and bromo substituents at the C-5 position of the isatin ring of the synthesized scaffolds resulted in 100% growth inhibition irrespective of the chain length as evident for compounds 4n–4w.
Table 1. Inhibitory activity of the compound library against T. vaginalis, G. lamblia and E. histolytica.
| Compound | Average % inhibition at 50 μM (T. vaginalis) | Average % inhibition at 50 μM (G. lamblia) | Average % inhibition at 50 μ M (E. histolytica) |
| 3a | 62.51 ± 29.87 | 47.22 ± 6.33 | 7.23 ± 4.97 |
| 3b | 59.93 ± 22.62 | 22.85 ± 8.13 | 1.32 ± 11.83 |
| 3c | 89.75 ± 9.49 | 20.85 ± 7.89 | 8.47 ± 7.68 |
| 3d | 71.44 ± 12.31 | 25.75 ± 6.33 | 100 |
| 3e | 86.63 ± 9.84 | 98.9 ± 1.51 | Not active |
| 3f | 100 | 100 | Not active |
| 4a | 96.77 ± 17.61 | 6.75 ± 12.44 | Not active |
| 4b | 96.77 ± 0.47 | 7.84 ± 1.46 | Not active |
| 4c | 61.29 ± 21.20 | 11.64 ± 9.82 | Not active |
| 4d | 90.90 ± 3.94 | 94.27 ± 1.55 | 100 |
| 4e | 45.45 ± 23.89 | 9.68 ± 0.75 | Not active |
| 4f | 30.30 ± 6.34 | 80.69 ± 6.98 | 100 |
| 4g | 78.78 ± 12.69 | 3.82 ± 5.35 | 73.58 ± 41.19 |
| 4h | 100 | Not active | Not active |
| 4i | 100 | Not active | Not active |
| 4j | 100 | 100 | 100 |
| 4k | 93.93 ± 3.94 | 94.52 ± 1.99 | Not active |
| 4l | 75.75 ± 12.29 | 8.56 ± 0.86 | Not active |
| 4m | 45.45 ± 6.34 | 8.15 ± 9.86 | Not active |
| 4n | 100 | 100 | Not active |
| 4o | 100 | 1.2 ± 0.68 | Not active |
| 4p | 100 | Not active | Not active |
| 4q | 100 | 30.9 ± 9.85 | Not active |
| 4r | 100 | Not active | 13.82 ± 2.70 |
| 4s | 100 | Not active | Not active |
| 4t | 100 | 19.27 ± 4.06 | Not active |
| 4u | 100 | Not active | Not active |
| 4v | 100 | Not active | Not active |
| 4w | 100 | 43.16 ± 1.68 | Not active |
| 4x | 96.77 ± 3.12 | 51.1 ± 0.90 | Not active |
The evaluation of the synthesized compounds against G. lamblia showed activity dependence upon the nature of the substituent at the C-5 position as well as the spacer length. Among the precursors, viz. N-alkylbromoisatins, the activity improved with the increase in chain length as evident for 3e (n = 6) and 3f (n = 8) with growth inhibition of 98.9 and 100%, respectively. Evaluation of the anti-giardial activity of bis-isatins 4a–4f (R H) further confirmed the improvement in activity profiles with the increase in spacer length with compounds 4d (n = 5) and 4f (n = 8) exhibiting 94.2 and 80.6% growth inhibition, respectively. Introduction of a chloro substituent at the C-5 position (4g–l) did not improve the activity while it was retained at longer alkyl chain length (4k, n = 6, 94.5%). Introduction of either a fluoro or a bromo substituent at the C-5 position substantially reduced the anti-giardial activity with most of the compounds being inactive even at longer alkyl chain length. Further, most of the synthesized compounds exhibited poor anti-amoebic activity with few exceptions, viz.3d, 4d, 4f and 4j which exhibited 100% growth inhibition.
The active compounds from the preliminary inhibition data were chosen in order to determine their IC50 which is the minimum concentration required for 50% growth inhibition and the results are tabulated in Table 2. As evident from Table 2, compound 4t having a bromo substituent at the C-5 position of the isatin ring with a propyl chain linker is the most active compound of the series exhibiting an IC50 value of 3.72 μM against T. vaginalis. For G. lamblia, the synthesized compounds exhibited IC50 values ranging from 18.4–38.0 μM, with compound 3f having an octyl spacer length proven to be the most potent. 3d, with a pentyl spacer length, proved to be the most potent against amoebiasis exhibiting an IC50 value of 23 μM. The synthesized compounds were also evaluated against Naegleria fowleri, a brain tropic amoeba causing a sudden and severe brain infection called naegleriasis. Even though the synthesized compounds were not as potent as the standard drug amphotericin B, they exhibited better activity than miltefosine. Compound 4j, with an optimum combination of a chloro substituent at the C-5 position and a pentyl chain introduced as a spacer, proved to be the most potent among bis-isatins exhibiting an IC50 value of 14.8 μM.
Table 2. IC50 of the synthesized hybrids against T. vaginalis, G. lamblia, N. fowleri and E. histolytica.
| Compound | T. vaginalis IC50 (μM) | G. lamblia IC50 (μM) | N. fowleri IC50 (μM) | E. histolytica IC50 (μM) |
| 3a | 23.6 | |||
| 3c | 14.5 | |||
| 3d | 23 | |||
| 3e | 24.4 | |||
| 3f | 18.4 | |||
| 4d | 26 | |||
| 4f | 38 | |||
| 4g | 15.0 | 28.3 | ||
| 4j | 23.9 | 14.8 | ||
| 4k | 31.5 | |||
| 4n | 12.5 | 28.8 | ||
| 4p | 27.3 | |||
| 4t | 3.72 | |||
| 4u | 25 | |||
| Metronidazole | 0.72 | 6.4 | 5 | |
| Amphotericin B | 0.2 | |||
| Miltefosine | 54.5 |
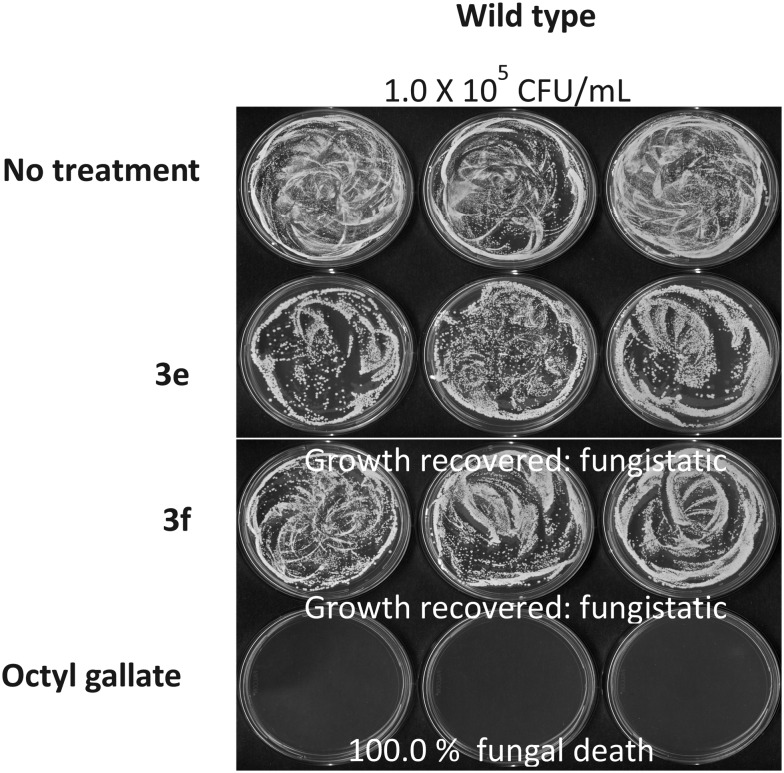
Further, the synthesized library of bis-isatins was also screened for their antifungal activity against Aspergillus parasiticus and Saccharomyces cerevisiae using octyl gallate as the positive control. Eleven of the synthesized compounds exhibited a certain level of antifungal activity against A. parasiticus 5862 or S. cerevisiae BY4741 wild type and the data is listed in Table 3. As is evident, S. cerevisiae showed higher susceptibility to the test compounds compared to A. parasiticus. Compounds 3c–f proved to be the most active exhibiting a growth score of 0 at 48 h during the microtiter plate liquid bioassay (Table 3). Fungal recovery bioassay on YPD plates, where S. cerevisiae wild type was treated with compounds 3e and 3f, showed that the growth could be recovered (Fig. 1), indicating that their antifungal activity is “fungistatic” but not “fungicidal.”
Table 3. Antifungal activity of compounds tested against A. parasiticus 5862 and S. cerevisiae wild type a .
| Compound | A. parasiticus 24 h | A. parasiticus 48 h | S. cerevisiae 24 h | S. cerevisiae 48 h |
| No treatment | 4 | 4 | 4 | 4 |
| 3a | 3 to 4 | 4 | 1 | 3 |
| 3b | 3 to 4 | 4 | 2 | 3 |
| 3c | 3 to 4 | 4 | 0 | 2 |
| 3d | 3 to 4 | 4 | 0 | 2 |
| 3e | 3 to 4 | 4 | 0 | 0 |
| 3f | 3 to 4 | 4 | 0 | 0 |
| 4g | 4 | 4 | 1 | 2 |
| 4i | 4 | 4 | 1 | 3 |
| 4p | 4 | 4 | 2 | 3 |
| 4t | 4 | 4 | 1 | 2 |
| 4v | 4 | 4 | 2 | 4 |
| Octyl gallate | 0 | 0 | 0 | 0 |
aGrowth scores (0 to 4) were determined based on the microtiter plate liquid bioassay.
Fig. 1. S. cerevisiae survival test following the determination of drug efficacy (3e and 3f at 500 μM) in liquid culture (microtiter wells).
Since these compounds had varying cytotoxic effects against the various parasitic strains, we wondered if the effects of these compounds were specific to parasites or were potentially broad spectrum. Of critical importance would be to evaluate the effect of these compounds on normal human flora since they occupy the same niche as these protozoal pathogens. Using the classic disc diffusion assay, we tested these compounds against a panel of normal human flora consisting of the non-pathogenic strains Escherichia coli K12 MG1655, Lactobacillus acidophilus, Lactobacillus rhamnosus LGG, and Lactobacillus reuteri. No cytotoxic effects from any of these synthesized compounds were observed for any of the normal flora. Therefore, the cytotoxic effects seen on these protozoa would suggest that these compounds are anti-parasitic.
The physicochemical and absorption, distribution, metabolism and excretion (ADME) properties of the synthesized library of compounds 3a–3f and 4a–4x were profiled in silico using the web-based applications ChemAxon (; http://www.chemaxon.com/) and PreADMET (; http://preadmet.bmdrc.org/). The basicity and lipophilicity of the new compounds were predicted with ChemAxon (Table 5), and Caco-2, MDCK, BBB, HIA, plasma protein binding and skin permeability data were predicted with PreADMET (Table 4). The synthesized hybrids are predicted to exhibit lower blood–brain barrier permeation and thus are less likely to cause neurotoxicity. The physicochemical properties of the synthesized library of compounds 3a–3f and 4a–4x were obtained using the ChemAxon software to assess their compliance with the Lipinski rule of five criteria (Table 5). The analysis of data revealed (a) that all molecules have molecular weight in the range of 252–482 which is below the limit of the accepted value (500), (b) a log P lower than 5.0 indicating that the compounds are not very lipophilic and (c) molar refractivity in the range of 55.92–120.75 which lies well within the limits of accepted values (40–130). All the derivatives comply with hydrogen bond properties. The results of this analysis suggest that all the synthesized compounds show compliance with the Lipinski rule and ADME properties which are known as the filters for drug-likeness in order to increase the efficiency of drug discovery and thus represent pharmacologically active frameworks that should be considered on progressing further potential hits.
Table 5. In silico physicochemical parameters of the synthesized compounds a .
| Compound | M. wt | No. of H bond acceptors | No. of H bond donors | Log P | Molar refractivity | No. of Lipinski violations |
| 3a | 252.08 | 4 | 0 | 1.31 | 55.92 | 0 |
| 3b | 266.10 | 4 | 0 | 1.36 | 60.78 | 0 |
| 3c | 280.14 | 4 | 0 | 1.82 | 65.43 | 0 |
| 3d | 294.16 | 4 | 0 | 2.21 | 70.03 | 0 |
| 3e | 308.01 | 4 | 0 | 2.61 | 74.63 | 0 |
| 3f | 336.06 | 4 | 0 | 3.00 | 79.23 | 0 |
| 4a | 320.07 | 8 | 0 | 1.28 | 80.02 | 0 |
| 4b | 334.09 | 8 | 0 | 0.96 | 90.08 | 0 |
| 4c | 348.11 | 8 | 0 | 1.42 | 94.72 | 0 |
| 4d | 362.12 | 8 | 0 | 1.81 | 99.32 | 0 |
| 4e | 376.14 | 8 | 0 | 2.21 | 103.92 | 0 |
| 4f | 404.17 | 8 | 0 | 3.00 | 113.13 | 0 |
| 4g | 354.04 | 8 | 0 | 1.80 | 84.83 | 0 |
| 4h | 368.05 | 8 | 0 | 1.48 | 94.88 | 0 |
| 4i | 382.07 | 8 | 0 | 1.93 | 99.53 | 0 |
| 4j | 396.08 | 8 | 0 | 2.33 | 104.13 | 0 |
| 4k | 410.10 | 8 | 0 | 2.73 | 108.73 | 0 |
| 4l | 438.13 | 8 | 0 | 3.52 | 117.93 | 0 |
| 4m | 338.07 | 8 | 0 | 1.42 | 80.24 | 0 |
| 4n | 352.08 | 8 | 0 | 1.10 | 90.29 | 0 |
| 4o | 366.10 | 8 | 0 | 1.56 | 94.94 | 0 |
| 4p | 380.11 | 8 | 0 | 1.95 | 99.54 | 0 |
| 4q | 394.13 | 8 | 0 | 2.35 | 104.14 | 0 |
| 4r | 422.16 | 8 | 0 | 3.14 | 113.34 | 0 |
| 4s | 397.99 | 8 | 0 | 2.07 | 87.65 | 0 |
| 4t | 412.00 | 8 | 0 | 1.76 | 97.70 | 0 |
| 4u | 426.02 | 8 | 0 | 2.21 | 102.35 | 0 |
| 4v | 440.03 | 8 | 0 | 2.60 | 106.95 | 0 |
| 4w | 454.05 | 8 | 0 | 3.00 | 111.55 | 0 |
| 4x | 482.04 | 8 | 0 | 3.79 | 120.75 | 0 |
aCalculator plugins were used for structure property prediction and calculation, Marvin 5.11.4, 2012, ChemAxon (http://www.chemaxon.com).
Table 4. In silico ADME profiling of 3a–4x.
| Compound | Absorption
a
|
Distribution
a
|
||||
| Human intestinal absorption (HIA) (percentage) | In vitro Caco-2 cell permeability (nm s–1) | In vitro MDCK cell permeability (nm s–1) | In vitro skin permeability (log Kp, cm h–1) | In vitro plasma protein binding (percentage) | In vivo blood–brain barrier penetration (Cbrain/Cblood) | |
| 3a | 98.05 | 21.25 | 5.33 | –3.07 | 41.75 | 1.94 |
| 3b | 98.02 | 9.30 | 58.36 | –3.20 | 83.67 | 2.00 |
| 3c | 98.00 | 20.72 | 45.82 | –2.93 | 86.28 | 2.45 |
| 3d | 98.00 | 21.50 | 21.61 | –2.82 | 91.90 | 2.46 |
| 3e | 98.01 | 22.03 | 7.14 | –2.70 | 95.48 | 0.41 |
| 3f | 98.06 | 22.88 | 0.41 | –2.45 | 100.0 | 0.21 |
| 4a | 99.01 | 21.06 | 80.91 | –4.26 | 74.12 | 0.26 |
| 4b | 99.01 | 20.55 | 5.15 | –4.22 | 66.53 | 0.21 |
| 4c | 98.95 | 21.62 | 0.05 | –4.07 | 74.90 | 0.23 |
| 4d | 98.85 | 22.24 | 0.04 | –3.94 | 87.20 | 0.23 |
| 4e | 98.73 | 21.28 | 0.09 | –3.80 | 86.20 | 0.12 |
| 4f | 98.42 | 24.17 | 149.0 | –3.46 | 90.84 | 0.01 |
| 4g | 98.62 | 21.18 | 2.65 | –4.29 | 87.74 | 0.30 |
| 4h | 98.46 | 21.22 | 0.74 | –4.26 | 80.64 | 0.34 |
| 4i | 98.30 | 21.56 | 0.05 | –4.13 | 85.64 | 0.42 |
| 4j | 98.14 | 21.95 | 0.04 | –3.99 | 93.90 | 0.26 |
| 4k | 98.00 | 22.52 | 0.06 | –3.85 | 91.41 | 0.09 |
| 4l | 97.76 | 24.44 | 73.79 | –3.53 | 93.76 | 0.01 |
| 4m | 99.01 | 21.16 | 3.09 | –4.44 | 77.61 | 0.20 |
| 4n | 99.00 | 20.40 | 3.46 | –4.41 | 72.34 | 0.24 |
| 4o | 98.94 | 21.48 | 0.06 | –4.30 | 80.03 | 0.31 |
| 4p | 98.84 | 22.09 | 0.04 | –4.19 | 88.11 | 0.23 |
| 4q | 98.72 | 21.70 | 0.07 | –4.07 | 87.68 | 0.11 |
| 4r | 98.41 | 25.00 | 72.17 | –3.80 | 91.07 | 0.01 |
| 4s | 98.09 | 21.23 | 0.15 | –4.21 | 89.18 | 0.33 |
| 4t | 97.95 | 21.40 | 0.06 | –4.17 | 82.93 | 0.37 |
| 4u | 97.83 | 21.68 | 0.02 | –4.02 | 87.08 | 0.44 |
| 4v | 97.73 | 22.07 | 0.03 | –3.88 | 94.90 | 0.27 |
| 4w | 97.65 | 22.64 | 0.02 | –3.72 | 92.74 | 0.09 |
| 4x | 97.56 | 24.33 | 0.46 | –3.38 | 94.73 | 0.01 |
aPredicted properties related to ADME using PreADMET using the web-based application PreADMET (http://preadmet.bmdrc.org).
In conclusion, a series of bis-isatins were synthesized and evaluated for their inhibitory activities against four different mucosal protozoal pathogens, one pathogenic fungus, and a number of different normal flora microbiota. The evaluation studies revealed the dependence of activity on the C-5 substituent of the isatin ring as well as the length of the alkyl chain, introduced as a spacer. The most active compound of the series, 4t, exhibited an IC50 value of 3.72 μM against T. vaginalis while 4j exhibited an IC50 value of 14.8 μM against N. fowleri, which is better than the standard drug miltefosine. Compound 3f exhibited an IC50 of 18.4 μM against G. lamblia while 3d exhibited an IC50 of 23 μM against E. histolytica. The compounds also exhibited potent activities against the fungal pathogen A. parasiticus, illustrating a possible broad-spectrum activity. The synthesized compounds were also evaluated against a panel of normal human flora consisting of the non-pathogenic strains and were found to be non-cytotoxic. The physicochemical studies further revealed that all the compounds are compliant with the Lipinski rule suggestive of the fact that the designed molecular framework can act as a therapeutic template for the synthesis of new antimicrobial drugs.
Experimental section
General
Melting points were determined by means of an open capillary using a Veego precision digital melting point apparatus (MP-D) and are uncorrected. 1H NMR spectra were recorded in DMSO-d6 and CDCl3 with a BRUKER AVANCE II (500 MHz) spectrometer using TMS as an internal standard. Chemical shift values are expressed as parts per million downfield from TMS and J values are in hertz. Splitting patterns are indicated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double doublet, ddd: doublet of doublets of doublets, and br: broad peak. 13C NMR spectra were recorded in DMSO-d6 and CDCl3 with a BRUKER AVANCE II (125 MHz) spectrometer using TMS as an internal standard. Mass spectra were recorded on a BRUKER high-resolution mass spectrometer (micrOTOF-QII). Elemental analyses were performed on a Heraeus CHN-O rapid elemental analyzer. Column chromatography was performed on a silica gel (60–120 mesh) using an ethyl acetate–hexane mixture as the eluent.
Typical procedure for the synthesis of C-5 substituted bis-isatins (4a–4x)
To a stirred suspension of sodium hydride (1.5 mmol) in dry DMF (10 ml) was added isatin 1 (1 mmol), resulting in the formation of a purple-coloured anion solution. The solution was stirred at room temperature till the evolution of hydrogen ceased. To this reaction mixture was then added N-alkylbromoisatins353 (1.1 mmol) in DMF and the resulting mixture was allowed to stir at room temperature for 2 hours. After completion of the reaction, as evidenced by TLC, the reaction mixture was quenched by dropwise addition of water (20 mL) and subsequently extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine solution, dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification of the reaction mixture via column chromatography using a hexane : ethyl acetate (8 : 2) mixture furnished the desired bis-isatins in good yields.
1-[2-(2,3-Dioxo-2,3-dihydro-indol-1-yl)ethyl]-1H-indole-2,3-dione (4a)
Red solid, yield 87%, m.pt. 274–275 °C. 1H NMR (500 MHz, DMSO-d6): δ 3.69 (t, J = 5.8 Hz, 4H, –CH2–); 7.10–7.13 (m, 2H, ArH); 7.20 (d, J = 7.9 Hz, 2H, ArH); 7.52 (d, J = 7.3 Hz, 2H, ArH); 7.62–7.65 (m, 2H, Ar–H). 13C NMR (125 MHz, DMSO-d6): 24.5, 79.6, 111.2, 117.9, 123.5, 124.9, 138.6, 151.0, 158.6, 183.8. HRMS calculated for C18H12N2O4 320.0797 [M+]; found 320.0803. Anal. calcd (%) for C, 67.50; H, 3.78; N, 8.75; found C, 67.43; H, 3.86; N, 8.81.
1-[3-(2,3-Dioxo-2,3-dihydro-indol-1-yl)propyl]-1H-indole-2,3-dione (4b)
Red solid, yield 82%, m.pt. 241–242 °C. 1H NMR (500 MHz, CDCl3): δ 2.15 (t, J = 6.0 Hz, 2H, –CH2–); 3.87 (t, J = 6.0 Hz, 4H, –CH2–); 7.11–7.15 (m, 4H, ArH); 7.55 (d, J = 7.5 Hz, 2H, ArH); 7.60 (d, J = 7.5 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): 24.7, 37.5, 110.3, 117.3, 123.2, 124.6, 138.0, 150.1, 158.0, 182.9. HRMS calculated for C19H14N2O4 334.0954 [M+]; found 334.0969. Anal. calcd (%) for C, 68.26; H, 4.22; N, 8.38; found C, 68.31; H, 4.15; N, 8.47.
1-[4-(2,3-Dioxo-2,3-dihydro-indol-1-yl)butyl]-1H-indole-2,3-dione (4c)
Red solid, yield 90%, m.pt. 205–206 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.83 (t, J = 6.0 Hz, 4H, –CH2–); 3.84 (t, J = 6.0 Hz, 4H, –CH2–); 7.03–7.11 (m, 4H, ArH); 7.48 (d, J = 7.5 Hz, 2H, ArH); 7.69 (t, J = 7.5 Hz, 2H, ArH). 13C NMR (125 MHz, DMSO-d6): 24.8, 39.7, 111.5, 117.8, 123.8, 124.2, 138.7, 150.4, 158.8, 182.7. HRMS calculated for C20H16N2O4 348.1110 [M+]; found 348.1126. Anal. calcd (%) for C, 68.96; H, 4.63; N, 8.04; found C, 68.88; H, 4.73; N, 8.12.
1,1′-(pentane-1,5-diyl)diindoline-2,3-dione (4d)
Red solid, yield 82%, m.pt. 198–199 °C. 1H NMR (500 MHz, CDCl3): δ 1.44–1.54 (m, 2H, –CH2–); 1.76–1.84 (m, 4H, –CH2–); 3.67–3.72 (m, 4H, –CH2–); 7.12–7.24 (m, 4H, ArH); 7.53 (d, J = 8.0 Hz, 2H, ArH); 7.65 (d, J = 7.5 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): 24.7, 26.7, 27.1, 39.4, 39.7, 110.8, 118.3, 123.8, 124.2, 138.9, 150.2, 158.5, 182.6. HRMS calculated for C21H18N2O4 362.1267 [M+]; found 362.1275. Anal. calcd (%) for C, 69.60; H, 5.01; N, 7.73; found C, 69.67; H, 5.08; N, 7.69.
1-[6-(2,3-Dioxo-2,3-dihydro-indol-1-yl)hexyl]-1H-indole-2,3-dione (4e)
Red solid, yield 79%, m.pt. 189–190 °C. 1H NMR (500 MHz, CDCl3): δ 1.35–1.38 (m, 4H, –CH2–); 1.56–1.62 (m, 4H, –CH2); 3.64 (t, J = 7.1 Hz, 4H, –CH2); 7.11 (t, J = 7.5 Hz, 2H, ArH); 7.16 (d, J = 7.9 Hz, 2H, ArH); 7.53 (d, J = 7.3, 4H, ArH), 7.64 (t, J = 7.8 Hz, 2H, Ar–H). 13C NMR (125 MHz, CDCl3): 26.3, 27.0, 28.1, 39.7, 111.1, 117.9, 123.5, 124.9, 138.6, 151.1, 158.5, 183.9. HRMS calculated for C22H20N2O4 376.1423 [M+]; found 376.1412. Anal. calcd (%) for C, 70.20; H, 5.36; N, 7.44; found C, 70.28; H, 5.28; N, 7.51.
1-[8-(2,3-Dioxo-2,3-dihydro-indol-1-yl)octyl]-1H-indole-2,3-dione (4f)
Red solid, yield 85%, m.pt. 168–169 °C. 1H NMR (500 MHz, CDCl3): δ 1.37–1.40 (m, 8H, –CH2–); 1.69 (t, J = 7.0 Hz, 4H, –CH2); 3.73 (t, J = 7.0 Hz, 4H, –CH2); 6.91 (d, J = 8.0 Hz, 2H, ArH); 7.13 (t, J = 7.5 Hz, 2H, ArH); 7.59–7.63 (m, 4H, ArH). 13C NMR (125 MHz, CDCl3): 26.7, 27.1, 29.0, 40.2, 110.1, 117.6, 123.6, 124.4, 138.4, 151.0, 158.2, 183.6. HRMS calculated for C24H24N2O4 404.1736 [M+]; found 404.1743. Anal. calcd (%) for C, 71.27; H, 5.98; N, 6.93; found C, 71.19; H, 5.92; N, 7.01.
5-Chloro-1-[2-(2,3-dioxo-2,3-dihydro-indol-1-yl)ethyl]-1H-indole-2,3-dione (4g)
Red solid, yield 89%, m.pt. 275–276 °C. 1H NMR (500 MHz, DMSO-d6): δ 4.05–4.15 (m, 4H, –CH2–); 7.09 (t, J = 7.5 Hz, 1H, ArH); 7.27 (d, J = 8.0 Hz, 1H, ArH); 7.30 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.41 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.51–7.59 (m, 2H, ArH); 7.78 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, DMSO-d6): 37.2, 37.9, 111.5, 112.3, 117.2, 118.1, 118.7, 124.9, 125.0, 128.5, 138.6, 139.1, 148.7, 151.2, 158.4, 159.6, 182.8, 183.5. HRMS calculated for C18H11ClN2O4 354.0407 [M+]; found 354.0413; [M + 2] 356.0378; found 356.0389. Anal. calcd (%) for C, 60.94; H, 3.13; N, 7.90; found C, 61.03; H, 3.20; N, 7.86.
5-Chloro-1-[3-(2,3-dioxo-2,3-dihydro-indol-1-yl)propyl]-1H-indole-2,3-dione (4h)
Red solid, yield 81%, m.pt. 247–248 °C. 1H NMR (500 MHz, DMSO-d6): δ 2.21 (t, J = 6.0 Hz, 2H, –CH2–); 3.63 (t, J = 6.0 Hz, 4H, –CH2–); 6.98 (t, J = 7.5 Hz, 1H, ArH); 7.17 (d, J = 8.0 Hz, 1H, ArH); 7.33 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.46 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.51–7.61 (m, 2H, ArH); 7.82 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, DMSO-d6): 24.9, 36.8, 111.2, 112.3, 116.9, 117.8, 118.2, 124.5, 125.7, 127.2, 136.6, 140.3, 149.1, 150.6, 158.1, 159.7, 182.6, 183.2. HRMS calculated for C19H13ClN2O4 368.0564 [M+]; found 368.0571; [M + 2] 370.0534; found 370.0540. Anal. calcd (%) for C, 61.88; H, 3.55; N, 7.60; found C, 61.79; H, 3.62; N, 7.68.
5-Chloro-1-[4-(2,3-dioxo-2,3-dihydro-indol-1-yl)butyl]-1H-indole-2,3-dione (4i)
Red solid, yield 87%, m.pt. 198–200 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.86 (t, J = 6.0 Hz, 2H, –CH2–); 3.82 (t, J = 6.0 Hz, 4H, –CH2–); 6.95 (t, J = 7.5 Hz, 1H, ArH); 7.20 (d, J = 7.5 Hz, 1H, ArH); 7.32 (dd, J = 3.5, 8.0 Hz, 1H, ArH); 7.48 (dd, J = 3.5, 8.0 Hz, 1H, ArH); 7.56–7.67 (m, 2H, ArH); 7.92 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 24.5, 25.4, 40.0, 40.3, 110.8, 111.6, 116.5, 117.9, 118.6, 124.4, 125.0, 128.3, 136.9, 138.9, 149.1, 151.1, 158.6, 160.4, 182.5, 183.6. HRMS calculated for C20H15ClN2O4 382.0720 [M+]; found 382.0705; [M + 2] 384.0691; found 384.0719. Anal. calcd (%) for C, 62.75; H, 3.95; N, 7.32; found C, 62.81; H, 3.87; N, 7.38.
5-Chloro-1-[5-(2,3-dioxoindolin-1-yl)pentyl]indoline-2,3-dione (4j)
Red solid, yield 78%, m.pt. 195–196 °C. 1H NMR (500 MHz, CDCl3): δ 1.49–1.54 (m, 2H, –CH2–); 1.79–1.87 (m, 4H, –CH2–); 3.76–3.83 (q, J = 7.0 Hz, 4H, –CH2–); 6.93 (d, J = 7.5 Hz, 2H, ArH); 7.24 (dt, J = 0.5 Hz, 7.5 Hz, 1H, ArH); 7.57–7.69 (m, 2H, ArH–); 7.77 (d, J = 7.5 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): 23.4, 26.8, 27.4, 40.3, 40.6, 110.6, 112.6, 116.5, 117.8, 118.9, 123.6, 124.5, 128.7, 138.4, 140.6, 150.8, 151.3, 157.9, 158.2, 182.8, 183.5. HRMS calculated for C21H17ClN2O4 396.0877 [M+]; found 396.0891; [M + 2] 398.0847; found 398.0836. Anal. calcd (%) for C, 63.56; H, 4.32; N, 7.06; found C, 63.61; H, 4.25; N, 7.15.
5-Chloro-1-[6-(2,3-dioxo-2,3-dihydro-indol-1-yl)hexyl]-1H-indole-2,3-dione (4k)
Red solid, yield 80%, m.pt. 183–184 °C. 1H NMR (500 MHz, CDCl3): δ 1.46 (s, 4H, –CH2–); 1.72 (t, J = 6.5 Hz, 4H, –CH2); 3.74 (t, J = 6.5 Hz, 4H, –CH2); 6.86–6.91 (m, 2H, ArH); 7.13 (t, J = 7.5 Hz, 1H, ArH); 7.28–7.33 (m, 2H, ArH); 7.59–7.63 (m, 2H, ArH). 13C NMR (125 MHz, CDCl3): 26.4, 27.0, 27.1, 28.6, 39.9, 40.1, 110.1, 111.2, 116.9, 117.6, 118.6, 124.7, 125.5, 128.2, 138.3, 140.9, 150.8, 151.0, 157.9, 158.2, 182.3, 183.4. HRMS calculated for C22H19ClN2O4 410.1033 [M+]; found 410.1052; [M + 2] 412.1004; found 412.1020. Anal. calcd (%) for C, 64.31; H, 4.66; N, 6.82; found C, 64.25; H, 4.73; N, 6.90.
5-Chloro-1-[8-(2,3-dioxo-2,3-dihydro-indol-1-yl)octyl]-1H-indole-2,3-dione (4l)
Red solid, yield 91%, m.pt. 165–166 °C. 1H NMR (500 MHz, CDCl3): δ 1.32 (s, 8H, –CH2–); 1.69 (t, J = 7.0 Hz, 4H, –CH2); 3.81 (t, J = 7.0 Hz, 4H, –CH2); 6.92 (t, J = 7.5 Hz, 1H, ArH); 7.21 (d, J = 8.0 Hz, 1H, ArH); 7.35 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.38 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.54–7.61 (m, 2H, ArH); 7.83 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 25.9, 26.7, 27.1, 27.8, 29.0, 29.3, 40.0, 40.2, 110.1, 111.6, 116.8, 117.6, 118.2, 123.9, 125.3, 127.8, 138.4, 139.7, 151.0, 151.5, 158.2, 159.0, 182.8, 183.3. HRMS calculated for C24H23ClN2O4 438.1346 [M+]; found 438.1358; [M + 2] 440.1317; found 440.1324. Anal. calcd (%) for C, 65.68; H, 5.28; N, 6.38; found C, 65.59; H, 5.35; N, 6.46.
1-[2-(2,3-Dioxo-2,3-dihydro-indol-1-yl)ethyl]-5-fluoro-1H-indole-2,3-dione (4m)
Red solid, yield 81%, m.pt. 270–271 °C. 1H NMR (500 MHz, DMSO-d6): δ 3.97–4.02 (m, 4H, –CH2–); 7.13 (t, J = 7.5 Hz, 1H, ArH); 7.21 (d, J = 8.0 Hz, 1H, ArH); 7.28 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.46 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.53–7.57 (m, 2H, ArH); 7.66 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 37.4, 37.6, 111.0, 112.1, 112.5, 117.8, 118.7, 123.8, 124.7, 125.0, 138.3, 138.7, 146.8, 150.6, 158.9, 182.7, 183.3. HRMS calculated for C18H11FN2O4 338.0703 [M+]; found 338.0718. Anal. calcd (%) for C, 63.91; H, 3.28; N, 8.28; found C, 63.85; H, 3.34; N, 8.37.
1-[3-(2,3-Dioxo-2,3-dihydro-indol-1-yl)propyl]-5-fluoro-1H-indole-2,3-dione (4n)
Red solid, yield 90%, m.pt. 239–240 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.95–2.01 (m, 2H, –CH2–); 3.79 (t, J = 7.3 Hz, 4H, –CH2–); 7.12 (t, J = 7.5 Hz, 1H, ArH); 7.22 (d, J = 8.0 Hz, 1H, ArH); 7.26 (dd, J = 3.6, 8.7 Hz, 1H, ArH); 7.44 (dd, J = 3.0 Hz, 7.0 Hz, 1H, ArH); 7.48–7.54 (m, 2H, ArH); 7.63 (t, J = 7.4 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 25.0, 37.9, 38.0, 111.1, 111.7, 111.9, 123.5, 124.2, 124.8, 138.4, 147.1, 150.9, 158.7, 183.1, 183.7. HRMS calculated for C19H13FN2O4 352.0859 [M+]; found 352.0874. Anal. calcd (%) for C, 64.77; H, 3.72; N, 7.95; found C, 64.69; H, 3.80; N, 7.87.
1-[4-(2,3-Dioxo-2,3-dihydro-indol-1-yl)butyl]-5-fluoro-1H-indole-2,3-dione (4o)
Red solid, yield 87%, m.pt. 208–209 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.89 (t, J = 6.0 Hz, 4H, –CH2–); 3.87 (t, J = 6.0 Hz, 4H, –CH2–); 7.05 (t, J = 7.5 Hz, 1H, ArH); 7.29 (d, J = 7.5 Hz, 1H, ArH); 7.37 (dd, J = 3.5, 8.0 Hz, 1H, ArH); 7.42 (dd, J = 3.5, 8.0 Hz, 1H, ArH); 7.49–7.58 (m, 2H, ArH); 7.82 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 24.2, 24.4, 39.3, 39.6, 110.1, 111.9, 116.8, 117.5, 118.6, 124.0, 125.6, 128.3, 138.6, 140.8, 149.4, 150.4, 157.7, 158.5, 182.0, 183.3. HRMS calculated for C20H15FN2O4 366.1016 [M+]; found 366.1022. Anal. calcd (%) for C, 65.57; H, 4.13; N, 7.65; found C, 65.65; H, 4.06; N, 7.73.
1-[5-(2,3-Dioxo-2,3-dihydro-indol-1-yl)pentyl]-5-fluoro-1H-indole-2,3-dione (4p)
Red solid, yield 80%, m.pt. 172–174 °C. 1H NMR (500 MHz, CDCl3): δ 1.45–1.51 (m, 2H, –CH2–); 1.77–1.84 (m, 4H, –CH2–); 3.74 (q, J = 7.0 Hz, 4H, –CH2–); 6.94 (d, J = 7.5 Hz, 2H, ArH); 7.16 (dt, J = 0.5 Hz, 7.5 Hz, 1H, ArH); 7.32–7.35 (m, 2H, ArH); 7.62 (d, J = 7.5 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): 23.8, 26.4, 26.5, 39.6, 39.9, 110.8, 111.3, 116.3, 117.2, 118.9, 123.8, 124.7, 127.9, 138.4, 139.7, 149.6, 150.8, 158.0, 158.3, 182.8, 183.1. HRMS calculated for C21H17FN2O4 380.1172 [M+]; found 380.1189. Anal. calcd (%) for C, 66.31; H, 4.50; N, 7.36; found C, 66.38; H, 5.56; N, 7.28.
1-[6-(2,3-Dioxo-2,3-dihydro-indol-1-yl)hexyl]-5-fluoro-1H-indole-2,3-dione (4q)
Red solid, yield 88%, m.pt. 186–187 °C. 1H NMR (500 MHz, CDCl3): δ 1.42 (s, 4H, –CH2–); 1.80 (t, J = 6.0 Hz, 4H, –CH2); 3.71 (t, J = 6.0 Hz, 4H, –CH2); 6.89–6.97 (m, 2H, ArH); 7.17 (t, J = 7.5 Hz, 1H, ArH); 7.31–7.38 (m, 2H, ArH); 7.54–7.60 (m, 2H, ArH). 13C NMR (125 MHz, CDCl3): 25.9, 26.8, 27.1, 29.5, 40.2, 40.9, 110.4, 112.1, 117.3, 118.3, 118.7, 124.5, 125.6, 129.3, 138.1, 140.4, 149.2, 150.4, 158.5, 160.1, 182.6, 183.1. HRMS calculated for C22H19FN2O4 394.1329 [M+]; found 394.1337. Anal. calcd (%) for C, 67.00; H, 4.86; N, 7.10; found C, 67.07; H, 4.78; N, 7.18.
1-[8-(2,3-Dioxo-2,3-dihydro-indol-1-yl)octyl]-5-fluoro-1H-indole-2,3-dione (4r)
Red solid, yield 85%, m.pt. 169–170 °C. 1H NMR (500 MHz, CDCl3): δ 1.35 (s, 8H, –CH2–); 1.73 (t, J = 7.0 Hz, 4H, –CH2); 3.65 (t, J = 7.0 Hz, 4H, –CH2); 6.81 (t, J = 7.5 Hz, 1H, ArH); 7.08 (d, J = 8.0 Hz, 1H, ArH); 7.38 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.42 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.50–7.61 (m, 2H, ArH); 7.78 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 24.7, 25.9, 27.8, 28.0, 29.1, 30.2, 39.8, 40.8, 110.4, 111.3, 117.2, 117.8, 118.6, 123.8, 125.5, 139.7, 140.4, 150.6, 151.8, 159.6, 160.2, 182.8, 183.1. HRMS calculated for C24H23FN2O4 422.1642 [M+]; found 422.1658. Anal. calcd (%) for C, 68.23; H, 5.49; N, 6.63; found C, 68.31; H, 5.42; N, 6.71.
5-Bromo-1-[2-(2,3-dioxo-2,3-dihydro-indol-1-yl)ethyl]-1H-indole-2,3-dione (4s)
Red solid, yield 91%, m.pt. 268–269 °C. 1H NMR (500 MHz, DMSO-d6): δ 3.97–4.09 (s, 4H, –CH2–); 7.18 (t, J = 7.5 Hz, 1H, ArH); 7.23 (d, J = 8.0 Hz, 1H, ArH); 7.36 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.49 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.55–7.63 (m, 2H, ArH); 7.72 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 36.9, 37.4, 111.9, 112.5, 117.9, 118.5, 118.9, 124.2, 125.8, 128.1, 138.2, 138.6, 149.2, 150.2, 158.4, 159.8, 182.9, 183.1. HRMS calculated for C18H11BrN2O4 397.9902 [M+]; found 397.9910. Anal. calcd (%) for C, 54.16; H, 2.78; N, 7.02; found C, 54.22; H, 2.69; N, 7.11.
5-Bromo-1-[3-(2,3-dioxo-2,3-dihydro-indol-1-yl)propyl]-1H-indole-2,3-dione (4t)
Red solid, yield 92%, m.pt. 249–250 °C. 1H NMR (500 MHz, DMSO-d6): δ 2.12 (t, J = 6.0 Hz, 2H, –CH2–); 3.74 (t, J = 6.0 Hz, 4H, –CH2–); 7.13 (t, J = 7.5 Hz, 1H, ArH); 7.24 (d, J = 8.0 Hz, 1H, ArH); 7.39 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.43 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.56–7.64 (m, 2H, ArH); 7.89 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 25.7, 37.9, 111.3, 112.6, 117.1, 118.6, 119.2, 123.8, 124.9, 127.6, 138.1, 140.8, 149.6, 151.4, 158.7, 159.1, 182.7, 183.1. HRMS calculated for C19H13BrN2O4 412.0059 [M+]; found 412.0075. Anal. calcd (%) for C, 55.23; H, 3.17; N, 6.78; found C, 55.16; H, 3.23; N, 6.73.
5-Bromo-1-[4-(2,3-dioxo-2,3-dihydro-indol-1-yl)butyl]-1H-indole-2,3-dione (4u)
Red solid, yield 87%, m.pt. 195–196 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.63–1.70 (m, 4H, –CH2–); 3.69 (t, J = 5.8 Hz, 4H, –CH2–); 7.12 (t, J = 7.4 Hz, 1H, ArH); 7.18–7.20 (m, 2H, ArH); 7.53 (d, J = 7.3, 1H, ArH); 7.62–7.67 (m, 2H, ArH); 7.78–7.80 (m, 1H, ArH). 13C NMR (125 MHz, CDCl3): 24.4, 24.5, 112.2, 113.3, 115.2, 117.9, 119.7, 123.5, 124.8, 127.0, 138.6, 140.1, 149.9, 151.0, 158.2, 158.6, 182.6, 183.8. HRMS calculated for C20H15BrN2O4 426.0215 [M+]; found 426.0228. Anal. calcd (%) for C, 56.22; H, 3.54; N, 6.56; found C, 56.29; H, 3.48; N, 6.66.
5-Bromo-1-[5-(2,3-dioxo-2,3-dihydro-indol-1-yl)pentyl]-1H-indole-2,3-dione (4v)
Red solid, yield 85%, m.pt. 189–190 °C. 1H NMR (500 MHz, CDCl3): δ 1.45–1.51 (m, 2H, –CH2–); 1.77–1.84 (m, 4H, –CH2–); 3.72–3.76 (q, J = 7.0 Hz, 4H, –CH2–); 6.94 (d, J = 7.5 Hz, 2H, ArH); 7.13 (dt, J = 0.5, 7.5 Hz, 1H, ArH); 7.32–7.34 (m, 2H, ArH–); 7.63 (d, J = 7.5 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): 23.8, 26.4, 26.5, 39.6, 39.9, 110.1, 111.4, 112.7, 117.5, 118.1, 123.8, 124.7, 124.9, 125.5, 138.4, 146.8, 150.7, 158.0, 158.3, 160.3, 182.8, 183.4. HRMS calculated for C21H17BrN2O4 377.1501 [M+]; found 377.1514. Anal. calcd (%) for C, 70.01; H, 5.61; N, 7.42; found C, 70.10; H, 5.53; N, 7.51.
5-Bromo-1-[6-(2,3-dioxo-2,3-dihydro-indol-1-yl)hexyl]-1H-indole-2,3-dione (4w)
Red solid, yield 86%, m.pt. 188–189 °C. 1H NMR (500 MHz, CDCl3): δ 1.35 (s, 4H, –CH2–); 1.77 (t, J = 6.0 Hz, 4H, –CH2); 3.74 (t, J = 6.0 Hz, 4H, –CH2–); 6.92 (t, J = 7.5 Hz, 1H, ArH); 7.13 (d, J = 7.5 Hz, 1H, ArH); 7.29 (dd, J = 3.0, 8.0 Hz, 1H, ArH); 7.47 (dd, J = 3.0, 8.0 Hz, 1H, ArH); 7.51–7.64 (m, 2H, ArH); 7.84 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 25.5, 26.3, 27.1, 28.4, 39.8, 40.4, 110.2, 111.7, 116.4, 117.3, 118.8, 123.9, 125.2, 128.8, 137.7, 139.7, 150.2, 151.6, 158.1, 159.9, 182.4, 183.3. HRMS calculated for C22H19BrN2O4 454.0528 [M+]; found 454.0537. Anal. calcd (%) for C, 58.04; H, 4.21; N, 6.15; found C, 58.11; H, 4.28; N, 6.05.
5-Bromo-1-[8-(2,3-dioxo-2,3-dihydro-indol-1-yl)octyl]-1H-indole-2,3-dione (4x)
Red solid, yield 89%, m.pt. 162–164 °C. 1H NMR (500 MHz, CDCl3): δ 1.28 (s, 8H, –CH2–); 1.63 (t, J = 7.0 Hz, 4H, –CH2); 3.72 (t, J = 7.0 Hz, 4H, –CH2); 6.98 (t, J = 7.5 Hz, 1H, ArH); 7.17 (d, J = 8.0 Hz, 1H, ArH); 7.31 (dd, J = 3.5, 8.5 Hz, 1H, ArH); 7.43 (dd, J = 3.0, 7.0 Hz, 1H, ArH); 7.55–7.64 (m, 2H, ArH); 7.89 (t, J = 8.0 Hz, 1H, ArH). 13C NMR (125 MHz, CDCl3): 25.2, 26.0, 27.3, 27.5, 29.2, 29.7, 40.1, 40.6, 110.9, 111.1, 117.3, 118.0, 118.6, 124.0, 125.1, 128.4, 138.8, 140.1, 150.9, 151.4, 158.9, 159.6, 182.5, 182.9. HRMS calculated for C24H23BrN2O4 482.0841 [M+]; found 482.0858. Anal. calcd (%) for C, 59.64; H, 4.80; N, 5.80; found C, 59.57; H, 4.88; N, 5.72.
Materials and methods
In vitro protozoal parasite susceptibility assay
Protozoal parasites were cultured for 24 h at 37 °C. To perform the initial susceptibility screens on T. vaginalis, compounds were suspended in DMSO to obtain concentrations of 100 μM; 5 mL aliquots of these suspensions were diluted in 5 mL of TYM diamond's media to obtain a final concentration of 100 mM. After 24 h, cells were counted using a hemacytometer. Cell counts were normalized to the DMSO controls, in order to allow direct comparison and averaging of the various trials. These data sets were then transformed using GraphPad Prism software, by taking the log of the drug concentrations for the trials and inputting this transform into a log (inhibitor) versus response-variable slope regression option. Within this non-linear regression, constraints were set to force the maximum value (top) to 1 and the minimum value (bottom) to 0. The slope was left variable, and then determined through which regression was performed. The sample size consists of 4 independent trials carried out on four different days (to account for possible variation in the parasite population). The assays were performed in 15 mL culture tubes, with both wild type parasites and 0.1% DMSO-only treated parasites serving as control tubes to normalize for the effects of the solvent and in vitro conditions. After 24 h, cells were counted using a hemacytometer. The IC50 values were determined by titration assays of increasing compound concentrations, 5–40 μM, and performing a regression analysis using Prism software from GraphPad. The calculated IC50 values of the compounds were then re-confirmed by assaying again using the same assay described above.
In vitro activity against E. histolytica and G. lamblia
E. histolytica (strain HM1:IMSS) and G. lamblia (WB strain) trophozoites were axenically maintained in TYI-S-33 medium supplemented with penicillin (100 mg mL–1) and streptomycin (100 mg mL–1).36 For primary screening, 0.5 μL of each 10 μM stock compound was transferred in duplicate to a 96-well microtiter plate to achieve a final concentration of 50 μM. 0.5% DMSO served as a vehicle control and 30 μM metronidazole as a positive control. For both E. histolytica and G. lamblia, 5000 trophozoites in 99.5 μL of TYI-S-33 medium were transferred to each well. Plates were incubated at 37 °C for 48 h in a GasPak EZ anaerobe gas-generating pouch systems (VWR) to maintain an anaerobic environment. After 48 h of incubation, the activity of the compounds was measured using a luminometer (EnVision multilabel plate reader, PerkinElmer) using an ATP bioluminescence-based CellTiter-Glo luminescent cell viability assay (Promega) technology.37 Compounds showing more than 50% inhibition in primary screening at 50 μM were identified as hits. Hits obtained in the primary screen underwent secondary screening to reconfirm the hits and also to determine EC50. For the secondary screening of hits, compounds were serially diluted in triplicate to test at 8-point concentrations ranging from 0.39 μM to 50 μM. The EC50 of the hits was determined after generating dose response curves using GraphPad Prism software 5.0.
In vitro activity against N. fowleri
Trophozoites of N. fowleri strain KUL were axenically cultured in Nelson's medium supplemented with 10% FBS at 37 °C.38 The primary and secondary screenings of compounds were performed following a similar protocol to that for E. histolytica and G. lamblia but with a few changes. N. fowleri screenings were carried out with 10 000 trophozoites in an oxygenated environment and in the absence of the GasPak EZ anaerobe gas-generating pouch system and 50 μM amphotericin B was used as a positive control.39 Both miltefosine and amphotericin B, which are current drugs for the treatment of PAM, were also used in triplicate for EC50 determination. The EC50 of the hits was determined after plotting the data in GraphPad Prism software 5.0.
In vitro anti-fungal evaluation
Microorganisms
Aspergillus parasiticus NRRL5862 (National Center for Agricultural Utilization and Research, USDA-ARS, Peoria, IL, USA), a producer of hepato-carcinogenic aflatoxins, was cultured at 35 °C on potato dextrose agar (PDA). The model yeast Saccharomyces cerevisiae BY4741 wild type (Mat a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) was procured from Open Biosystems (Huntsville, AL, USA).40 Yeast strains were cultured on Synthetic Glucose (SG; yeast nitrogen base without amino acids 0.67%, glucose 2% with appropriate supplements: 0.02 mg mL–1 uracil, 0.03 mg mL–1 amino acids) or Yeast Peptone Dextrose (YPD; Bacto yeast extract 1%, Bacto peptone 2%, glucose 2%) medium at 30 °C. All chemicals for culturing fungi were procured from Sigma Co. (St. Louis, MO, USA).
Chemicals
Test compounds and octyl gallate (OG; positive control for antifungal activity; Sigma Co., St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO; Sigma Co., St. Louis, MO, USA; absolute DMSO amount: <2% in media) before incorporation into culture media. Throughout this study, control wells (no treatment) in microtiter plates contained DMSO at levels equivalent to those of cohorts receiving antifungal agents, within the same set of experiments.
Antifungal bioassay: microtiter plate liquid bioassay
To determine the antifungal activity of the test compounds in filamentous fungi, bioassays (triplicate wells; 100 μL per well) (3 × 104 to 5 × 104 CFU per mL) were performed in microtiter plates at 35 °C (RPMI 1640 medium; Sigma Co., St. Louis, MO, USA). Compounds were examined at 500 μM, where fungal growth was monitored at 24 to 48 h after inoculation. A numerical score from 0 to 4 was provided to each well as follows: 0 = optically clear/no visible growth, 1 = slight growth (25% of no treatment control), 2 = prominent growth reduction (50% of no treatment control), 3 = slight growth reduction (75% of no treatment control), and 4 = no growth reduction [according to the Clinical and Laboratory Standards Institute (CLSI) M38-A protocol].41
To test the antifungal activity of the test compounds in S. cerevisiae strains, bioassays were performed [500 μM in SG liquid; triplicate wells (100 μL per well) (1 × 105 CFU per mL) in microtiter plates] using the modified protocols outlined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).42 Antifungal activity was assessed 24 to 48 h after inoculation. A numerical score from 0 to 4 was provided to each well as described above. Fungal survival was determined following completion of liquid bioassays in microtiter plates by spreading an entire volume of microtiter wells onto individual YPD (recovery) plates and culturing for additional 48 to 72 h (30 °C).
Cytoxicity assay for normal flora microbiota
Cultures of non-pathogenic strains such as Lactobacillus reuteri (ATCC 23272), Lactobacillus acidophilus (ATCC 43560), and Lactobacillus rhamnosus (ATCC 53103) were grown in Lactobacilli MRS at 37 °C under anaerobic conditions. Non-pathogenic Escherichia coli K12 MG1655 was grown in Luria broth at 37 °C under aerobic conditions. 100 μM stock solutions of the compounds and vehicle control DMSO were diluted to 100 μM in media to generate working dilutions. Empty BDL-sensi-discs (6 mm) were incubated in the working dilutions for 20 min at room temperature. Bacterially streaked agar plates were incubated with discs containing the vehicle control, compounds, or various antibiotic discs [levofloxacin (5 μg), gentamicin (10 μg), and gentamicin (120 μg)] and incubated overnight at 37 °C. Sensitivity to the vehicle control, compounds, and antibiotics was determined via measurement of zones of inhibition around each disc in mm. All organisms were purchased from the American Type Culture Collection (ATCC).
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
V. K. acknowledges the Council of Scientific and Industrial Research (CSIR) New Delhi (grant no. 02(0293)/17/EMR-I) for providing financial assistance. C. T. and L. W. C. were funded by the U. S. Department of Agriculture, Agricultural Research Service (National Program 108, Project CRIS 5325-42000-049-00D). K. M. L. was supported by the Department of Biological Sciences at the University of the Pacific. A. D. was supported by the National Institutes of Health (grant no. 1KL2TR001444).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00434f
References
- World Health Organization Global incidence and prevalence of selected curable sexually transmitted infections–2008, WHO, Geneva, Switzerland, 2012.
- World Health Organization Prevalence and incidence of selected sexually transmitted infections: Chlamydia trachomatis, Neisseria gonorrhoea, syphilis and Trichomonas vaginalis: Methods and results used by WHO to generate 2005 estimates, WHO, Geneva, Switzerland, 2011.
- Soper D. Am. J. Obstet. Gynecol. 2004;190:281. doi: 10.1016/j.ajog.2003.08.023. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Practice Bulletins-Gynaecology Obstet. Gynecol. 2006;107:1195. [Google Scholar]
- Krieger J. N. Sex. Transm. Dis. 1995;22:83. [PubMed] [Google Scholar]
- Madico G., Quinn T. C., Rompalo A. J. Clin. Microbiol. 1998;36:3205. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova R. N. J. Reprod. Immunol. 2009;83:185. doi: 10.1016/j.jri.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley P., Wilkinson D., Connolly C., Moodley J., Sturm A. W. Clin. Infect. Dis. 2002;34:519. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- Zhang Z. F., Graham S., Yu S. Z., Marshall J., Zielezny M., Chen Y. X., Sun M. Ann. Epidemiol. 1995;5:325. doi: 10.1016/1047-2797(94)00101-x. [DOI] [PubMed] [Google Scholar]
- Guenthner P. C., Secor W. E., Dezzutti C. S. Infect. Immunol. 2005;73:4155. doi: 10.1128/IAI.73.7.4155-4160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Liao Y., Gomez A. M., Gaydos C. A., Mellow D. D. J. Infect. Dis. 2008;197:503. doi: 10.1086/526497. [DOI] [PubMed] [Google Scholar]
- McClelland R. S., Sangare L., Hassan W. M. J. Infect. Dis. 2007;195:698. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- Laga M., Manoka A., Kivuvu M., Malele B., Tuliza M., Nzila N., Goeman J., Behets F., Batter V., Alary M. AIDS. 1993;7:95. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- Moodley P., Wilkinson D., Connolly C., Moodley J., Sturm A. W. Clin. Infect. Dis. 2002;34:519. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- (a) Upcroft P., Upcroft J. A. Clin. Microbiol. Rev. 2001;14:150. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Walsh J. A. Rev. Infect. Dis. 1986;8:228. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- Lal A., Baker M. G., Hales S., French N. P. Trends Parasitol. 2013;29:83. doi: 10.1016/j.pt.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Huang D. B., White A. C. Clin. North Am. 2006;35:291. doi: 10.1016/j.gtc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Leggett H. C., Cornwallis C. K., West S. A. PLoS Pathog. 2012;8:1002512. doi: 10.1371/journal.ppat.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrad A. M., Debnath A., Miyamoto Y., Hansford K. A., Pelingon R., Butler M. S., Bains T., Karoli T., Blaskovich M. A. T., Eckmann L., Cooper M. A. Eur. J. Med. Chem. 2016;120:353. doi: 10.1016/j.ejmech.2016.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft J. A., Campbell R. W., Banekli K., Upcroft P., Vanelle P. Antimicrob. Agents Chemother. 1999;43:73. doi: 10.1128/aac.43.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Kim D. W., Park J. M., Yoon B. W., Baek M. J., Kim J. E., Kim S. J. Neurol. Sci. 2004;224:107. doi: 10.1016/j.jns.2004.06.012. [DOI] [PubMed] [Google Scholar]; (b) el-Nahas A. F., el-Ashmawy I. M. Basic Clin. Pharmacol. Toxicol. 2004;94:226. doi: 10.1111/j.1742-7843.2004.pto940505.x. [DOI] [PubMed] [Google Scholar]; (c) Wright J. M., Webb R. I., O'donoghue P., Upcroft P., Upcroft J. A. J. Eukaryotic Microbiol. 2010;57:171. doi: 10.1111/j.1550-7408.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- Kidwai M., Jahan A., Mishra N. K. Med. Chem. 2014;4:451. [Google Scholar]
- Roth G. J., Heckel A., Colbatzky F., Handschuh S., Kley J., Lehmann-Lintz T., Lotz R., Tontsch-Grunt U., Walter R., Hilberg F. J. Med. Chem. 2009;52:4466. doi: 10.1021/jm900431g. [DOI] [PubMed] [Google Scholar]
- Jr R. R. Biochem. Biophys. Res. Commun. 2007;356:323. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Michaelson M. D., Redman B. G., Hudes G. R., Wilding G., Figlin R. A., Ginsberg M. S., Kim S. T., Baum C. M., DePrimo S. E., Li J. Z., Bello C. L., Theuer C. P., George D. J., Rini B. I. J. Clin. Oncol. 2006;24:16. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- Prenen H., Cools J., Mentens N., Folens C., Sciot R., Schoffski P., Van Oosterom A., Marynen P., Debiec-Rychter M. Clin. Cancer Res. 2006;12:2622. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. S., Abou-seri S. M., Ismail N. S. M., Elaasser M. M., Aly M. H., Abdel-Aziz H. A. Eur. J. Med. Chem. 2016;108:415. doi: 10.1016/j.ejmech.2015.11.047. [DOI] [PubMed] [Google Scholar]
- Jautelat R., Brumby T., Schafer M., Briem H., Eisenbrand G., Schwahn S., Kruger M., Lucking U., Prien O., Siemeister G. ChemBioChem. 2005;6:531. doi: 10.1002/cbic.200400108. [DOI] [PubMed] [Google Scholar]
- Polychronopoulos P., Magiatis P., Skaltsounis A. L., Myrianthopoulos V., Mikros E., Tarricone A., Musacchio A., Roe S. M., Pearl L., Leost M. J. Med. Chem. 2004;47:935. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- Raj R., Singh P., Haberkern N. T., Faucher R. M., Patel N., Land K. M., Kumar V. Eur. J. Med. Chem. 2013;63:897. doi: 10.1016/j.ejmech.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Nisha, Mehra V., Hopper M., Patel N., Hall D., Wrischnik L. A., Land K. M., Kumar V. MedChemComm. 2013;4:1018. [Google Scholar]
- Nisha, Kumar K., Bhargava G., Land K. M., Chang K.-H., Arora R., Sen S., Kumar V. Eur. J. Med. Chem. 2014;74:657. doi: 10.1016/j.ejmech.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisha, Tran R., Yang D., Hall D., Hopper M. J., Wrischnik L. A., Land K. M., Kumar V. Med. Chem. Res. 2014;23:4570. doi: 10.1007/s00044-014-0956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Kumar K., Liu N., Yang D., Na D., Thompson J., Wrischnik L. A., Land K. M., Kumar V. Bioorg. Med. Chem. 2015;23:5190. doi: 10.1016/j.bmc.2015.04.075. [DOI] [PubMed] [Google Scholar]; (b) Singh A., Gut J., Rosenthal P. J., Kumar V. Eur. J. Med. Chem. 2017;125:269. doi: 10.1016/j.ejmech.2016.09.044. [DOI] [PubMed] [Google Scholar]; (c) Singh A., Rani A., Gut J., Rosenthal P. J., Kumar V. Chem. Biol. Drug Des. 2017 doi: 10.1111/cbdd.12982. [DOI] [PubMed] [Google Scholar]; (d) Kumar S., Saini A., Gut J., Rosenthal P. J., Raj R., Kumar V. Eur. J. Med. Chem. 2017;138:993. doi: 10.1016/j.ejmech.2017.07.041. [DOI] [PubMed] [Google Scholar]; (e) Raj R., Biot C., Kremer S. C., Kremer L., Guerardel Y., Gut J., Rosenthal P. J., Forge D., Kumar V. Chem. Biol. Drug Des. 2014;83:622. doi: 10.1111/cbdd.12273. [DOI] [PubMed] [Google Scholar]
- Singh P., Kaur S., Kumar V., Bedi P. M. S., Mahajan M. P., Sehar I., Pal H. C., Saxena A. K. Bioorg. Med. Chem. Lett. 2011;21:3017. doi: 10.1016/j.bmcl.2011.03.043. [DOI] [PubMed] [Google Scholar]
- (a) Diamond L. S., Harlow D. R., Cunnick C. C. Trans. R. Soc. Trop. Med. Hyg. 1978;72:431. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]; (b) Keister D. B. Trans. R. Soc. Trop. Med. Hyg. 1983;77:487. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- (a) Debnath A., Shahinas D., Bryant C., Hirata K., Miyamoto Y., Hwang G., Gut J., Renslo A. R., Pillai D. R., Eckmann L., Reed S. L., McKerrow J. H. Antimicrob. Agents Chemother. 2014;58:4138. doi: 10.1128/AAC.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Debnath A., Parsonage D., Andrade R. M., He C., Cobo E. R., Hirata K., Chen S., García-Rivera G., Orozco E., Martínez M. B., Gunatilleke S. S., Barrios A. M., Arkin M. R., Poole L. B., McKerrow J. H., Reed S. L. Nat. Med. 2012;18:956. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kim J. H., Sohn H. J., Yang H. J., Na B. K., Chwae Y. J., Park S., Kim K., Shin H. J. Parasitol. Res. 2014;113:2765. doi: 10.1007/s00436-014-3936-3. [DOI] [PubMed] [Google Scholar]
- Debnath A., Tunac J. B., Galindo-Gomez S., Silva-Olivares A., Shibayama M., McKerrow J. H. Antimicrob. Agents Chemother. 2012;56:5450. doi: 10.1128/AAC.00643-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccharomyces Genome Database, Available online: http://www.yeastgenome.org (accessed on 29 December 2015).
- Clinical and Laboratory Standards Institute (CLSI) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: Approved standard–Second edition. CLSI document M38-A2. Clinical and Laboratory Standards Institute: Wayne, PA, 2008, 28.
- Arendrup M. C., Cuenca-Estrella M., Lass-Florl C., Hope W. Clin. Microbiol. Infect. 2012;18:246. doi: 10.1111/1469-0691.12148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.