Abstract
Background: Artesunate has recently been used in some pharmacological preparation to induce tumor cell apoptosis. The drug is a semi-synthetic derivative of artemisinin, traditionally used for its antimalarial. However, up to now, its anticancer mechanism against different types of tumors is not known.
Objectives: The most important purposes of the present research was firstly investigating induction of apoptosis on human breast cancer MCF-7 cells by the drug and, in the second place, introducing its possible mechanism of action.
Materials and Methods: The MTT assay was used to investigate the inhibitory effect of artesunate on growth of breast cancer MCF-7 cells. For this aim, different concentrations of artesunate were used to treat the cells and flow cytometry assay was done followed by annexin V-FITC/PI staining. The activities of caspase-3, -8 and -9 were then determined by relative assay kits.
Results: Based on the results from MTT assay, it was found that artesunate could significantly inhibit the growth of MCF-7 cells in a dose- and time-dependent manner. On the other hand, the flow cytometry findings showed that the anti-proliferative activity of artesunate on MCF-7 cells is due to apoptosis. Besides, caspase colorimetric assays revealed a significant rise in cellular levels of the initiators (caspase-8 and -9) and effector (caspase-3) in the cells treated by artesunate.
Conclusions: According to our results, it could be concluded that artesunate could inhibit the growth of MCF-7 breast cancer cells through induction of apoptosis by intrinsic and extrinsic caspase-dependent pathways. Therefore, we claim that artesunate could be introduced as a suitable candidate for the treatment of the breast cancer.
Keywords: Apoptosis, Artesunate, Breast cancer, Caspases, MCF-7 Cells
1. Background
Cancer is one of the most leading causes of human death, worldwide, and breast cancer is the most common type of malignancy in women (1). In Iran, breast cancer ranks the first among cancers diagnosed in females with an increasing trend in the breast cancer related mortality rate in the recent decade (2). The growth and survival of cancer cells is correlated with a reduced level of genetically programmed cell death; the apoptosis (3). Therefore, cytotoxic agents which induce apoptosis in the cancer cells are used as anticancer chemotherapeutic agents (4). Therefore, all of the conventional therapies including chemotherapy, radiation, and hormonal treatments rely heavily on apoptosis for destroying breast cancer cells (5, 6).
Apoptosis is characterized by both morphological and biochemical changes. The most important biochemical event during an early apoptosis is translocation of the phosphatidylserine from the inner to the outer cell membrane. In addition, there are various biochemical pathways during apoptosis which finally converge and result in the activation of a family of cysteine proteases called caspases (cysteine aspartate-specific proteases) (7). Although, apoptosis can be mediated through caspase-dependent or caspase-independent pathways, the latter is the common mechanism, since most cells undergo apoptosis through the involvement of caspases.
Therefore, caspase pathways are considered as suitable candidate targets for anticancer drug discovery. It has been reported that some commonly used plant-derived anticancer drugs such as tamoxifen, doxorubicin, etoposide and cisplatin promote apoptosis in tumor cells through caspase pathways (8, 9). Despite significant pharmacological advances in treating breast cancer, it still remains an incurable disease for most patients, therefore, the quest to discover new treatments must continue (10). In addition, there are several undesirable side effects associated with many of the present breast cancer chemotherapeutic agents (11). One major challenge to overcome this problem is to develop the potent compounds with specificity on the tumor cells but without the harmful side effects on normal mammalian cells (12).
Natural products, especially plants’ products, have been used for treating cancers for over 3,500 years. As an important natural source of new anticancer drugs with the minimal side effects (13-15), several of such natural products still play an important role in the modern medicine. Plant secondary metabolites have proven to be excellent resources of new medications for cancer treatment (16), as evidenced by effective plant-derived anticancer agents including taxol and etoposide (17, 18). Sesquiterpene lactones (SLs), a subfamily of terpenoids, are a group of plants’ secondary metabolites which often are used in the traditional medicine for cancer treatment. These compounds are among the promising candidates in cancer drug discovery (19).
Artemisinin is a sesquiterpene lactone isolated in 1972 from the Chinese medicinal herb, Artemisia annua L., used for treating fever and malaria for over two millennia (20, 21). Although, artemisinin is one of the very few drugs used as antimalarials with minimal side effects (22), it has pharmacokinetic limitations such as low solubility, a short half-life, as well as poor bioavilability (23). Thus, in efforts to overcome some of these problems, several semi-synthetic derivatives have designed and developed (12). Besides their antimalarial activity, artemisinin and its derivatives have also shown to have significant antitumor activity (24, 25), antimicrobial (26), antioxidant (27), and anti-inflammatory activity (28, 29). Research on the anticancer properties of artemisisnin and its derivatives were started by the studies of Lai and Singh in 2004 when for the first time, they showed that artemisinin induces apoptosis in human cancer cells and inhibits tumor growth (30). In the recent years, there are many reports regarding the potent and broad anticancer activities of the artemisinin and its licensed semisynthetic derivatives in cell lines (30-32), animal models (33), and in clinical trials (19, 34) against various types of tumors including breast cancer. Considerable research has been focused on the most active water soluble semi-synthetic derivative, artesunate. Analysing 55 human cancer cell lines, the Developmental Therapeutic Program of the NCI (National Cancer Institute) has shown that artesunate displays anticancer activity against different cancerous cell lines (35). In addition, it has been proven that artesunate exerts its anticancer activity through induction of apoptosis in the breast cancer cells (36). On the other hand, because of its wide application as an anti-malarial drug with few side effects and excellent clinical safety (37), there is growing evidence supporting the use of artesunate in cancer therapy (30-35, 38).
Although, it is currently in phase I–II clinical trials against breast, colorectal and non-small-cell lung cancers, the real potential and benefits of the artesunate for cancer treatment remains yet to be elucidated and the rationale for its use in anticancer therapy must be evaluated following to a deeper understanding of the underlying mechanisms involved in its cytotoxic effects (12). However, despite the profound cytotoxic action of the artesunate against a wide spectrum of tumor cells, its anticancer signaling mechanism is still unclear (39). Furthermore, the mechanisms by which artesunate and other artemisinin derivatives induce apoptosis, as well as the role of caspases to such effect, are not well understood. Therefore, further investigation is required in this field. Due to the ability of artesunate to employ a variety of as yet undefined mechanisms for inducing apoptosis in the breast cancer cells, it might be useful to explore the exact mechanism that it plays in the breast tumors. The present study was designed to elucidate the anticancer activity of the most potent derivative of artemisinin, artesunate, on human breast cancer MCF-7 cells through determining the cytotoxicity and apoptosis induction by flow cytometry. This would, then, lead us to investigate the mechanism involved in the artesunate-induced apoptosis of MCF-7 cells based on the assessment of caspase activity.
2. Objective
The aim of this study was to determine if artesunate could induce apoptosis in human breast cancer cell line MCF-7 cells, and to explore the apoptotic signaling pathway underlying it. The possible mechanism of action of artesunate in breast cancer cells was explored by focusing on its ability to induce apoptosis as well as activation of the key apoptosis enzymes, caspases.
Materials and Methods
3.1. Chemicals
All culture media including DMEM (Dulbecco’s Modified Eagle Medium)/F12 medium, trypsin-EDTA, penicillin-streptomycin solution and fetal bovine serum were obtained from Gibco (Invitrogen, South America). Trypan blue, 3-(4, 5 dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT) and artesunate powders were supplied from Sigma-Aldrich, USA. All chemicals, unless otherwise indicated, were purchased from Merck, Germany. In addition, annexin V-Fluorescein Isothiocyanate(FITC)/Propidium Iodide(PI) kit as well as Caspase-3, -8, -9 colorimetric assay kits were obtained from Abnova, Germany.
3.2. Cell Culture
Human breast cancer cell line, MCF-7, was purchased from the Iranian Biological Resource Center (IBRC™, Tehran, Iran) and cultured in DMEM/F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin. MCF-7 cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 throughout the study and were routinely grown in 25 cm2 culture flasks. In order to harvest, cells were trypsinized after reaching to the growth confluence, followed by centrifugation (1300 ×g for 7 min), and re-suspension in the culture medium. Cells in the log-phase of the growth were used for the following study.
3.3. Cytotoxicity Test-MTT Assay
To evaluate the cytotoxic effect of artesunate on the MCF-7 cells, MTT colorimetric assay was applied (40, 41). Briefly, MCF-7 cells at a density of approximately 1 × 104 cells per well were seeded into 96-well plates. Stock solutions of artesunate were prepared in acetone with a final vehicle concentration that did not exceed 0.1% (vol/vol) for preparing different concentrations of the artesunate (1, 5, 10, 25, 50, 75, 100, and 200 µg.mL-1). MCF-7 cells were treated for 24, 48, and 72h and 10 μL of MTT (5 mg.mL-1) was then added to each well and the plate was further incubated. After 4h, the supernatant in each well was carefully removed and 100 μL of DMSO (Dimethyl sulfoxide) was added in order to dissolve the resulting formazan crystals, the amount of which could be quantified by determining absorbance at 570 nm using an ELISA (Enzyme-Linked Immunosorbent Assay) microplate reader (BioTek, USA). Then, the IC50 value (inhibitory concentration; a concentration of a compound inhibiting 50% of the cell growth) was calculated and compared to that of the untreated control group.
3.4. Assessment of the Morphological Changes
The untreated MCF-7 cells (2×105) or cells treated with artesunate at a concentration of 25 µg.mL-1 for 24 h, were observed under an inverted microscope (Nikon, Japan).
3.5. Detection of Apoptosis by Flow Cytometry
Apoptosis was assessed by flow cytometry using an annexin V-FITC apoptosis detection kit, according to the manufacturer’s instructions. Briefly, MCF-7 cells (5 × 105) were treated with artesunate solution prepared to the final concentrations of 5, 25, 50, 75, and 100 μg.mL-1. After 24 h cells were gently trypsinized, washed once with serum-containing the medium and re-suspended in 500 μL of 1X binding buffer. Then, 5 μL of annexin V-FITC and 5 μL of propidium iodide (50 μg.mL-1) were added. After incubation at room temperature for 15 min in the dark, annexin V-FITC binding and propidium iodide staining were analyzed by flow cytometer (BD Falcon, USA) using the FITC signal detector (FL1) and phycoerythrin emission signal detector (FL2). Annexin V apoptosis detection assay is based on the key feature of apoptotic cells, which is translocation of phosphatidylserine (PS) from the inner leaflets of the plasma membrane to the cell surface where it can be detected by labeling with a fluorescent conjugate of annexin V, a Ca2+-dependent phospholipid-binding protein with a high affinity for the phosohatidylserin. This assay can distinguish apoptosis and necrosis when performing both annexin V-FITC and PI staining since PI would detect necrotic cells with permeabilized plasma membrane.
3.6. Measurement of Caspase-3, -8, and -9 Activities
Caspase activation is a common apoptotic mechanism by which anticancer agents induce apoptosis. Thus, the level of caspase activation was determined to further elucidate the artesunate’s mode of action. For this purpose, the cultivated MCF-7 cells (1 × 106) in 25 cm2 flasks were treated with 5, 25, 50, and 100 μg.mL-1artesunate for 24 h or with a fixed dose of 25 μg.mL-1for 0, 4, 8, 12, 18, and 24 h. The activities of caspase-3, -8, and -9 were determined by using caspase colorimetric assay kits according to the manufacturer’s recommended protocol. Briefly, cells were washed with ice-cold PBS and lysed with 50 μL of chilled cell lysis buffer and incubated on ice for 10 minutes. After centrifuging for 1 min in a Microcentrifuge (10,000 ×g), the supernatant (cytosolic extract) was transferred to a fresh tube and put on ice for immediate assay. Protein concentrations of the supernatants were assessed by the Bradford method and 200 μg protein was then diluted to 50 μL cell lysis buffer for each assay. In addition, 50 μL of 2X reaction buffer (containing 10 mM DTT) and 4 mM DEVD-pNA substrate were added to each sample. After incubation at 37 °C for 2 h, samples were read at 405 nm using an ELISA microplate reader (BioTek, USA). The change in caspases’ activities involved in the apoptosis) 3, 8, and 9) were determined by comparing these results with the level of the uninduced control.
3.7. Statistical Analysis
Each concentration was assayed in triplicates (n=3) and repeated in three independent experiments. SPSS statistical software (version 23.0, SPSS) was used for statistical analysis and values with p<0.05 were considered as statistically significant. The results were analyzed using one-way ANOVA followed by Dunnett’s test to compare groups with the control and were expressed as mean±SD.
4. Results
4.1. Effects of Artesunate on MCF-7 Cell Proliferation
To examine the in vitro anticancer activity of artesunate, breast cancer MCF-7 cells were treated with various doses of the artesunate (0, 1, 5, 10, 25, 50, 75, 100, and 200 µg.mL-1) for 24, 48, and 72 h and then cell were subjected to MTT assay. The result was statistically significant (p<0.05) and indicated that artesunate inhibited the proliferation of MCF-7 cells in a dose- and time-dependent manner, producing IC50 values of 43.78 ± 1.73, 28.25±0.98, and 18.11±0.26 μg.mL-1for artesunate treatments by 24, 48, and 72 h, respectively (Fig. 1).
Figure 1.
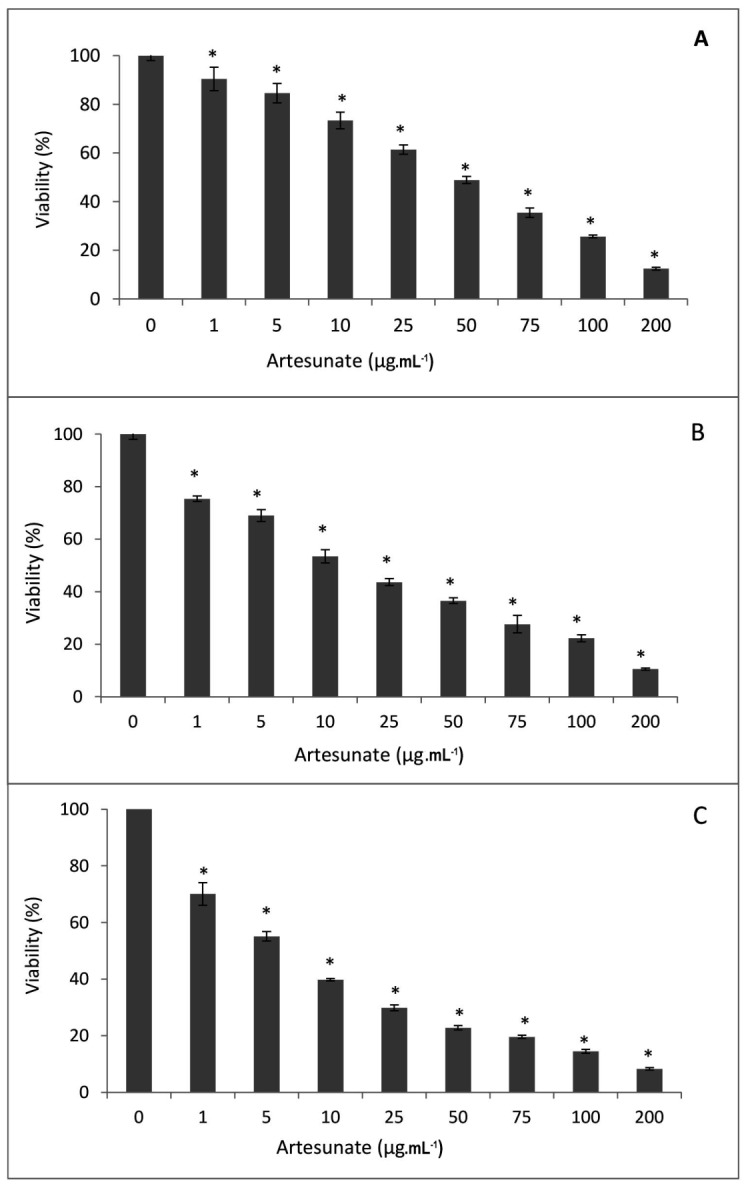
Effects of artesunate on MCF-7 cells’ proliferation. MCF-7 cells viability (%) was measured following to the treatment with 0, 1, 5, 10, 25, 50, 75, 100, or 200 µg.mL-1 of the artesunate for 24 h (A), 48 h (B), or 72 h (C) as were determined by MTT assay. The results are presented as means ± standard error of the mean (SEM) from three independent experiments. * Indicates significant difference in comparison to control group (cells treated only with medium) (p< 0.05).
4.2. Effects of Artesunate on MCF-7 Cell Viability
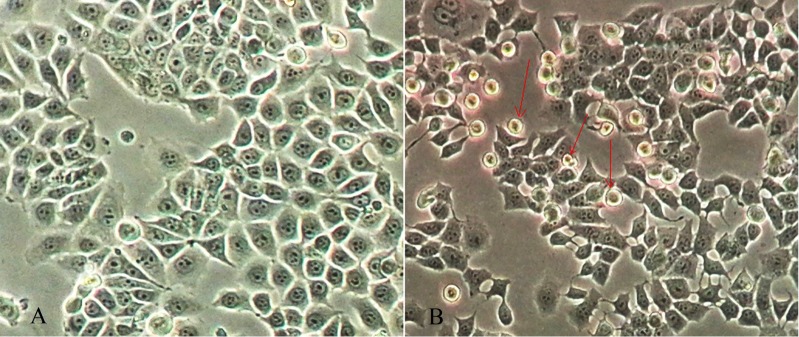
Applying inverted microscopy, our observations show that artesunate inhibits proliferation of MCF-7 cells since there was a significant difference in the cell viability between the treated and control cells. Following to exposure to artesunate, MCF-7 cells became round, small, and then were detached from the flask (Fig. 2).
Figure 2.
Morphological examination of MCF-7 cells by inverted microscope (×400). Control untreated cells (A), and cells treated with a fixed concentration of artesunate (25 µg.mL-1) for 24 h (B) are shown. Arrows indicate dying apoptotic cells that became round and small in size.
4.3. Effects of Artesunate on MCF-7 Cells Apoptosis
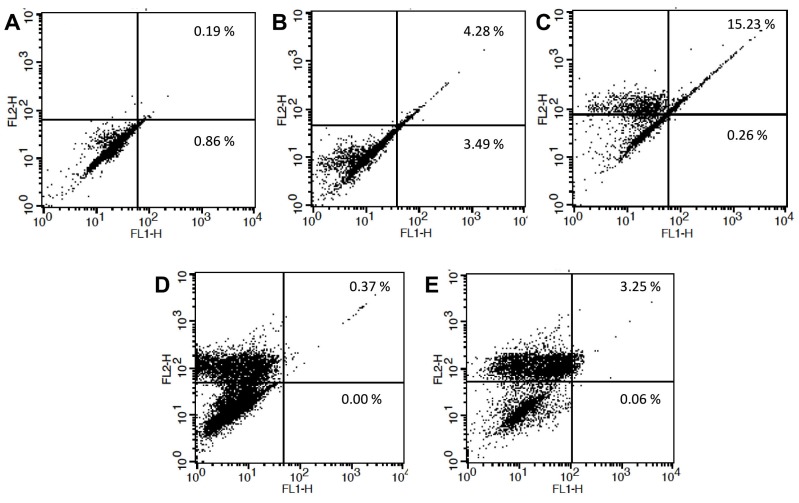
Annexin V/PI staining and flow cytometry were used to evaluate whether artesunate could induce cell death in MCF-7 cells via apoptosis. Flow cytometry analysis has positivity indicated that after treatment with artesunate at concentrations of 0 (as control), 5, 25, 50, and 100 µg.mL-1for 24 h, cellular apoptosis rate increases by a concentration dependent manner to the certain concentrations, above which artesunate acts as a cytotoxic agent. The rate of induced apoptosis was according to the following order: 1.05 ± 0.21, 8.07 ± 0.13, 15.49 ± 1.46, 0.37 ± 0.33, and 3.31±0.51 for 0 to 100 µg.mL-1of artesunate, respectively (Fig. 3. A to E). Thus, the apoptotic cell population has increased in a dose-dependent manner, peaking at 25 µg.mL-1of artesunate and reaching a significant difference to the control at 5 µg.mL-1of the artesunate (p<0.05). Although, there was no difference between the artesunate of 50 and 100 µg.mL-1compared to the control, however, cell death yet was increased as explained above (P>0.05).
Figure 3.
Detection of early and late apoptosis by flow cytometry assay through annexin V and PI staining. MCF-7 cells were exposed to 5, 25, 50, and 100 µg.mL-1 of the artesunate for 24 h and then were subjected to the assessment by flow cytometry, (B-E, respectively). Also, MCF-7 cells were treated with medium only without artesunate as a control. Control (A).
4.4. Effects of Artesunate on the Activity of Caspase-3, -8 and -9
In most cases, different anticancer agents eventually mediate a common apoptotic pathway through the activation of caspases (42). In order to study the involvement of caspases in artesunate-induced apoptosis, activation of important key caspases (caspase-3, -8 and -9) in apoptotic pathways were investigated in MCF-7 cells upon exposure to the different concentrations of artesunate (5-200 µg.mL-1) for 24h. In addition, in order to evaluate the time course of caspase activation, cells were treated with a fixed concentration of 25 µg.mL-1for the number of incubation time sets (i.e., 4, 8, 12, 18, and 24 h). Enzyme assay indicated that the activities of caspase-8 and -9 in the artesunate treated MCF-7 cells were increased significantly in a dose- and time-dependent manner. However, exposure to 25 µg.mL-1artesunate displayed the largest increase in the activity of both caspase-8 and -9 (Figs. 4A and B). Interestingly, a brisk increase in the activity of caspase-8 was observed after 8h exposure to the effective dose of artesunate (25 µg.mL-1) which was decreased thereafter. A Similar result was obtained in case of caspase-9 but after 12 h (Fig. 5a and B). Furthermore, treatment of the cells with 25 µg.mL-1artesunate also activated the caspase-3, but to a lesser extent and in the different doses and times, such that a prominent increase in the level of activation of effector caspase-3 was observed at 50 µg.mL-1 (Fig. 4C) and after 24 h of exposure to the artesunate (Fig. 5C).
Figure 5.
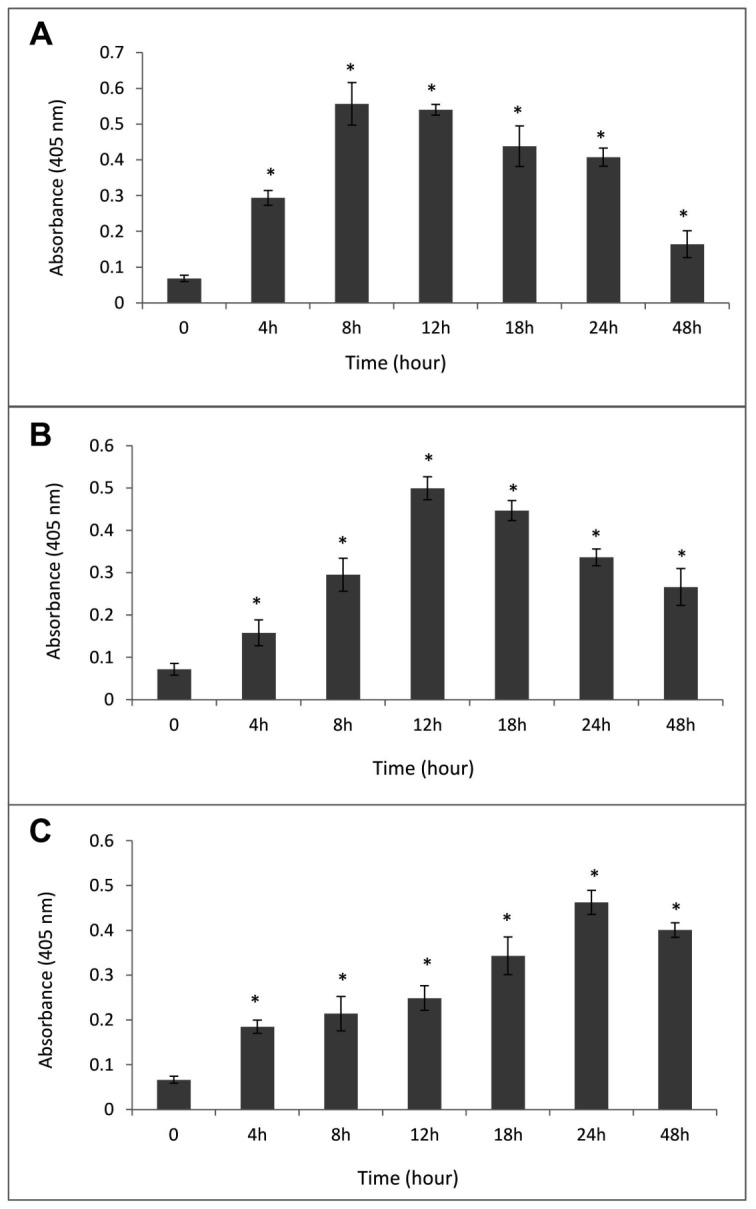
Activities of caspase-8 (A), caspase-9 (B), and caspase-3 (C) in the MCF-7 cells after treatment with 25 µg.mL-1 artesunate at the different incubation times. Each value is the mean ± SD of the three experiments. * Indicates a significant difference between the untreated and artesunate-treated cells, p<0.05.
Figure 4.
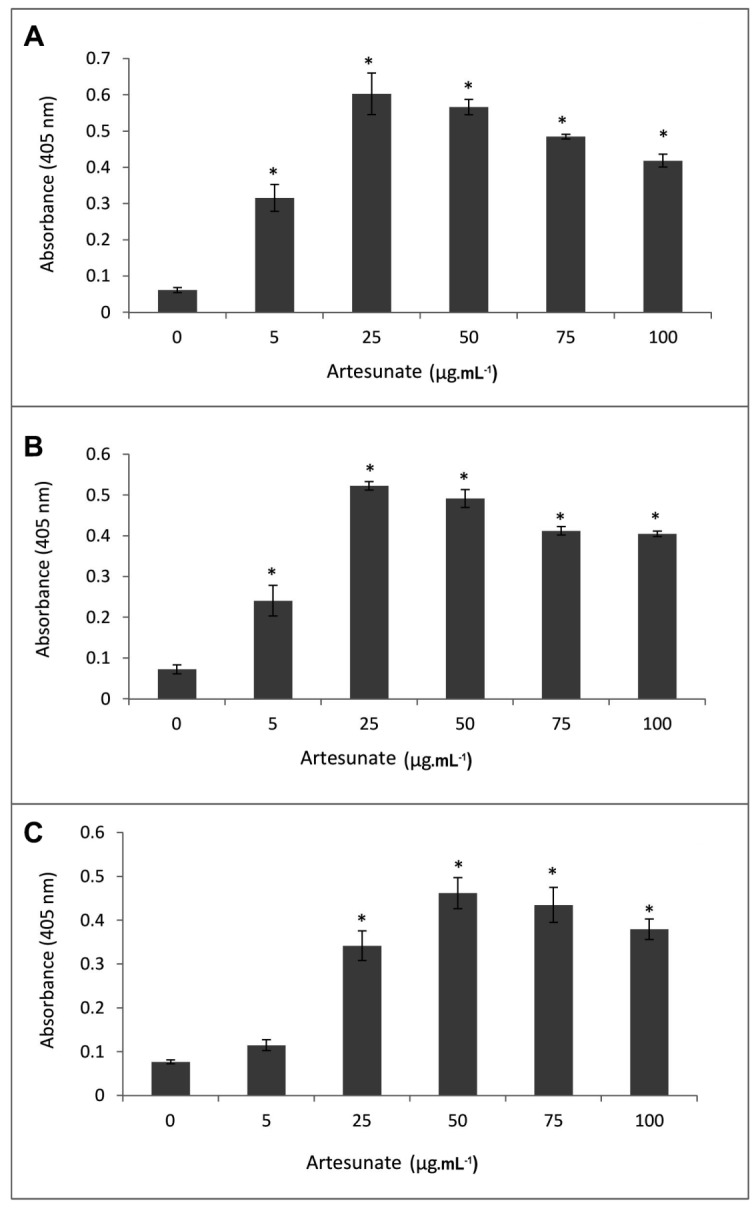
Activities of caspase-8 (A) caspase-9 (B) and caspase-3 (C) in the MCF-7 breast cancer cells after treatment with the various concentrations of artesunate (0, 5, 25, 50, 75, 100, and 200 µg.mL-1) for 24 h. Each value is the mean ± SD of the three experiments. * Indicates a significant difference between the untreated and artesunate-treated cells, p<0.05.
5. Discussion
Breast cancer is the leading cause of cancer death (1) which despite numerous treatment options including the conventional therapies, radiation, and surgery, still remains an incurable disease and a significant public health problem (10). Therefore, it is of high importance to search new effective drugs with specificity on cancers but little or no side effects on normal mammalian cells.
Fortunately, artesunate is one among such few drugs. Widely used as an antimalarial drug, it doesn’t show any significant side effects and more importantly, its cytotoxic effect is specific to cancer cells (32). Previous studies have demonstrated the profound anti-proliferative activity of artesunate in different cancer cell lines (35) including breast cancer cells as well as various types of animal cancer models (33, 38) In addition, several cases of significant improvement in the breast cancer treatment after receiving artesunate have been reported (34).
Despite the many recent reports of the potent anticancer activity of artesunate, both in vitro and in vivo, and even in the clinical trials against different tumors, its molecular mechanism of action toward cancer cells (39) and apoptosis induction is still unexplored (43). Also, to the best of our knowledge, there are no further reports on its mechanism of action in MCF-7 cells. Therefore, in the present study, we investigated the mechanism by which artesunate induced cell death in human breast cancer MCF-7 cell line.
The possible mechanism of action of artesunate in breast cancer cells was explored by focusing on its ability to induce apoptosis as well as activation of the key apoptosis enzymes, caspases. Our results showed that artesunate exerted a cytotoxic effect on MCF-7 cell line and inhibited the proliferation of the cells in a dose- and time-dependent manner. Inverted microscopic observation also showed that floating cells increased in the breast cancer cells’ culture treated with artesunate, suggesting that artesunate has inhibited the growth of MCF-7 cells. In addition, according to the result of flow cytometry, artesunate can induce apoptosis such that in doses lower than IC50 (i.e. 5 and 25 μg.mL-1) mainly induced apoptosis, while at doses upper than IC50 (i.e. 50 and 100 μg.mL-1) it is toxic toward the cells, increased the rate of cell death but not apoptosis. These findings, which suggest apoptosis induction in the breast cancer cells, are consistent with the several other studies demonstrating in vitro anticancer activity of artesunate (44-46).
However, there are limited reports regarding the mechanisms of artesunate-induced cell death in MCF-7 cells. On the other hand, apoptosis can be mediated through caspase-dependent or caspase-independent pathways. Thus, caspase activation was assessed to further elucidate the artesunate’s mode of action in breast cancer cells. Caspases are the key regulatory proteins in apoptosis, which, based on their order in apoptotic pathways, could be classified into two groups. These are initiator caspases (e.g. caspase-8 and -9) which mediate extrinsic and intrinsic apoptotic pathways, respectively, and effector caspases (e.g. caspase-3, -6 and -7) which are shared by the both apoptotic pathways following to the activation.
Among the effector caspases, caspase-3 is absolutely crucial for induction of apoptosis as by cleaving the majority of caspase substrates, this enzyme contributes to the typical morphological as well as biochemical changes associated with the apoptosis (47). Our results from caspase assay showed that activity of the caspases-3, -8, and -9 increased up to 25 μg.mL-1of the artesunate and then it decreases upon application of higher concentrations. Moreover, the effect of artesunate on the level of caspase activation through the course of time and timing of apoptosis was different. It was observed that artesunate exposure initiated the extrinsic pathway of the apoptosis with a brisk increase in levels of activated caspase-8 at 8 h followed by activation of caspase-9 at 12 h. Moreover, a prominent increase in the levels of effector caspase-3 was evident after 24 h of the artesunate exposure. These findings clearly follow the established order of caspase activation cascade in caspase-dependent pathway in which upon activation, caspasae-8 initiates the mitochondrial pathway to apoptosis resulting in activation of caspase-9, and activated caspase-9 then instigates a caspase activation cascade by processing caspases-3 which propagates further caspase processing events, as depicted (48). Thus, it seems that artesunate induces apoptosis in MCF-7 cells through both intrinsic and extrinsic caspase-dependent pathways and, therefore, exerts its anticancer effect.
The results of this study may provide evidence of the beneficial effect of artesunate in the treatment of the breast tumors since it increases apoptosis in breast cancer cells. Also, considering the long-term administration of this drug as anti-malaria and its excellent safety profile as well as outstanding cases of breast cancer treatment success, it could be concluded that artesunate may be a promising candidate as a cytotoxic chemotherapeutic agent for the treatment of breast cancer that can improve treatment options for these currently incurable diseases.
Acknowledgments
A part of the financial support of this study was provided by the University of Guilan.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Taghavi A, Fazeli Z, Vahedi M, Baghestani AR, Pourhoseingholi A, Barzegar F. et al. Increased trend of breast cancer mortality in Iran. Asian Pac J Cancer Prev. 2012;13(1):367–70. doi: 10.7314/APJCP.2012.13.1.367. [DOI] [PubMed] [Google Scholar]
- 3.Waxman DJ, Schwartz PS. Harnessing apoptosis for improved anticancer gene therapy. Cancer Res. 2003;63(24):8563–72. [PubMed] [Google Scholar]
- 4.Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11(2):121–39. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 5.Parton M, Dowsett M, Smith I. Studies of apoptosis in breast cancer. BMJ. 2001;322(7301):1528. doi: 10.1136/bmj.322.7301.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edward Chu, Alan C Sartorelli. Cancer Chemotherapy. In: Katzung BG, Masters SB, Trevor AJ. Basic & clinical pharmacology. 7th ed. New York: MC Grow Hill; 2004. p. 898-9330.
- 7. Alison M. The cancer handbook. 2th ed. New Jersey: John Wiley & Sons; 2007.
- 8.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431–41. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 10.Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117(11):3155–3163. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azim HA, De Azambuja E, Colozza M, Bines J, Piccart M. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011;22(9):1939–47. doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 12.Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. Biomed Res Int. 2011;2012(247597):149–167. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartwell J. Plants used against cancer. 15th ed. Chicago: Lawrence MA. Quarterman Publications; 1982.
- 14.Kaur R, Kapoor K, Kaur H. Plants as a source of anticancer agents. J Nat Prod Plant Resour. 2011;1(1):119–24. [Google Scholar]
- 15.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–95. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulda S, Efferth T. Selected Secondary Plant Metabolites for Cancer Therapy. J Tradit Chin Med. 2015;1(1):1–5. doi: 10.15806/j.issn.2311-8571.2014.0005. [DOI] [Google Scholar]
- 17.Nirmala MJ, Samundeeswari A, Sankar PD. Natural plant resources in anti-cancer therapy-A review. Res Plant Biol. 2011;1(3):01–14. [Google Scholar]
- 18.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E. et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59(6):365–78. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today. 2010;15(15):668–78. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Liao F. Discovery of artemisinin (qinghaosu) Molecules. 2009;14(12):5362–6. doi: 10.3390/molecules14125362. [DOI] [Google Scholar]
- 21.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G. et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6(27):1–15. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordi T, Lepist E-I. Artemisinin derivatives: toxic for laboratory animals, safe for humans? Toxicol Lett. 2004;147(2):99–107. doi: 10.1016/j.toxlet.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Weina PJ, Milhous WK. Pharmacokinetic and pharmacodynamic profiles of rapid-acting artemisinins in the antimalarial therapy. Curr Drug Ther. 2007;2(3):210–23. doi: 10.2174/157488507781695649. [DOI] [Google Scholar]
- 24.Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11(32):1–15. doi: 10.1017/S1462399409001239. [DOI] [PubMed] [Google Scholar]
- 25.Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer lett. 1995;91(1):41–46. doi: 10.1016/0304-3835(94)03716-V. [DOI] [PubMed] [Google Scholar]
- 26.Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin--from bench to bedside. Planta Med. 2007;73(4):299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira JF, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15(5):3135–70. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim W-S, Choi WJ, Lee S, Kim WJ, Lee DC, Sohn UD. et al. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J Physiol Pharmacol. 2015;19(1):21–7. doi: 10.4196/kjpp.2015.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konkimalla VB, Blunder M, Korn B, Soomro SA, Jansen H, Chang W. et al. Effect of artemisinins and other endoperoxides on nitric oxide-related signaling pathway in RAW 2647 mouse macrophage cells. Nitric Oxide. 2008;19(2):184–91. doi: 10.1016/j.niox.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh NP, Lai HC. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004;24(4):2277–80. [PubMed] [Google Scholar]
- 31.Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70(1):49–56. doi: 10.1016/S0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- 32.Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354(1):28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Moore JC, Lai H, Li J-R, Ren R-L, McDougall JA, Singh NP. et al. Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett. 1995;98(1):83–7. doi: 10.1016/S0304-3835(06)80014-5. [DOI] [PubMed] [Google Scholar]
- 34. Rowen RJ, Fang HBJ. From the Townsend Letter for Doctors & Patients. http://www.townsendletter. com/Dec2002/artemisinin1202. htmH; 2002.
- 35.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18(4):767–73. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 36.Stein HA. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem. 2011;286(8):6587–6601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis TM, Phuong HL, Ilett KF, Hung NC, Batty KT, Phuong VDB. et al. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob Agents Chemother. 2001;45(1):181–6. doi: 10.1128/AAC.45.1.181-186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai H, Nakase I, Lacoste E, Singh NP, Sasaki T. Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer Res. 2009;29(10):3807–10. [PubMed] [Google Scholar]
- 39.Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO. et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64(2):382–94. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 40.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH. et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48(17):4827–33. [PubMed] [Google Scholar]
- 41.Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Drug Des Dis. 2011;716(9):157–168. doi: 10.1007/978-1-61779-012-6-9. [DOI] [PubMed] [Google Scholar]
- 42.Fulda S, Debatin K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 43.Tin AS, Sundar SN, Tran KQ, Park AH, Poindexter KM, Firestone GL. Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anti-cancer Drugs. 2012;23(4):370–9. doi: 10.1097/CAD.0b013e32834f6ea8. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Yang J, Chen L, Wang J, Wang Y, Luo J. et al. Artesunate induces apoptosis through caspase-dependent and-independent mitochondrial pathways in human myelodysplastic syndrome SKM-1 cells. Chem Biol Interact. 2014;219:28–36. doi: 10.1016/j.cbi.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Wu G-D, Zhou H-J, Wu X-H. Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vascul Pharmacol. 2004;41(6):205–12. doi: 10.1016/j.vph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Lijuan W, Yucong Y, Wenli G. Effect of artesunate on human endometrial carcinoma HEC-1B cells. Mil Med Res. 2010;25(3):143–51. doi: 10.1016/S1000-1948(10)60033-0. [DOI] [Google Scholar]
- 47. Los M, Walczak H. Caspases: their role in cell death and cell survival In: Molecular Biology Intelligence Unit. New York: Springer; 2002.
- 48.Cullen S, Martin S. Caspase activation pathways: some recent progress. Cell Death Differ. 2009;16(7):935–8. doi: 10.1038/cdd.2009.59. [DOI] [PubMed] [Google Scholar]