Abstract
Background: The transversion of G to T (G894T) in human endothelial nitric oxide synthase (eNOS) gene has profound effects such as male infertility, recurrent miscarriage, multiple sclerosis and cardiovascular diseases.
Objectives: Development of a new Multiplex Tetra-Primer Amplification Refractory Mutation System - Polymerase Chain Reaction (T-ARMS-PCR) for detection of rs1799983 (G894T) in the human eNOS was sought.
Materials and Methods: A T-ARMS-PCR for rs1799983 polymorphism in a single-step PCR was carried out, and the results were confirmed by PCR-RFLP technique in 82 infertile men with varicocele.
Results: The results showed that GG (varicocele infertile men), GT and TT genotypes appear to be 53.65%, 34.14%, and 12.19%, respectively. Full accordance between PCR-RFLP and T-ARMS-PCR methods for genotyping of rs1799983 polymorphism was found.
Conclusions: This is the first work that describes a rapid, relatively cheap, high throughput detection of G894T polymorphism in eNOS that can be used in large scale clinical studies.
Keywords: Endothelial nitric oxide synthase (eNOS) , rs1799983, T-ARMS-PCR, Varicocele
1. Introduction
Inflammation of the pampiniform venous plexus in scrotum is the main identifiable causes of varicocele. A majority of idiopathic varicocele generally occurs on the left side (1). Varicocele occurs in approximately 30-40% of infertile males (2). Although the pathogenic mechanisms by which varicocele leads to changes in spermatogenesis are not clear, some of these mechanisms may possess a known cause, such as generation of reactive oxygen species (ROS) (3, 4). ROS may damage morphology, motility and sperm concentrations, leading to loss of fertility. Nitric oxide (NO) is an important antioxidant found in seminal plasma (5). NO is being produced by endothelial nitric oxide synthase (eNOS) (6-8).
Several SNPs of the eNOS (GenBank ID: 4846) were described in various populations including G894T (rs1799983), T-786C (rs20707044) and 4a4b (rs1722009) (9, 10). The common eNOS source of polymorphism observed in various populations is G894T in exon seven that leads to Glu298Asp, (9). Several reports are indicative of reduced eNOS activity in substituted individuals (11). So far genotyping of G894T has been performed via polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), real time-PCR and direct sequencing.. These techniques are relatively slow and very expensive in comparison to Tetra primer-amplification refractory mutation system-PCR (T-ARMS-PCR) (12-15).
In conventional ARMS-PCR, the amplification of normal and mutant allele is carried out in two separate reactions. Here in T-ARMS-PCR, both normal and mutant alleles can be run altogether with a control fragment in a single reaction. In T-ARMS-PCR, a pair of common (outer) primers produces a non–allele-specific PCR product and in combination with two inner, allele-specific primers (in opposite orientation to each other) produces 2 PCR products.
The outer primers amplify a large fragment of the target gene contains variant nucleotide as a control fragment and smaller allele-specific amplicons with different sizes. The product can easily be discriminated on gel electrophoresis either as homozygous or heterozygous. A deliberate mismatch at position -2 or -3 from the 3' terminal end of the inner primers can improve allele specificity (16).
2. Objectives
The aim of this study was to develop a rapid single-step method using T-ARMS-PCR for detection of rs1799983 (G894T) polymorphism in eNOS, a possible SNP for male infertility.
Materials and Methods
3.1. Subjects and DNA Extraction
A case-control study of 82 male patients with clinical varicocele and 80 male controls (healthy volunteers) were recruited from the Yazd Infertility Center (17). Physical examination in standing position and via scrotal palpation in a temperature controlled room (23 °C) was carried out to confirm varicocele. Non-obstructive azoospermic individuals were not included in this study. Semen analysis was performed according to the WHO laboratory manual (18). Patients with varicocele were divided into three grades: Grade I (n = 13), Grade II (n = 31) and Grade III (n = 38). The control group (healthy volunteers) consisted of 80 fertile and normospermic men from Yazd Infertility Center who fathered at least one child. All participants were fully informed of the objectives of the study and those that signed the consent form were assigned to the study. The examination of blood samples was approved by the local ethics review. Peripheral blood samples (2 mL) were obtained from varicocele patients and the DNA was extracted using a standard salting-out procedure (19). Purified DNA samples from peripheral blood samples were used for PCR reactions. DNA pellets were dissolved in 50 mL of TE (20 mM Tris-HCl, 0.1 mM EDTA, pH 8.0) buffer (approximately 20-30 ng.µL-1DNA concentration) and stored at 20 ºC until the genotype analysis was performed.
3.2. Primer Design and T-ARMS-PCR Analysis
Primer1was used for primer design (1) and the specificity of the primers was checked by BLAST. PCR was performed in a total volume of 25 µL containing 50 ng of template DNA, 5 pmol of each outer primers, 10 pmol of each inner primers and 1× PCR Master Mix (YektaTajhizAzma, Tehran, Iran). PCR amplification (touchdown) was carried out at 95 ºC for 2 min, followed by denaturation at 95 ºC for 20 sec, first annealing at 68 ºC (10 cycles). In the remaining cycles (25 cycles), annealing was carried out at 69 ºC for 1 min and extension at 72 ºC for 50 sec, followed by a final extension for 5 min. The PCR products were electrophoresed on an ethidium bromide-stained 2% agarose gel.
3.3. PCR-RFLP Analysis
G894T substitution (exon 7 NOS3) introduces the restriction site for MboI endonuclease.
To validate genotyping of eNOS polymorphism by T-ARMS-PCR, conventional PCR was carried out by F3 and R3 primers and followed by restriction endonuclease digestion. PCR amplification (95 °C: 2 min; followed by 35 cycles [95 °C: 30 s, 64 °C: 40 s, 72 °C: 30 s] and a final extension at 72 °C for 5 min) in a final volume of 25 μL containing 100 ng total DNA, primers (10 pmol), 1× Master Mix PCR (YektaTajhizAzma, Tehran, Iran). A 10 µL of the PCR reaction was mixed with 1× buffer and 1.5 U of MboI (Fermentas, Germany) and incubated at 37 °C for 16 h. The digestion products were electrophoresed on an ethidium bromide-stained 2.5% (w/v) agarose gel.
3.4. Statistical Analysis
Statistical analysis was performed with the GraphPad Prism software (GraphPad Software, Inc. USA). The Chi-square goodness-of-fit test was used for the association between patient and control groups. Values of p< 0.05 were regarded as statistically significant.
4. Results
The age difference between the 82 Iranian infertile men with varicocele (mean age of 31.54 ± 6.35) and 80 normal controls (mean age: 29.3 ± 6.54) was not significant (p = 0.765).
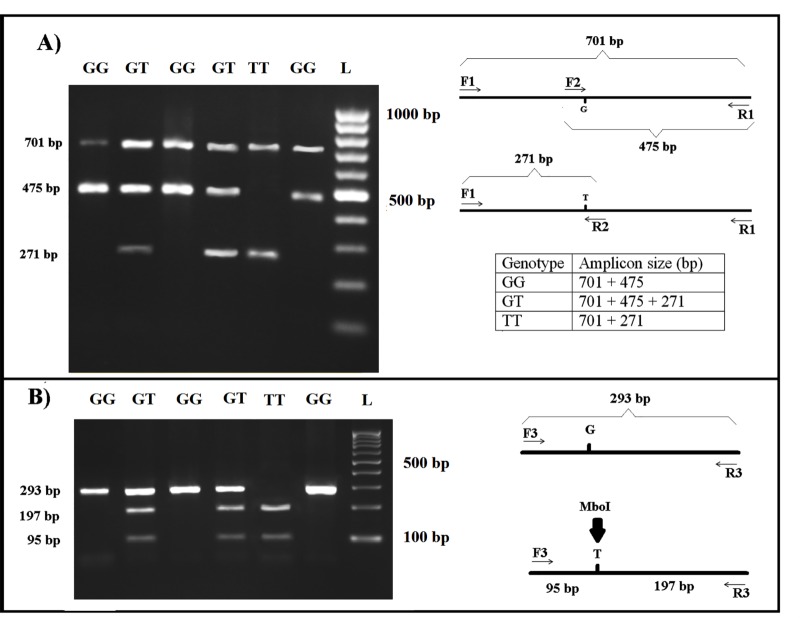
This technique produced 3 fragments in heterozygotes (701, 475 and 271bp) and 2 fragments in homozygotes (GG resulting in 701 and 475 bp, and TT resulting in 701 and 271 bp) (Fig. 1).
Figure 1.
A) Results of T-ARMS-PCR of eNOS polymorphism (rs1799983). F1 and R1: outer primers for control PCR product (701 bp); F2 and R2: inner primers for two allele-specific PCR products G allele (475bp) and T allele (271bp). GG genotype results 2 bands (701 and 475 bp) and TT genotype results 2 bands (701 and 271 bp) and 3 bands in GT genotype (701, 475 and 271bp). B) Validation using PCR-RFLP by MboI restriction endonuclease
The frequencies of allele G (wild type) of rs1799983 SNP among infertile men with varicocele and controls were 70.73 % and 88.75 %, respectively. For the T allele (mutant), the percentages were 29.26 and 11.25, respectively. An association between allele T and the incidence of Varicocele (p<0.0001) was found. A significant difference was found between the patients (GG: 53.65%; GT: 34.14%; TT: 12.19%) and the control group (GG: 80.0%; GT: 17.50%; TT: 2.50%) (p = 0.0319).
As it is shown in Figure 1, G894 allele produced an undigested fragment (292 bp) that showed the homozygote state for genotype GG and digested fragments (197 and 95 bp) showed the genotype TT and genotype GT produced both undigested and digested fragments (292, 197 and 95 bp).
The results of DNA analysis with T-ARMS-PCR and PCR-RFLP using restriction enzyme MboI were exactly identical.
5. Discussion
The relationship of the rs1799983 SNP with the prevalence of male infertility has been reported elsewhere. (12). The standard PCR instruments are highly desirable for scientific studies of large numbers of patients and for diagnostic analyses, economical and fast assays.
The majority of genotyping studies have utilized MboI digestion to identify the rs1799983 polymorphism (13). However, this technique is very expensive and relatively slow. Yun et al. used pyrosequencing to genotype of the rs1799983 in Korean population(14).
Here, we designed a sensitive, rapid and single-step T-PCR-ARMS method for rs1799983 SNP detection. Primer design was the most important step for successful development of a new multiplex T-ARMS-PCR method. Moreover, primers’ concentrations and the PCR conditions were optimized.
This T-ARMS-PCR is more advantageous than allele-specific PCR and RFLP because this method allows the simultaneous detection of the both alleles (wild type and mutant) in one tube. T-ARMS-PCR reduces cost by up to 37%, compared to PCR-RFLP test since fewer chemicals and less time are required (Table 2). Besides, it needs only traditional PCR reagents and with no special equipment requirements.
Table 2. Benefit and cost details in use to T-ARMS-PCR and PCR-RFLP methods for genotyping the eNOS rs1799983 polymorphism.
| Method | T-ARMS-PCR | PCR-RFLP |
| Materials for reaction | ● Enzyme and buff ers in PCR | ● Enzyme and buff ers in PCR Restriction enzyme and buffers (MboI enzyme) |
| Time | ● 2.5 h for PCR |
● 1.5 h for PCR 3 h for RFLP reaction |
| Cost | ● Cost 3.00 US Dollar per sample | ● Cost 4.00 US Dollar per sample |
| Problems |
● Fast, no digestion problems ● Common agarose gel electrophoresis ● No restriction enzyme ● No digestion |
● Lengthy, incomplete digestion
problems ● High resolution agarose gel electrophoresis ● MboI restriction enzyme ● Digestion for 3 h |
Table 1. PCR primers and conditions. F1 and R1 (outer primers), F2 and R2 (inner primers) were designed for T-ARMSPCR. To enhance the specificity of inner primers (F2 and R2), at the 3rd nucleotide from the 3´-terminus changes to destabilizing mismatch (the bold underlined bases indicate the extra mismatched bases introduced). F3 and R3 primers were used for PCR- RFLP reaction.
| Primer sequence | Tm (ºC) | Amplicon size (bp) |
|
F1: 5'-AGCCTCGGTGAGATAAAGGATG-3' R1: 5'-CCTGGACCTGCTCTGATTGTC-3' F2: 5'-GCTGCTGCAGGCCCCAGATAAG-3' R2:5'-GCAGAAGGAAGAGTTCTGGGAGA-3' F3: 5'-TCACGGAGACCCAGCCAATGAG-3' R3: 5'-TCCATCCCACCCAGTCAATCCC-3' |
66 66 72 70 70 70 |
G allele (475) T allele (271) Control band (701) G allele undigested (293) T allele digest with MboI (197+95) |
Acknowledgements
This research was funded by Yazd University. We thank all the patients for providing blood samples for the scientific research. We also would like to appreciate the Research and Clinical Center for Infertility at Shahid Sadughi University of Medical Sciences, Yazd, Iran. The study was approved by Yazd University Human Research Ethics Committee.
References
- 1.Poongothai J, Gopenath TS, Manonayaki S. Genetics of human male infertility. Singapore Med J. 2009;50(4):336–347. [PubMed] [Google Scholar]
- 2.Oliveira A, Neto A, Almeida C, Silva-Ramos M, Versos R, Barros A. et al. Comparative study of gene expression in patients with varicocele by microarray technology. Andrologia. 2012;44 Suppl 1:260–265. doi: 10.1111/j.1439-0272.2011.01173.x. [DOI] [PubMed] [Google Scholar]
- 3.Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23(6):737–752. doi: 10.1002/j.1939-4640.2002.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Saleh RA. Utility of oxidative stress test in the male infertility clinic. Zhonghua Nan Ke Xue. 2002;8(1):1–9. [PubMed] [Google Scholar]
- 5.Taylor CT. Antioxidants and reactive oxygen species in human fertility. Environ Toxicol Pharmacol. 2001;10(4):189–198. doi: 10.1016/s1382-6689(01)00099-0. [DOI] [PubMed] [Google Scholar]
- 6.Shamsi MB, Venkatesh S, Kumar R, Gupta NP, Malhotra N, Singh N. et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010;47(1):38–43. doi: 10.1007/978-1-4939-2140-9_10. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 8.Felaco M, Grilli A, De Lutiis MA, Patruno A, Libertini N, Taccardi AA. et al. Endothelial nitric oxide synthase (eNOS) expression and localization in healthy and diabetic rat hearts. Ann Clin Lab Sci. 2001;31(2):179–186. [PubMed] [Google Scholar]
- 9.Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW. et al. The Glu298Asp polymorphism of the NOS3 gene as a determinant of the baseline production of nitric oxide. J Hypertens. 2002;20(10):2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto Y, Saito Y, Kajiyama N, Yoshimura M, Shimasaki Y, Nakayama M. et al. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension. 1998;32(1):3–8. doi: 10.1161/01.HYP.32.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Wang XL, Mahaney MC, Sim AS, Wang J, Wang J, Blangero J. et al. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol. 1997;17(11):3147–3153. doi: 10.1161/01.atv.17.11.3147. [DOI] [PubMed] [Google Scholar]
- 12.Bianco B, Ghirelli-Filho M, Cavalheiro CM, Cavalcanti V, Peluso C, Gava MM. et al. Variants in endothelial nitric oxide synthase (eNOS) gene in idiopathic infertile Brazilian men. Gene. 2013;519(1):13–17. doi: 10.1016/j.gene.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Safarinejad MR, Shafiei N, Safarinejad S. The role of endothelial nitric oxide synthase (eNOS) T-786C, G894T, and 4a/b gene polymorphisms in the risk of idiopathic male infertility. Mol Reprod Dev. 2010;77(8):720–727. doi: 10.1002/mrd.21210. [DOI] [PubMed] [Google Scholar]
- 14.Yun YJ, Park JH, Song SH, Lee S. The association of 4a4b polymorphism of endothelial nitric oxide synthase (eNOS) gene with the sperm morphology in Korean infertile men. Fertil Steril. 2008;90(4):1126–1131. doi: 10.1016/j.fertnstert.2007.07.1382. [DOI] [PubMed] [Google Scholar]
- 15.Buldreghini E, Mahfouz RZ, Vignini A, Mazzanti L, Ricciardo-Lamonica G, Lenzi A. et al. Single nucleotide polymorphism (SNP) of the endothelial nitric oxide synthase (eNOS) gene (Glu298Asp variant) in infertile men with asthenozoospermia. J Androl. 2010;31(5):482–488. doi: 10.2164/jandrol.109.008979. [DOI] [PubMed] [Google Scholar]
- 16.Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29(17):E88–88. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidari MM, Khatami M, Talebi AR, Moezzi F. Mutation analysis of TNP1 gene in infertile men with varicocele. Iran J Reprod Med. 2014;12(4):257–262. [PMC free article] [PubMed] [Google Scholar]
- 18.World Health O. Laboratory manual of the WHO for the examination of human semen and sperm-cervical mucus interaction. Ann Ist Super Sanita. 2001;37(1):I–XII, 1. [PubMed] [Google Scholar]
- 19.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]