 An unprecedented gold-catalyzed formal [3+2] cycloaddition between ynamides and isoxazoles has been developed, allowing rapid and practical access to a wide range of synthetically useful 2-aminopyrroles.
An unprecedented gold-catalyzed formal [3+2] cycloaddition between ynamides and isoxazoles has been developed, allowing rapid and practical access to a wide range of synthetically useful 2-aminopyrroles.
Abstract
The generation of gold carbenes via the gold-catalyzed intermolecular reaction of nucleophiles containing relatively labile N–O or N–N bonds with alkynes has received considerable attention during recent years. However, this protocol is not atom-economic as the reaction produces a stoichiometric amount of pyridine or quinoline waste, the cleaved part of the N–O or N–N bonds. In this article, we disclose an unprecedented gold-catalyzed formal [3+2] cycloaddition between ynamides and isoxazoles, allowing rapid and practical access to a wide range of synthetically-useful 2-aminopyrroles. Most importantly, mechanistic studies and theoretical calculations revealed that this reaction presumably proceeds via an α-imino gold carbene pathway, thus providing a strategically novel, atom-economic route to the generation of gold carbenes. Other significant features of this approach include the use of readily-available starting materials, high flexibility, simple procedure, mild reaction conditions, and in particular, no need to exclude moisture or air (“open flask”).
Introduction
Catalytic transformations involving gold carbenes are arguably the most important aspect of homogeneous gold catalysis.1 Recently, the possibility of forming an α-oxo gold carbenoid species via gold-catalyzed intramolecular or intermolecular alkyne oxidation by a N–O bond oxidant (initially a sulfoxide), pioneered by Toste and Zhang,2a,b represents a significant advance in gold carbene chemistry, and various efficient synthetic methods have been developed based on this strategy.2 Compared with intramolecular alkyne oxidation, the intermolecular approach offers much greater flexibility as no tethering of the oxidant is required, and therefore it is more synthetically useful.3 However, this intermolecular approach is obviously not atom-economic as the reaction produces a stoichiometric amount of pyridines or quinolines, the reduced form of the corresponding pyridine N-oxides or quinoline N-oxides, as waste (eqn (1)),4 which may even deactivate the gold catalyst via coordination.5
 |
1 |
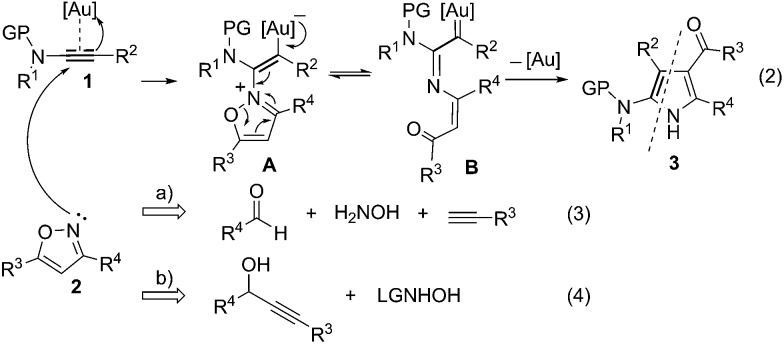
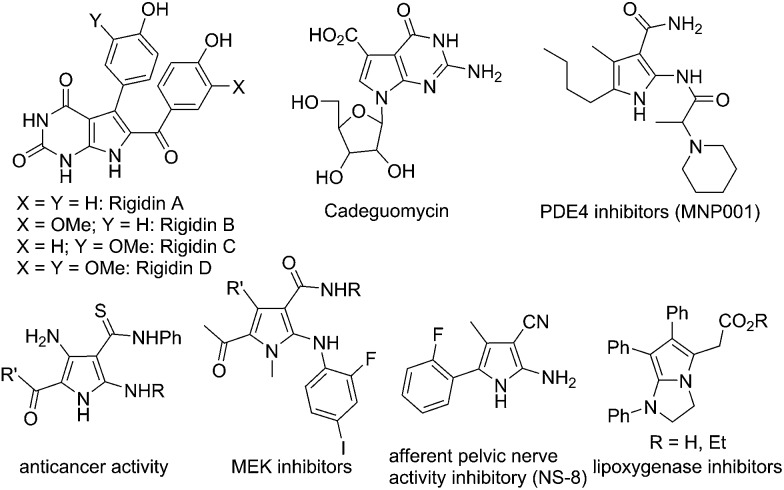
Access to the related α-imino gold carbenes via gold-catalyzed nitrene transfer to alkynes, however, remains a highly challenging task. Here, it should be noted that: (1) the nitrene moiety is delivered via an outer sphere attack and no gold nitrene complex6 is involved in this case; this mode of nitrene transfer is distinctively different from many well-established nitrene transfer reactions;7 (2) this protocol would present alkynes as equivalents of α-diazo imines, which are difficult to access as α-diazo imines can readily cyclize into the corresponding 1,2,3-triazoles. To date, only limited success has been achieved in this type of gold-catalyzed nitrene transfer, mainly by the intramolecular reaction of alkyne and azide.8 For example, Toste and co-workers used azide as an effective nitrene equivalent and realized the first protocol for the generation of α-imino gold carbenes in 2005.8a Later, elegant studies on the synthesis of indoles from alkynyl azides were demonstrated by Gagosz8c and Zhang,8d independently. Recently, several studies have invoked the intermolecular transfer of nitrene to alkynes by the use of iminopyridinium ylides as nitrene-transfer reagents, as disclosed by the groups of Zhang,9a Davies,9b,c and Liu.9d However, similar to those of the above-mentioned gold-catalyzed intermolecular alkyne oxidations, a stoichiometric amount of pyridine was produced as the waste in these cases. Therefore, the exploration of intermolecular approaches to the generation of α-imino gold carbenes, especially in an atom-economic way, is very attractive to researchers. We envisioned that the α-imino gold carbene intermediate B might be generated through the gold-catalyzed intermolecular reaction of ynamides 1 10 with isoxazoles 2, which could be obtained in an efficient and modular manner following the synthetic routes shown in eqn (3) and (4) in Scheme 1.11 The carbene B, likely highly electrophilic, could then undergo an electrophilic cyclization to yield the final 2-aminopyrroles 3, thus constituting a gold-catalyzed formal [3+2] cycloaddition (Scheme 1, eqn (2)). Herein, we report the successful implementation of this mechanistic design to a facile and practical synthesis of a wide range of polysubstituted 2-aminopyrroles, which are common structural motifs found in natural products and pharmacologically active molecules (Fig. 1)12 and are difficult to access via traditional methods for pyrrole synthesis.13 Most importantly, an α-imino gold carbene is most likely generated as the key intermediate on the basis of both mechanistic studies and theoretical calculations, thereby providing a strategically-novel, atom-economic route to the generation of gold carbenes.
Scheme 1. Synthetic design for the atom-economic generation of α-imino gold carbenes: formation of 2-aminopyrroles 3 through gold-catalyzed formal [3+2] cycloaddition between ynamides 1 and isoxazoles 2.
Fig. 1. 2-Aminopyrrole subunit in natural products and bioactive molecules.
Results and discussion
At the outset, ynamide 1a and 3,5-dimethylisoxazole 2a were used as the reacting species and a series of experiments were performed in order to validate our approach. To our delight, the expected product 3a was indeed formed in 70% 1H NMR yield in the presence of 5 mol% IPrAuNTf2 (Table 1, entry 1). Then, various typical gold catalysts with a range of electronic and steric characteristics were screened (Table 1, entries 2–7), and (ArO)3PAuNTf2 (Ar = 2,4-di-tert-butylphenyl) gave the best yield of the desired product (Table 1, entry 7). Somewhat surprisingly, AgNTf2 could also catalyze this reaction in 50% yield (Table 1, entry 8). Notably, without a metal catalyst, the reaction failed to give even a trace of 3a, and PtCl2 and Zn(OTf)2 were not effective in promoting this reaction (Table 1, entries 9–10).14 The reaction proved to be less efficient when it was performed at a reduced temperature (Table 1, entry 11). In addition, the use of 2 equiv. of 2a also gave the desired pyrrole 3a in 90% yield (Table 1, entry 12).
Table 1. Optimization of reaction conditions a .
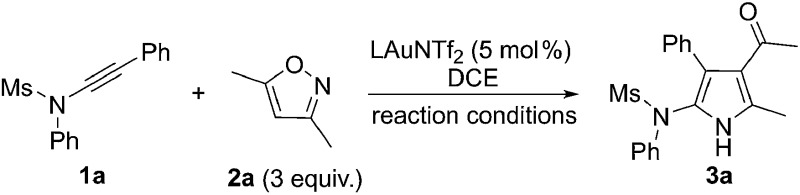
| |||
| Entry | Metal catalyst | Conditions | Yield b (%) |
| 1 | IPrAuNTf2 | DCE, 80 °C, 3 h | 70 |
| 2 | Ph3PAuNTf2 | DCE, 80 °C, 3 h | 69 |
| 3 | Et3PAuNTf2 | DCE, 80 °C, 3 h | 54 |
| 4 | Cy-JohnPhosAuNTf2 | DCE, 80 °C, 3 h | 71 |
| 5 | BrettPhosAuNTf2 | DCE, 80 °C, 12 h | 27 |
| 6 | Au(III) c | DCE, 80 °C, 3 h | 34 |
| 7 | (ArO)3PAuNTf2 d | DCE, 80 °C, 3 h | 95 |
| 8 | AgNTf2 | DCE, 80 °C, 3 h | 50 |
| 9 e | PtCl2 | toluene, 80 °C, 3 h | <5 |
| 10 e | Zn(OTf)2 (10 mol%) | DCE, 80 °C, 3 h | <5 |
| 11 | (ArO)3PAuNTf2 d | DCE, 60 °C, 5 h | 75 |
| 12 f | (ArO)3PAuNTf2 d | DCE, 80 °C, 3 h | 90 |
aReaction conditions: [1a] = 0.05 M; DCE = 1,2-dichloroethane.
bMeasured by 1H NMR using diethyl phthalate as the internal standard.
cDichloro(2-picolinato)gold(iii).
d Ar = 2,4-di-tert-butylphenyl.
e 1a was decomposed.
f2.0 equiv. of 2a was used.
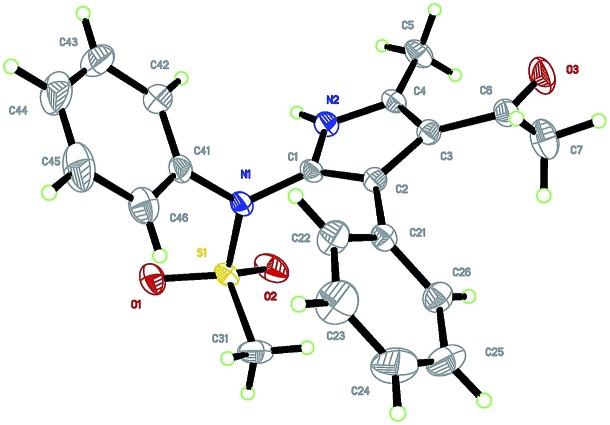
With the optimized reaction conditions in hand, the scope of the transformation was explored. As seen from the results collected in Table 2, the reaction proceeded smoothly with various ynamide substrates 1, and the yields ranged from 58% to 96%. For example, ynamides with different protecting groups, even the Ns group (Table 2, entries 4–5), readily gave the desired 2-aminopyrroles 3a–f (Table 2, entries 1–6). Of note, an excellent yield could be achieved in the case of an ynamide with an oxazolidinone moiety and no dimerization reaction was observed (Table 2, entry 6).15 When R1 is an allyl group, the desired 3j could also be formed in 86% yield, and no cyclopropanation product was formed (Table 2, entry 10).16,5g Other aryl-substituted ynamides were also suitable substrates for this reaction, giving the corresponding functionalized pyrroles 3k–l in excellent yields (Table 2, entries 11–12). Interestingly, for styryl or cyclopropyl-substituted ynamides, this reaction still led to 75% yield and 58% yield, respectively (Table 2, entries 13–14). The molecular structure of 3a was further confirmed by X-ray diffraction (Fig. 2).17
Table 2. Reaction scope for different ynamides 1 a .
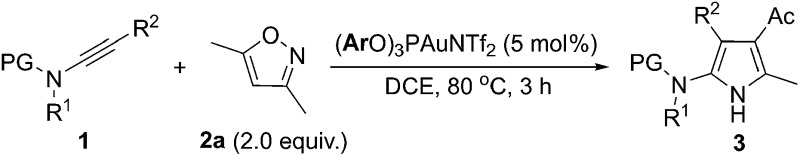
|
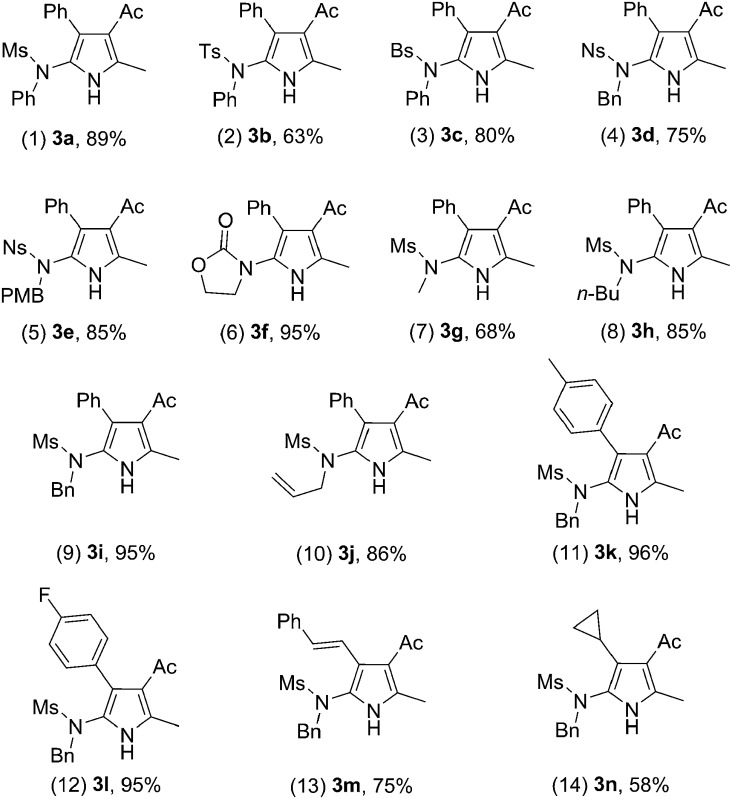
|
aReactions run in vials; [1] = 0.05 M; isolated yields are reported.
Fig. 2. Crystal structure of compound 3a.
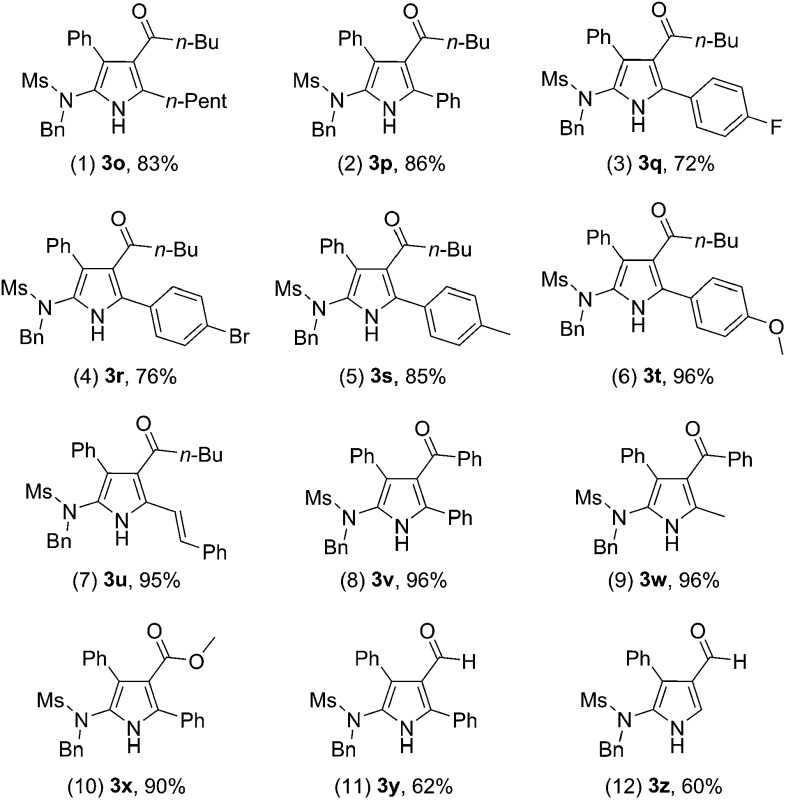
We next extended the reaction to different 3,5-disubstituted isoxazoles 2. To our delight, the reaction of ynamide 1i with various isoxazole substrates 2 worked well under the above optimized reaction conditions, giving versatile polysubstituted 2-aminopyrroles 3o–z in generally good to excellent yields. As summarized in Table 3, a range of aryl-substituted isoxazoles 2c–g were successful (R2 = aryl), delivering the desired 3p–t in 72–96% yield (Table 3, entries 2–6). In addition, when R1 is an aryl group, the reaction also worked well to afford the corresponding pyrroles 3v–w in excellent yields (Table 3, entries 8–9). Pleasingly, methyl 3-pyrrolecarboxylate 3x was formed in 90% yield from the corresponding isoxazole (Table 3, entry 10). It should be mentioned that 3-formylpyrroles 3y–z could also be prepared in serviceable yields (Table 3, entries 11–12).
Table 3. Reaction scope for different isoxazoles 2 a .
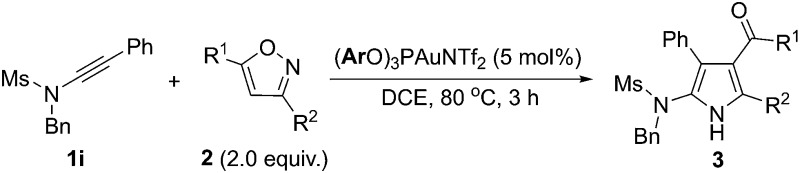
|
|
aReactions run in vials; [1i] = 0.05 M; isolated yields are reported.
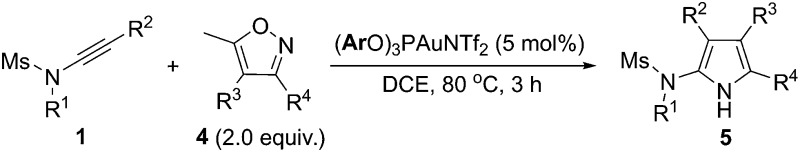
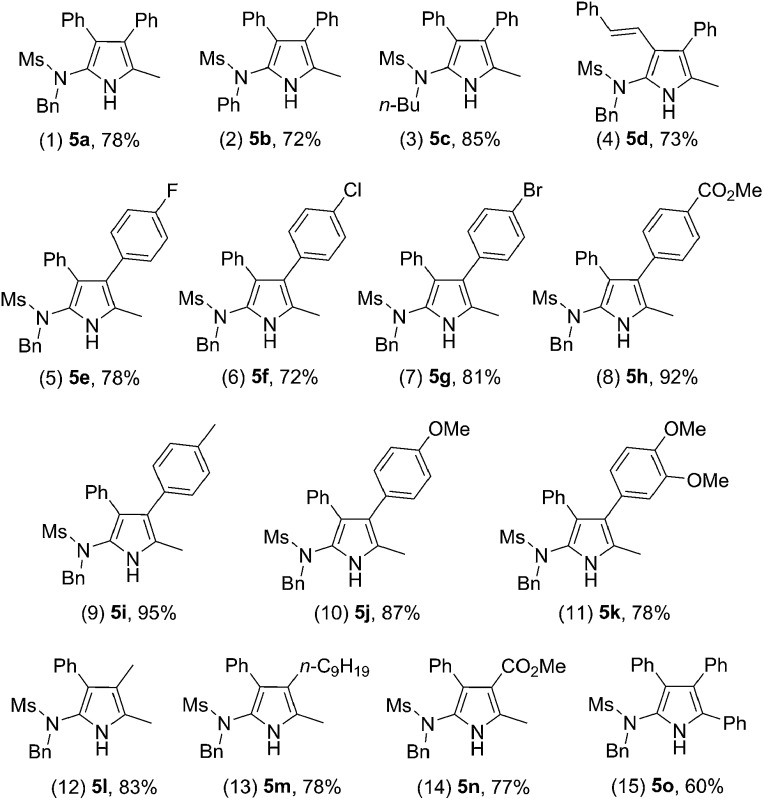
Interestingly, when the scope of the method was extended to fully-substituted isoxazoles 4, the reaction also proceeded well, allowing the convenient synthesis of deacylated polysubstituted 2-aminopyrroles 5. A series of readily-available substituted ynamides was first examined. The corresponding pyrroles 5a–d were obtained in 72–85% yield (Table 4, entries 1–4). Then, isoxazoles 4 with substituents at the 4-position were also investigated, giving the products 5e–m in mostly good to excellent yields (Table 4, entries 5–13). Notably, methyl 3-pyrrolecarboxylate 5n could also be obtained in 77% yield from the corresponding 4-substituted isoxazole, which is complementary to the above protocol based on the 3,5-disubstituted isoxazoles 2 (Table 4, entry 14 vs. Table 3, entry 10). In particular, the 3,4-diphenyl substituted isoxazole also reacted smoothly, delivering the 3,4,5-triphenyl substituted pyrrole 5o in a respectable 60% yield (Table 4, entry 15).
Table 4. Reaction scope for different ynamides 1 and 4-substituted isoxazoles 4 a .
|
|
aReactions run in vials; [1] = 0.05 M; isolated yields are reported.
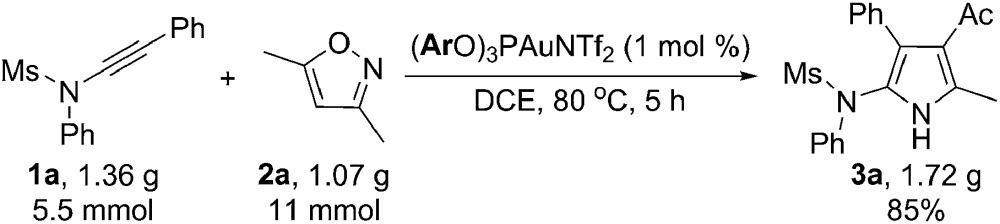
To further test the practicality of the current catalytic system, a gram-scale reaction of 1.36 g of 1a and 1.07 g of 2a was carried out with a much lower catalyst loading (1 mol%), and 1.72 g of the desired pyrrole 3a was formed in 85% yield, highlighting the synthetic utility of this chemistry (eqn (5)). Interestingly, the reaction could also be performed well even in water to afford the desired product 3a in 80% yield and no hydration of the ynamide was observed (eqn (6)),10a–c thus making this protocol more practical and environmentally benign.
 |
5 |
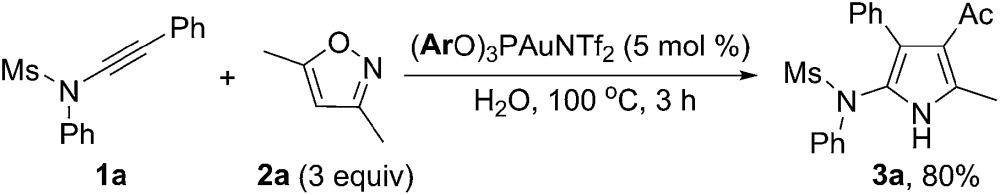 |
6 |
This chemistry can also be used to construct N-heteropyrrolizines, which are present in a variety of bioactive molecules.18,12k For example, treatment of ynamide 1p with isoxazole 4a under the optimized reaction conditions gave the pyrrole 5p, which could be converted into fused 2-aminopyrrole 6 in basic conditions in a one-pot process (63% two-step overall yield, eqn (7)). Compound 6 might serve as a precursor for the synthesis of lipoxygenase inhibitors (Fig. 1).12k
 |
7 |
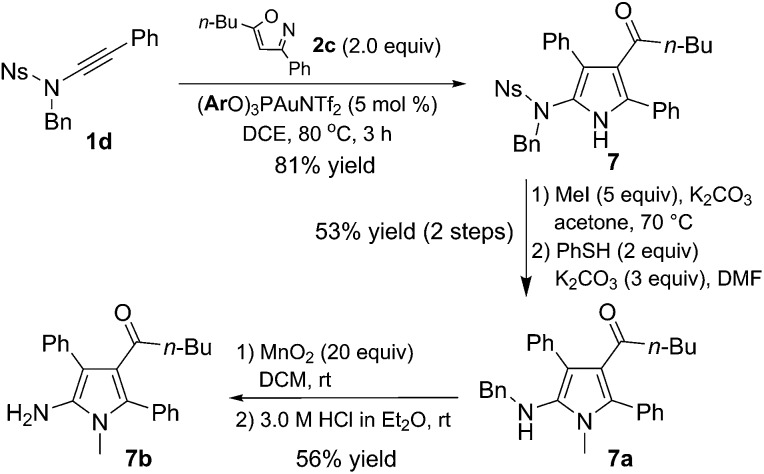
The sulfonamide could be readily transformed into a free amine (Scheme 2). For example, the reaction of ynamide 1d with isoxazole 2c under the optimized reaction conditions furnished pyrrole 7 in 81% yield. Nitrogen protection of 7 with a methyl group and subsequent removal of the Ns group using the standard conditions (PhSH, K2CO3) resulted in the formation of species 7a (53% two-step overall yield). Subsequent deprotection of the benzyl group in 7a could be realized by performing MnO2-mediated oxidation followed by hydrolysis to afford 7b in 56% yield.13e
Scheme 2. Transformation of a sulfonamide into an amine.
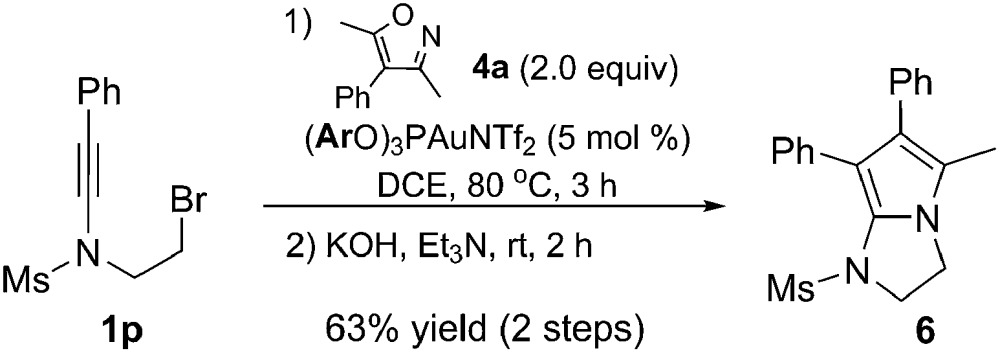
To probe the mechanism of this reaction, we first synthesized the alkyl-substituted ynamide 1q as the alkyl-substituted gold carbene is well-known in the gold-catalyzed cycloisomerizations of enynes; [1,2] hydride shift followed by elimination of the gold catalyst was involved as the critical deauration step.1e,19 Indeed, as depicted in eqn (8), when ynamide 1q reacted with 2a under the standard reaction conditions, none of the desired pyrrole was detected and α,β-unsaturated amide 8 was isolated in 25% yield. Amide 8 is supposed to be derived from [1,2] hydride shift followed by elimination of the gold catalyst and subsequent hydrolysis. This result indicated that a gold carbene is most likely generated as the key intermediate in this process. On the other hand, the low chemoselectivity in the case of n-butyl substituted ynamide shows the importance of aryl substituents on the ynamides to keep a high reactivity for the reactions in Tables 2–4.20
 |
8 |
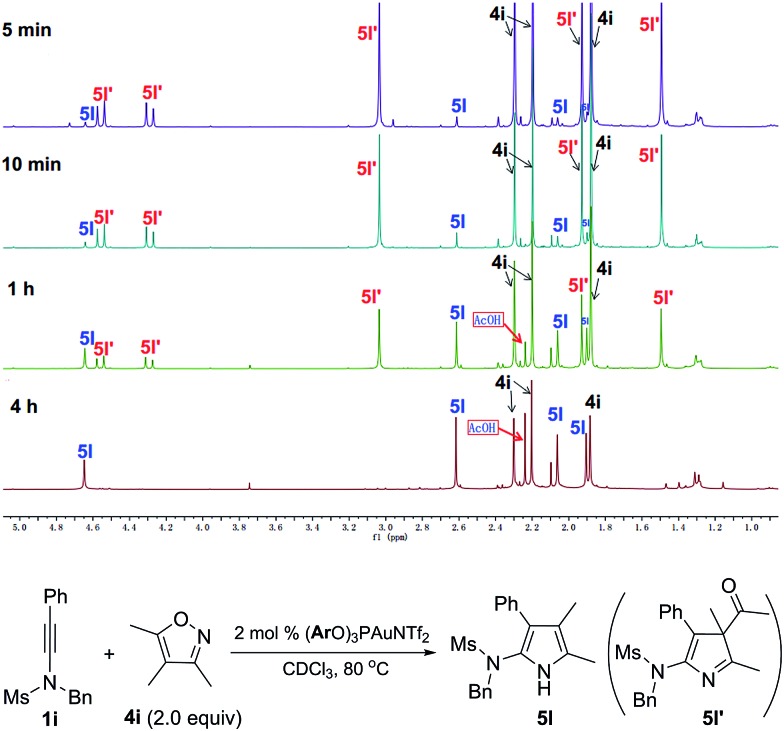
In addition, it was found that a key intermediate 3H-pyrrole 5l′ could be detected and isolated in the case of the reaction of ynamide 1i with fully-substituted isoxazole 4i (Table 4, entry 12). To further demonstrate this process, we monitored the reaction by 1H NMR spectroscopy, as depicted in Fig. 3. Here, the reaction was performed in the presence of 2 mol% (ArO)3PAuNTf2 in CDCl3 in order to better track the reaction intermediates. At the early stage of the reaction, we could clearly observe the formation of the 3H-pyrrole 5l′, which was gradually transformed into the final 1H-pyrrole 5l.
Fig. 3. 1H NMR monitoring of the reaction of ynamide 1i with fully-substituted isoxazole 4i.
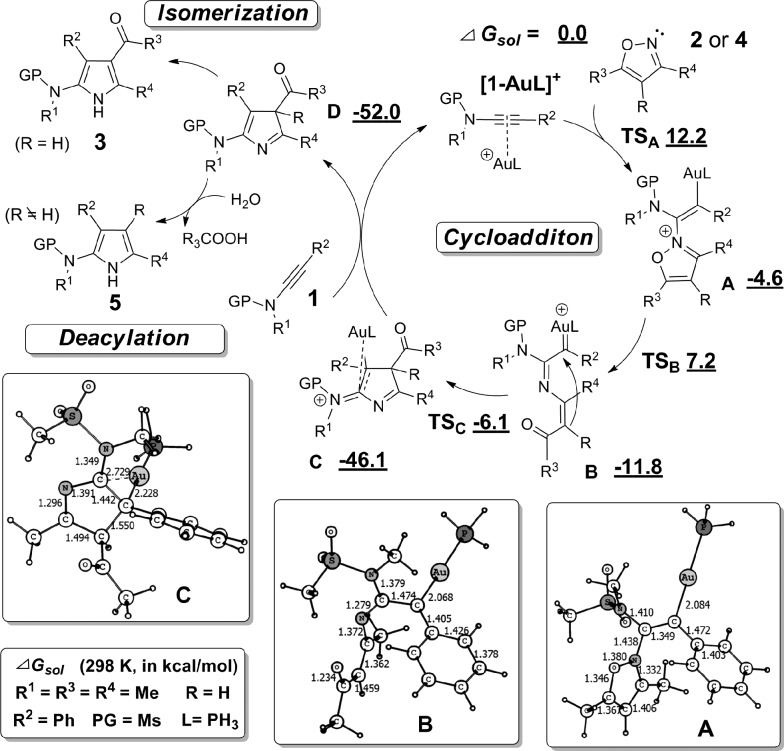
A plausible mechanism to rationalize the formation of pyrrole 3 or 5 is illustrated in Scheme 3, in light of the above experimental observations and density functional theory (DFT) computations (see ESI† for details).21 Initially, nucleophilic attack of isoxazole 2 or 4 to the Au(i)-ligated alkyne of ynamide 1 forms vinyl gold intermediate A by overcoming a moderate barrier (12.2 kcal mol–1). Intermediate A isomerizes into the gold carbene intermediate B upon breakage of the isoxalic N–O bond,22 again requiring an activation energy around 12.0 kcal mol–1. Subsequent 1,5-cyclization23 within intermediate B readily occurs to afford the Au(i)-ligated 3H-pyrrole C, which upon ligand exchange with another ynamide 1 releases 3H-pyrrole D. The whole process is highly exothermic with free energy release amounting to 52 kcal mol–1. For 3,5-disubstituted isoxazoles 2, 3H-pyrrole D readily isomerizes into the final aromatic 1H-pyrrole 3 by sigmatropic H-migrations.24 In the case of fully-substituted isoxazole substrates 4, D is ultimately transformed into the final 1H-pyrrole 5, presumably by a water-assisted deacylative aromatization.25
Scheme 3. Plausible reaction mechanism. Theoretical investigations on the reaction pathways for the formation of product 3g (Table 2, entry 7): relative free energies (ΔG sol, in kcal mol–1) of key intermediates and transition states were computed at the M06/6-31+G(d)/SDD level in 1,2-dichloroethane at 298 K.
Conclusions
In summary, we have developed a novel gold-catalyzed formal [3+2] cycloaddition between ynamides and isoxazoles, leading to the concise and flexible synthesis of polysubstituted 2-aminopyrroles. This methodology makes it possible to introduce four substituents onto a pyrrole ring very freely with high efficiency. Of particular interest, fully substituted isoxazoles also react under deacylation, closing a further gap in the reaction scope. Moreover, an α-imino gold carbene is the most likely intermediate based on both mechanistic studies and theoretical calculations, thus providing a new strategy for the generation of gold carbenes, especially in an atom-economic way. Studies to elucidate the detailed mechanism and further synthetic applications of the current protocol are in progress in our laboratory.
Acknowledgments
We are grateful for the financial support from NSFC (no. 21102119, 21273177 and 21272191), RFDP (20130121110004), NFFTBS (no. J1310024), and the Program for Changjiang Scholars and Innovative Research Team in University.
Footnotes
References
- For reviews, see: ; (a) Fensterbank L., Malacria M. Acc. Chem. Res. 2014;47:953. doi: 10.1021/ar4002334. [DOI] [PubMed] [Google Scholar]; (b) Obradors C., Echavarren A. M. Acc. Chem. Res. 2014;47:902. doi: 10.1021/ar400174p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Obradors C., Echavarren A. M. Chem. Commun. 2014;50:16. doi: 10.1039/c3cc45518a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hashmi A. S. K. Angew. Chem., Int. Ed. 2010;49:5232. doi: 10.1002/anie.200907078. [DOI] [PubMed] [Google Scholar]; (e) Jiménez-Núñez E., Echavarren A. M. Chem. Rev. 2008;108:3326. doi: 10.1021/cr0684319. [DOI] [PubMed] [Google Scholar]; (f) Hashmi A. S. K. Chem. Rev. 2007;107:3180. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; (g) Fürstner A., Davies P. W. Angew. Chem., Int. Ed. 2007;46:3410. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- (a) Shapiro N. D., Toste F. D. J. Am. Chem. Soc. 2007;129:4160. doi: 10.1021/ja070789e. [DOI] [PubMed] [Google Scholar]; (b) Li G., Zhang L., Angew. Chem., Int. Ed., 2007, 46 , 5156 , ; for reviews, see: . [DOI] [PubMed] [Google Scholar]; (c) Xiao J., Li X. Angew. Chem., Int. Ed. 2011;50:7226. doi: 10.1002/anie.201100148. [DOI] [PubMed] [Google Scholar]; (d) Zhang L. Acc. Chem. Res. 2014;47:877. doi: 10.1021/ar400181x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yeom H.-S., Shin S. Acc. Chem. Res. 2014;47:966. doi: 10.1021/ar4001839. [DOI] [PubMed] [Google Scholar]
- For recent representative examples on gold-catalyzed intermolecular alkyne oxidation, see: ; (a) Schulz J., Jašíková L., Škríba A., Roithová J. J. Am. Chem. Soc. 2014;136:11513. doi: 10.1021/ja505945d. [DOI] [PubMed] [Google Scholar]; (b) Li L., Shu C., Zhou B., Yu Y.-F., Xiao X.-Y., Ye L.-W. Chem. Sci. 2014;5:4057. [Google Scholar]; (c) Pan F., Liu S., Shu C., Lin R.-K., Yu Y.-F., Zhou J.-M., Ye L.-W. Chem. Commun. 2014;50:10726. doi: 10.1039/c4cc05115g. [DOI] [PubMed] [Google Scholar]; (d) Karad S. N., Liu R.-S. Angew. Chem., Int. Ed. 2014;53:5444. doi: 10.1002/anie.201403015. [DOI] [PubMed] [Google Scholar]; (e) Wang T., Shi S., Hansmann M. M., Rettenmeier E., Rudolph M., Hashmi A. S. K. Angew. Chem., Int. Ed. 2014;53:3715. doi: 10.1002/anie.201310146. [DOI] [PubMed] [Google Scholar]; (f) Wang T., Shi S., Rudolph M., Hashmi A. S. K. Adv. Synth. Catal. 2014;356:2337. [Google Scholar]; (g) Santos M. D., Davies P. W. Chem. Commun. 2014;50:6001. doi: 10.1039/c4cc01059k. [DOI] [PubMed] [Google Scholar]; (h) Li J., Ji K., Zheng R., Nelson J., Zhang L. Chem. Commun. 2014;50:4130. doi: 10.1039/c4cc00739e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wu G., Zheng R., Nelson J., Zhang L. Adv. Synth. Catal. 2014;356:1229. [Google Scholar]; (j) Nösel P., dos Santos Comprido L. N., Lauterbach T., Rudolph M., Rominger F., Hashmi A. S. K. J. Am. Chem. Soc. 2013;135:15662. doi: 10.1021/ja4085385. [DOI] [PubMed] [Google Scholar]; (k) Wang L., Xie X., Liu Y. Angew. Chem., Int. Ed. 2013;52:13302. doi: 10.1002/anie.201304700. [DOI] [PubMed] [Google Scholar]; (l) Pawar S. K., Wang C.-D., Bhunia S., Jadhav A. M., Liu R.-S. Angew. Chem., Int. Ed. 2013;52:7559. doi: 10.1002/anie.201303016. [DOI] [PubMed] [Google Scholar]; (m) Ji K., Zhao Y., Zhang L. Angew. Chem., Int. Ed. 2013;52:6508. doi: 10.1002/anie.201301601. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Henrion G., Chava T. E. J., Le Goff X., Gagosz F. Angew. Chem., Int. Ed. 2013;52:6277. doi: 10.1002/anie.201301015. [DOI] [PubMed] [Google Scholar]; (o) Ghorpade S., Su M.-D., Liu R.-S. Angew. Chem., Int. Ed. 2013;52:4229. doi: 10.1002/anie.201210313. [DOI] [PubMed] [Google Scholar]
- For examples on the utilization of the side products, see: ; (a) Talbot E. P. A., Richardson M., McKenna J. M., Toste F. D. Adv. Synth. Catal. 2014;356:687. doi: 10.1002/adsc.201300996. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huple D. B., Ghorpade S., Liu R.-S. Chem.–Eur. J. 2013;19:12965. doi: 10.1002/chem.201302533. [DOI] [PubMed] [Google Scholar]; (c) Mukherjee A., Dateer R. B., Chaudhuri R., Bhunia S., Karad S. N., Liu R.-S. J. Am. Chem. Soc. 2011;133:15372. doi: 10.1021/ja208150d. [DOI] [PubMed] [Google Scholar]
- To avoid catalyst deactivation by the byproduct pyridine or quinoline, acid has to be used as the additive in some cases. See: ; (a) Shu C., Li L., Xiao X.-Y., Yu Y.-F., Ping Y.-F., Zhou J.-M., Ye L.-W. Chem. Commun. 2014;50:8689. doi: 10.1039/c4cc03565h. [DOI] [PubMed] [Google Scholar]; (b) Shu C., Li L., Yu Y.-F., Jiang S., Ye L.-W. Chem. Commun. 2014;50:2522. doi: 10.1039/c3cc49238a. [DOI] [PubMed] [Google Scholar]; (c) Shi S., Wang T., Yang W., Rudolph M., Hashmi A. S. K. Chem.–Eur. J. 2013;19:6576. doi: 10.1002/chem.201300518. [DOI] [PubMed] [Google Scholar]; (d) Hashmi A. S. K., Wang T., Shi S., Rudolph M. J. Org. Chem. 2012;77:7761. doi: 10.1021/jo301381z. [DOI] [PubMed] [Google Scholar]; (e) Xu M., Ren T.-T., Li C.-Y. Org. Lett. 2012;14:4902. doi: 10.1021/ol302238t. [DOI] [PubMed] [Google Scholar]; (f) He W., Xie L., Xu Y., Xiang J., Zhang L. Org. Biomol. Chem. 2012;10:3168. doi: 10.1039/c2ob25235j. [DOI] [PubMed] [Google Scholar]; (g) Qian D., Zhang J. Chem. Commun. 2011;47:11152. doi: 10.1039/c1cc14788a. [DOI] [PubMed] [Google Scholar]; (h) Luo Y., Zhang G., Hwang E. S., Wilcoxon T. A., Zhang L. Beilstein J. Org. Chem. 2011;7:596. doi: 10.3762/bjoc.7.69. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Ye L., He W., Zhang L. J. Am. Chem. Soc. 2010;132:8550. doi: 10.1021/ja1033952. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ye L., Cui L., Zhang G., Zhang L. J. Am. Chem. Soc. 2010;132:3258. doi: 10.1021/ja100041e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Li Z., Capretto D. A., Rahaman R. O., He C. J. Am. Chem. Soc. 2007;129:12058. doi: 10.1021/ja0724137. [DOI] [PubMed] [Google Scholar]; (b) Li Z., Ding X., He C. J. Org. Chem. 2006;71:5876. doi: 10.1021/jo060016t. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see: ; (a) Fantauzzi S., Caselli A., Gallo E. Dalton Trans. 2009:5434. doi: 10.1039/b902929j. [DOI] [PubMed] [Google Scholar]; (b) Diaz-Requejo M. M., Perez P. J. Chem. Rev. 2008;108:3379. doi: 10.1021/cr078364y. [DOI] [PubMed] [Google Scholar]; (c) Mueller P., Fruit C. Chem. Rev. 2003;103:2905. doi: 10.1021/cr020043t. [DOI] [PubMed] [Google Scholar]
- (a) Gorin D. J., Davis N. R., Toste F. D. J. Am. Chem. Soc. 2005;127:11260. doi: 10.1021/ja053804t. [DOI] [PubMed] [Google Scholar]; (b) Shapiro N. D., Toste F. D. J. Am. Chem. Soc. 2007;129:4160. doi: 10.1021/ja070789e. [DOI] [PubMed] [Google Scholar]; (c) Wetzel A., Gagosz F. Angew. Chem., Int. Ed. 2011;50:7354. doi: 10.1002/anie.201102707. [DOI] [PubMed] [Google Scholar]; (d) Lu B., Luo Y., Liu L., Ye L., Wang Y., Zhang L. Angew. Chem., Int. Ed. 2011;50:8358. doi: 10.1002/anie.201103014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xiao Y., Zhang L. Org. Lett. 2012;14:4662. doi: 10.1021/ol302102h. [DOI] [PubMed] [Google Scholar]; (f) Yan Z.-Y., Xiao Y., Zhang L. Angew. Chem., Int. Ed. 2012;51:8624. doi: 10.1002/anie.201203678. [DOI] [PubMed] [Google Scholar]; (g) Tokimizu Y., Oishi S., Fujii N., Ohno H. Org. Lett. 2014;16:3138. doi: 10.1021/ol5012604. [DOI] [PubMed] [Google Scholar]; (h) During the preparation of this manuscript, an intramolecular reaction between alkyne and 2H-azirine groups was reported: Prechter A., Henrion G., dit Bel P. F., Gagosz F., Angew. Chem., Int. Ed., 2014, 53 , 4959 . [DOI] [PubMed] [Google Scholar]
- (a) Li C., Zhang L. Org. Lett. 2011;13:1738. doi: 10.1021/ol2002607. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Davies P. W., Cremonesi A., Dumitrescu L. Angew. Chem., Int. Ed. 2011;50:8931. doi: 10.1002/anie.201103563. [DOI] [PubMed] [Google Scholar]; (c) Chatzopoulou E., Davies P. W. Chem. Commun. 2013;49:8617. doi: 10.1039/c3cc45410j. [DOI] [PubMed] [Google Scholar]; (d) Hung H.-H., Liao Y.-C., Liu R.-S. J. Org. Chem. 2013;78:7970. doi: 10.1021/jo401161h. [DOI] [PubMed] [Google Scholar]
- For recent reviews on ynamide reactivity, see: ; (a) Wang X.-N., Yeom H.-S., Fang L.-C., He S., Ma Z.-X., Kedrowski B. L., Hsung R. P. Acc. Chem. Res. 2014;47:560. doi: 10.1021/ar400193g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeKorver K. A., Li H., Lohse A. G., Hayashi R., Lu Z., Zhang Y., Hsung R. P. Chem. Rev. 2010;110:5064. doi: 10.1021/cr100003s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Evano G., Coste A., Jouvin K., Angew. Chem., Int. Ed., 2010, 49 , 2840 , ; for recent selected examples on the gold-catalyzed reactions of ynamides, see: . [DOI] [PubMed] [Google Scholar]; (d) Pawar S. K., Vasu D., Liu R.-S. Adv. Synth. Catal. 2014;356:2411. [Google Scholar]; (e) Rettenmeier E., Schuster A. M., Rudolph M., Rominger F., Gade C. A., Hashmi A. S. K. Angew. Chem., Int. Ed. 2013;52:5880. doi: 10.1002/anie.201301382. [DOI] [PubMed] [Google Scholar]; (f) Heffernan S. J., Beddoes J. M., Mahon M. F., Hennessy A. J., Carbery D. R. Chem. Commun. 2013;49:2314. doi: 10.1039/c3cc00273j. [DOI] [PubMed] [Google Scholar]; (g) Karad S. N., Bhunia S., Liu R.-S. Angew. Chem., Int. Ed. 2012;51:8722. doi: 10.1002/anie.201203723. [DOI] [PubMed] [Google Scholar]; (h) Dateer R. B., Shaibu B. S., Liu R.-S. Angew. Chem., Int. Ed. 2012;51:113. doi: 10.1002/anie.201105921. [DOI] [PubMed] [Google Scholar]; (i) Dateer R. B., Pati K., Liu R.-S. Chem. Commun. 2012;48:7200. doi: 10.1039/c2cc33030j. [DOI] [PubMed] [Google Scholar]
- (a) Pinho e Melo T. M. V. D. Curr. Org. Chem. 2005;9:925. [Google Scholar]; (b) Hansen T. V., Wu P., Fokin V. V. J. Org. Chem. 2005;70:7761. doi: 10.1021/jo050163b. [DOI] [PubMed] [Google Scholar]; (c) Tang S., He J., Sun Y., He L., She X. Org. Lett. 2009;11:3982. doi: 10.1021/ol901626n. [DOI] [PubMed] [Google Scholar]; (d) Debleds O., Gayon E., Ostaszuk E., Vrancken E., Campagne J.-M. Chem.–Eur. J. 2010;16:12207. doi: 10.1002/chem.201001461. [DOI] [PubMed] [Google Scholar]; (e) Griesbeck A. G., Franke M., Neudörfl J., Kotaka H. Beilstein J. Org. Chem. 2011;7:127. doi: 10.3762/bjoc.7.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see: ; (a) Kumar T. B., Sumanth C., Vaishaly S., Rao M. S., Sekhar K. B. C., Meda C. L. T., Kandale A., Rambabu D., Krishna G. R., Reddy C. M., Kumar K. S., Parsa K. V. L., Pal M. Bioorg. Med. Chem. Lett. 2012;22:5639. doi: 10.1016/j.bmcl.2012.06.100. [DOI] [PubMed] [Google Scholar]; (b) Frolova L. V., Evdokimov N. M., Hayden K., Malik I., Rogelj S., Kornienko A., Magedov I. V. Org. Lett. 2011;13:1118. doi: 10.1021/ol103149b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jalani H. B., Pandya A. N., Baraiya A. B., Kaila J. C., Pandya D. H., Sharma J. A., Sudarsanam V., Vasu K. K. Tetrahedron Lett. 2011;52:6331. [Google Scholar]; (d) Wallace M. B., Adams M. E., Kanouni T., Mol C. D., Dougan D. R., Feher V. A., O'Connell S. M., Shi L., Halkowycz P., Dong Q. Bioorg. Med. Chem. Lett. 2010;20:4156. doi: 10.1016/j.bmcl.2010.05.058. [DOI] [PubMed] [Google Scholar]; (e) Onnis V., De Log A., Cocco M. T., Fadda R., Meleddu R., Congiu C. Eur. J. Med. Chem. 2009;44:1288. doi: 10.1016/j.ejmech.2008.08.003. [DOI] [PubMed] [Google Scholar]; (f) Migawa M. T., Drach J. C., Townsend L. B. J. Med. Chem. 2005;48:3840. doi: 10.1021/jm0402014. [DOI] [PubMed] [Google Scholar]; (g) Lauria A., Bruno M., Diana P., Barraja P., Montalbano A., Cirrincione G., Dattolo G., Almerico A. M. Bioorg. Med. Chem. 2005;13:1545. doi: 10.1016/j.bmc.2004.12.027. [DOI] [PubMed] [Google Scholar]; (h) Cocco M. T., Congiu C., Onnis V. Bioorg. Med. Chem. 2003;11:495. doi: 10.1016/s0968-0896(02)00465-0. [DOI] [PubMed] [Google Scholar]; (i) Tanaka M., Sasaki Y., Kimura Y., Fukui T., Ukai Y. BJU Int. 2003;92:1031. doi: 10.1111/j.1464-410x.2003.04512.x. [DOI] [PubMed] [Google Scholar]; (j) Stephens C. E., Felder T. M., Sowell Sr J. W., Andrei G., Balzarini J., Snoeck R., Clercq E. D. Bioorg. Med. Chem. 2001;9:1123. doi: 10.1016/s0968-0896(00)00333-3. [DOI] [PubMed] [Google Scholar]; (k) Laufer S., Striegel H. G. and Dannhardt G., Ger. Offen. DE 44 19 315A1, 1995.; (l) Bennet S. M., Nguyen-Ba N., Ogilvie K. K. J. Med. Chem. 1990;33:2162. doi: 10.1021/jm00170a019. [DOI] [PubMed] [Google Scholar]; (m) Kobayshi J., Cheng J., Kikuchi Y., Ishibashi M., Yamamura S., Ohizumi Y., Ohta T., Nozoe S. Tetrahedron Lett. 1990;31:4617. [Google Scholar]
- For recent examples on 2-aminopyrrole synthesis, see: ; (a) Qi X., Xiang H., He Q., Yang C. Org. Lett. 2014;16:4186. doi: 10.1021/ol5018855. [DOI] [PubMed] [Google Scholar]; (b) Wang X., Xu X.-P., Wang S.-Y., Zhou W., Ji S.-J. Org. Lett. 2013;15:4246. doi: 10.1021/ol401976w. [DOI] [PubMed] [Google Scholar]; (c) Yu W., Chen W., Liu S., Shao J., Shao Z., Lin H., Yu Y. Tetrahedron. 2013;69:1953. [Google Scholar]; (d) Frolova L. V., Evdokimov N. M., Hayden K., Malik I., Rogelj S., Kornienko A., Magedov I. V. Org. Lett. 2011;13:1118. doi: 10.1021/ol103149b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Fontaine P., Masson G., Zhu J. Org. Lett. 2009;11:1555. doi: 10.1021/ol9001619. [DOI] [PubMed] [Google Scholar]; (f) Barnea E., Majumder S., Staples R. J., Odom A. L. Organometallics. 2009;28:3876. [Google Scholar]; (g) Chien T.-C., Meade E. A., Hinkley J. M., Townsend L. B. Org. Lett. 2004;6:2857. doi: 10.1021/ol049207d. [DOI] [PubMed] [Google Scholar]; (h) Nair V., Vinod A. U., Rajesh C. J. Org. Chem. 2001;66:4427. doi: 10.1021/jo001714v. [DOI] [PubMed] [Google Scholar]
- For recent Zn(OTf)2-catalyzed metathesis reactions between 3-en-1-ynamides and nitrosoarenes, see: Gawade S. A., Huple D. B., Liu R.-S., J. Am. Chem. Soc., 2014, 136 , 2978 . [DOI] [PubMed] [Google Scholar]
- Kramer S., Odabachian Y., Overgaard J., Rottländer M., Gagosz F., Skrydstrup T. Angew. Chem., Int. Ed. 2011;50:5090. doi: 10.1002/anie.201100327. [DOI] [PubMed] [Google Scholar]
- For examples on gold-catalyzed oxidative-cyclopropanation, see: ; (a) Wang K.-B., Ran R.-Q., Xiu S.-D., Li C.-Y. Org. Lett. 2013;15:2374. doi: 10.1021/ol4007629. [DOI] [PubMed] [Google Scholar]; (b) Vasu D., Hung H.-H., Bhunia S., Gawade S. A., Das A., Liu R.-S. Angew. Chem., Int. Ed. 2011;50:6911. doi: 10.1002/anie.201102581. [DOI] [PubMed] [Google Scholar]
- ESI.
- (a) Li Z., Xu X., Ye Z., Qian X., Shao X., Xu Z., Zeng B. and Song G., PCT Int. Appl. WO 2013007168A1, 2013.; (b) Lowman H. B. and Liu S., PCT Int. Appl. WO 2013192546A1, 2013.; (c) Kerr W. G., PCT Int. Appl. WO 2010045199A2, 2010.; (d) Lagrange A., PCT Int. Appl. WO 2007071686A1, 2007.; (e) Cotteret J. and Lagrange A., Eur. Pat. Appl. EP 1428510A1, 2004.; (f) Mckee T. D. and Suto R. K., PCT Int. Appl. WO 2003073999A2, 2003.; (g) Lang G., PCT Int. Appl. WO 2001066071A1, 2001.; (h) Audousset M. P., Eur. Pat. Appl. EP 1002520A1, 2000.; (i) Vidal L. and Malle G., PCT Int. Appl. WO 9735554A1, 1997.
- (a) Zhang L., Sun J., Kozmin S. A. Adv. Synth. Catal. 2006;348:2271. [Google Scholar]; (b) Davies P. W., Cremonesi A., Martin N. Chem. Commun. 2011;47:379. doi: 10.1039/c0cc02736g. [DOI] [PubMed] [Google Scholar]; (c) Lu B., Li C., Zhang L. J. Am. Chem. Soc. 2010;132:14070. doi: 10.1021/ja1072614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DFT studies on substituent effects are given in the ESI.
- For theoretical calculations involving α-oxo gold carbenes, see: ; (a) Schulz J., Jašíková L., Škríba A., Roithová J. J. Am. Chem. Soc. 2014;136:11513. doi: 10.1021/ja505945d. [DOI] [PubMed] [Google Scholar]; (b) Lu B., Li Y., Wang Y., Aue D. H., Luo Y., Zhang L. J. Am. Chem. Soc. 2013;135:8512. doi: 10.1021/ja401343p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima C., Mukai N., Yamamoto Y., Tsuda Y., Omote Y. Heterocycles. 1977;7:241. [Google Scholar]
- Manning J. R., Davies H. M. L. J. Am. Chem. Soc. 2008;130:8602. doi: 10.1021/ja803139k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen W., Traulsen T., Elguero J., Katritzky A. R. Adv. Heterocycl. Chem. 2000;76:85. [Google Scholar]
- For selected examples of acid-catalyzed deacylation of pyrroles, see: ; (a) Ondrus A. E., Kaniskan H. Ü., Movassaghi M. Tetrahedron. 2010;66:4784. doi: 10.1016/j.tet.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barrero F., Sánchez J. F., Oltra J. E., Teva D. J. J. Heterocycl. Chem. 1991;28:939. [Google Scholar]; (c) Smith K. M., Miura M., Tabba H. D. J. Org. Chem. 1983;48:4779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.