Abstract
Background and aims
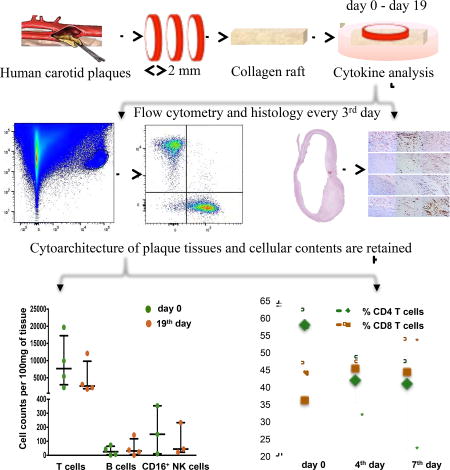
The mechanisms that drive atherosclerotic plaque progression and destabilization in humans remain largely unknown. Laboratory models are needed to study these mechanisms under controlled conditions. The aim of this study was to establish a new ex vivo model of human atherosclerotic plaques that preserves the main cell types in plaques and the extracellular components in the context of native cytoarchitecture.
Methods
Atherosclerotic plaques from carotid arteries of 28 patients undergoing carotid endarterectomy were dissected and cultured. At various time-points, samples were collected and analysed histologically. After enzymatic digestion, single cells were analysed with flow cytometry. Moreover, tissue cytokine production was evaluated.
Results
We optimised the plaque dissection protocol by cutting plaques into circular segments that we cultured on collagen rafts at the medium–air interface, thus keeping them well oxygenated. With this technique, the relative presence of T and B lymphocytes did not change significantly during culture, and the sizes of lymphocyte subsets remained stable after day 4 of culture. Macrophages, smooth muscle cells, and fibroblasts with collagen fibres, as well as both T and B lymphocyte subsets and CD16 natural killer cells, remained largely preserved for 19 days of culture, with a continuous production of inflammatory cytokines and chemokines.
Conclusions
Our new model of ex vivo human atherosclerotic plaques, which preserves the main subsets of immune cells in the context of tissue cytoarchitecture, may be used to investigate important aspects of atherogenesis, in particular, the functions of immune cells under controlled laboratory conditions.
Keywords: Atherosclerosis, atherosclerotic plaque, tissue culture, immune system, lymphocyte, cytokine
Graphical abstract
Introduction
Atherosclerosis and its cardiac and cerebral complications are the leading causes of death from cardiovascular diseases. For a long time, accumulation of modified lipoproteins within the arterial wall was considered to be the main cause of atherosclerotic disease [1]. More recently, however, cells of various types, such as smooth muscle cells, macrophages, and T cells, have been found to play an important role in atherosclerotic plaque formation [2]. Moreover, a currently accepted theory of atherogenesis emphasizes the role of immune system activation caused by oxidized lipoproteins, which activate endothelial cells (as do other foreign agents within the vascular wall) [3,4]. It is thought that immune cells are attracted by chemokines, which are produced by activated endothelial cells, and migrate into the subendothelium, where they proliferate, leading to atherosclerotic plaque progression [2,5–7].
The role of immune cells in the growth of plaques has been confirmed in experimental models on immunodeficient mice, as well as from the presence of autologous antibodies against oxidized low density lipoproteins in atherosclerotic plaques [8,9]. In our earlier work, we demonstrated T lymphocyte activation in human atherosclerotic plaques in comparison with the blood of the same patients [10], thus providing further evidence for the involvement of the immune system in atherogenesis.
Despite plentiful evidence for the critical role of the immune system in atherosclerosis, many important aspects of this phenomenon remain unknown. The lack of this knowledge is in part due to limitations on access to human atherosclerotic plaques in vivo, while animal models are often not adequate because of differences in structures of arterial walls [11,12]. In vitro laboratory-controlled systems are required for the study of atherosclerotic plaque formation and rupture. Several such models with cells of only one or two types cultured together have been suggested [13,14]; however, none of them faithfully reproduces the whole range of intercellular interactions within human atherosclerotic plaques [15,16].
Here, we describe a new ex vivo model of human atherosclerotic plaques, which preserves the main cell types of plaques in vivo, together with the general tissue cytoarchitecture. We think that this model may prove useful for investigation of immune cell function in atherogenesis and for development of novel therapeutic approaches to atherosclerosis treatment.
Materials and methods
For a detailed description of the Materials and methods see Supplementary Data.
Patients
We collected atherosclerotic plaques from carotid arteries of 28 patients with peripheral artery disease, undergoing carotid endarterectomy because of extended atherosclerosis (19 men and 9 women; mean age ± standard deviation = 65.4 ± 8.5 years). The degree of carotid artery stenosis varied from 65% to 90% (median 90.0%, interquartile range (IQR) 73.8% to 90.0%). Ten patients suffered from transient ischemic attack or stroke within 5 years before surgery, and more than half of all plaque specimens (60.7%) were ruptured, as determined from macroscopic evaluation. All patients’ characteristics are presented in Supplementary Table1.
This protocol was approved by the A.I. Yevdokimov Moscow State University of Medicine and Dentistry Ethics Committee. All the participants provided written informed consent.
Tissue processing
Our work was based on the pioneer work of Dr. Hoffman [17,18], who developed the technique of histoculture that makes possible maintenance of blocks of mammalian tissues for weeks at the air–liquid interface.
According to the protocol, surgical atherosclerotic plaque samples were dissected and divided into three parts: one part of the material was fixed in 4% formaldehyde (Pierce, Thermo Fisher Scientific, Waltham, MA, USA, cat. 28908) and embedded in paraffin for histological examination, the second part was digested with an enzymatic cocktail into a single-cell suspension for flow cytometry. The third part was dissected, placed on a wetted collagen sponge raft (Pfizer, New York, NY, USA, cat. 0315-08) at the medium–air interface, and cultured. After one day of culture, and then every 3rd day, culture medium was collected and replaced with fresh medium. Every 3rd day, several tissue blocks were analysed histologically and by means of flow cytometry.
Histology
Histology, histochemistry, and immunohistochemistry were performed according to standard techniques. We focused on several cell types that could not be properly isolated from plaques by enzymatic treatment and thus were not analysed with flow cytometry. In particular, we assessed fibroblasts and collagen tissue, macrophages, and smooth muscle cells, using Masson’s Trichrome staining (Agilent Technologies, Santa Clara, CA, USA, cat. AR17392-2), antibodies against CD68 (clone KP1, Agilent Technologies) and α-smooth muscle actin (α-SMA) (clone 1A4, Agilent Technologies), respectively.
We analysed the concentration of 34 cytokines and chemokines in culture medium from plaque explants using an original multiplex bead array assay developed by us. Briefly, samples were serially diluted in assay buffer; 50 µL of each sample was added to the well with 50 µL of bead mixture and incubated overnight at 4°C in 96-well flat bottom plates. The plates were washed twice with 200 µL of assay buffer. The beads were then resuspended in 50 µL of assay buffer containing biotinylated polyclonal antibodies against the measured proteins for 1 hour at RT. The plates were washed twice with PBS, the beads were resuspended in 50 µL per well of assay buffer with streptavidin-PE at 16 µg/mL (Molecular Probes, Thermo Fisher Scientific, cat. S866). The plates were read on a Luminex-100 platform and analysed with the Bioplex Manager software (Bio-Rad, Hercules, CA, USA).
Flow cytometry
To isolate T cells from atherosclerotic plaques with minimal stripping off of cell surface markers, we digested plaques with an enzymatic cocktail according to our previously developed method with minor changes [10]. Briefly, blocks of cultured plaques were weighed and then cut into cubes with 2-mm sides. Tissues were digested by an enzymatic mixture: RPMI 1640 Advanced (Gibco, Thermo Fisher Scientific, cat. 12633012) with collagenase XI from Clostridium histolyticum at 1.25 mg/ml (Sigma-Aldrich, Saint-Loius, MS, USA, cat. C9697) and desoxyribonuclease I at 0.2 mg/ml (Sigma-Aldrich, cat. D5319). Isolated cells were stained with monoclonal antibodies against clusters of differentiation (CD) 45 (PE-Cy7, eBioscience, cat. 25-0459), CD3 (PerCP-Cy5.5, eBioscience, Thermo Fisher Scientific, cat. 45-0037), CD19 (PE, eBioscience, cat. 12-0199), CD4 (APC-eFluor 780, eBioscience, cat. 47-0048), CD8α (eFluor 450, eBioscience, cat. 25-0087), and CD16 (FITC, eBioscience, cat. 11-0168). We estimated the number of cells per 100 mg of tissue using AccuCheck Counting Beads (Thermo Fisher Scientific, cat. PCB100). Flow cytometry was performed on BD FACS Canto II and FACS Aria II (Becton Dickinson Biosciences, San Jose, CA, USA). Data were acquired with Diva 6 and 8 and analysed with FlowJo 9.4 and 10 (Tree Star, Ashland, OR, USA). A flowchart of experiments is provided in Supplementary Fig.2.
Statistical analyses
The data obtained in the present study were not normally distributed, according to the Shapiro-Wilk test, and are presented as medians and IQR. Since distributions were not normal, for comparison of two independent groups we used the Mann-Whitney rank test, and for dependent groups we used the Wilcoxon matched pair test, Friedman ANOVA, and Kendall test. To assess between-group effects, we used a multiple comparisons rank test. For the age distribution, we made the assumption of its normality. Statistical analysis was performed with Statistica 10.0 (Statsoft, Tulsa, OK, USA) and SPSS Statistics 21.0 (IBM, Armonk, NY, USA). Values of p <0.05 were considered statistically significant.
Results
Histology of ex vivo plaques
Initially, we separated atherosclerotic plaques from normal artery tissue and dissected them into ~2-mm cubic blocks for culture, similarly to what was successfully used earlier to culture various human tissues ex vivo [17,19]. However, analysis of stained histological sections showed that the viability of cultured tissue blocks of this size decreased over 8–12 days, and cultured blocks contained only ~20 live cells per 100 mg of tissue (Supplementary Fig.3).
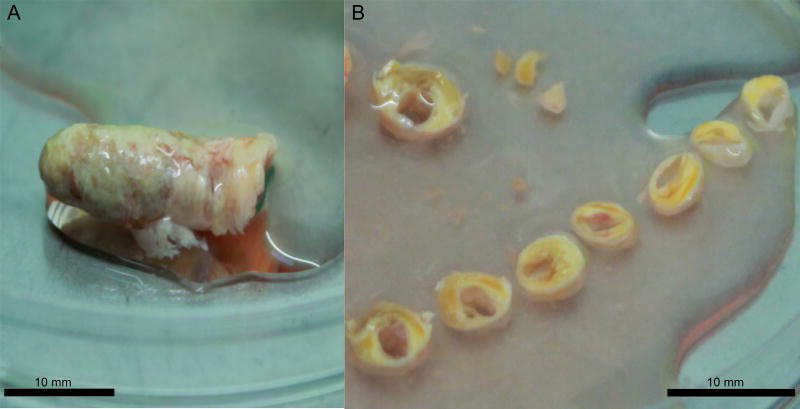
We thought that the tissues might be damaged during dissection into small blocks. Therefore, to diminish tissue injury during preparation, we modified our protocol and instead of dissecting into small blocks, we sliced tissue into ring-shaped 2-mm thick segments, and with a diameter depending of the carotid artery size (Fig. 1). To verify tissue viability, every 3rd day several dissected segments were analysed histologically and by means of flow cytometry.
Figure 1.
Preparation of plaque tissue as ring-shaped segments.
(A) Atherosclerotic plaque obtained from carotid endarterectomy. (B) Circular plaque sections prepared for culture.
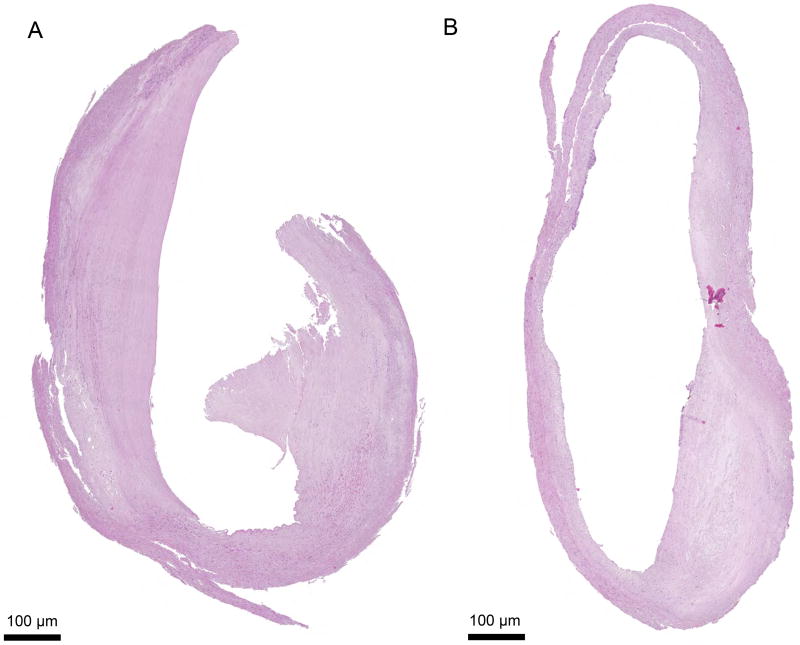
Analysis of histological sections showed that the dissection of plaques into large circular segments significantly increased cell survival: tissues were preserved for 19 days. For histological evaluation, tissue segments were stained with hematoxylin and eosin, Masson’s Trichrome, anti-CD68, and anti-α-SMA antibodies and their morphology was assessed as described in Materials and methods. We found that these plaque segments retained their gross morphology and appeared viable for more than 19 days of culture. In particular, the integrity of the endothelium and internal elastic membrane, which are most sensitive to the culture conditions, were preserved over 19 days of culture without a significant increase in the necrotic core area (Fig. 2).
Figure 2.
Histology sections of circular plaque segments.
Histology sections of plaque explants at days (A) 0 and (B) 19 of culture in circular blocks. At day 19 of culture, plaque tissues preserved their structure and the endothelial layer of the vessel. A typical example of tissue morphology, out of 16 replicates, is shown.
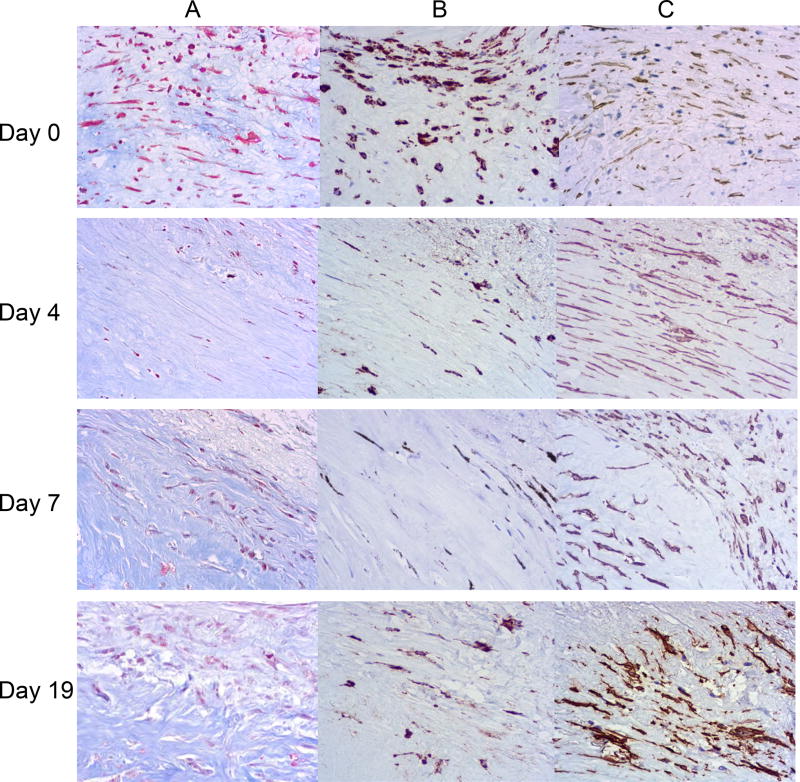
Furthermore, in 6 plaques, we quantified areas reacting with aniline blue, anti-CD68, and anti-α-SMA at days 0, 4, 7, and 19 (Fig. 3). We identified macrophages, fibroblasts, and smooth muscle cells, along with the endothelium, until day 19 of culture. We found no statistically significant changes (p >0.05) in the fraction of these cells during the entire culture period (Table 1).
Figure 3.
Immune and histochemical staining of plaque explants during culture. Histology staining of plaque explants at days 0, 4, 7, and 19 with (A) Masson’s Trichrome, (B) anti-CD68 antibodies, and (C) anti-α-SMA antibodies. A typical example of tissue morphology, out of 6 replicates, is shown.
Table 1. Immuno- and histochemical quantification of plaque cells in culture.
Indexed area of cells from the total section area in percentages for populations of α-SMA+ smooth muscle cells, CD68+ macrophages, and aniline blue+ collagen and fibroblasts at days 0, 4, 7, and 19; indexed quantity of cells per 1 cm of vessel circumference for H&E endothelial cells at days 0, 4, 7, and 19. Medians and IQR for plaques from six donors cultured for 19 days are presented.
| Cell type/day of culture | α-SMA+, % from total area |
aniline blue+, % from total area |
CD68+, % from total area |
H&E, per 1 cm of vessel circle |
|---|---|---|---|---|
| Day 0, Me [IQR] | 11.5 [8.8–17.5] | 82.9 [74.2–86.8] | 11.9 [5.2–13.7] | 13.9 [5.3–22.3] |
| Day 4, Me [IQR] | 19.6 [16.4–20.3] | 79.4 [72.5–85.1] | 4.9 [4.6–5.1] | 4.3 [2.5–9.5] |
| Day 7, Me [IQR] | 11.9 [10.5–14.8] | 81.1 [71.1–83.3] | 8.3 [4.3–14.2] | 4.6 [1.2–11.7] |
| Day 19, Me [IQR] | 15.6 [13.9–17.0] | 84.4 [73.9–88.7] | 4.1 [2.0–5.9] | 10.1 [2.9–11.5] |
| p-level | 0.066 | 0.204 | 0.133 | 0.294 |
Flow cytometry of plaque cells
In plaque samples from 16 donors, we assessed tissue viability using flow cytometry by analysing immune cells extracted from plaque tissue. We compared flow cytometry results at day 0 with those at days 4, 7, and 19. Towards this goal, we digested plaque segments with an enzymatic cocktail containing collagenase XI and desoxyribonuclease I, washed isolated cells, and stained them with live/dead staining and monoclonal antibodies against CD45, CD3, CD19, CD4, CD8, and CD16. Two plaques were excluded from the analysis because of a low cellularity at day 0 (lower than 500 live cells per 100 mg of tissue).
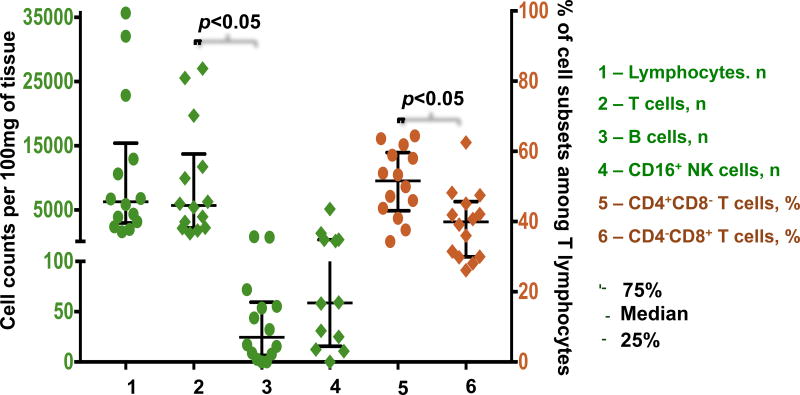
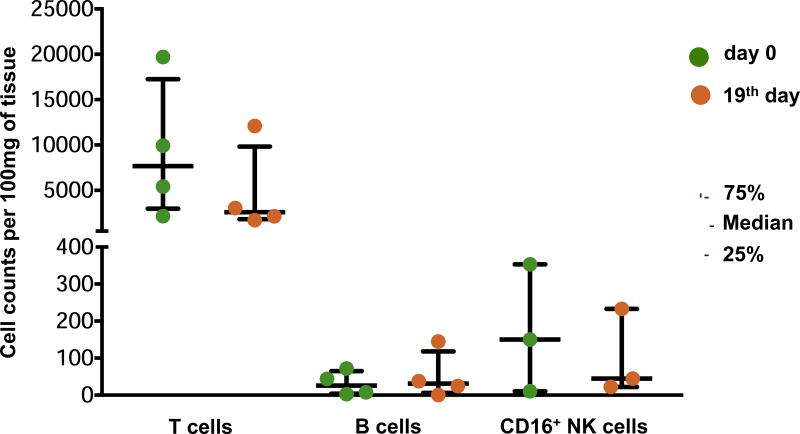
Analysis of tissue at day 0 revealed a median of 6,286.0, IQR [3,172.1–12,918.9] lymphocytes per 100 mg of plaque tissue. The absolute numbers and percentages of B lymphocytes among all lymphocytes at day 0 were significantly lower than those of T lymphocytes (24.6 [7.8–55.3] cells/100 mg vs. 5,694.1 [2,26.7–11,726.6] cells/100 mg; 0.4% [0.1%–0.5%] vs. 89.6% [84.3%–91.2%], p=0.001). At day 0, among T lymphocytes, the fraction of CD4+CD8− cells was larger than the fraction of CD4−CD8+ cells (51.6% [43.8%–58.9%] vs. 39.9% [30.0%–45.1%], p=0.041). The median amount of CD16 NK cells at day 0 was 58.6 [18.7–360.9] per 100 mg of plaque tissue (Fig. 4). Consecutive flow cytometry after day 0 was performed in plaques from 8 patients, four of which were cultured until day 19. We showed that, after a decrease during the first 4 days of culture (n=8, 4,125.8 [2,771.4–6,286.0] cells/100 mg at day 0 vs. 2,619.3 [1,360.8–3,712.2] cells/100 mg at day 4, p=0.036), the amounts of lymphocytes stabilized and did not change significantly until the 7th day of culture (n=8, 1,249.6 [445.5–3,706.0] cells/100 mg; p=0.161). A similar pattern was found in T cells, with a stabilization of their amounts after a reduction during the first 4 days of culture (n=8, 3,546.5 [2,194.2–5,694.1] cells/100 mg at day 0 vs. 2,123.4 [1,210.5–3,280.1] cells/100 mg at day 4, p=0.036 vs. 949.5 [375.1–2,378.3] cells/100 mg at day 7, p=0.124). In addition, we found no significant changes in the amounts of B cells during the first days of culture (n=8, 12.2 [5.3–35.3] cells/100 mg at day 0 vs. 5.6 [3.3–37.2] cells/100 mg at day 4 vs. 7.9 [0.0–11.4] cells/100 mg at day 7, p=0.798). Furthermore, both T and B cells were also preserved in plaque tissues during 19 days of culture, although their ratio changed slightly because of the decrease in the number of T lymphocytes (n=4, 7,673.4 [3,789.0–14,813.2] cells/100 mg vs. 2,594.5 [1,926.8–7,569.0] cells/100 mg for T cells, and 25.8 [5.3–57.9] cells/100 mg vs. 31.0 [12.2–91.2] cells/100 mg for B cells). CD16 NK cells were found at day 19 as well: the median cell count at the last day of culture constituted 44.9 [21.9–233.0] cells/100 mg (Fig. 5). We presume that the initial fall in T cell count may originate from the intense effect of tissue dissection, while the statistically significant decrease in the amounts of B cells and CD16 NK cells may not have been revealed because of the small size of these cell subsets. These changes were followed by a subsequent system stabilization. This was also evident by the lack of significant changes (p=0.417) in the fraction of dead cells, which even at day 19 remained at the level of on average 11.2% [10.1%–14.6%].
Figure 4.
Comparison of populations of lymphocytes in atherosclerotic plaques before culture.
Populations of lymphocytes in atherosclerotic plaques at day 0. For lymphocytes, T cells, B cells, and CD16+ NK cells data are shown as absolute cell counts (green); data for CD4+ and CD8+ T cells are shown as percentages of the T lymphocyte population (brown), as determined from flow cytometry. Medians and IQR for plaques from 14 donors are presented.
Figure 5.
Populations of lymphocytes in plaques at day 0 and day 19 of culture. Absolute cell counts for populations of T cells, B cells, and CD16+ NK cells at day 0 (green) and day 19 (brown) of culture, as determined from flow cytometry. Medians and IQR for plaques from 4 donors cultured for 19 days are presented.
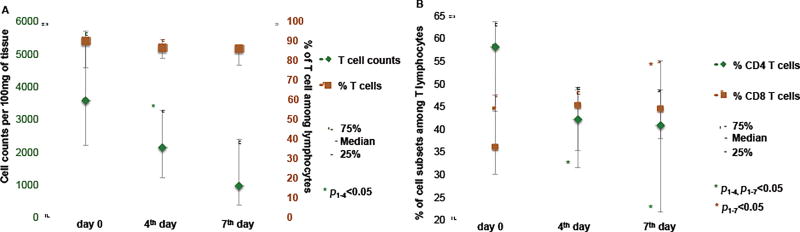
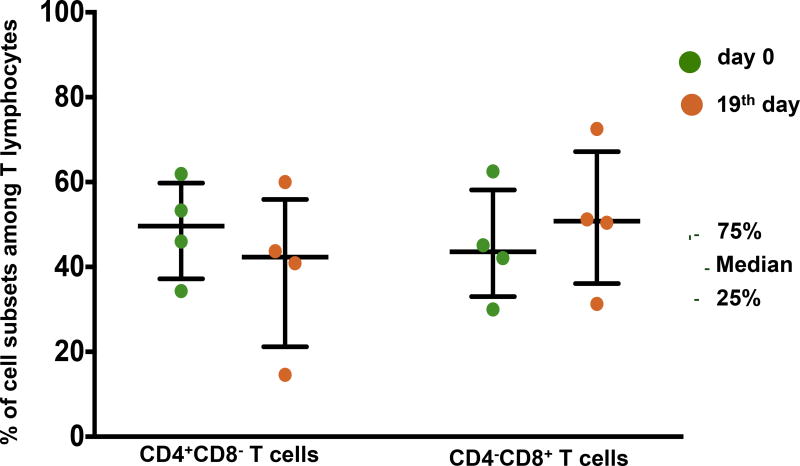
Importantly, the initial drop in T cell count was not accompanied by significant changes in the fraction of T cells among all lymphocytes (n=8, 89.6% [76.3%–91.2%] at day 0 vs. 86.2% [81.1%–90.8%] at day 4, p=0.779; vs. 86.0% [77.5%–86.9%] at day 7, p=0.674) (Fig. 6A). The decrease in T cell numbers during the first days of culture was predominantly associated with the reduction of the fraction of CD4 T cells (n=7, 58.0% [43.8%–63.6%] at day 0 vs. 42.0% [31.4%–49.2%] at day 4, p=0.018; vs. 40.8% [21.6%–48.7%] at day 7, p=0.018), accompanied by a minor rise in the fraction of CD8 T cells (n=7, 36.0% [30.0%–47.5%] at day 0 vs. 45.2% [35.3%–48.6%] at day 4, p=0.063; vs 44.4% [37.9%–55.1%] at day 7, p=0.018) (Figure 6B). As a result of these changes, the CD4+CD8−/CD4−CD8+ ratio decreased slightly during culture. As of the 19th day of culture, the fraction of CD4 T cells was reduced with a concurrent increase in the fraction of CD8 T cells (49.7% [40.2%–57.6%] vs. 42.3% [27.8%–51.9%] for CD4 T cells, and 43.6% [36.1%–53.8%] vs. 50.8% [40.9%–61.9%] for CD8 T cells) (Figure 7). Nevertheless, both CD4 and CD8 T cells were also preserved in culture for at least 19 days.
Figure 6.
Dynamics of T cell counts during first days of culture.
(A) Changes in T cell absolute counts (green) and percentages (brown) among all lymphocytes. (B) Changes in CD4+ (green) and CD8+ (brown) T cell fractions of lymphocytes between day 0 and day 7 of culture, as determined from flow cytometry. Medians and IQR for cultured plaques from 8 donors are presented.
Figure 7.
Subpopulations of lymphocytes in plaques at day 0 and day 19 of culture. The fractions of CD4+CD8− T cells and CD4−CD8+ T cells at day 0 (green) and day 19 (brown) of culture, as determined from flow cytometry. Medians and IQR for plaques from 4 donors cultured for 19 days are presented.
Cytokine production by plaques ex vivo
We analysed the concentrations of cytokines and chemokines released by six cultured plaques and accumulated in the culture medium from day 1 to day 4 and from day 16 to day 19 when the medium was changed. We found that in our system plaques produce substantial amounts of interleukin (IL)-1α, IL-6, IL-8, IL-16, IL-18, IL-21, IL-22, eotaxin, interferon-λ, granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage-CSF, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, growth related oncogene (GRO)-α, interferon gamma-inducible protein-10, monocyte chemoattractant protein-1, monokine induced by gamma interferon, macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES. In contrast, the concentrations of the other measured cytokines and chemokines were lower than the detection limit of the Luminex platform for these analytes.
Within the panel of cytokines and chemokines that were detectable in the culture medium, we found no significant changes during culture in most of the cytokines, except for IL-16 and several cytokines whose concentration decreased, in particular IL-8 (n=6, from 25,698.7 [14,817.3–52,553.3] pg/ml on day 4 to 4,273.1 [2,695.6–6,274.2] pg/ml on day 19), and to a lesser extent GM-CSF, TNF-α, and GRO-α. At the same time, the concentrations of other cytokines (including eotaxin, TGF-β, and MIP-1β) increased during culture (Supplementary Table 2).
Discussion
Historically, atherosclerosis was considered as a storage disease induced by accumulation and further oxidation of lipids within the arterial wall [1]. However, the simple accumulation of lipids cannot explain the structural complexity of atherosclerotic plaques, which has been known for a long time: it was first described by R. Virchow [20]. Nowadays it is thought that the deposition in the vascular wall of a foreign substance, namely modified lipoproteins, leads to activation of the immune system in an attempt to destroy it [5,21,22]. Therefore, innate and adaptive immunity play significant roles in atherogenesis, with both local and systemic inflammatory processes [5,7,23]. It was also found that different subsets of T lymphocytes, both in atherosclerotic plaques and in blood, are associated with a risk of cardiovascular events in patients with coronary artery disease [7,24]. Advanced plaques contain more dendritic cells and lymphocytes, and in particular more activated oligoclonal T cells, than stable ones [25–27]. In the present work, we have also shown that atherosclerotic plaques from human carotid arteries are enriched with lymphocytes, in particular with T cells [10].
Detailed study of these complex processes requires authentic laboratory models. The first experimental model developed to study atherosclerotic processes used animals exposed to a high-lipid diet. In particular, the first model of cholesterol-fed rabbit for atherogenesis studies was established as early as in 1913 by N. Anitschkow [28]. Later, hyperlipidemic miniature swine, hamsters, and mice were introduced [12,16].
However, it was discovered that atherosclerotic plaques in these animal models have different cellular content and lack some intracellular components, such as myeloperoxidase, in comparison with human plaques, and therefore these experimental models fail faithfully to reflect important intercellular interactions in human atherosclerotic plaques [12,15,16,29]. Nowadays, experimental studies are often carried out in mice with a considerably smaller content of CD8+ T cells than in humans [11].
A number of in vitro models have been developed to study atherosclerosis. In particular, researchers used monocultures of endothelial cells, of smooth muscle cells, and of foam cells [30–32]. However, because these cells are isolated from their native microenvironment, the single-cell cultures do not reflect the complexity of intercellular interactions in atherosclerotic plaques. In attempts to overcome these limitations, co-cultures of endothelial cells and smooth muscle cells or monocytes have been used [33,34]. Although more adequate than single-type cell cultures, these co-cultures lack the extracellular components typical of plaque formation and also do not contain all the cell types present in atherosclerotic plaques.
In other studies, similar co-cultures were embedded into vascular grafts or collagen and polyethylene gels to form extracellular matrixes in order to analyse the interaction between cells and matrix [35,36]. This made it possible to simulate the three-dimensional system and introduce medium flow to mimic the situation in an in vivo artery [13,14,37,38]. However, all these models used only two or three cell types to investigate atherogenesis, without an effect from different types of lymphocytes, which, as mentioned earlier, are important in the atherosclerotic process.
Attempts were made to culture solid tissue samples from intact carotid arteries and aortas of mice [39,40] as well as from intact parts of human internal thoracic and renal arteries [41,42]. The samples were cut into ring-shaped blocks perpendicular to the vessel lumen, with thicknesses from 2 to 5 mm. These large blocks of intact sections of arteries survived for up to 56 days [43].
These results with intact vessels gave hope that plaque tissues may also be maintained ex vivo for significant periods, but tissues of animal and human atherosclerotic lesions have been reported to remain alive for only 4–24 hours [44–46]. Nevertheless, these short-term cultures were useful for investigating the effects of exogenous T lymphocyte activation on production of different cytokines and chemokines in atherosclerotic plaques [47–49]. However, to investigate the mechanisms of plaque maturation and of immune cells in this process, longer-living ex vivo plaque tissues are necessary.
In the present study, we attempted to develop long-term cultures of human atherosclerotic plaques. Initially, we used a technique that has been described for other tissue cultures [18,19]: dissecting plaques into 2-mm cubic blocks. However, we found that such blocks maintained poorly during culture and underwent necrosis.
Instead, we dissected atherosclerotic plaques into circular segments and cultured them on collagen rafts for better oxygenation of tissue layers [18]. With this protocol, plaques obtained from human carotid arteries survived in culture for at least 19 days, as determined from both histological analysis and flow cytometry. To our knowledge, such long-term cultures of human atherosclerotic plaques, as opposed to intact artery samples, have not been described before.
Upon histological examination, we found macrophages, fibroblasts, and smooth muscle cells preserved along with the endothelial cells within 19 days of culture without significant changes in their numbers. Also, flow cytometry revealed that during 19 days of culture both T and B lymphocytes, as well as CD16 NK cells, are preserved in the explants of human plaques. The initial reduction in the numbers of lymphocytes in the first days can probably be attributed to the cell damage during tissue dissection. Nevertheless, counts of these cells stabilized with continued culture. Moreover, the relative presence of both T and B lymphocytes was constant during tissue culture. Within the T cell subsets we noticed a change in the CD4/CD8 ratio during culture that was due to a reduction of the CD4+ T cell fraction, accompanied by a minor rise in the CD8+ T cell fraction. This shift may be of interest for further studies, as it may reflect T cell activation that occurs also in vivo, albeit more slowly [50]. The results of the present study allow investigators to choose the time window of the culture at which the experiments may be performed, depending on the problems that are addressed in this system.
The viability of the plaque cultures was also confirmed from measurements of cytokines that the tissues release into the culture medium. Most of the cytokines released in significant quantities at first days of culture continued to be produced in similar quantities during the entire 19-day period of culture, and their amounts were constant. The fact that some cytokines continued to be produced and others were not produced at all indicates tight regulation of cytokine production by the cells in plaques. Variability of cytokine production and of the relative presence of different cell subsets may be related to the differences in plaques’ evolution.
Although the system of ex vivo atherosclerotic plaque provides the opportunity to study the functions of individual plaque cells under controlled laboratory conditions and may provide a platform for pre-clinical drug testing, this system has significant limitations: (i) the early stages of the disease could not be adequately reflected in this model because carotid endarterectomy is usually performed for advanced obstructive atherosclerotic plaques with a high degree of stenosis or complications; (ii) a highly complicated plaque structure with atheromatosis and intraplaque hemorrhage limits the amount of explants derived from one specimen; (iii) although an almost 3-week period of plaque viability, proven in our system, is longer than in most other published models of human atherosclerotic plaque culture, it does not permit study of plaque maturation in real-time. The ability to maintain plaques for months would provide such an opportunity.
In conclusion, we have established an adequate ex vivo model of human atherosclerotic plaques that can now be used to investigate important aspects of atherogenesis, in particular the function of immune cells in the context of complex cell–cell interactions within tissue.
Supplementary Material
Highlights.
A new ex vivo model of human atherosclerotic plaques has been established.
The cytoarchitecture of the plaques was well preserved for 19 days in culture.
The major plaque cell types were preserved for the entire culture period.
Plaque tissue continued to release cytokines and chemokines during culture.
This model can be used to study complex immunological aspects of atherogenesis.
Acknowledgments
We thank Dr. Barry Alpher for assistance in editing and improving the English style.
Financial support
The work of A.L., D.V., M.V., O.I., E.F., A.S., and E.V. was supported by Russian Federation Government grant #14.B25.31.0016 and RFBR grant #16-04-017/16. The work of W.F. and L.M. was supported by the Intramural Program of the National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
Anna Lebedeva - designed and performed the experiments, analysed the data, and contributed to the writing of all sections of the manuscript.
Daria Vorobyeva – collected atherosclerotic plaques, performed the experiments analysed the data, and contributed to the writing of the manuscript.
Murad Vagida – measured cytokine production and analysed the data.
Oxana Ivanova - performed the experiments.
Eugeny Felker - performed the experiments.
Wendy Fitzgerald - analysed cytokine production
Natalya Danilova - performed histological and immunohistochemical study
Vladimir Gontarenko – performed endarterectomy, and collected and evaluated atherosclerotic plaques.
Alexander Shpektor - conceived and designed the experiments, analysed the data.
Elena Vasilieva - conceived and designed the experiments, analysed the data, and contributed to the writing of the manuscript.
Leonid Margolis - conceived and designed the experiments, analysed the data, and contributed to the writing of all sections of the manuscript.
References
- 1.Shaikh M, Martini S, Quiney JR, Baskerville P, La Ville AE, Browse NL, et al. Modified plasma-derived lipoproteins in human atherosclerotic plaques. Atherosclerosis. 1988;69:165–72. doi: 10.1016/0021-9150(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 2.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–8. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Santos-Gallego CG, Picatoste B, Badimón JJ. Pathophysiology of Acute Coronary Syndrome. Curr. Atheroscler. Rep. 2014;16:401. doi: 10.1007/s11883-014-0401-9. [DOI] [PubMed] [Google Scholar]
- 4.Nikitskaya E, Lebedeva A, Ivanova O, Maryukhnich E, Shpektor A, Grivel J, et al. Cytomegalovirus-Productive Infection Is Associated With Acute Coronary Syndrome. J. Am. Heart Assoc. 2016;5:e003759. doi: 10.1161/JAHA.116.003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 6.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr. Opin. Pharmacol. 2010;10:197–202. doi: 10.1016/j.coph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ. J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–22. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 9.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler. Thromb. a J. Vasc. Biol. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 10.Grivel J-C, Ivanova O, Pinegina N, Blank PS, Shpektor A, Margolis LB, et al. Activation of T lymphocytes in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2011;31:2929–2937. doi: 10.1161/ATVBAHA.111.237081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am. J. Pathol. 1996;149:359–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Getz GS, Reardon CA. Animal Models of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorweiler B, Torzewski M, Dahm M, Ochsenhirt V, Lehr H-A, Lackner KJ, et al. A novel in vitro model for the study of plaque development in atherosclerosis. Thromb. Haemost. 2006;95:182–9. [PubMed] [Google Scholar]
- 14.Robert J, Weber B, Frese L, Emmert MY, Schmidt D, von Eckardstein A, et al. A three-dimensional engineered artery model for in vitro atherosclerosis research. PLoS One. 2013;8:e79821. doi: 10.1371/journal.pone.0079821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross R, Agius L. The process of atherogenesis--cellular and molecular interaction: from experimental animal models to humans. Diabetologia. 1992;35(Suppl 2):S34–40. doi: 10.1007/BF00586277. [DOI] [PubMed] [Google Scholar]
- 16.Badimon L. Atherosclerosis and thrombosis: lessons from animal models. Thromb. Haemost. 2001;86:356–65. [PubMed] [Google Scholar]
- 17.Li LN, Margolis LB, Hoffman RM. Skin toxicity determined in vitro by threedimensional, native-state histoculture. Proc. Natl. Acad. Sci. U. S. A. 1991;88:1908–12. doi: 10.1073/pnas.88.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman RM. Three-dimensional histoculture: origins and applications in cancer research. Cancer Cells. 1991;3:86–92. [PubMed] [Google Scholar]
- 19.Grivel J-C, Margolis L. Use of human tissue explants to study human infectious agents. Nat. Protoc. 2009;4:256–269. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virchov RLK, Virchow RLK. Cellular Pathology. special. Vol. 1859. Churchill, John; London, UK: 1978. [Google Scholar]
- 21.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:1421–8. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruth HS. Sequestration of aggregated low-density lipoproteins by macrophages. Curr. Opin. Lipidol. 2002;13:483–488. doi: 10.1097/00041433-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hartvigsen K, Chou M-Y, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, et al. The role of innate immunity in atherogenesis. J. Lipid Res. 2008;50:S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J. Am. Coll. Cardiol. 2007;50:1450–1458. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 25.De Palma R, Del Galdo F, Abbate G, Chiariello M, Calabró R, Forte L, et al. Patients with acute coronary syndrome show oligoclonal T-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation. 2006;113:640–646. doi: 10.1161/CIRCULATIONAHA.105.537712. [DOI] [PubMed] [Google Scholar]
- 26.Rossmann A, Henderson B, Heidecker B, Seiler R, Fraedrich G, Singh M, et al. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp. Gerontol. 2008;43:229–37. doi: 10.1016/j.exger.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B Cells) Immunity and Control by Dendritic Cells in Atherosclerosis. Circ. Res. 2014;114:1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 28.Finking G, Hanke H, Anitschkow N, Chalatow S, Anitschkow N, Bocan T, et al. Nikolaj Nikolajewitsch Anitschkow (1885–1964) established the cholesterol-fed rabbit as a model for atherosclerosis research. Atherosclerosis. 1997;135:1–7. doi: 10.1016/S0021-9150(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins PN. Molecular Biology of Atherosclerosis. Physiol. Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 30.Antonov AS, Nikolaeva MA, Klueva TS, Romanov YuA, Babaev VR, Bystrevskaya VB, et al. Primary culture of endothelial cells from atherosclerotic human aorta. Part 1. Identification, morphological and ultrastructural characteristics of two endothelial cell subpopulations. Atherosclerosis. 1986;59:1–19. doi: 10.1016/0021-9150(86)90027-4. [DOI] [PubMed] [Google Scholar]
- 31.MacLeod DC, Strauss BH, de Jong M, Escaned J, Umans VA, van Suylen RJ, et al. Proliferation and extracellular matrix synthesis of smooth muscle cells cultured from human coronary atherosclerotic and restenotic lesions. J. Am. Coll. Cardiol. 1994;23:59–65. doi: 10.1016/0735-1097(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 32.Tume RK, Bradley TR, Day AJ. An investigation by tissue culture techniques on the growth of foam cells isolated from rabbit atherosclerotic lesions. J. Atheroscler. Res. 1969;9:151–7. doi: 10.1016/s0368-1319(69)80050-5. [DOI] [PubMed] [Google Scholar]
- 33.Davies PF, Truskey GA, Warren HB, O’Connor SE, Eisenhaure BH. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J. Cell Biol. 1985;101:871–9. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napoleone E, Di Santo A, Lorenzet R. Monocytes upregulate endothelial cell expression of tissue factor: a role for cell-cell contact and cross-talk. Blood. 1997;89:541–9. [PubMed] [Google Scholar]
- 35.Takaku M, Wada Y, Jinnouchi K, Takeya M, Takahashi K, Usuda H, et al. An in vitro coculture model of transmigrant monocytes and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 1999;19:2330–2339. doi: 10.1161/01.atv.19.10.2330. [DOI] [PubMed] [Google Scholar]
- 36.van Buul-Wortelboer MF, Brinkman HJ, Dingemans KP, de Groot PG, van Aken WG, van Mourik JA. Reconstitution of the vascular wall in vitro. A novel model to study interactions between endothelial and smooth muscle cells. Exp. Cell Res. 1986;162:151–8. doi: 10.1016/0014-4827(86)90433-7. [DOI] [PubMed] [Google Scholar]
- 37.Chiu J-J, Chen L-J, Lee P-L, Lee C-I, Lo L-W, Usami S, et al. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101:2667–2674. doi: 10.1182/blood-2002-08-2560. [DOI] [PubMed] [Google Scholar]
- 38.Rainger GE, Nash GB. Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ. Res. 2001;88:615–22. doi: 10.1161/01.res.88.6.615. [DOI] [PubMed] [Google Scholar]
- 39.Ni C-W, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, et al. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho Y-E, Choi J-E, Alam MJ, Lee M-H, Sohn H-Y, Beattie JH, et al. Zinc deficiency decreased cell viability both in endothelial EA.hy926 cells and mouse aortic culture ex vivo and its implication for anti-atherosclerosis. Nutr. Res. Pract. 2008;2:74–9. doi: 10.4162/nrp.2008.2.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang B, Dreyer T, Heidt M, Yu JCM, Philipp M, Hehrlein FW, et al. Insulin and local growth factor PDGF induce intimal hyperplasia in bypass graft culture models of saphenous vein and internal mammary artery. Eur. J. Cardiothorac. Surg. 2002;21:1002–8. doi: 10.1016/s1010-7940(02)00111-2. [DOI] [PubMed] [Google Scholar]
- 42.Poppert S, Schlaupitz K, Marre R, Voisard R, Roessler W, Weckermann D, et al. Chlamydia pneumoniae in an ex vivo human artery culture model. Atherosclerosis. 2006;187:50–6. doi: 10.1016/j.atherosclerosis.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 43.Voisard R, von Eicken J, Baur R, Gschwend JE, Wenderoth U, Kleinschmidt K, et al. A human arterial organ culture model of postangioplasty restenosis: results up to 56 days after ballooning. Atherosclerosis. 1999;144:123–34. doi: 10.1016/s0021-9150(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang BY, Ho HK, Lin PS, Schwarzacher SP, Pollman MJ, Gibbons GH, et al. Regression of atherosclerosis: role of nitric oxide and apoptosis. Circulation. 1999;99:1236–41. doi: 10.1161/01.cir.99.9.1236. [DOI] [PubMed] [Google Scholar]
- 45.Moreno JA, Ortega-Gómez A, Delbosc S, Beaufort N, Sorbets E, Louedec L, et al. In vitro and in vivo evidence for the role of elastase shedding of CD163 in human atherothrombosis. Eur. Heart J. 2012;33:252–63. doi: 10.1093/eurheartj/ehr123. [DOI] [PubMed] [Google Scholar]
- 46.Fortunato G, Di Taranto MD, Bracale UM, Del Guercio L, Carbone F, Mazzaccara C, et al. Decreased paraoxonase-2 expression in human carotids during the progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:594–600. doi: 10.1161/ATVBAHA.107.154658. [DOI] [PubMed] [Google Scholar]
- 47.Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler. Thromb. Vasc. Biol. 1998;18:1498–505. doi: 10.1161/01.atv.18.9.1498. [DOI] [PubMed] [Google Scholar]
- 48.Lebastchi AH, Qin L, Khan SF, Zhou J, Geirsson A, Kim RW, et al. Activation of human vascular cells decreases their expression of transforming growth factor-beta. Atherosclerosis. 2011;219:417–424. doi: 10.1016/j.atherosclerosis.2011.07.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erbel C, Okuyucu D, Akhavanpoor M, Zhao L, Wangler S, Hakimi M, et al. A Human Ex Vivo Atherosclerotic Plaque Model to Study Lesion Biology. J Vis Exp. 2014:50542. doi: 10.3791/50542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sainz T, Serrano-Villar S, Díaz L, Tomé MIG, Gurbindo MD, de José MI, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. 2013;27:1513–1516. doi: 10.1097/QAD.0b013e32835faa72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.