Abstract
Transmissible vaccines have the potential to revolutionize infectious disease control by reducing the vaccination effort required to protect a population against a disease. Recent efforts to develop transmissible vaccines focus on recombinant transmissible vaccine designs (RTVs) because they pose reduced risk if intra-host evolution causes the vaccine to revert to its vector form. However, the shared antigenicity of the vaccine and vector may confer vaccine-immunity to hosts infected with the vector, thwarting the ability of the vaccine to spread through the population. We build a mathematical model to test whether a RTV can facilitate disease management in instances where reversion is likely to introduce the vector into the population or when the vector organism is already established in the host population, and the vector and vaccine share perfect cross-immunity. Our results show that a RTV can autonomously eradicate a pathogen, or protect a population from pathogen invasion, when cross-immunity between vaccine and vector is absent. If cross-immunity between vaccine and vector exists, however, our results show that a RTV can substantially reduce the vaccination effort necessary to control or eradicate a pathogen only when continuously augmented with direct manual vaccination. These results demonstrate that estimating the extent of cross-immunity between vector and vaccine is a critical step in RTV design, and that herpesvirus vectors showing facile reinfection and weak cross-immunity are promising.
Keywords: Epidemiology, Mathematical model, Recombinant, Vectored vaccine, Transmissible vaccine, Vaccine, Infectious vaccine
1. Introduction
Vaccines have had a wide range of positive impacts on the health of human and animal populations. In many cases, however, the full potential of vaccination cannot be realized because it is difficult or impossible to vaccinate a substantial proportion of the host population. Particularly challenging scenarios for efficient vaccine delivery include human diseases in regions with poorly developed public health infrastructure and diseases of wild animal populations. An important consequence of the challenges associated with vaccinating large proportions of wild animal populations is that we may be missing opportunities to eliminate or reduce reservoir populations of diseases that occasionally spill over into human populations (e.g., Ebola, Rabies; [1–5]), or that provide the raw material for full scale host shifts into the human population (e.g., SARS; [6]). This problem is particularly pressing in light of evidence suggesting that the incidence of such host-shifts is increasing due to the greater prevalence of humans in regions with high levels of wildlife diversity [7]. One promising new technology for dealing with the challenges associated with these difficult-to-immunize animal populations is the development of transmissible vaccines.
In the most general sense, transmissible vaccines are vaccines that can spread from one individual to the next, with the benefit being that for every individual that is immunized directly, additional individuals are immunized indirectly. One way in which a transmissible vaccine can be developed is through the process of attenuation [8]. Although attenuation can be accomplished in many ways, the goal in all cases is the transformation of the original viral disease into a benign yet transmissible vaccine. This approach has been used to develop transmissible vaccines both unintentionally, as with the oral polio vaccine [8,9], and intentionally, as with myxoma virus in rabbits [10–12]. However, there are limitations and risks associated with transmissible vaccines produced through attenuation. For instance, because attenuated vaccines are built from the pathogen itself, the ability of the vaccine to spread between hosts is bounded by the transmissibility of the pathogen. More worrisome is the possibility of reversion to wild type virulence, as has been observed in the oral polio vaccine [9,13,14]. For this reason, and the constraints imposed by the process of attenuation itself, transmissible vaccines developed through attenuation will generally be only weakly transmissible. Despite these limitations, recent theoretical work has demonstrated that weakly transmissible attenuated vaccines could be highly effective tools, particularly against infectious diseases with relatively low transmission rates [15].
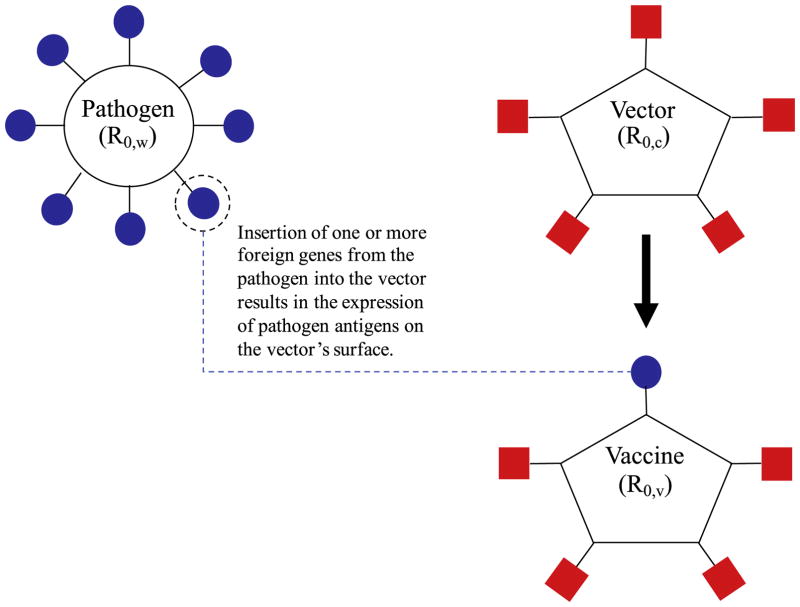
An alternative approach to designing a transmissible vaccine relies on recombinant genetic engineering rather than traditional attenuation. Specifically, rather than weakening the pathogen itself through a process of attenuation, recombinant transmissible vaccines (RTVs), also called transmissible recombinant vectored vaccines [16], are developed by inserting one or more pathogen genes with antigenic activity into the genome of a benign but transmissible vector organism. Infection with the modified vector, now termed the vaccine, exposes the host immune system to pathogen antigens and prompts a pathogen-specific immune response (Fig. 1). This approach has been used to develop a transmissible vaccine against Sin Nombre Virus within deer mice [17], and is now being used to develop a transmissible vaccine against Ebola within mice and nonhuman primate models [18,19].
Fig. 1. The design of a recombinant transmissible vaccine (RTV).
Pathogen genes are inserted into a benign vector organism, resulting in the expression of pathogen antigen(s) on the viral vector, now termed the vaccine. Infection with the vaccine exposes the host immune system to pathogen antigen(s), and prompts a pathogen-specific immune response. R0,c, R0,v, and R0,w represent the basic reproduction numbers of the vector, vaccine, and pathogen, respectively. Because the vaccine is produced by inserting non-beneficial foreign genes into the vector organism, the vaccine R0 is likely bounded above by the vector’s R0 (R0,v < R0,c ).
In principle, RTVs offer several advantages over vaccines produced by attenuation. For instance, evolutionary reversion in a RTV is likely to produce the benign vector organism rather than the virulent pathogen itself. Also, because the transmission rate of the vaccine is related to the transmission rate of the vector rather than the pathogen, there are fewer limitations on the vaccine transmission rate. However, because a RTV necessarily integrates components of both disease and vector, it may struggle to spread through a host population where substantial cross-immunity to either the disease or vector already exists [20,21]. This reduction in effectiveness is important in cases where the vector organism is already present in the population, or where vaccine-reversion produces free vector. For these reasons, it is currently unclear whether RTVs that generate cross-immunity can serve as effective tools in the battle against infectious disease.
The limitations imposed by cross-immunity have focused research efforts on vectors thought to largely circumvent existing host immunity [4]. Cytomegalovirus (CMV), for example, is a type of herpesvirus that can evade an existing immune response, in part by down-regulating the presentation of MHC antigens [22,23]. RTV’s that use CMV as a backbone are seemingly capable of reinfecting hosts that have had previous exposure to the vaccine, and superinfecting hosts with previous exposure to the CMV vector from which the vaccine was built [17,24]. These studies suggest that under natural conditions, CMV-based vaccines may experience little or no cross-immunity with the vector, and therefore largely escape the detrimental consequences of competition with the vector.
For RTVs in general, levels of cross-immunity are likely to lie somewhere between the extremes of perfect host exclusion, in which infection with the vaccine or vector always precludes infection with the other, and the complete absence of cross-immunity. Our goal is to establish bounds on the role of cross-immunity in hindering the effectiveness of a RTV. We begin by evaluating the ability of a RTV to facilitate pathogen control when the vector is absent from the host population. This first scenario also applies to the extreme case for which cross-immunity with the vector is absent. We compare this baseline case to the other extreme case where cross-immunity is perfect and the vector is circulating in the population or likely to be introduced through sporadic reversion. We direct part of our analysis towards current RTV designs which envision a vaccine that, when administered to a small number of ‘founder’ animals in a population, will spread autonomously and sufficiently to vaccinate the remaining population. We use a mathematical model to address the following specific questions: (1) can a RTV autonomously eradicate and/or protect a population that has previously experienced vector infection? and (2) if autonomous vaccination by a RTV is impossible, can augmenting the vaccine with direct, manual vaccinations make a RTV an effective tool for the control of infectious disease? Our results indicate that supplementation of a RTV through direct vaccination is critical for disease control if reversion to the vector form is likely and vector-vaccine cross-immunity is present. Our results identify key criteria that must be satisfied for a RTV to be an effective tool in infectious disease management, and outline quantities that must be estimated during the process of vaccine development to evaluate these criteria.
2. Results
2.1. Cross-immunity prevents autonomous vaccination and pathogen eradication
Ideally, it would be possible to engineer a RTV that, when introduced into the host population, would spread autonomously and sufficiently to eradicate an existing pathogen or prevent future infection by a pathogen not yet present. If the vector used to construct the vaccine remains absent from the population, or exhibits no cross-immunity with the vaccine, this ideal situation can be realized. Specifically, if vaccine-infected individuals transmit the vaccine to more than one additional unvaccinated individual (R0,v > 1), and the vaccine’s R0 is larger than the pathogen’s (R0,v > R0,w), then a host population is cleared of an endemic pathogen and/or protected from future infection (SI, Section 2).
In some cases, however, it will be impossible to engineer a RTV that exhibits no cross-immunity with its corresponding vector. In the extreme case where the vector and vaccine share perfect cross-immunity, model analyses show that the vector will always outcompete the vaccine and drive the vaccine to extinction (SI Section 2). This occurs regardless of the initial number of vaccinated individuals, and is a direct consequence of the cross-immunity between the vector and vaccine, and the vaccine’s reduced rate of transmission relative to the vector (R0,v < R0,c, Fig. 1). The reduction in transmission of the vaccine relative to its vector renders the vaccine unable to repel the spread of its associated vector should reversion occur, while perfect cross-immunity ensures that the vector will drive the vaccine to extinction by imparting vaccine-immunity to a substantial portion of the population. These results suggest that the viability of autonomous vaccination depends critically on the degree of cross-immunity between the vector and vaccine. In light of this result, we next investigate the extent to which vaccine transmission, when coupled with a steady program of direct vaccination, can facilitate pathogen control even when the vaccine and vector share perfect cross-immunity.
2.2. Supplementation eliminates the threat of vector competition
In most cases, sporadic reversion events will inevitably introduce the vector into an otherwise vector-naïve population, possibly resulting in significant competition between the vaccine and its associated vector. A simple strategy for protecting the RTV against the threat of competition with its vector is to manually vaccinate individuals at a rate sufficient to drive the vector to extinction. Model analyses show that if the vector and vaccine share perfect cross-immunity, any vector incidence in the population will decrease to zero if the fraction of newborns vaccinated (σ) satisfies
| (1) |
Given that direct vaccination meets or exceeds expression (1), spontaneous reversion events will be unable to spread through the population.
If vaccine supplementation exceeds the critical value in expression (1), a pathogen-naïve population is protected from a pathogenic threat and a pathogen-infected population is cleared of the pathogen when the fraction of newborns vaccinated satisfies
| (2) |
Therefore, if it is possible to supplement the vaccine at a rate that exceeds both of the critical values defined by expressions (1) and (2), the threat of pathogenic disease in a vector-naïve population will be eliminated, as will be the potential for interference from competition with the vector (SI, Section 3). Though pathogen prophylaxis can be achieved with lower levels of direct vaccination, supplementing at the maximum of expressions (1) and (2) avoids any risks of competition with the associated vector, and still outperforms a traditional non-transmissible vaccine if the ratio of the vaccine’s R0 relative to the vector’s is not too small (Fig. S1).
2.3. Vaccine transmission augments direct vaccination and promotes eradication
Even if the vector organism is endemic in the host population prior the vaccination program, pathogen control can still be achieved with supplemental direct vaccination. In the absence of cross-immunity between vector and vaccine, a pathogen-naïve population is protected against the threat of an invading pathogen when a minimal fraction, , of newborns are vaccinated [SI, Sections 4, 15]. If, on the other hand, vector infection imparts perfect vaccine immunity, a fraction
| (3) |
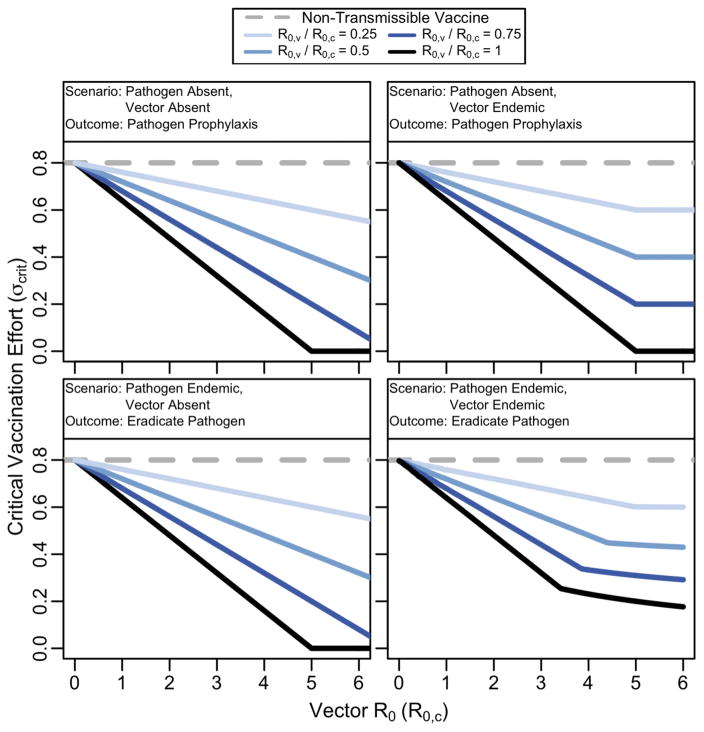
of newborns must be continually vaccinated (SI, Section 4 and Fig. 2). The necessary vaccination effort for prophylaxis depends critically on the relative R0′s of the vector and pathogen. Eq. (3) shows that the critical level of supplementation is unaffected by vector-vaccine cross-immunity as long as the vector is less transmissible than the pathogen (R0,c < R0,w). Given perfect cross-immunity and fixed ratio , the benefit in vaccination effort that occurs when increasing the vector’s R0 plateaus when R0,c = R0,w (Fig. 2, top right panel).
Fig. 2. Vaccination effort required for protection against pathogen invasion (top row), or pathogen eradication (bottom row) in different vector scenarios.
The RTV outperforms a non-transmissible vaccine, even when the vector is circulating in the population and vector/vaccine cross-immunity is perfect. The “Vector Absent” panes describe scenarios in which the vector remains absent from the host population, or when vector/vaccine cross-immunity is absent. When the vector is endemic, cross-immunity is perfect, and is constant, the benefits of increasing vector transmission plateau when the vector’s transmission approaches the pathogen’s. In each case, parameters were set to R0,w = 5, b = 10, d = 0.01, δc = 2 and δw = 2.
By comparison, a traditional, non-transmissible vaccine requires a threshold fraction of newborns to be vaccinated to prevent a pathogen from spreading through the population. Thus, if the vector organism has reached endemic levels in the population, has a larger R0 than the pathogen, and imparts vaccine cross-immunity, the use of a RTV reduces the rate of direct vaccination required for prophylactic protection from a pathogen by a factor
| (4) |
Eq. (4) indicates that, should the vector become endemic in the host population, substantial savings in vaccination effort are still realized as long as the ratio of the vaccine’s transmissibility relative to its vector is not too small. Again, the ratio of the vaccine’s R0 relative to the vector’s is a key quantity for gauging a vaccine’s potential when competing against its associated vector. To our knowledge, this ratio has not been measured in any empirical experiments involving RTVs, despite its fundamental importance for predicting the vaccine’s effectiveness in pathogen control when vector-vaccine cross-immunity is present (Figs. 2 and S1).
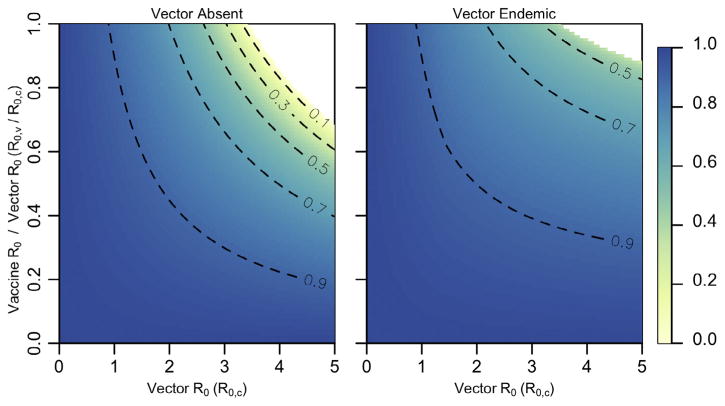
If both the vector and the pathogen are endemic to the population prior to the administration of the vaccination program, the use of a RTV results in a substantial reduction in the fraction of new-borns that must be vaccinated to eradicate the pathogen relative to a non-transmissible vaccine (Figs. 2 and S2, SI: Section 4). As before, the role of vector-vaccine cross-immunity has a limited effect on the vaccine’s effectiveness as long as the vector is less transmissible than the pathogen (R0,c < R0,w, Fig. S2). Fig. S2 shows that the ratio is most influential when the vector is more transmissible than the pathogen (R0,c < R0,w). Even when achieving the levels of continuous, manual vaccinations necessary for pathogen-eradication exceed the logistics or budget of the vaccination program, a RTV can still greatly reduce the pathogen incidence in vector-infected population relative to that achieved by a non-transmissible vaccine (Fig. 3).
Fig. 3. Fractional steady-state pathogen incidence.
Ratio of pathogen-infected individuals at steady-state with a RTV relative to the case of a non-transmissible vaccine. The left panel describes scenarios where the vector remains absent from the host population, or when vector/vaccine cross-immunity is absent. The right panel describes scenarios in which the vector is endemic at steady-state levels prior to the start of the vaccination program, and vector/vaccine cross-immunity is perfect. In all cases, σ = 0.25, R0,w = 5, b = 10, d = 0.01, δc = 2, δw = 2. Areas in white result in disease eradication.
3. Discussion and conclusions
Our mathematical results demonstrate that recombinant transmissible vaccines (RTVs) can be powerful tools for the management of infectious pathogens under some circumstances. For instance, if infection with the vector does not confer vaccine immunity to hosts, or if the vector is absent from the population altogether, our results show that a RTV can autonomously sweep through a host population and eradicate an endemic infectious pathogen or provide prophylaxis against a future epidemic. Alternatively, if the vector organism is already circulating within the host population and vector infection imparts perfect vaccine cross-immunity, the benefits of a RTV are more modest, and require that autonomous vaccination be supplemented with continuous direct vaccination. The level of direct vaccination that is required, and the benefits that a RTV provides relative to a non-transmissible vaccine, depend critically on levels of cross-immunity between vector and vaccine, the likelihood of evolutionary reversion to free vector, and the relative transmission rates of vector, vaccine, and pathogen. Unfortunately, because these key parameters are largely unknown in natural infection scenarios, it is challenging to predict how well RTV’s currently under development [17,18,25] will work.
Although reliable estimates for key model parameters are not yet available, the biology of the transmissible vaccines currently under development is promising. For instance, the RTV’s being developed for Sin Nombre virus and Ebola use Cytomegalovirus (CMV) as a vector [17,18]. Available evidence suggests that reinfection and superinfection by CMV is possible, such that cross-immunity between vector and vaccine may be weak or absent [17,24]. In addition, at least within the lab, it is possible to engineer a genetically modified herpesvirus similar to CMV that does not revert, suggesting it may be evolutionarily stable, at least over relatively short time periods [26]. Finally, at least under some circumstances, CMV is highly transmissible [27], suggesting that it could be a useful vector against even infectious pathogens with relatively high R0. In short, the biology of the RTV’s currently being developed provides reason for optimism, but without accurate estimates for the strength of cross-immunity, and rates of reversion and transmission, their likely efficacy remains uncertain.
Recombinant transmissible vaccines have the potential to revolutionize control of infectious disease but their successful engineering and deployment will require estimating key parameters governing their epidemiology and evolution. A more accurate description of the population dynamics of a RTV and vector that potentially compete and coevolve will require a model that allows intermediate levels of cross-immunity. However, the predictions of such multi-strain models are highly sensitive to assumptions made regarding the presence of strain competition within a co-infected host, the rate of strain re-infection, as well as the level of cross-immunity between strains [28]. Once estimates of these specific parameters are found, our theory allows the potential advantage provided by vaccine transmission to be quantified. Because RTV’s carry with them the risks associated with the release of any genetically modified organism [29,30], their use should only be entertained in cases where the advantages can be quantified and demonstrated to be substantial relative to a traditional, directly administered vaccine.
4. Methods
To quantify the effectiveness of a RTV-based vaccination program, we developed a mathematical model that describes the spread of a RTV in a population that is susceptible to the associated vector and an infectious pathogen. The model consists of ordinary differential equations based on a standard SIR framework that tracks transitions between 11 classes of individuals that describe infectious and immune states of hosts in the presence of the vector, vaccine, and pathogen. The vaccine is administered by continual and direct vaccination of a fraction of newborns, and can also transmit between individuals resulting in indirect vaccinations.
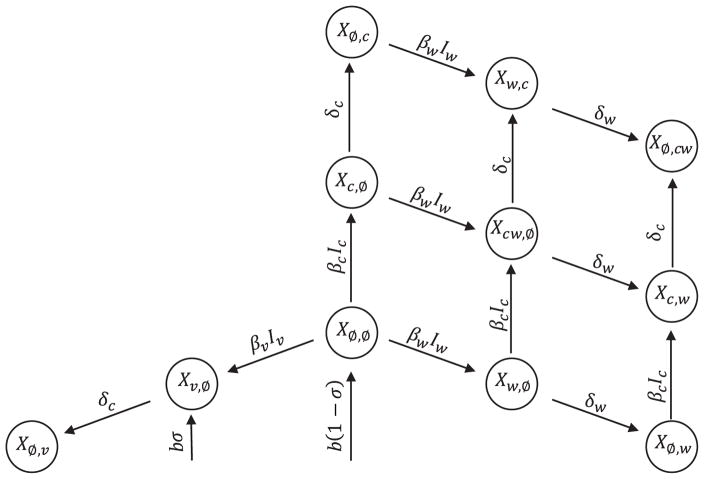
State variables are notated XI,J, where infection and immunity status are described by the subsets I, J ⊂ {Ø, c, v, w}; subscripts c, v, and w refer to the vaccine vector, the recombinant transmissible vaccine (RTV), and the pathogen respectively. Susceptible individuals are denoted XØ,Ø because they are not currently infected and have no immunity. Individuals may be infected by, or recovered from, the vaccine vector, the RTV, or the pathogen; the current infection statuses are indicated with a c, v, or w respectively in the I set: Xcw,Ø, for example, describes individuals infected with both vector and pathogen but no immunity, whereas XØ,cw describes individuals that are currently free of infection, but have recovered from both the vector and pathogen. Standard mass action terms describe disease transmission; infectious agent I ∈ {c, v, w} has transmission rate βI, and recovery rate δI. Individuals are born at a constant rate b, a fraction σ of which are vaccinated at birth (and placed in class Xv,Ø), and die at rate d. We assume disease-induced mortality is negligible and can be ignored.
Because a RTV is engineered by inserting a foreign, non-beneficial antigenic region into a vector organism, it is likely that the RTV’s ability to transmit between hosts will be reduced relative to the vector organism; we incorporate this key biological constraint by assuming βv < βc. For simplicity, we assume that a host infected with the vaccine recovers at the same rate as a host infected with the vector (δv = δc ). Defining the basic reproduction number of infectious agent I ∈ {c,v,w} as , these latter two assumptions imply R0,v < R0,c.
Cross-immunity between infectious agents is assumed to be either perfect or absent altogether. In the scenarios that describe the vector circulating in the population, we assume perfect cross-immunity exists between the vector and vaccine, as well as between the pathogen and vaccine, but that cross-immunity between the vector and pathogen is absent. These assumptions allow us to describe the epidemiological dynamics of vector, vaccine, and pathogen using the following system of ordinary differential equations (ODEs):
| (5a) |
| (5b) |
| (5c) |
| (5d) |
| (5e) |
| (5f) |
| (5g) |
| (5h) |
| (5i) |
| (5j) |
| (5k) |
where Ic and Iw are defined as the total number of vector infected and pathogen infected individuals,
| (5l) |
| (5m) |
The biological interpretations of the model variables and parameters are described in Tables 1 and 2, respectively. A flow diagram of the model is presented in Fig. 4.
Table 1.
State variables of the model. XI,J denotes individuals that are infected by elements of set I, and have recovered from elements in set J: I, J ⊂ {Ø, c, v, w}.
| Variables | Meaning |
|---|---|
| XØ,Ø | Number of susceptible individuals |
| Xc,Ø | Number of vector infected individuals |
| Xv,Ø | Number of recombinant transmissible vaccine infected individuals |
| Xw,Ø | Number of pathogen infected individuals |
| Xcw,Ø | Number of individuals co-infected with the pathogen & vector |
| Xw,c | Number of individuals infected with the pathogen & recovered from infection by the vector |
| Xc,w | Number of individuals infected with the vector & recovered from infection by the pathogen |
| XØ,w | Number of individuals recovered from infection by the pathogen |
| XØ,c | Number of individuals recovered from infection by the vector |
| XØ,v | Number of individuals recovered from infection by the vaccine |
| XØ,cw | Number of individuals recovered from infection by vector & pathogen |
Table 2.
Parameters used in the model. Parameters are subscripted with the infectious agent they describe, I ∈ {c,v,w} for the vector, recombinant transmissible vaccine, and pathogen respectively.
| Parameters | Description |
|---|---|
| βI | Transmission rates |
| δI | Recovery rates |
| d | Death rate |
| b | Birth rate |
| σ | Proportion of individuals vaccinated at birth |
| R0,c | Basic reproduction number of the vaccine vector |
| R0,v | Basic reproduction number of the recombinant vaccine |
| R0,w | Basic reproduction number of the pathogen |
Fig. 4. Flow diagram of the model describing the vaccine, vector, and pathogen when vector/vaccine cross-immunity is perfect.
State variables are notated XI,J, where infection and immunity status are described by the subsets I, J ⊂ {Ø, c, v, w}. We assume individuals infected with the vaccine recover at the same rate as those infected with the vector. Note that death rates are omitted.
Current RTV research is focused on vector organisms that impart little or no vaccine cross-immunity upon host infection [17,24]. We use the subsystem consisting of equations 5a, 5c, 5d, 5h, 5k to describe cases in which the vector does not impart vaccine immunity to hosts, and the full model to describe cases in which vector/vaccine cross-immunity is perfect. By exploring these two extreme descriptions of cross-immunity, our model captures the limits of a broad range of RTVs.
Supplementary Material
Acknowledgments
This work was funded by NIH grant number R01GM122079 (S.L. N.).
Abbreviations
- RTV
recombinant transmissible vaccine
Appendix A. Supplementary material
Details on all analyses can be found in the supporting information (SI). Conditions 1–4 were derived using standard steady-state analysis. In cases where analysis was intractable, numerical solutions of system (5) were used to estimate disease management criteria. Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.12.037.
Footnotes
Data, code, and materials
All R scripts and Mathematica code mentioned in the main text and supplementary materials are available upon request. Inquiries should be directed to Andrew Basinski (abasinski@uidaho.edu).
Conflict of Interest
We declare we have no conflicts of interest
Author Contributions
A.J.B., C.H.R., and S.L.N. conceived of the study; A.J.B., S.L.N. derived analytical solutions; A.J.B., T.J.V., M.S, R.H.M., C.H.R. and S.L.N. helped draft the manuscript. All authors gave final approval for publication.
References
- 1.Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95:1619–24. doi: 10.1099/vir.0.067199-0. https://doi.org/10.1099/vir.0.067199-0. [DOI] [PubMed] [Google Scholar]
- 2.Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7:462–71. doi: 10.1371/journal.pbio.1000053. https://doi.org/10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. doi: 10.1038/438575a. https://doi.org/10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 4.Murphy AA, Redwood AJ, Jarvis MA. Self-disseminating vaccines for emerging infectious diseases. Expert Rev Vacc. 2016;15:31–9. doi: 10.1586/14760584.2016.1106942. https://doi.org/10.1586/14760584.2016.1106942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slate D, Rupprecht CE, Rooney JA, Donovan D, Lein DH, Chipman RB. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. 2005;111:68–76. doi: 10.1016/j.virusres.2005.03.012. https://doi.org/10.1016/j.virusres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Hu B, Ge X, Wang LF, Shi Z. Bat origin of human coronaviruses. Virol J. 2015;12:221–30. doi: 10.1186/s12985-015-0422-1. https://doi.org/10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–3. doi: 10.1038/nature06536. https://doi.org/10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151:420–36. doi: 10.1093/infdis/151.3.420. https://doi.org/10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- 9.Fine PEM, Carneiro IAM. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol. 1999;150:1001–21. doi: 10.1093/oxfordjournals.aje.a009924. https://doi.org/10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- 10.Bárcena J, Morales M, Vázquez B, Boga JA, Parra F, Lucientes J, et al. Horizontal transmissible protection against myxomatosis and rabbit hemorrhagic disease by using a recombinant myxoma virus. J Virol. 2000;74:1114–23. doi: 10.1128/jvi.74.3.1114-1123.2000. https://doi.org/10.1128/JVI.74.3.1114-1123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira C, Ramírez E, Castro F, Ferreras P, Alves PC, Redpath S, et al. Field experimental vaccination campaigns against myxomatosis and their effectiveness in the wild. Vaccine. 2009;27:6998–7002. doi: 10.1016/j.vaccine.2009.09.075. https://doi.org/10.1016/j.vaccine.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Torres JM, Sánchez C, Ramırez MA, Morales M, Bárcena J, Ferrer J, et al. First field trial of a transmissible recombinant vaccine against myxomatosis and rabbit hemorrhagic disease. Vaccine. 2001;19:4536–43. doi: 10.1016/s0264-410x(01)00184-0. https://doi.org/10.1016/S0264-410X(01)00184-0. [DOI] [PubMed] [Google Scholar]
- 13.Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis. 2014;210:S283–93. doi: 10.1093/infdis/jiu295. https://doi.org/10.1093/infdis/jiu295. [DOI] [PubMed] [Google Scholar]
- 14.Kew O. Reaching the last one per cent: progress and challenges in global polio eradication. Curr Opin Virol. 2012;2:188–98. doi: 10.1016/j.coviro.2012.02.006. https://doi.org/10.1016/j.coviro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Nuismer SL, Althouse BM, May R, Bull JJ, Stromberg SP, Antia R. Eradicating infectious disease using weakly transmissible vaccines. Proc R Soc B. 2016;283:20161903–909. doi: 10.1098/rspb.2016.1903. https://doi.org/10.1098/rspb.2016.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull JJ, Smithson MW, Nuismer SL. Transmissible Viral Vaccines. Trends Microbiol. 2017 doi: 10.1016/j.tim.2017.09.007. Advance online publication https://doi.org/10.1016/j.tim.2017.09.007. [DOI] [PMC free article] [PubMed]
- 17.Rizvanov AA, Khaiboullina SF, van Geelen AG, Jeor SC. Replication and immunoactivity of the recombinant Peromyscus maniculatus cytomegalovirus expressing hantavirus G1 glycoprotein in vivo and in vitro. Vaccine. 2006;24:327–34. doi: 10.1016/j.vaccine.2005.07.070. https://doi.org/10.1016/j.vaccine.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Marzi A, Murphy AA, Feldmann F, Parkins CJ, Haddock E, Hanley PW, et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci Rep. 2016:6. doi: 10.1038/srep21674. https://doi.org/10.1038/srep21674. [DOI] [PMC free article] [PubMed]
- 19.Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, et al. A replicating cytomegalovirus-based vaccine encoding a single ebola virus nucleoprotein CTL epitope confers protection against ebola virus. PLoS Negl Trop Dis. 2011;5:e1275. doi: 10.1371/journal.pntd.0001275. https://doi.org/10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. https://doi.org/10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey A, Singh N, Vemula SV, Couëtil L, Katz JM, Donis R, et al. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS One. 2012;7:e33428. doi: 10.1371/journal.pone.0033428. https://doi.org/10.1371/journal.pone.0033428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328:102–6. doi: 10.1126/science.1185350. https://doi.org/10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pande NT, Powers C, Ahn K, Früh K. Rhesus cytomegalovirus contains functional homologues of US2, US3, US6, and US11. J Virol. 2005;79:5786–98. doi: 10.1128/JVI.79.9.5786-5798.2005. https://doi.org/10.1128/JVI.79.9.5786-5798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. doi: 10.1038/nm.1935. https://doi.org/10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beverley PC, Ruzsics Z, Hey A, Hutchings C, Boos S, Bolinger B, et al. A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis. J Immunol. 2014;193:2306–16. doi: 10.4049/jimmunol.1302523. https://doi.org/10.4049/jimmunol.1302523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Ward C, Yeasmin R, Skiena S, Krug LT, Forrest JC. A codon-shuffling method to prevent reversion during production of replication-defective herpesvirus stocks: implications for herpesvirus vaccines. Sci Rep. 2017:7. doi: 10.1038/srep44404. https://doi.org/10.1038/srep44404. [DOI] [PMC free article] [PubMed]
- 27.Farroway LN, Gorman S, Lawson MA, Harvey NL, Jones DA, Shellam GR, et al. Transmission of two Australian strains of murine cytomegalovirus (MCMV) in enclosure populations of house mice (Mus domesticus) Epidemiol Infect. 2005;133:701–10. doi: 10.1017/s0950268805003717. https://doi.org/10.1017/S0950268805003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipsitch M, Colijn C, Cohen T, Hanage WP, Fraser C. No coexistence for free: neutral null models for multistrain pathogens. Epidemics. 2009;1:2–13. doi: 10.1016/j.epidem.2008.07.001. https://doi.org/10.1016/j.epidem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn KJ, Mitra A, Greenwell HC, Sui J. Monster potential meets potential monster: pros and cons of deploying genetically modified microalgae for biofuels production. Interface Focus. 2013;3:20120037–047. doi: 10.1098/rsfs.2012.0037. https://doi.org/10.1098/rsfs.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolia A, Manzo A, Veronesi F, Rosellini D. An overview of the last 10 years of genetically engineered crop safety research. Crit Rev Biotechnol. 2014;34:77–88. doi: 10.3109/07388551.2013.823595. https://doi.org/10.3109/07388551.2013.823595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.