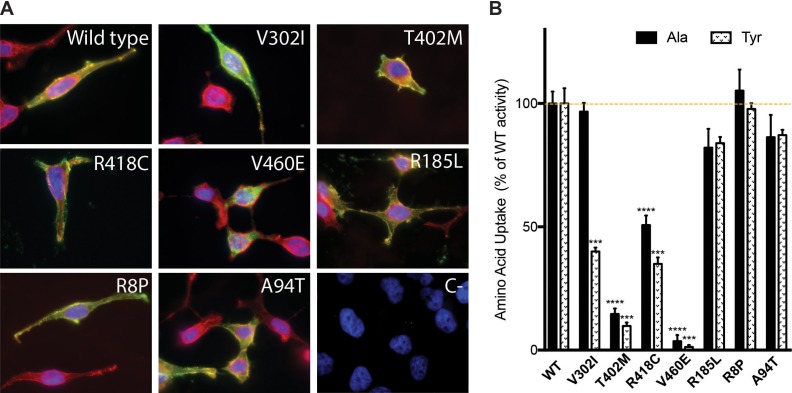
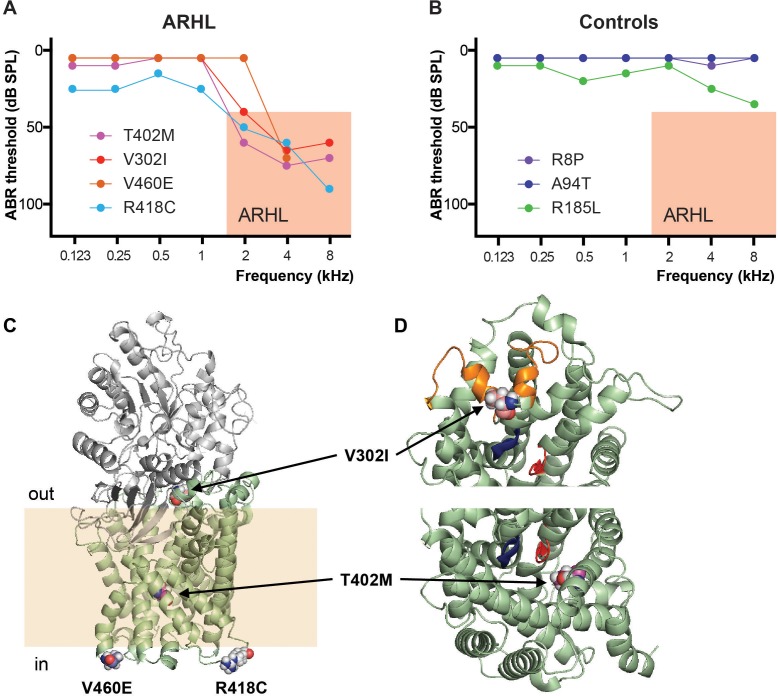
(A and B) Graphs representing the pure-tone audiometry performed using standard audiometers in ARHL patients and controls carrying SLC7A8 mutations. The analysis of hearing functions was performed by determining the pure tone average of air conduction (PTA) at different frequencies: lower (0.125, 0.25 and 0.5 kHz), medium (1 and 2 kHz), and high (4, 8 kHz). Audiometry of the best hearing ear is shown for each individual. Salmon box indicates the inclusion criteria considered for ARHL phenotype. (A) ARHL cases (threshold >40 dB at PTA-H) and (B) Controls (threshold <25 dB at PTA-H). (C–D) Cartoon representation of the actual structural model of SLC7A8/CD98hc heterodimer. (C) The ectodomain of human CD98hc (gray) and human SLC7A8 (pale-green) in an outward-facing conformation are shown. The transmembrane (TM) domain of CD98hc is not shown because there is no structural information about its localization. Residues involved in sequence variants identified in patients with ARHL are highlighted (atoms represented by spheres). Atoms are colored according to: O (red), N (blue) and C-atoms depending on the residue (V302, pale-pink; T402, magenta; R418, gray and V460, slate-blue). The pale-yellow band is shown to visualize the insertion of SLC7A8 in the membrane. Residue R418 is located in the intracellular loop between TM domains 10 and 11, and residue V460 is located at the end of the TM domain 12, just before the intracellular C-terminus that is not depicted. (D) Top view close-up from outside the cell showing the localization of residues V302 and T402, respectively. To facilitate the view, the extracellular domain of CD98hc has been deleted. Unwound segments of TM domains 1 and 6 that interact with the α-amino-carboxyl end of the amino acid substrates are colored in blue and red respectively. Residue V302 is located in the extracellular loop 4 (EL4) (orange), which contains a double α helix structure that closes the substrate binding cavity in the inward-facing conformations. Residue T402 is located in TM domain 10 facing the substrate binding cavity.