Supplemental Digital Content is available in the text
Keywords: Cirrhosis, Chronic Liver Failure-Sequential Organ Failure Assessment, liver failure, model for end-stage liver disease, mortality, organ failure, Sequential Organ Failure Assessment
Abbreviations: MV, mechanical ventilation, NLR, neutrophil-to-lymphocyte ratio, SOFA, Sequential Organ Failure Assessment Score
ABSTRACT
The neutrophil-to-lymphocyte ratio (NLR) is an inflammation score recognized as associated with outcome. Although inflammation has been shown to correlate with the development of acute-on-chronic liver failure (ACLF), we sought to investigate the role of NLR in predicting 90-day mortality in cirrhotic patients experiencing ACLF. We performed a retrospective cohort study involving a total of 108 consecutive cirrhotic patients admitted in the intensive care unit (ICU). NLR, clinical and biological data were recorded. Of the total, 75 patients had ACLF. The 90-day mortality rate was 53%. ACLF patients displayed higher NLR values in comparison with cirrhotic patients without ACLF throughout the ICU stay. NLR proved more elevated in nonsurvivors ACLF patients, with mortality correlating with increasing quartiles of NLR. On multivariable Cox regression analysis, NLR was found to be a predictor of mortality along with the Sequential Organ Failure Assessment (SOFA) score and mechanical ventilation requirement. The model for end-stage liver disease (MELD) score was not predictive of 90-days mortality. Performance analysis revealed an area under curve of 0.71 [95% confidence interval: 0.59–0.82] regarding NLR capacity to predict 90-days mortality. When including NLR, SOFA score, and mechanical ventilation requirement into the final model, the area under curve was significantly higher (0.81 [95% confidence interval: 0.72–0.91]).
These findings suggest that NLR is associated with mortality in ACLF patients admitted to the ICU. Combining NLR, SOFA score, and the need for mechanical ventilation could be a useful prognostic tool to identify ACLF patients at a higher risk of mortality.
INTRODUCTION
The neutrophil-to-lymphocyte ratio (NLR) is an easily accessible biomarker for assessing inflammatory status. It has been shown to be predictive of poor outcome in a variety of diseases, such as cardiovascular disease (1), cancer (2), and postoperative infection (3). In cirrhosis, NLR is a recognized predictor of survival in patients with hepatocarcinoma (4) or hepatitis B virus (HBV) infection, as well as in patients awaiting transplantation (5, 6). Moreover, it has been shown that in uncomplicated cirrhosis, higher NLR could predict mortality independently of the model for end-stage liver disease (MELD) and Child-Pugh scores (7).
Although acute decompensation (AD) refers to the development of 1 or more cirrhotic complications, acute-on-chronic liver failure (ACLF) is defined as an acute liver function deterioration leading to extra-hepatic organ failure (OF) and short-term mortality (8, 9). Despite presenting similar precipitating events, some patients recover whereas others progress to ACLF. The underlying mechanisms of ACLF are not yet fully understood (10). In the CANONIC study (CLIF Acute-On-Chronic Liver Failure in Cirrhosis), white blood cell (WBC) count and plasma C-reactive protein (CRP) level were shown to be higher in patients with ACLF than in those without (11). In addition, WBC count was independently associated with ACLF occurrence, number of failing organs, and mortality rate. Based on these findings, the body's systemic inflammatory response seems to play a pivotal role in ACLF development.
The pathogenesis of systemic inflammation in ACLF is indeed complex. The release of pathogen-associated-molecular patterns (PAMPs) by bacteria has been suggested as a possible mechanism, yet only a third of ACLF patients have been documented harboring bacterial infections (8). In addition to this, excessive inflammation may also result from nonbacterial endogenous mediators (8, 12). Hence, the initial level of systemic inflammation following the precipitating event could favor a shift toward excessive persistent inflammation, leading to organ dysfunction (13–15). Supporting this hypothesis, evidence is growing of systemic inflammatory response correlating with the outcome (16–18).
Despite its recognized role in ACLF pathogenesis, systemic inflammation proves difficult to assess in cirrhotic patients, while identifying readily available surrogate markers is still challenging (19–20). Furthermore, given that clinical and biological signs can overlap between AD and ACLF, early assessment and risk stratification is crucial.
This study sought to investigate whether initial inflammation measured by NLR is associated with the ACLF occurrence and predictive of 90 days mortality in a cohort of consecutive cirrhotic patients hospitalized in an ICU due to cirrhosis-related complications.
PATIENTS AND METHODS
We performed a retrospective observational cohort study in a tertiary, 22-bed, mixed intensive care unit (ICU) at Saint-Luc university hospital. This report was written in accordance with the STrengthening the Reporting of OBservational checklist for observational studies in Epidemiology (STROBE) statement (21). The study sample consisted of consecutive patients admitted to the ICU within the preceding 18 months for cirrhosis-related complications. Institutional approval was provided by the Saint Luc University Hospital Ethics Committee (Reference: 2014/546; Chairman: Prof J-M Maloteaux), and our study complied with the Helsinki Declaration. A waiver was obtained for written informed consent in view of the study's retrospective design. To ensure confidentiality, patient data were anonymously recorded in the final database, in accordance with Belgian law.
Clinical diagnosis and variable selection
Patients were included if the diagnosis of cirrhosis was histologically proven or clearly considered on the basis of biological, clinical, and radiological features. Patients with documented chronic hematological disorder, aged younger than 18 years, presenting with human immunodeficiency virus (HIV) infection or active malignancy, including hepatic carcinoma, were excluded from the analysis. Medical histories, including any relevant comorbidities and causes and history of liver disease, were taken on inclusion. Reasons for admission and all following medical events during the ICU hospitalization were carefully noted. Survival data were recorded at 28 days, 90 days and 1 year.
Liver failure severity on admission was assessed using the MELD and Child-Pugh scores, with critical illness evaluated using the SOFA score. Likewise, as proposed by Arroyo et al. (8), ACLF was defined as an AD of cirrhosis accompanied by OF. AD was determined according to the CANONIC study criteria and refers to development of ascites over 2 weeks, new-onset encephalopathy, gastrointestinal hemorrhage or bacterial infection (11). Organ dysfunctions (hemodynamics, respiratory, coagulation, brain, kidney, liver) were identified using the CLIF consortium organ failure score (8) as follows: hypotension requiring vasopressors, PaO2/FiO2 ratio <200, international normalized ratio (INR) ≥2.5, grade (West-Haven) 3–4 encephalopathy, creatinine ≥2 mg/dL (or requirement of renal replacement therapy), bilirubin >12 mg/dL.
Finally, severity was stratified by taking into account the number of OFs: grade 1 (1 OF), grade 2 (2 OFs) and grade 3 (3 to 6 OFs) (11). Bacterial infection was diagnosed when a focus of infection was clinically identified on ICU admission associated with positive microbiological samples or biological signs of infection (polymorphonuclear leucocytes >250/mm3 in ascitic fluid) in the case of spontaneous bacterial peritonitis. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the Saint Luc university hospitals (22).
Laboratory diagnosis
Leukocyte count was part of the routine ICU analyses. All venous blood samples were processed in a blood analyzer (Sysmex [TOA Medical Electronics, Kobe, Japan]) to determine complete blood cell counts and differential leukocyte counts. We recorded neutrophil and lymphocyte counts for the first 5 days after admission and calculated the NLR by dividing the neutrophil count by the lymphocyte count. Other laboratory tests were also recorded, such as blood chemistry, CRP, and hemostasis. Serum CRP level was assessed by immunoturbidimetric assay (Beckman Coulter DxC, Inc., Fullerton, Calif). All results from microbiological samples (blood, urine, ascites, and swab cultures) performed during hospitalization were registered.
Statistical analysis
All analyses were conducted using SPSS 21 software (SPSS software [IBM Corp. 2011, IBM SPSS Statistics for Windows, Version 21.0, Armonk, NY: IBM Corp]), with graphs drawn using Graphpad Prism 5.0 (GraphPad Software, La Jolla, Calif). Continuous variables were expressed as mean ± 1 standard deviation (SD) or median with interquartile ratio (IQR), and categorical variables as counts and percentages. Categorical variables were analyzed using Chi-squared test or Fisher's exact test. Continuous variables were analyzed using unpaired t test or Mann-Whitney U test, according to statistical distribution. The data were subjected to the Kolmogorov-Smirnov normality test and Bartlett's test for homogeneity of variance. To test the difference between means in the AD and ACLF groups according to the presence of infection or survival, 2-way analysis of variance (ANOVA) with F test was performed, followed by post-hoc multiple comparisons with Bonferroni correction. Survival curves were generated across NLR quartiles using the Kaplan-Meier method, compared by log-rank test. In order to find factors influencing survival, a Cox proportional hazards model was built. Variables with a P < 0.20 in univariable analysis were entered into a forward selection procedure based on the likelihood ratio, after checking the proportional hazard assumption for each variable and log-linearity. Finally, receiver-operating characteristic (ROC) curves were generated to assess the accuracy of NLR in predicting mortality. To this end, a logistic regression model was built as follows: all the variables significant in the univariable analyses were entered into a multivariable logistic regression with a backward elimination procedure, based on the likelihood ratio. The results were expressed as odds ratio (OR) with 95% confidence intervals (95% confidence interval [CI]). Areas under the receiving operator curve (AUROC) were compared using the method of Hanley and McNeil (23). All tests were 2-sided, with significance set at the 0.05 probability level.
RESULTS
Patient features
In total, 108 cirrhotic patients were enrolled in the study. Three were excluded from analysis, owing to chronic lymphoid leukemia. The overall characteristics of the included patients are presented in Table 1. The main cause of cirrhosis was alcohol abuse, and the foremost clinical events for ICU admission were bacterial infection (n = 45) followed by gastrointestinal hemorrhage (n = 43). The majority of patients had severe liver disease with a mean ± SD MELD score of 22 ± 9, AD patients exhibiting 14.7 ± 4, and ACLF 24.6 ± 9.5 (P < 0.001). Infection source was documented in 41 patients (91%) and microorganisms were detected in 77% of patients (Supplemental Digital Content, Appendix 1). Spontaneous bacterial peritonitis and pneumonia were the most reported infections and blood cultures were positive in 13 patients (29%). Escherichia coli was the most prevalent bacteria (27%) followed by Staphylococcus aureus (25%). Depending on the number of failing organs (11), ACLF was classified as Grade 1 (n = 48), Grade 2 (n = 21), or Grade 3 (n = 6). The mean ± SD SOFA score was 8.4 ± 2.9, being significantly higher in the ACLF group compared with the AD group (Table 1). Upon admission, respiratory failure was observed in 31 patients (29%) displaying a PaO2/FiO2 ratio <300 mmHg and in 11 patients (10.5%) with severe hypoxemia (PaO2/FiO2 ratio <200). In total, mechanical ventilation was provided to 55 patients (51%) during their ICU hospitalization. Initially, 37 patients presented acute renal failure, whereas renal replacement therapy was required in 26 patients (25%).
Table 1.
Clinical characteristics of total cohort and patients with acute decompensation and acute with acute-on-chronic liver failure
| Variables | Total cohort (n = 105) | AD (n = 30) | ACLF (n = 75) | P |
| Age (year) | 58 ± 10 | 60 ± 11 | 58 ± 11 | 0.36 |
| Male sex, n (%) | 72 (69) | 20 (66) | 52 (70) | 0.79 |
| Cause of cirrhosis (n) | ||||
| Alcohol | 65 | 18 | 47 | 0.8 |
| HBV virus | 6 | 1 | 5 | 0.67 |
| HCV virus | 11 | 4 | 7 | 0.72 |
| Alcohol + virus | 8 | 0 | 8 | 0.1 |
| Other* | 15 | 2 | 13 | 0.22 |
| MELD score | 22 ± 9 | 14.7 ± 4 | 24.6 ± 9.5 | <0.001 |
| SOFA score | 8.4 ± 2.9 | 6 ± 2 | 9.3 ± 2.7 | <0.001 |
| SOFA CLIF score | 9.8 ± 1.9 | 8 ± 1.2 | 10.8 ± 1.6 | <0.001 |
| Child-Pugh classes (A–B–C), n | 5–42–58 | 3–16–11 | 2–26–47 | 0.03 |
| Child-Pugh score | 10 ± 2 | 8.8 ± 1.7 | 10.2 ± 2 | 0.001 |
| Ascites, n (%) | 75 (72) | 14 (46) | 60 (80) | 0.005 |
| Precipiting event (n) | ||||
| Bacterial infection | 45 | 6 | 39 | 0.002 |
| Gastrointestinal bleeding | 43 | 20 | 23 | 0.001 |
| Toxic or alcoholic hepatitis | 4 | 1 | 3 | 0.87 |
| Encephalopathy | 7 | 2 | 5 | 0.99 |
| Other† | 6 | 3 | 3 | 0.20 |
| Organ failure‡ | ||||
| Liver, n (%) | 20 (19) | — | 20 (19) | |
| Hemodynamic, n (%) | 15 (14) | — | 15 (14) | |
| Lung, n (%) | 11 (10.5) | — | 11 (10.5) | |
| Kidney, n (%) | 39 (37) | — | 39 (37) | |
| Cerebral, n (%) | 12 (11) | — | 12 (11) | |
| Coagulation, n (%) | 13 (12) | — | 13 (12) | |
| Renal replacement therapy, n (%) | 26 (25) | 1 (3.3) | 25 (33) | <0.01 |
| Global mortality, n (%) | 47 (45) | 7 (23) | 40 (53) | 0.005 |
Values are mean ± standard deviation or number of patients (n) and percentages (%).
ACLF indicates acute-on-chronic liver failure; AD, acute decompensation; SOFA CLIF, Sequential Organ Failure Assessment Chronic Liver Failure.
*Refers to nonalcoholic fatty liver disease, biliary, auto-immune.
†Ascites with paracentesis and occurrence of acute renal failure.
‡Organ failure defined according to CLIF consortium organ failure score.
Association between NLR and mortality
The median NLR value for the entire cohort was high, notably 8.5 (IQR: 5–17). In total, 42 patients (40%) had died by Day 28. The overall mortality rate was 44% (n = 46) at 90 days, and 45% (n = 47) at 1 year post-ICU admission. Overall, 40 patients died in the ACLF group (53%), of whom 38 by day 28, versus 7 in the AD group. In those with ACLF, nonsurvivors exhibited higher SOFA score values in comparison with survivors whereas MELD score was not statistically different between both the groups (Fig. 1A). NLR at admission was higher in those admitted for bacterial infection (median [IQR]: 16 [8–28] vs. 8 [5–16], P < 0.001). For patients with bacterial infection on admission, CRP was higher in both the AD and ACLF groups, compared with those without infection (Fig. 1B). However, CRP values were not significantly different between survivors and non survivors (Fig. 1C).
Fig. 1.
Severity scores, neutrophil-to-lymphocyte ratio evolution and C-reactive protein.
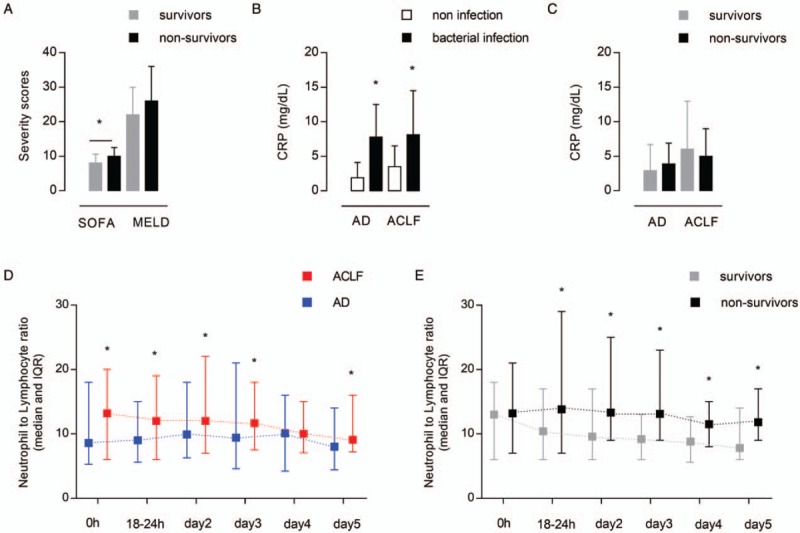
(A) SOFA and MELD scores among survivors and nonsurvivors, “∗” indicates values statistically different between survivors and nonsurvivors; (B) CRP values in patients with AD and ACLF, “∗" indicates values statistically different between patients presenting a bacterial infection or not; (C) CRP values in survivors and nonsurvivors; (D) evolution of NLR over the first 5 days after admission in patients with AD and ACLF—0 h: value at admission, 18–24 h: 18 to 24 hours after admission, “∗” indicates values statistically different between AD and ACLF; (E) evolution of NLR over the first 5 days after admission according to the survival in ACLF patients, “∗” indicates values statistically different between survivors and nonsurvivors. ACLF indicates acute-on-chronic liver failure; AD, acute decompensation; CRP, C-reactive protein; MELD, model for end-stage liver disease; NLR, neutrophil-to-lymphocyte ratio; SOFA, Sequential Organ Failure Assessment.
Patients presenting with ACLF displayed significantly higher NLR than the AD patients throughout the ICU stay except for day 4 (Fig. 1D). In the ACLF patients, comparing survivors from nonsurvivors, a lowering of NLR values was observed in the survivors group. The median NLR values decreased from 13 (IQR: 6–18) to 7.8 (IQR: 6–13) at day 5. In contrast, NLR was significantly higher in nonsurvivors from 18 to 24 hours following ICU admission and remained higher until day 5 (Fig. 1E). Since a significant difference of NLR values was found from this time and because this was the highest NLR value, we therefore predefined this window of interest to compare survivors from non survivors. Additional laboratory data are provided in Supplemental Digital Content (Appendix 2).
For the entire cohort, there was a significant increase in mortality depending on the NLR quartile, as shown in the survival curves (Fig. 2). When considering the ACLF group, a similar observation could be made with patients in the first quartile, who displayed the highest probability of survival, whereas patients in the fourth quartile exhibited the lowest survival probability.
Fig. 2.
Kaplan-Meier survival analysis across quartiles of neutrophil-to-lymphocyte ratio.
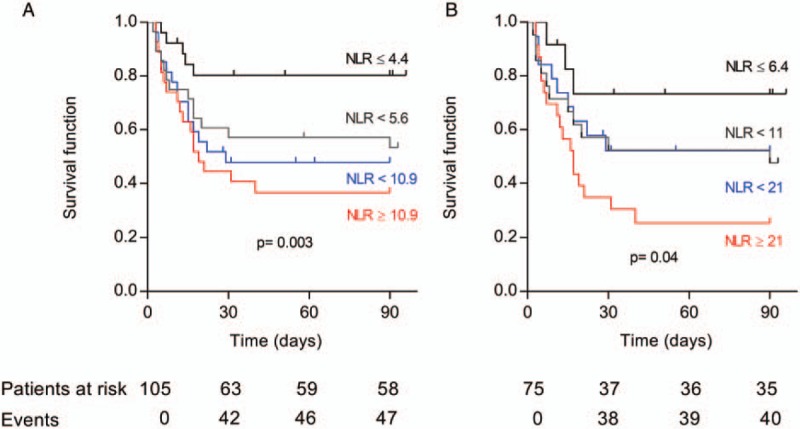
Survival was estimated with Kaplan-Meier method stratified on the basis of NLR in all cohort (A) and ACLF patients (B). Quartiles of NLR are indicated on the graph. ACLF indicates acute-on-chronic liver failure; NLR, neutrophil-to-lymphocyte ratio.
Additionally, in order to account for potential confounders, we conducted a Cox regression analysis for 90-day mortality, adjusting for relevant co-variables. In univariate analysis, NLR, SOFA score, renal replacement therapy and mechanical ventilation were identified as significant risk factors associated with a shorter time to death (Table 2). Multivariable model revealed that NLR was an independent predictor of mortality (adjusted hazard ratio [HR]: 1.02; [95% CI: 1.01–1.03], P = 0.009). Other variables associated with death in the multivariable analysis were the SOFA score and mechanical ventilation (Table 2).
Table 2.
Cox regression analysis for variables associated with 90 days mortality in patients with acute-on-chronic liver failure
| Univariable | Multivariable | |||||
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| NLR | 1.02 | 1.01–1.03 | 0.01 | 1.02 | 1.01–1.03 | 0.009 |
| MELD score | 1.03 | 0.99–1.07 | 0.09 | |||
| SOFA score | 1.30 | 1.12–1.50 | <0.001 | 1.23 | 1.07–1.41 | 0.004 |
| Age | 0.98 | 0.96–1.01 | 0.22 | |||
| Sex | 0.99 | 0.51–1.95 | 0.98 | |||
| Bacterial infection | 1.37 | 0.74–2.56 | 0.31 | |||
| Bleeding | 1.02 | 0.51–2.10 | 0.96 | |||
| Encephalopathy | 0.69 | 0.34–1.38 | 0.29 | |||
| RRT | 2.10 | 1.10–3.80 | 0.02 | |||
| Mechanical ventilation | 4.10 | 1.93–8.64 | <0.001 | 3.11 | 1.43–6.73 | 0.004 |
| Hemoglobin | 0.96 | 0.81–1.15 | 0.71 | |||
| CRP | 0.97 | 0.91–1.03 | 0.27 | |||
CI indicates confidence interval; CRP, C-reactive protein; HR, hazard ratio; MELD, model for end-stage liver disease; NLR, neutrophil-to-lymphocyte ratio; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment.
In unadjusted logistic regression, there was a statistical relationship between high NLR and 90 days mortality (Fig. 3A). Following multivariate adjustment, NLR along with the SOFA score and mechanical ventilation requirement were independently associated with 90 days mortality (Fig. 3B).
Fig. 3.
Logistic regression model predicting 90-days mortality and receiver-operating characteristic curves for estimated models.
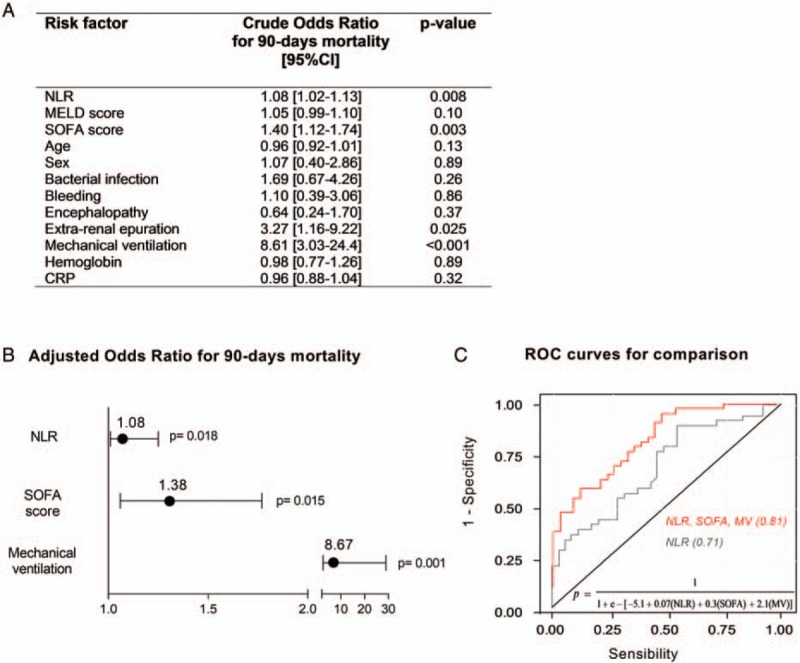
(A) univariable logistic regression analysis; (B) multivariable logistic regression analyses in ACLF patients; and (C) ROC curves were obtained to quantify the performance of multivariable logistic regression models for 90-days mortality. The curves demonstrated better accuracy for the model combining NLR, SOFA score and mechanical ventilation requirement (red line)—AUC 0.81 [95% CI: 0.72–0.91] P = 0.001—than the model using only NLR (gray line)—AUC of 0.73 [95% CI: 0.59–0.82] P = 0.002). The logistic regression equation for the model combining NLR, SOFA score, and mechanical ventilation and resulting in the red ROC curve is shown in the graph. ACLF indicates acute-on-chronic liver failure; AUC, area under curve; CI, confidence interval; MV, mechanical ventilation; NLR, neutrophil-to-lymphocyte ratio; ROC, receiver operating characteristic; SOFA, Sequential Organ Failure Assessment score.
Performance analysis of NLR
A performance analysis revealed an area under curve (AUC) of 0.71 ([95% CI: 0.59–0.82], P = 0.002) as to the NLR capacity to predict mortality at 90 days (Fig. 3C). The most discriminant NLR value to predict 90-day mortality was 6.2, with a sensitivity of 90% and specificity of 60%. The AUCs for other variables, such as MELD score or CRP, were not significant (0.59 [95% CI: 0.46–0.72], P = 0.18 and 0.51 [95% CI: 0.56–0.80], P = 0.92), whereas the AUC for the SOFA score was 0.69 (95% CI: 0.57–0.81; P = 0.004). The AUC for a predictive model associating NLR, SOFA score, and mechanical ventilation requirement was 0.81 ([95% CI: 0.72–0.91]; P = 0.001). The pairwise comparison of AUROCs revealed a statistically significant difference between the model associating the 3 variables and that, which included solely NLR (P = 0.001).
DISCUSSION
In this study, we examined NLR's ability to predict prognosis in a cohort of cirrhotic patients admitted to the ICU for acute cirrhotic complications. The main finding of our analysis revealed that a high NLR value was associated with mortality among those developing ACLF, whereas SOFA score and mechanical ventilation requirement were also significant independent predictors of mortality. A second crucial finding was that the NLR value was higher in nonsurvivor ACLF patients compared with AD patients. In ACLF patients, this independent association was not seen with CRP levels.
Association between NLR and outcome
Numerous studies have highlighted the association between high NLR values and outcome, irrespective of whether patients underwent AD or ACLF (7, 24), whereas our study was specifically focused on ACLF needing to be admitted in the ICU. To date, only few studies have investigated whether early NLR is predictive of short and medium-term mortality, specifically during ACLF. Those studies have focused on particular conditions such as HBV infection, hepatocarcinoma (25) or patients with low severity awaiting transplantation (26). One of those has so far identified the relationship between NLR and outcome in patients who developed ACLF in an HBV setting (5). This study compared NLR values between patients displaying chronic hepatitis, defining ACLF according to the recommendations of the Asian Pacific Association for the study of the liver. In contrast, we determined ACLF according to the new classification proposed by Arroyo et al. (8) taking into account organ failure, with our entire cohort comprising critically ill patients. Moreover, main causes for decompensation in our study were bleeding or bacterial infection. Our results demonstrate that NLR was higher in ACLF group in comparison with the AD patients. In addition, we showed that patients developing ACLF with NLR values >6.5 within the first 24 hours displayed a higher risk for mortality at 90 days. This observation is consistent with previous reports analyzing cases of both acute-complication and clinically stable cirrhosis. As such, an NLR value >5 was found correlating with high mortality in patients listed for liver transplantation (6). In another retrospective study, stable cirrhotic patients exhibiting NLR values exceeding 4.39 had a lower survival probability (7). It has also been shown that patients experiencing cirrhosis-related complications, such as uncontrolled ascites or variceal bleeding, exhibit NLR values ranging from 4.9 to 8.1 (according to the presence of infection), and these values were predictive of early mortality in Child-Pugh C patients (24). More broadly speaking, NLR on admission in a large cohort of unselected ICU patients was shown to correlate with mortality. Survival was stratified according to NLR quartiles, with increased mortality found correlating with increasing quartiles of NLR (27, 28). In our study, this trend was also observed across the distribution of NLR. Patients exhibiting values in the highest NLR quartile experienced the worst survival.
Acute-on-chronic liver failure and inflammation
Although our data suggest that NLR proves to be a predictive biomarker in a setting of cirrhosis-related critical illness, the association between elevated NLR and poor prognosis is still poorly understood. The current hypothesis is that NLR reflects the severity of the underlying acute systemic inflammation following the primary damage. In the setting of ACLF, Moreau et al. showed that leukocyte count was an independent predictor of mortality in ACLF (11, 17). Accordingly, systemic inflammation is common during stable cirrhosis independently of overt infection. This could be explained by high circulatory endotoxin levels activating the inflammatory pathways and recruiting neutrophils (29, 30). Furthermore, inflammation plays a role in exacerbation of cirrhosis, with systemic inflammation response shown to correlate with portal-hypertension-related complications and organ dysfunction (13). This supports the hypothesis that the relationship between elevated NLR and mortality could reflect the contribution of systemic inflammation to the ACLF outcome. In this setting, NLR could indicate the severity of the primary damage and provide information on how the immune system reacts. In our cohort, the difference in NLR values was due to the significantly higher neutrophil count in the ACLF group. This seems in line with the trend between increased leucocytes count and severity of ACLF demonstrated by Moreau et al. (11). Given this is in line with an excessive immune response, it is hypothesized that patients displaying a more significant immune response are more at risk for developing ACLF (8, 17, 31). Similarly, it has recently been shown in stable cirrhotic patients with low MELD scores that NLR correlated with low-density granulocytes having proinflammatory features (26). Such immature neutrophils have been recognized as a hallmark of sepsis (32, 33).
Impact of infection
In line with previous works proving the capacity of NLR and CRP to predict infection (24, 27), our study revealed both markers to be enhanced in the group with bacterial infection at admission. However, CRP proved unable to predict mortality in ACLF patients. The independent prognostic added value of the NLR is also in agreement with results from noncirrhotic postoperative patients (3). Nevertheless, it is crucial to note that CRP was examined only at admission. Contrarily to our data, CRP has already been shown to predict short-term mortality when persistently elevated throughout ICU hospitalization (16, 18, 34).
NLR as risk factor independent of the MELD score
Finally, we observed that the prognostic value of NLR in ACLF patients was independent of the MELD score. This raises concern about inflammation staging in acutely ill patients developing ACLF. In our cohort, patients undergoing ACLF were predominantly admitted to the ICU following bacterial infection or an alteration of tissue homeostasis during hemorrhage. In both cases, ACLF and organ failure occur through an exaggerated inflammatory response (8, 17).
In this line, NLR was recognized as a risk factor. Since SOFA score and the need for mechanical ventilation were also identified as risk factors, combining them with NLR interestingly improved the accuracy of the prediction, as shown by the ROC curves analysis. Thus, such a model could helpfully help clinicians to stratify the prognosis of ACLF patients. While the Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score has been developed to take into account specific cirrhotic features (11), we carried out a second multivariable analysis introducing CLIF-SOFA instead of just the SOFA score as a co-variable. With this adjustment, NLR, CLIF-SOFA, and mechanical ventilation requirement were still recognized as independent risk factors for mortality (Supplemental Digital Content, Appendix 3).
Strength and limitations
Some limitations of our study must be considered. First, the diagnostic performance of NLR alone assessed by an AUC of 71% is relatively limited. In addition to this, we showed that NLR level could vary on a wide range and then be difficult to interpret as a single value. However, NLR needs to be considered as a part of various elements used to establish the outcome, as shown in our model where we combined NLR with MV and SOFA. We also demonstrated that in nonsurvivors ACLF patients, NLR remained elevated in the time-course of ICU stay. This confirms that repeated values are also of interest.
Second, owing to our retrospective design used, our study findings would benefit from an external validation using a prospective approach. Nonetheless, our findings point out the relationship between NLR and early mortality in a cohort of advanced cirrhosis patients, the large majority of whom experienced ACLF.
Third, although we emphasized that NLR was higher in the group of patients with ACLF, as well as those who did not survive, we did not clarify whether the systemic inflammatory response directly contributed to hepatic function deterioration. Moreover, the relationship between NLR and mechanisms of organ dysfunction is not strongly supported solely on the basis of our data. Indeed, given the retrospective design of our work, the available inflammation markers are CRP and WBC count. Identifying the cytokines involved in the immune response leading to NLR disturbances, such as interleukin-17, could be of interest (35).
CONCLUSIONS
NLR may have some potential as an additional tool in combination with SOFA score and requirement of mechanical ventilation, to identify ACLF patients with a higher risk of early mortality.
In this way, our data suggest that this prognostic marker could be regarded as an indicator of excessive inflammation underlying advanced cirrhosis.
Nevertheless, further prospective studies are warranted to shed light on the relationship with systemic inflammatory response.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
The authors declare that they have no competing interests.
REFERENCES
- 1.Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, Jeffrey RR, Buchan KG, El-Shafei H, Hillis GS. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J 2007; 154 5:995–1002. [DOI] [PubMed] [Google Scholar]
- 2.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106 6:dju124. [DOI] [PubMed] [Google Scholar]
- 3.Forget P, Dinant V, De Kock M. Is the neutrophil-to-lymphocyte ratio more correlated than C-reactive protein with postoperative complications after major abdominal surgery? Peer J 2015; 13 3:e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao F, Li X, Geng M, Ye X, Liu H, Liu Y, Wan G, Wang X. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Medicine (Baltimore) 2015; 94 11:e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Lou Y, Chen Y, Yang J. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with acute-on-chronic liver failure. Int J Clin Pract 2014; 68 8:1034–1040. [DOI] [PubMed] [Google Scholar]
- 6.Leithead JA, Rajoriya N, Gunson BK, Ferguson JW. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int 2015; 35 2:502–509. [DOI] [PubMed] [Google Scholar]
- 7.Biyik M, Ucar R, Solak Y, Gungor G, Polat I, Gaipov A, Cakir OO, Ataseven H, Demir A, Turk S, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2013; 25 4:435–441. [DOI] [PubMed] [Google Scholar]
- 8.Arroyo V, Moreau R, Jalan R, Ginès P. EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol 2015; 62 suppl 1:S131–S143. [DOI] [PubMed] [Google Scholar]
- 9.Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care 2012; 16 6:R227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers 2016; 2:16041. [DOI] [PubMed] [Google Scholar]
- 11.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144 7:1426–1437. [DOI] [PubMed] [Google Scholar]
- 12.Moreau R. The pathogenesis of ACLF: the inflammatory response and immune function. Semin Liver Dis 2016; 36 2:133–140. [DOI] [PubMed] [Google Scholar]
- 13.Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 2007; 46 6:1872–1882. [DOI] [PubMed] [Google Scholar]
- 14.Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol 2009; 51 3:475–482. [DOI] [PubMed] [Google Scholar]
- 15.Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet 2015; 386 10003:1576–1587. [DOI] [PubMed] [Google Scholar]
- 16.Cervoni JP, Amorós À, Bañares R, Luis Montero J, Soriano G, Weil D, Moreau R, Pavesi M, Thévenot T, Di Martino V. EASL-CLIF Consortium. Prognostic value of C-reactive protein in cirrhosis: external validation from the CANONIC cohort. Eur J Gastroenterol Hepatol 2016; 28 9:1028–1034. [DOI] [PubMed] [Google Scholar]
- 17.Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, et al. CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 2016; 64 4:1249–1264. [DOI] [PubMed] [Google Scholar]
- 18.Di Martino V, Coutris C, Cervoni JP, Dritsas S, Weil D, Richou C, Vanlemmens C, Thevenot T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl 2015; 21 6:753–760. [DOI] [PubMed] [Google Scholar]
- 19.Tu KH, Jenq CC, Tsai MH, Hsu HH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Outcome scoring system for short-term prognosis in critically ill cirrhotic patients. Shock 2011; 36 5:445–450. [DOI] [PubMed] [Google Scholar]
- 20.Gacouin A, Locufier M, Uhel F, Letheulle J, Bouju P, Fillatre P, Le Tulzo Y, Tadié JM. Liver cirrhosis is independently associated with 90-day mortality in ARDS patients. Shock 2016; 45 1:16–21. [DOI] [PubMed] [Google Scholar]
- 21.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42 2:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148 3:839–843. [DOI] [PubMed] [Google Scholar]
- 24.Kwon JH, Jang JW, Kim YW, Lee SW, Nam SW, Jaegal D, Lee S, Bae SH. The usefulness of C-reactive protein and neutrophil-to-lymphocyte ratio for predicting the outcome in hospitalized patients with liver cirrhosis. BMC Gastroenterol 2015; 15:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SD, Kim SH, Kim YK, Lee SA, Park SJ. Prognostic significance of preoperative peripheral blood monocyte ratio in patients with hepatocellular carcinoma. World J Surg 2014; 38 9:2377–2385. [DOI] [PubMed] [Google Scholar]
- 26.Kalra A, Wedd JP, Bambha KM, Gralla J, Golden-Mason L, Collins C, Rosen HR, Biggins SW. Neutrophil-to-lymphocyte ratio correlates with proinflammatory neutrophils and predicts death in low model for end-stage liver disease patients with cirrhosis. Liver Transpl 2017; 23 2:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SY, Shin TG, Jo IJ, Jeon K, Suh GY, Lee TR, Yoon H, Cha WC, Sim MS. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am J Emerg Med 2017; 35:234–239. [DOI] [PubMed] [Google Scholar]
- 28.Salciccioli JD, Marshall DC, Pimentel MA, Santos MD, Pollard T, Celi LA, Shalhoub J. The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: an observational cohort study. Crit Care 2015; 19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Wang LK, Wang LW, Han XQ, Yang F, Gong ZJ. Blockade of high-mobility group box-1 ameliorates acute on chronic liver failure in rats. Inflamm Res 2013; 62 7:703–709. [DOI] [PubMed] [Google Scholar]
- 30.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol 2014; 60 1:197–209. [DOI] [PubMed] [Google Scholar]
- 31.Dirchwolf M, Podhorzer A, Marino M, Shulman C, Cartier M, Zunino M, Paz S, Muñoz A, Bocassi A, Gimenez J, et al. Immune dysfunction in cirrhosis: distinct cytokines phenotypes according to cirrhosis severity. Cytokine 2016; 77:14–25. [DOI] [PubMed] [Google Scholar]
- 32.Taneja R, Sharma AP, Hallett MB, Findlay GP, Morris MR. Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock 2008; 30 6:618–622. [DOI] [PubMed] [Google Scholar]
- 33.Seok Y, Choi JR, Kim J, Kim YK, Lee J, Song J, Kim SJ, Lee KA. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock 2012; 37 3:242–246. [DOI] [PubMed] [Google Scholar]
- 34.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol 2012; 56 6:1299–1304. [DOI] [PubMed] [Google Scholar]
- 35.Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013; 58 1:58–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


