Abstract
Purpose of review
There is growing clinical interest for the use of cardiopulmonary exercise testing (CPET) to evaluate patients with or suspected coronary artery disease (CAD). With mounting evidence, this concise review with relevant teaching cases helps to illustrate how to integrate CPET data into real world patient care.
Recent findings
CPET provides a novel and purely physiological basis to identify cardiac dysfunction in symptomatic patients with both obstructive-CAD and nonobstructive-CAD (NO-CAD). In many cases, abnormal cardiac response on CPET may be the only objective evidence of potentially undertreated ischemic heart disease. When symptomatic patients have NO-CAD on coronary angiogram, they are still at increased risk for cardiovascular events. This problem appears to be more common in women than men and may warrant more aggressive risk factor modification. As the main intervention is lifestyle (diet, smoking cessation, exercise) and medical therapy (statins, angiotensin-converting enzyme inhibitors, beta-blockers), serial CPET testing enables close surveillance of cardiovascular function and is responsive to clinical status.
Summary
CPET can enhance outpatient evaluation and management of CAD. Diagnostically, it can help to identify physiologically significant obstructive-CAD and NO-CAD in patients with normal routine cardiac testing. CPET may be of particular value in symptomatic women with NO-CAD. Prognostically, precise quantification of improvements in exercise capacity may help to improve long-term lifestyle and medication adherence for this chronic condition.
Keywords: cardiopulmonary exercise testing, coronary artery disease, therapeutic monitoring
INTRODUCTION
In the past decade, cardiopulmonary exercise testing (CPET) has seen an exponential increase in its evidence base for specific patient populations [1▪]. CPET for coronary artery disease (CAD) assessment is an area of growing clinical interest in which different parameters provide both diagnostic and prognostic insight for evaluation and management. The traditional diagnostic role of exercise stress testing has been to identify obstructive CAD (O-CAD) with the intent to revascularize culprit coronary lesions. Current strategies have focused on anatomical imaging with coronary computed tomography angiography (CCTA) with or without fractional flow reserve measurement (CCTA-FFR) and functional methods including stress ECG, stress echocardiography, and myocardial perfusion imaging (MPI) with ionizing radiation along with emergence of hybrid imaging of positron emission tomography (PET) together with CT (cardiac PET-CT). In outcomes analysis, direct coronary imaging with CCTA has shown reduced myocardial infarction (MI) compared with functional testing but without a reduction in mortality or hospitalizations at the expense of more frequent use of invasive procedures [2▪,3,4▪]. With the growing challenge of symptomatic nonobstructive CAD (NO-CAD) in clinical practice, these modalities have limited value. CPET helps to expand the role of exercise stress testing beyond identifying flow-limiting lesions. Diagnostically, it can help to confirm presence of cardiac dysfunction in symptomatic patients with 50% or less coronary stenosis which should act as a trigger for more aggressive risk factor modification to treat CAD. Prognostically, it enables close surveillance of cardiovascular status with serial testing whereby 10% increments in change in exercise capacity [i.e., peak oxygen consumption (volume of oxygen metabolized during exercise (VO2))] can be quantified to measure response to lifestyle and medical therapy to ensure patients are responding to therapy and remain adherent long term. Estimating exercise capacity lacks the precision needed to track longitudinal changes in patients acting as their own controls. This review addresses the clinical approach to utilize CPET in the evaluation and management of CAD.
Box 1.
no caption available
DIAGNOSTIC UTILITY
The diagnostic utility of CPET to detect exercise-induced cardiac dysfunction lies in its’ ability for key variables to serve as surrogates for cardiac output (CO) (i.e., VO2) and stroke volume (SV) (i.e., O2-pulse) as well as a direct measure of the heart-rate (HR) response, in real-time per the Fick equation. Breath-by-breath analysis during a linear ramp protocol on a cycle ergometer enables detection of a normal vs. pathological response caused by CAD [5]. As described by the ischemic cascade, mechanical dysfunction precedes electrical changes and symptoms (Fig. 1) [6]. Myocardial oxygen deficit during exertion causes mechanical dysfunction past the ischemic threshold resulting in SV to decrease with progressively increasing workload. To maintain peripheral perfusion to the exercising skeletal muscles, the autonomic nervous system upregulates sympathetic activity to accelerate HR as a compensation mechanism. This compensatory response in late exercise has been quantified as the change in HR to work-rate slope parameter (ΔHR–WR slope), comparing the HR slope in the last 2 min of exercise to that in middle of exercise. This parameter has been demonstrated to be abnormal in symptomatic patients with NO-CAD as well O-CAD [7▪]. Although healthy individuals have no change or deceleration of the HR response in late exercise (zero or negative ΔHR–WR slope), symptomatic patients with varying degrees of coronary plaque have acceleration of their HR response (positive ΔHR–WR slope; values >15% are pathological) in late exercise (Fig. 2) (Met-test database). In patients not capable of augmenting, the HR response (advanced CAD, autonomic dysfunction and HR limiting medications causing chronotropic incompetence), the abrupt plateau or decrease in SV is accompanied by a decrease in CO, reflected by VO2, relative to work-rate (ΔVO2/ΔWR slope flattens) [8,9] and minute ventilation (i.e., the oxygen uptake efficiency slope) [10–12]. A more blunted VO2 response is consistent with more severe disease.
FIGURE 1.
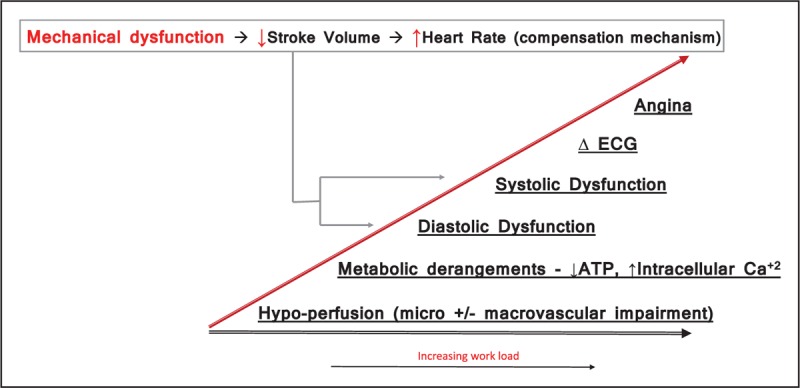
The figure demonstrates the sequence of metabolic derangements that occur when the myocardium becomes oxygen deprived with progressively increasing workload. ATP, adenosine triphosphate; Ca2+, calcium.
FIGURE 2.
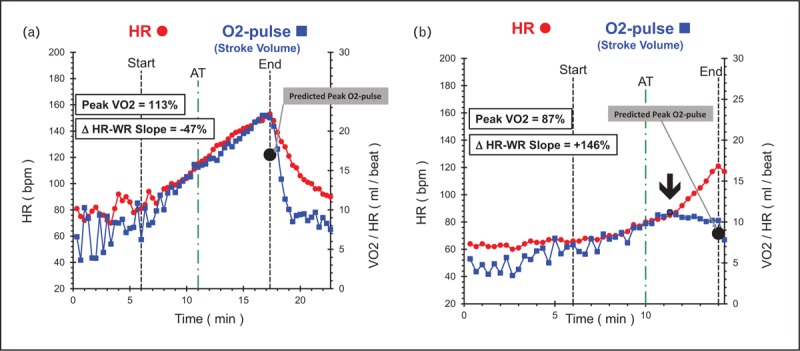
(a) Heart-rate and stroke-volume assessments in a healthy 45-year-old male with no heart disease and no cardiovascular risk factors. Note the high-peak linear stroke volume response (blue line) with deceleration of heart-rate slope (negative ΔHR–WR slope – red line) after the anaerobic threshold. (b) Heart-rate and stroke-volume assessments in a 58-year-old female patient after myocardial infarction, multiple stent placements, and prior to entry into cardiac rehab program. This study was performed on beta-blocker therapy. She achieves a normal peak stroke volume but has a decreasing trend with acceleration of heart-rate slope (positive ΔHR–WR slope) after the anaerobic threshold demonstrating persistent cardiac dysfunction after revascularization. ΔHR–WR slope, change in heart-rate slope in last 2 min of exercise compared with heart-rate slope at anaerobic threshold; AT, anaerobic threshold; bpm, beats per minute; HR, heart rate; O2-pulse, oxygen pulse (volume of oxygen metabolized during exercise/heart rate).
The recognition of NO-CAD as an insidious condition that raises the risk for cardiovascular events has increased the urgency for a new approach to manage this growing dilemma, particularly in women [13▪,14,15▪–17▪]. Traditional functional stress testing (stress ECG, stress echocardiography, and MPI) is not effective in detecting NO-CAD caused by diffuse microvascular ischemia [18,19▪,20,21]. Stress-imaging studies were designed to detect relatively intense regional hypo-perfusion abnormalities but lose their sensitivity when the global ischemic burden becomes diffuse via multiple mechanisms including coronary endothelial dysfunction and decreased coronary flow reserve (CFR) [22]. Sara et al.[23] reported that approximately two-thirds of men and women with NO-CAD and normal routine stress studies had microvascular dysfunction proven with functional coronary angiograms over a 20-year period. Cardiac PET can identify decreased CFR in patients without significant obstruction and decreased CFR predicts increased cardiovascular risk [24▪,25▪]. Whether or not the abnormal SV and HR patterns observed in symptomatic patients with NO-CAD on CPET is due to microvascular ischemia is an area in need of further evaluation and correlation with CFR and coronary endothelial dysfunction is necessary.
The main clinical advantage that CPET offers over other cardiac tests to diagnose ischemic heart disease (IHD) is its’ ability to prove that NO-CAD is causing physiologically significant inducible myocardial dysfunction, implying that symptoms are cardiac and likely due to undertreated CAD in symptomatic patients with normal routine testing. Relying on the body's natural compensation mechanism to identify undertreated atherosclerotic heart disease is a novel concept and has potential to improve preventive care as this information can be used to implement more aggressive exercise and medical treatment for CAD, particularly when symptoms are vague (anginal equivalents). Figure 3 demonstrates cardiac dysfunction on CPET in a patient with MI with NO-CAD (MINOCA) (Met-test database). She had a 3-year history of shortness of breath with normal routine outpatient cardiac testing. Her symptoms were not recognized as an anginal equivalent and the window to intensify atherosclerosis modifying therapy was missed resulting in preventable morbidity to the patient and increased costs to the healthcare system. Interest in augmenting outpatient exercise intolerance evaluation with CPET is growing. The Mayo Clinic now offers CPET in conjunction with nuclear MPI + CPET as a single test. The main motivation was not to enhance the evaluation of IHD but to identify nonischemic causes of symptoms (deconditioning, pulmonary, diastolic dysfunction). They reported that almost three-fourths of normal nuclear MPI studies had abnormal CPET findings that helped improve clinical care [26▪▪].
FIGURE 3.
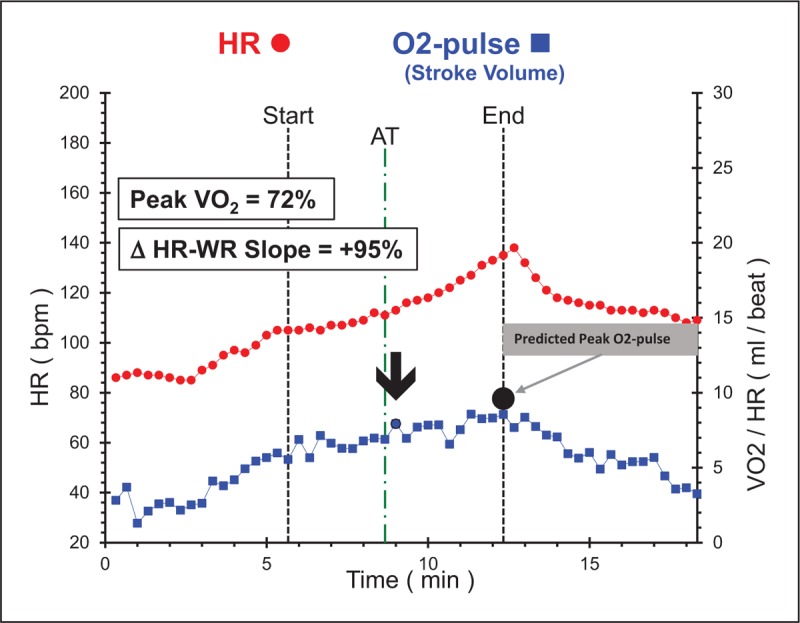
Cardiac dysfunction in a 53-year-old African-American female who presented to the emergency room with non-ST elevation myocardial infarction. Cardiopulmonary exercise testing abnormalities include plateau of O2-pulse with low peak value with corresponding acceleration of heart-rate–work-rate slope in late exercise. Coronary angiogram revealed only minor irregularities. This study was performed on beta-blocker therapy upon entry into cardiac rehab. The dysfunction pattern would be more pronounced off of beta-blockers. ΔHR–WR slope, change in heart-rate slope in last 2 min of exercise compared with heart-rate slope at anaerobic threshold; AT, anaerobic threshold; bpm, beats per minute; HR, heart rate; O2-pulse, oxygen pulse (volume of oxygen metabolized during exercise/heart rate).
PROGNOSTIC UTILITY FOR THERAPEUTIC MONITORING
The main aim of any intervention is to improve prognosis, limit morbidity, and maintain a higher quality of life, all of which are markers associated with high peak VO2 values. In fact, due to a robust evidence base, peak VO2, also defined as cardiorespiratory fitness (CRF), has been proposed as a clinical vital sign [27▪▪]. There is a strong, inverse, and independent association between peak VO2, or CRF, and the first nonfatal MI and subsequent heart failure risk, with significant risk reclassification by this primary CPET variable [28]. CRF, as determined by direct measurement of peak VO2, exerts a major long-term influence on prognosis in men after MI, coronary artery bypass surgery, or IHD and can play a valuable role in risk stratification and counseling [29]. In women with CAD, considered as a continuous variable, a 1 ml O2/kg/min advantage in initial peak VO2 was associated with 10% lower cardiac mortality [30]. In absolute terms, patients with a peak VO2 less than 16 ml O2/kg/min at time of discharge after MI and post-percutaneous coronary intervention (PCI) have been shown to be at increased risk for adverse events over 2 years [31]. With excellent reproducibility of peak VO2 measurements in the CAD population [32], the goal in each individual should be to increase this vital sign from baseline for longevity [27▪▪]. Peak VO2 is a variable that is responsive to therapy, and serial measures are potentially valuable in close surveillance of cardiovascular health status. Individuals whose peak VO2 increases between examinations have a lower risk of adverse health and clinical outcomes than those whose peak VO2 decreases, and this should be communicated to patients [27▪▪].
Exercise as a therapeutic modality
Exercise has a multitude of physiological benefits in cardiac patients (Table 1). It improves cardiac function (i.e., SV and HR response) as well as skeletal muscle perfusion and oxygen utilization [peripheral extraction = C(a − v)O2]. A large meta-analysis recently confirmed that exercise-based cardiac rehabilitation reduces cardiovascular mortality, hospital admissions (along with associated healthcare costs), and improves quality of life [33▪]. However, cardiac rehabilitation remains underutilized and more favorable outcomes are equated to larger increases in peak VO2[34,35]. In general, the greater the activity amount or intensity, the greater the increase in peak VO2. CAD-patients who perform regular moderate physical exercise at least 150 min/week have significantly better left ventricular (LV) diastolic function and higher peak VO2 than patients with less than 150 min/week exercise and higher weekly physical exercise outweighs the other modifiable cardiovascular risk factors of obesity, diabetes and hypertension [36▪]. Exercise training may constitute a relevant therapeutic strategy in patients with microvascular angina. A recent pilot study demonstrated that exercise training that increased mean peak VO2 by 12% was associated with significant reduction of reversible ischemic myocardial perfusion defects on single photon emission computed tomography in patients with angiographically normal coronaries along with improvement in quality of life [37▪]. Figure 4 highlights key exercise physiology parameters to monitor with serial testing and demonstrates a dramatic response to cardiac rehabilitation in a highly motivated 57-year-old male after 3-vessel coronary bypass graft surgery (Met-test database).
Table 1.
Benefits of formal cardiac rehabilitation and exercise training programs
| Improvement in exercise capacity |
| Estimated metabolic equivalents, +35% |
| Peak oxygen consumption, +15% |
| Peak anaerobic threshold, +11% |
| Improvement in lipid profiles |
| Total cholesterol, −5% |
| Triglycerides, −15% |
| HDL-C, +6% (higher in patients with a low baseline) |
| LDL-C, −2% |
| LDL-C/HDL-C, −5% (higher in certain subgroups) |
| Reduction in inflammation |
| hs-CRP, −40% |
| Reduction in indices of obesity |
| BMI, −1.5% |
| Fat, −5% |
| Metabolic syndrome, −37% |
| Improvements in behavioral characteristics |
| Depression |
| Anxiety |
| Hostility |
| Somatization |
| Overall psychological distress |
| Reduction in stress-related increased mortality |
| Improvements in quality of life and components |
| Increased heart-rate recovery |
| Increased heart-rate variability |
| Reduced resting pulse |
| Improvements in blood rheology |
| Reduction in hospital costs |
| Reduction in major morbidity and mortality |
hs-CRP, high-sensitive C-reactive protein; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol. Reprinted from Swift et al. with permission of the publisher. Copyright © 2013, The Japanese Circulation Society.
FIGURE 4.
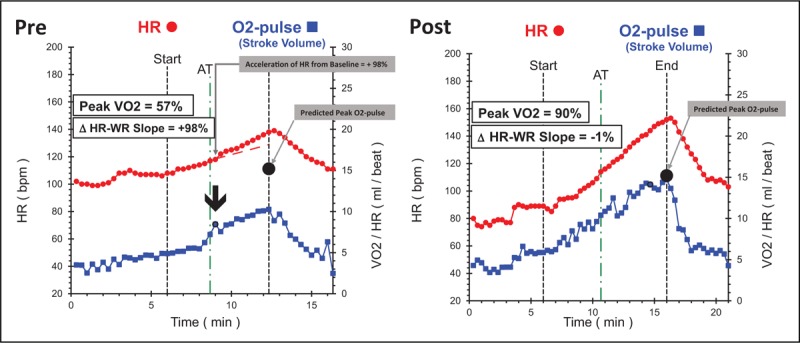
Precardiac and postcardiac rehab snapshot of a 57-year-old male who underwent three-vessel bypass surgery. This individual had hypertension, hyperlipidemia and was sedentary prior to surgery. He was highly motivated during rehab and was jogging by end of 3-month rehab. Changes: resting heart rate decreased from 100 to 80 bpm; acceleration in heart-rate–work-rate slope completely normalized in Test 2; peak volume of oxygen metabolized during exercise increased 57%; peak O2-pulse increased 42% to predicted normal value; peak heart rate increased 11% and anaerobic threshold, an effort independent measure of aerobic capacity increased by 42%. ΔHR–WR slope, change in heart-rate slope in last 2 min of exercise compared with heart-rate slope at anaerobic threshold; AT, anaerobic threshold; bpm, beats per minute; HR, heart rate; O2-pulse, oxygen pulse (volume of oxygen metabolized during exercise/heart rate).
Precise exercise prescriptions
Defining the moderate intensity exercise HR zones for CAD patients as the starting point of an exercise rehabilitation program is crucial. This can be a daunting task considering that more severe CAD patients have intrinsic chronotropic incompetence and many are on HR-limiting agents. CPET offers the unique ability to define individualized specific HR zones that correspond to moderate intensity exertion (40–60% of VO2 reserve) thereby targeting work zones that are safe and therapeutically effective.
Monitoring medical therapy
A recent observational study in 9136 patients (61% women) with MINOCA reported long-term outcomes results on medical therapy for secondary prevention. Findings revealed significant benefit from statin (23% reduction) and angiotensin-converting enzyme (ACE) inhibitor (ACE-I)/angiotensin II receptor blocker (ARB) therapy (18% reduction) and borderline benefit from beta-blocker therapy (14% reduction) [38▪▪]. Current American College of Cardiology/American Heart Association guidelines recognize the therapeutic benefits of various pharmacological interventions to improve functional capacity. Exercise time has proven to be a discriminating test for many antianginal therapies and is recommended for this purpose by both the US Food and Drug Administration and the European Medicines Agency. Statins, ACE-Is, and beta-blockers have reduced morbidity and mortality in patients with atherosclerotic heart disease and are the cornerstones of preventive medical therapy for this population. In theory, any therapy that improves coronary microcirculation should result in improved myocardial perfusion, contractility, higher peak SV, and therefore higher peak VO2 over time. Ranolazine has been shown to improve CFR and peak VO2 in patients with microvascular angina [39,40]. Coronary microvascular function quantified by CFR has been independently associated with peak VO2 in obese CAD patients [41]. Enhanced external counter-pulsation therapy decreases angina episodes and improves quality of life in patients with systolic dysfunction and one study elucidating the mechanism of these findings demonstrated improved endothelial function and dramatic increase in peak VO2 (+36%) after 35 sessions [42]. ACE-Is improve CFR in symptomatic women with NO-CAD over 4 months [43] and ACE-I along with ARBs significantly improve peak VO2 on their own and have led to greater enhancements when taken together [44]. Rosiglitazone improves endothelial function and peak VO2 in people with diabetes after 4 months of therapy [45]. Although beta-blockers blunt the HR response to physical exertion, previous research has found a significant increase in peak VO2 still occurs in post MI and heart failure patients who participate in an exercise training program [46]. Metformin improves ventilatory efficiency in nondiabetic heart failure patients with insulin resistance [47]. Of note, therapies that improve resting parameters but not exercise capacity are of questionable clinical value. In the ALDO-DHF trial, long-term aldosterone receptor blockade improved resting LV diastolic function but did not affect maximal exercise capacity and hence patient symptoms, or quality of life in patients with heart failure with preserved ejection fraction [48].
Given the associated improvements in key prognostic CPET variables with the initiation of pharmacological therapy, it may be interesting to explore the feasibility of titrating medications based on CPET ‘responders’ or ‘nonresponders’ in future clinical trials. The frequency at which a patient should perform CPET evaluation to monitor the effectiveness of interventions is not well defined. In clinically stable patients, CPET might be considered at 2-year to 4-year intervals, whereas in patients with signs and symptoms, the test might occur in a time frame that has been reported to cause significant improvements in the variable of interest [49▪].
Figure 5 illustrates changes in key CPET parameters with serial testing for longitudinal tracking in an at-risk asymptomatic individual found to have significant cardiac dysfunction and low-peak VO2 on baseline CPET. After 3.3 years of medical therapy on statin and niacin, there was significant improvement in cardiac dysfunction and peak VO2; addition of exercise rehab almost completely normalized these parameters by year 7 (Met-test database). Figure 6 demonstrates the distribution of change in peak VO2 in 225 firefighters tested 1 year apart. The 20 patients with more than 20% decrease in peak VO2 are of particular concern and should be singled out for further evaluation and treatment (Met-test database). Exercise therapy alone has potential to change the trajectory of these individuals with rapidly worsening prognosis.
FIGURE 5.
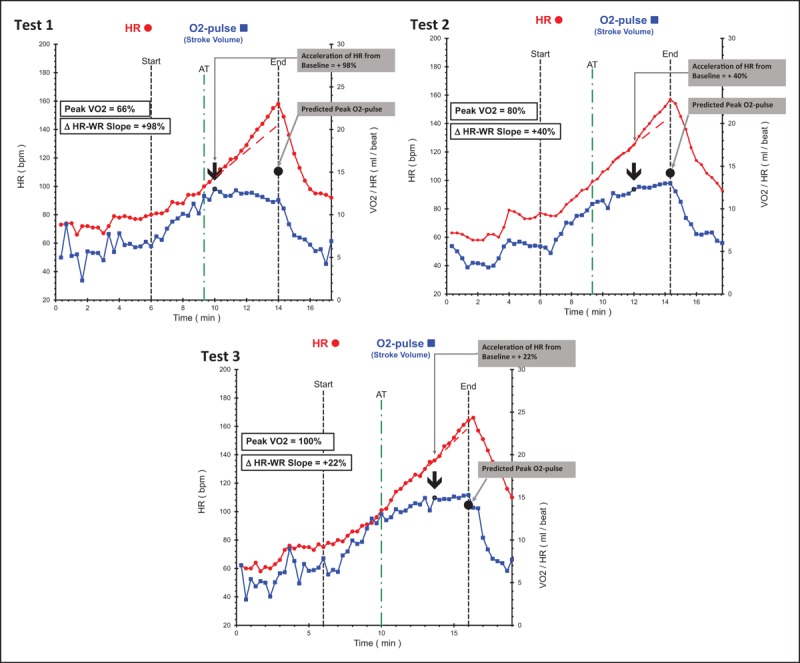
Serial comparison data of an individual acting as his own control; cardiovascular risk factors included a strong family history, hyperlipidemia and sedentary lifestyle. Test 1: baseline study at age 36 without symptoms demonstrating pronounced cardiac dysfunction with low peak volume of oxygen metabolized during exercise. Note the pronounced drop in stroke volume response just after the anaerobic threshold resulting significantly reduced peak O2-pulse. Heart-rate–work-rate response accelerates concurrently with 98% increase in slope from baseline. Test 2: repeat study after 3.3 years of medical therapy with statin + niacin with no change in lifestyle. Lipids improved dramatically and repeat cardiopulmonary exercise testing demonstrates less cardiac dysfunction with improved stroke volume response resulting in 12% higher peak volume of oxygen metabolized during exercise (ml/kg/min) and peak O2-pulse (ml/min) with less acceleration of heart-rate–work-rate slope (compensatory response has diminished). Test 3: motivated by improvement in Test 2, this person started regular exercise with cross-fit regimen. Test 3 is 4.5 years after Test 2 and represents effect of exercise in addition to continuing lipid therapy. Absolute peak volume of oxygen metabolized during exercise increased 30%, peak O2-pulse increased 15% and there is borderline left ventricular dysfunction with marginal acceleration of heart-rate response after the anaerobic threshold. This individual has better cardiovascular function at age 42 than he did at 36 and has likely improved his long-term prognosis, quality of life, and healthcare costs. ΔHR–WR slope, change in heart-rate slope in last 2 min of exercise compared with heart-rate slope at anaerobic threshold; AT, anaerobic threshold; bpm, beats per minute; HR, heart rate; O2-pulse, oxygen pulse (volume of oxygen metabolized during exercise/heart rate); SV, stroke volume.
FIGURE 6.
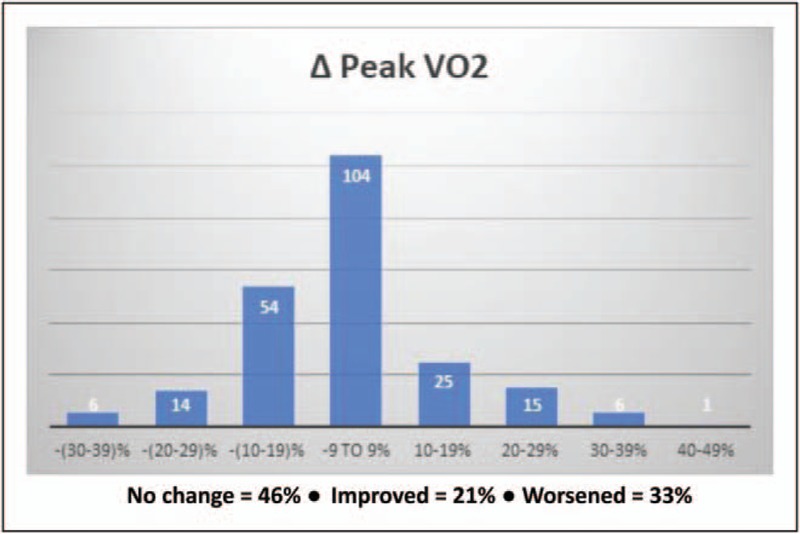
Change in peak volume of oxygen metabolized during exercise in 225 firefighters tested 1 year apart. The 20 individuals that had more than 20% decrease should be of particular concern and should warrant more aggressive risk assessment with intervention. Δ Peak volume of oxygen metabolized during exercise, change in peak volume of oxygen metabolized during exercise between two serial tests approximately 1 year apart.
OBSTRUCTIVE CORONARY ARTERY DISEASE AND REVASCULARIZATION
Cardiac dysfunction detected by CPET is a function of global ischemic burden given that the abnormalities in SV and HR are seen in symptomatic patients with both NO-CAD and O-CAD [7▪]. Men had the highest rate of revascularization in this study and their mean peak VO2 was 68% of predicted. ‘Balanced Ischemia’ seen in patients with diffuse O-CAD (triple vessel and left main disease) is a challenging condition for imaging-based stress testing to detect due to the nonregionalized nature of the ischemic burden. These patients are some of the highest risk patients and tend to have decreased peak VO2. Coronary angiogram should be considered in symptomatic patients with normal stress imaging and cardiac dysfunction with reduced peak VO2 (<70% of predicted) on CPET. Coronary CTA with FFR [50] may be the ideal next study in such individuals as patients in need of revascularization can be singled out and patients with NO-CAD can undergo exercise and medical therapy with close surveillance to ensure that peak VO2 and prognosis is improving over time. Continuous regular feedback with set goals for future peak VO2 values has the potential to improve patient adherence with lifestyle changes and medications. The effect of revascularization on peak VO2 would be an area of interest with the current paucity of information. The results of ORBITA, the only blinded, randomized placebo-controlled trial of PCI, show that in patients with angina and single vessel coronary stenosis, exercise capacity (measured by CPET) and symptoms were not improved significantly compared with placebo intervention [51▪▪]. In patients with multivessel CAD, one study comparing complete vs. incomplete revascularization with PCI in patients after MI did not show significant short-term differences on CPET between the two approaches [52]. These data lend credence to the hypothesis that increasing peak VO2 is more likely a function of other mechanisms (including the microcirculation) rather than macrocirculation and that therapeutic interventions should move beyond the stenosis-centric frame to optimize outcomes.
CONCLUSION
In conclusion, there is a robust body of evidence demonstrating the clinical value of CPET in a number of patient populations, including those with cardiovascular disease. Key CPET variables hold powerful diagnostic and prognostic utility in patients with cardiovascular disease. CPET also holds considerable promise in gauging the response to a broad range of therapies, including pharmacologic, surgical and lifestyle interventions. Improving the CPET response, in particular peak VO2, may evolve into a primary treatment goal in patients with cardiovascular disease if future randomized trials support this approach.
Acknowledgements
None.
Financial support and sponsorship
There was no funding associated with this article.
Conflicts of interest
S.C. is an employee and owns equity at Met-test. N.K. is an employee and owns equity at Whitby Cardiovascular Institute. D.L.B. discloses the following relationships – Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (Clinical Trial Steering Committees), Harvard Clinical Research Institute (Clinical Trial Steering Committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (Clinical Trial Steering Committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME Steering Committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. The remaining authors have no conflict of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016; 133:e694–e711. [DOI] [PubMed] [Google Scholar]; Most recent review article outlining the use of cardiopulmonary exercise testing (CPET) parameters for different clinical applications.
- 2▪.Foy AJ, Dhruva SS, Peterson B, et al. Coronary computed tomography angiography vs functional stress testing for patients with suspected coronary artery disease: a systematic review and meta-analysis. JAMA Intern Med 2017; 177:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]; Most recent data summarizing the clinical impact of different testing strategies for stable coronary artery disease (CAD).
- 3.Hwang I-C, Choi SJ, Choi JE, et al. Comparison of mid- to long-term clinical outcomes between anatomical testing and usual care in patients with suspected coronary artery disease: a meta-analysis of randomized trials. Clin Cardiol 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Blankstein R, Bittencourt MS, Bhatt DL. Coronary CTA in the evaluation of stable chest pain: clear benefits, but not for all. J Am Coll Cardiol 2017; 69:1771–1773. [DOI] [PubMed] [Google Scholar]; Editorial highlighting that most of the clinical gains with computed tomography angiography over functional testing likely come from enhanced utilization of preventive medications.
- 5.Chaudhry S, Arena R, Wasserman K, et al. Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. Am J Cardiol 2009; 103:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upton MT, Rerych SK, Newman GE, et al. Detecting abnormalities in left ventricular function during exercise before angina and ST-segment depression. Circulation 1980; 62:341–349. [DOI] [PubMed] [Google Scholar]
- 7▪.Chaudhry S, Kumar N, Behbahani H, et al. Abnormal heart-rate response during cardiopulmonary exercise testing identifies cardiac dysfunction in symptomatic patients with nonobstructive coronary artery disease. Int J Cardiol 2017; 228:114–121. [DOI] [PubMed] [Google Scholar]; First article to describe abnormal acceleration of heart-rate response during late exercise in symptomatic patients with suspected CAD compared with a normal cohort. This abnormal compensatory response is physiologically indistinguishable between patients with obstructive and nonobstructive CAD (NO-CAD) and is consistent with undertreated atherosclerotic heart disease.
- 8.Belardinelli R, Lacalaprice F, Carle F, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J 2003; 24:1304–1313. [DOI] [PubMed] [Google Scholar]
- 9.Belardinelli R, Lacalaprice F, Tiano L, et al. Cardiopulmonary exercise testing is more accurate than ECG-stress testing in diagnosing myocardial ischemia in subjects with chest pain. Int J Cardiol 2014; 174:337–342. [DOI] [PubMed] [Google Scholar]
- 10.Pinkstaff S, Peberdy MA, Kontos MC, et al. Usefulness of decrease in oxygen uptake efficiency slope to identify myocardial perfusion defects in men undergoing myocardial ischemic evaluation. Am J Cardiol 2010; 106:1534–1539. [DOI] [PubMed] [Google Scholar]
- 11.Coeckelberghs E, Buys R, Goetschalckx K, et al. Prognostic value of the oxygen uptake efficiency slope and other exercise variables in patients with coronary artery disease. Eur J Prev Cardiol 2016; 23:237–244. [DOI] [PubMed] [Google Scholar]
- 12.Uliari S, Myers J, Bernardi E, et al. Oxygen uptake attenuation at ventilatory threshold in men with coronary artery disease. J Cardiopulm Rehabil Prev 2016; 36:258–262. [DOI] [PubMed] [Google Scholar]
- 13▪.Alzuhairi KSM, Soegaard PS, Ravkilde JR, et al. Long-term prognsis of nonst-elevation myocardial infarction patients with no obstructive coronary artery disease is worse than patients with one- or two-vessel disease. Eur Heart J 2017; 38 Suppl_1: ehx504.P4686-ehx4504.P4686. [Google Scholar]; Outcomes data showing that patients with non-ST elevation MI and NO-CAD have a comparable prognosis to patients with one-vessel or two-vessel disease and patients with diffuse atherosclerosis have worse prognosis than those with angiographically normal coronary arteries.
- 14.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012; 33:734–744. [DOI] [PubMed] [Google Scholar]
- 15▪.Pizzi C, Xhyheri B, Costa GM, et al. Nonobstructive versus obstructive coronary artery disease in acute coronary syndrome: a meta-analysis. J Am Heart Assoc 2016; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]; Patients with NO-CAD are potentially undertreated and require more specific management.
- 16▪.Tavella R, Cutri N, Tucker G, et al. Natural history of patients with insignificant coronary artery disease. Eur Heart J 2016; 2:117–124. [DOI] [PubMed] [Google Scholar]; Symptomatic patients with NO-CAD often have persistent chest pain and impaired quality of life requiring novel strategies to improve health outcomes.
- 17▪.Williams MJA, Barr PR, Lee M, et al. Myocardial Infarction with non-obstructive coronary artery disease: not a benign condition. Heart Lung Circ 2017; 26:S23. [DOI] [PubMed] [Google Scholar]; Myocardial infarction without obstructive coronary disease is common (∼one in nine patients) and has an adverse outcome rate 12 times that of age and sex-matched patients without CAD.
- 18.Cassar A, Chareonthaitawee P, Rihal CS, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv 2009; 2:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: The iPOWER Study. J Am Heart Assoc 2016; 5:e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Impaired coronary flow reserve (CFR) is common in patients with angina and is independent of traditonal cardiovascular risk factors.
- 20.Rozanski A, Gransar H, Hayes SW, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013; 61:1054–1065. [DOI] [PubMed] [Google Scholar]
- 21.Shaw LJ, Mieres JH, Hendel RH, et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation 2011; 124:1239–1249. [DOI] [PubMed] [Google Scholar]
- 22.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015; 131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015; 8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 24▪.Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017; 135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]; Impaired CFR in women identifies increased cardiovascular risk and is a potential target for future noninterventional therapeutics.
- 25▪.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017; 10.1161/circulationaha.117.029992. [DOI] [PMC free article] [PubMed] [Google Scholar]; In patients that underwent cardiac PET scans, impaired CFR was a stronger predictor of cardiovascular mortality than maximal myocardial blood flow indicating that impaired coronary microcirculation is the main driver of cardiovascular events.
- 26▪▪.Christopoulos G, Bois J, Allison TG, et al. The impact of combined cardiopulmonary exercise testing and SPECT myocardial perfusion imaging on downstream evaluation and management. J Nucl Cardiol 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; First combined modality study with CPET and MPI performed as one test. Diagnostic yield was enhanced by identification of nonischemic mechanism of exercise intolerance resulting in improved patient care. This study did not attempt to analyze inducible cardiac dysfunction from CAD on CPET, an analysis that would likely have reclassified a number of studies.
- 27▪▪.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016; 134:e653–e699. [DOI] [PubMed] [Google Scholar]; Excellent review article highlighting extensive data in the literature that cardiorespiratory fitness (CRF) is a powerful clinical vital sign for all-cause mortality and accurately reclassifies risk for adverse outcomes independent of traditional cardiovascular risk factors. The underlying premise is that the addition of CRF for risk classification presents health professionals with unique opportunities to improve patient management and to encourage lifestyle-based strategies.
- 28.Khan H, Jaffar N, Rauramaa R, et al. Cardiorespiratory fitness and nonfatalcardiovascular events: a population-based follow-up study. Am Heart J 2016; 184:55–61. [DOI] [PubMed] [Google Scholar]
- 29.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002; 106:666–671. [DOI] [PubMed] [Google Scholar]
- 30.Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol 2003; 42:2139–2143. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto W, Oishi S, Kawai H. The prognostic significance of cardiopulmonary exercise testing at discharge for the patients with acute myocardial infarction. J Card Fail 2016; 22:S174. [Google Scholar]
- 32.Coeckelberghs E, Buys R, Goetschalckx K, et al. Test-retest reliability of maximal and submaximal gas exchange variables in patients with coronary artery disease. J Cardiopulm Rehabil Prev 2016; 36:263–269. [DOI] [PubMed] [Google Scholar]
- 33▪.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67:1–12. [DOI] [PubMed] [Google Scholar]; The study confirms that exercise-based cardiac rehabilitation reduces cardiovascular mortality and provides important data showing reductions in hospital admissions and improvements in quality of life that are consistent across patient and intervention types.
- 34.Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65:2079–2088. [DOI] [PubMed] [Google Scholar]
- 35.Martin B-J, Arena R, Haykowsky M, et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc 2013; 88:455–463. [DOI] [PubMed] [Google Scholar]
- 36▪.Boscheri A, Haller B, Christle J, et al. Physical exercise- mediated effects on left ventricular diastolic function outweigh other modifiable risk factors in coronary artery disease patients. Eur Heart J 2017; 38 Suppl_1: ehx493.P6036-ehx6493.P6036. [Google Scholar]; CAD patients who perform regular physical exercise at least 150 min/week have significantly better resting left ventricular diastolic function and peak volume of oxygen metabolized during exercise (VO2) independent of many traditional cardiovascular risk factors.
- 37▪.Simoes MV, Carvalho EEV, Crescencio JC, et al. Prospective controlled trial testing the effect of physical training over myocardial perfusion disturbance and quality of life in patients with primary microvascular angina: a pilot study. Eur Heart J 2017; 38 Suppl_1: ehx504.P3428-ehx3504.P3428. [Google Scholar]; Physical training is associated with reduction of reversible ischemic myocardial perfusion defects in patients with microvascular angina. This positive effect was accompanied by significant improvement of the functional capacity and quality of life. The results of this pilot study indicate that physical training may constitute a relevant therapeutic strategy in this population.
- 38▪▪.Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017; 135:1481–1489. [DOI] [PubMed] [Google Scholar]; The long-term observational study in myocardial infarction with NO-CAD (MINOCA) patients indicates beneficial effects of treatment with statins and angiotensin-converting enzyme inhibitors/AT1 blockers on outcome in patients with MINOCA, a trend toward a positive effect of β-blocker treatment, and a neutral effect of dual antiplatelet therapy. Medical management is the mainstay for improving outcomes in patients with symptomatic NO-CAD just as it is in obstructive CAD.
- 39.Chaudhry S, Arena R, Wasserman K, et al. The utility of cardiopulmonary exercise testing in the assessment of suspected microvascular ischemia. Int J Cardiol 2011; 148:e7–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagliamonte E, Rigo F, Cirillo T, et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography 2015; 32:516–521. [DOI] [PubMed] [Google Scholar]
- 41.Jürs A, Pedersen LR, Olsen RH, et al. Coronary microvascular function, insulin sensitivity and body composition in predicting exercise capacity in overweight patients with coronary artery disease. BMC Cardiovasc Disord 2015; 15:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck DT, Martin JS, Casey DP, et al. Enhanced external counterpulsation improves endothelial function and exercise capacity in patients with ischaemic left ventricular dysfunction. Clin Exp Pharmacol Physiol 2014; 41:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J 2011; 162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatia V, Bhatia R, Mathew B. Angiotensin receptor blockers in congestive heart failure: evidence, concerns, and controversies. Cardiol Rev 2005; 13:297–303. [DOI] [PubMed] [Google Scholar]
- 45.Regensteiner JG, Bauer TA, Reusch JEB. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care 2005; 28:2877–2883. [DOI] [PubMed] [Google Scholar]
- 46.Pavia L, Orlando G, Myers J, et al. The effect of beta-blockade therapy on the response to exercise training in postmyocardial infarction patients. Clin Cardiol 1995; 18:716–720. [DOI] [PubMed] [Google Scholar]
- 47.Wong AKF, Symon R, AlZadjali MA, et al. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur J Heart Fail 2012; 14:1303–1310. [DOI] [PubMed] [Google Scholar]
- 48.Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the ALDO-DHF randomized controlled trial. JAMA 2013; 309:781–791. [DOI] [PubMed] [Google Scholar]
- 49▪.Guazzi M, Bandera F, Ozemek C, et al. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol 2017; 70:1618–1636. [DOI] [PubMed] [Google Scholar]; The review highlights modern CPET use as a single or combined test that allows the pathophysiological bases of exercise limitation to be translated into clinical practice.
- 50.Cook CM, Petraco R, Shun-Shin MJ, et al. Diagnostic accuracy of computed tomography-derived fractional flow reserve: a systematic review. JAMA Cardiol 2017; 2:803–810. [DOI] [PubMed] [Google Scholar]
- 51▪▪.Al-Lamee R, Thompson D, Dehbi H-M, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; First trial comparing percutaneous coronary intervention (PCI) with placebo (sham procedure) to relieve angina and increase exercise capacity in individuals with angina and single vessel disease with CPET as the primary endpoint. Surprisingly, the results revealed that CPI did not improve symptoms or increase exercise time or peak VO2 compared with placebo and called into question the clinical value of PCI in patients with single vessel disease. The results cannot be generalized to multivessel disease.
- 52.Zhao W, Bai J, Zhang F, et al. Impact of completeness of revascularization by coronary intervention on exercise capacity early after acute ST-elevation myocardial infarction. J Cardiothorac Surg 2014; 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]


