Abstract
Purpose of review
The current review discusses the integration of guideline and evidence-based palliative care into heart failure end-of-life (EOL) care.
Recent findings
North American and European heart failure societies recommend the integration of palliative care into heart failure programs. Advance care planning, shared decision-making, routine measurement of symptoms and quality of life and specialist palliative care at heart failure EOL are identified as key components to an effective heart failure palliative care program. There is limited evidence to support the effectiveness of the individual elements. However, results from the palliative care in heart failure trial suggest an integrated heart failure palliative care program can significantly improve quality of life for heart failure patients at EOL.
Summary
Integration of a palliative approach to heart failure EOL care helps to ensure patients receive the care that is congruent with their values, wishes and preferences. Specialist palliative care referrals are limited to those who are truly at heart failure EOL.
Keywords: advance care planning, heart failure, palliative care, shared decision-making
INTRODUCTION
Heart failure is a chronic illness with a median survival of 2.1 years after diagnosis [1]. Patients with heart failure typically experience a progressive decline in physical functioning and a gradual increase in symptom severity. Patients are considered to have Stage D heart failure when, despite optimal medical management, they continue to have symptoms of shortness of breath and fatigue at rest [2]. Once heart failure progresses to Stage D, patients experience poor quality of life, high symptom burden and face a median life expectancy of only 6–12 months [3]. The last 6 months of life for a heart failure patient is often characterized by frequent hospital admissions, procedures and intensive care use, often culminating in a hospital death [4,5]. This occurs despite the majority of heart failure patients expressing a preference to die at home and wanting less invasive care at end of life (EOL) [6,7]. The unpredictable course and life-limiting nature of heart failure suggest patients with heart failure would benefit from palliative care.
Palliative care is a multidisciplinary approach to patient management that focuses on relieving unpleasant symptoms and improving quality of life for all patients diagnosed with a life-limiting illness [8]. Historically, heart failure palliative care referrals were initiated when the patient was felt to have a life expectancy 6 months or less (Stage D heart failure). This model proved ineffective, as many clinicians would defer or delay palliative care referral until they were certain a patient was dying [9–11,12▪]. Even if the transition to Stage D heart failure was accepted as the trigger for palliative care referral, there are simply too few palliative care providers to meet the needs of this large patient population. Recommendations from North American and European heart failure societies support shifting the focus from being prognosis-driven to a symptom-centered model whereby symptom distress and poor quality of life trigger a referral to palliative care regardless of the patient's estimated survival time [13–15]. The heart failure specialist with palliative care consultancy model is an effective way to integrate palliative care into a heart failure program (Fig. 1) [16▪]. The heart failure team remains responsible for delivering and evaluating patient care, advance care planning (ACP) and goals of care discussions. Palliative care providers support the heart failure team's management, by helping to manage patient symptoms that have not responded to the team's interventions or are beyond their scope of practice. Patients continue to receive care from the heart failure team who they trust and referrals to a specialized palliative care program are reserved for those patients who truly need it. The purpose of this article is to provide practical advice on implementing a guideline and evidence-based palliative approach to heart failure EOL care. This is achieved by focusing on four key areas: ACP, shared decision-making, routine measurement of symptoms and quality of life, and specialist palliative care at heart failure EOL.
FIGURE 1.
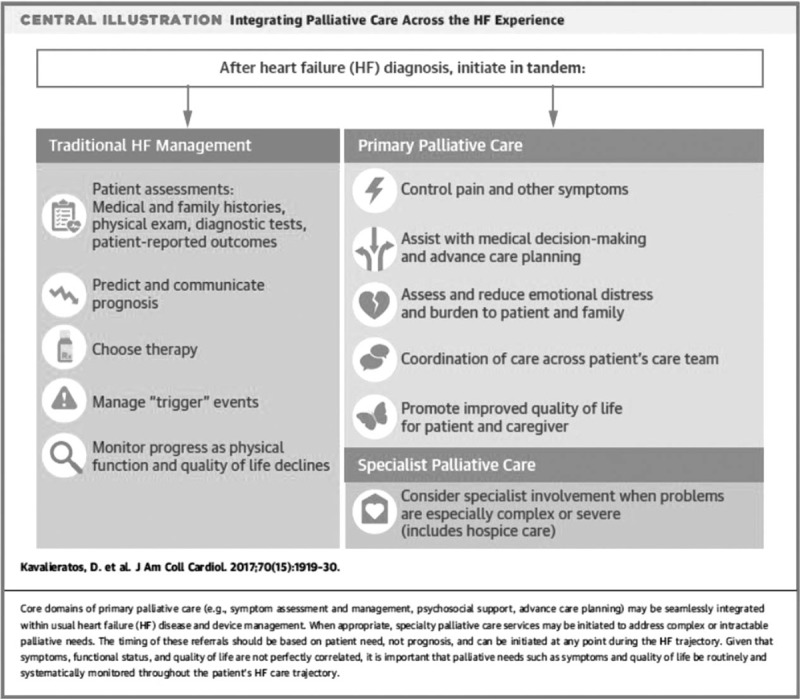
Specialist heart failure care with palliative care consultancy. Reproduced with permission [16▪].
Box 1.
no caption available
ADVANCE CARE PLANNING
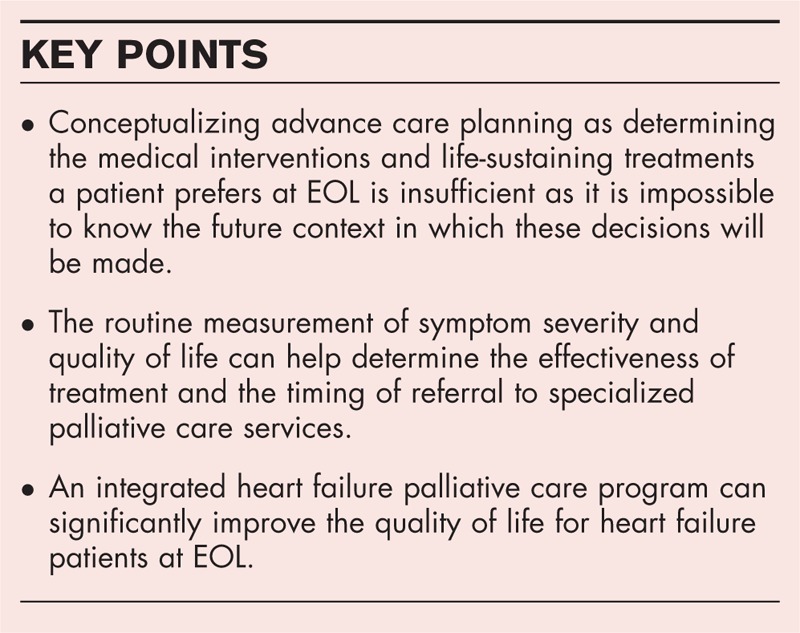
The Canadian Cardiovascular Society, American College of Cardiology and American Heart Association recommend that heart failure patients engage in ACP early in their illness [13,14,17]. Effective ACP is associated with improved quality of life and satisfaction with care, lower rates of depression and anxiety amongst bereaved family members and lower healthcare costs [18]. ACP is the process a person uses to reflect on their values and beliefs to establish wishes that guide decision-making in the future, particularly at EOL [19▪,20]. There are three main elements [1] identification of the substitute decision maker (SDM) [2] reflecting on values, wishes and beliefs as they relate to health care and [3] preparing SDMs for future decision-making by discussing these values and wishes. Once established, the wishes inform future consent or refusal of treatment. The purpose of this process is to support patient autonomy and involvement in decision-making by requiring all care decisions to be based on the expressed wishes or best interest of the patient, regardless of whether the decision is made by a capable patient or the SDM of an incapable patient. Traditionally, ACP has been thought of as determining the medical interventions and life-sustaining treatments that are preferred at EOL. There is now evidence that this method is largely ineffective as it is impossible to know the future context in which these decisions will be made [21]. The value-based model of ACP represents a new paradigm in heart failure EOL care. By engaging with this process, the patient and their SDM acquire the information and develop the skills needed to participate in the complex medical decisions that may be needed as their medical condition worsens. This approach is more likely to ensure that the care an individual receives is concordant with their values, goals and wishes. At present, there is no evidence to determine if the value-based model of ACP is effective. Future research is needed to determine if this approach actually increases ACP completion rates for patients living with heart failure.
SHARED DECISION-MAKING
Patients who transition to Stage D heart failure have, on average, less than 6 months to live and are considered to be at EOL. The American Heart Association endorses a model of shared decision-making for patients at EOL [22]. Shared decision-making is enacted through a series of meetings or discussions between the patient, family/SDM and heart failure team. The patient and their family are responsible for sharing their understanding of their current condition, symptoms, quality of life, goals of treatment and the outcomes of ACP [22]. Clinicians are responsible for summarizing the patient's prognosis, including information on potential treatment outcomes, quality of life, symptom burden, caregiver burden and the types of challenges and decisions the patient may face in the next 6–12 months [22]. Team-based palliative care providers can attend the meeting, participate in the discussion and suggest treatments that may help achieve the patient's goals of care. Together the patient, family and heart failure team determine the goals of care and document it in the patient's electronic medical record. Discussions should include preferences and timing of implantable cardioverter deactivation, life-sustaining treatments such as cardiopulmonary resuscitation, ventilation and dialysis as well as any preferences for palliative or hospice care. Decisions may be enacted immediately or in the future and are subject to change based on the patient's condition. The shared decision-making process is proactive and iterative with any significant change in the patient's health, for example a hospitalization or ICD shock, triggering another discussion about the goals of care.
Currently, there is no evidence to support the effectiveness of shared decision-making for heart failure EOL care. However, the benefits are implicit. When the palliative care provider is presented as a member of the heart failure team, patients and team members may be more receptive to their interventions. It eliminates a referral to the palliative care consult team which can cause fragmentation of care and increase emotional distress for the patient and family as they perceive their EOL care may no longer be provided by a team they trust. Trainees can participate and lead these discussions which will help them build their palliative care and communication skills. As trainees transition to their own practice, they will carry these skills with them which will have an impact on future patients and trainees. Finally, patients, family members and the clinical team are aware of the patient's wishes and plan of care which is a helpful way to ensure, as much as possible, that the care a patient receives is congruent with their values, beliefs and wishes and facilitates future decision-making.
ROUTINE MEASUREMENT OF SYMPTOMS AND QUALITY OF LIFE
The symptom-centered model of palliative care recommends that unpleasant symptoms and poor quality of life trigger a palliative care referral. However, there is very little evidence on how to operationalize this in clinical practice. In our practice, we have found it helpful to use standardized instruments to measure quality of life and symptom severity [23,24▪]. The Edmonton Symptom Assessment System (ESAS) can be used to measure symptom severity in heart failure [23,25,26]. Patients rate the severity of 10 symptoms on individual 10-cm visual analog scales. The scores can be summed to provide an overall symptom distress score with higher scores representing higher symptom severity. The ESAS takes about 5 min to complete. Once unpleasant symptoms are identified, interventions can be initiated to reduce the symptom distress. The ESAS can be readministered at follow-up to determine if interventions are effective. Persistent individual symptom scores over 7 (severe distress), or the presence of multiple symptoms, should trigger a referral to the heart failure team-based palliative care provider. Reducing symptom severity can improve quality of life.
The Minnesota Living with Heart Failure Questionnaire (MLHFQ), Kansas City Cardiomyopathy Questionnaire (KCCQ) are heart failure specific tools to measure quality of life. Scores on the MLHFQ range from 0 to 105 with higher scores representing poorer quality of life. Scores for the KCCQ range from 0 to 100 with higher scores representing better quality of life. The EQ5D visual analog scale is a generic measure of quality of life with scores ranging from 0 to 100 with higher scores representing better quality of life. The EQ-5D-5L has documented reliability and validity in heart failure [27]. All three scales are responsive to change associated with heart failure palliative care intervention [28,29]. Patients who have persistent MLHFQ score more than 60, KCCQ or EQ5D score less than 40 should be referred to palliative care. The quality of life instruments take about 10 min to complete. It is important to note that the MLHFQ, KCCQ and EQ5D are licensed tools, and the cost of using them might be beyond the resources of the program. The ESAS is not licensed and widely available on the internet. ‘General wellbeing’ is measured on the ESAS and could be used as a surrogate for quality of life.
SPECIALIST PALLIATIVE CARE AT HEART FAILURE END OF LIFE
Patients who continue to deteriorate despite the interventions of the heart-failure-team-based palliative care provider may benefit from hospice care. Hospice care is specialized palliative care that uses a multidisciplinary team approach to provide patients and families with comprehensive EOL care. Care can be provided either at home or within a residential hospice. Historically, this was the type of care many cardiologists understood as palliative care [12▪]. The results of the palliative care in heart failure (PAL-HF) trial suggest specialist heart failure palliative care is an effective method to support heart failure patients at EOL [30▪▪]. PAL-HF randomized 150 patients, from one site, to usual care (n = 75) or heart failure palliative care intervention (n = 75). The palliative care intervention consisted of interdisciplinary, guideline-driven heart failure palliative care to manage symptoms, psychosocial and spiritual needs and quality of life for patients with advanced heart failure. Patients were eligible for enrollment if they were at high risk for rehospitalization or death based on their Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness score [31]. Consistent with a palliative approach to heart failure EOL care, the primary outcomes were quality of life scores; KCCQ and the Functional Assessment of Chronic Illness Therapy – Palliative Care scale (FACIT-PAL); FACIT spiritual and well-being scores and Hospital Anxiety and Depression (HADs) scores. Patients were followed for 6 months. There were no differences in baseline characteristics between the two groups. The average age of the sample was 71 years, 47% were women and 45% had a diagnosis of heart failure with preserved ejection fraction (HFpEF). The average duration of heart failure was 67 months. At 6 months, patients in the intervention arm showed significant improvement in the KCCQ and FACIT-PAL scores, HADs scores and spiritual well-being scores. There was no difference in hospitalization rates (30%) or mortality (29%). Importantly, the sample included almost 50% women and patients with HFpEF, often under-represented groups in heart failure trials, which improves the generalizability of results to clinical care.
Intravenous inotropes can help improve symptoms at heart failure EOL. At present, most patients receiving intravenous inotropes must remain in hospital. This practice pattern evolved from evidence that patients on home intravenous inotrope therapy had higher 6 month mortality rates than patients with no intravenous inotropes [32–34]. However, a recent study evaluating the effectiveness of a home intravenous inotrope program for heart failure palliation reported a median survival of 9 months [35]. We are currently collaborating with a specialist palliative care program to provide and evaluate outpatient (home or residential hospice) intravenous inotrope therapy. Prior to hospital discharge, patients must understand that the role of the intravenous inotropes is to improve quality of life and not prolong life. Patients receive their care through the palliative care program with heart failure consultancy for inotrope or heart-failure-specific concerns. Although it is too early to tell if the program is effective, we do feel it addresses the needs of patients by allowing patients to spend their final days, weeks or months at home.
CONCLUSION
We have been working on our palliative approach to heart failure EOL for over 10 years. At the outset, we were already meeting annually with patients to discuss the results of prognostic testing, review their preferences and develop a plan of care. We reinforced the progressive and life-limiting nature of heart failure and encouraged patients to engage in the ACP process at time of diagnosis, with the annual review and with any significant change in a patient's condition. We are currently revising our approach to ACP to a values-based model. In 2012, we implemented the routine measurement of quality of life and symptom severity for patients being considered for heart transplantation or mechanical circulatory support. A natural progression was to implement this with our Stage D patients who were not eligible for transplant of mechanical support. We found that many patients were unable to complete the battery of tests, so our current practice is to have them complete the ESAS [24▪]. We formally implemented our palliative approach to heart failure care in 2014. Our palliative care physician attends heart failure clinic 1 day/week. Patients with palliative care needs, regardless of prognosis or disease severity, are scheduled to attend clinic on that day. The palliative care physician may see patients with the team or meet with them individually. This partnership has helped the heart failure team improve their overall knowledge of palliative care and enhance communication skills for difficult conversations. Palliative care providers have a broader knowledge of community supports that might assist heart failure patients and families manage at home. Our patients are now receiving referrals to these programs. The number of referrals to the specialized palliative care program has decreased as we manage patient care issues we previously would have referred out. Referrals to specialized palliative care services are limited to those who are truly at EOL and facilitated by our palliative care physician. In our experience, the implementation of a palliative approach to heart failure EOL care has had helped us to ensure the care we provide to our patients is congruent with their preferences.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ko DT, Alter DA, Austin PC, et al. Life expectancy after an index hospitalization for patients with heart failure: a population-based study. Am Heart J 2008; 155:324–331. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:1977–2016. [DOI] [PubMed] [Google Scholar]
- 3.Costanzo MR, Mills RM, Wynne J. Characteristics of ‘Stage D’ heart failure: insights from the acute decompensated heart failure national registry longitudinal module (ADHERE LM). Am Heart J 2008; 155:339–347. [DOI] [PubMed] [Google Scholar]
- 4.Unroe KT, Grenier MA, Hernandez AF, et al. Resource use in the last 6 months of life among Medicare beneficiaries with heart failure, 2000–2007. Arch Int Med 2011; 171:196. [DOI] [PubMed] [Google Scholar]
- 5.Kaul P, McAlister FA, Ezekowitz J, et al. Resource use in the last 6 months of life among patients with heart failure in Canada. Arch Int Med 2011; 171:211–217. [DOI] [PubMed] [Google Scholar]
- 6.Fried TR, van Doorn C, O’Leary JR, et al. Older persons’ preferences for site of terminal care. Ann Intern med 1999; 131:109–112. [DOI] [PubMed] [Google Scholar]
- 7.Formiga F, Ortega CC, Casas S, et al. End-of-life preferences in elderly patients admitted for heart failure. Qual J Med 2004; 97:803–808. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization, World Health Organization. WHO definition of palliative care. 2011; Available at: http://www.who.int/cancer/palliative/definition/en/. [Accessed 14 September 2017]. [DOI] [PubMed] [Google Scholar]
- 9.Kavalieratos D, Mitchell EM, Carey TS, et al. Not the ‘grim reaper service’: an assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc 2014; 3:e000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauptman PJ, Swindle J, Hussain Z, et al. Physician attitudes toward end-stage heart failure: a national survey. Am J Med 2008; 121:127–135. [DOI] [PubMed] [Google Scholar]
- 11.Dunlay SM, Foxen JL, Cole T, et al. A survey of clinician attitudes and self-reported practices regarding end-of-life care in heart failure. Palliat Med 2015; 29:260–267. [DOI] [PubMed] [Google Scholar]
- 12▪.McIlvennan CK, Allen LA. Palliative care in patients with heart failure. BMJ 2016; 353:i1010. [DOI] [PubMed] [Google Scholar]; An excellent review article on heart failure palliative care that includes a discussion of the barriers and opportunities for integration of palliative care onto the heart failure team.
- 13.McKelvie RS, Moe GW, Cheung A, et al. The 2011 Canadian Cardiovascular Society Heart Failure Management Guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol 2011; 27:319–338. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e239. [DOI] [PubMed] [Google Scholar]
- 15.Jaarsma T, Beattie JM, Ryder M, et al. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2009; 11:433–443. [DOI] [PubMed] [Google Scholar]
- 16▪.Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative care in heart failure. J Am Coll Cardiol 2017; 70:1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review of heart failure palliative care that includes a discussion of symptom management and inpatient support.
- 17.Howlett J, Morrin L, Fortin M, et al. End-of-life planning in heart failure: it should be the end of the beginning. Can J Cardiol 2010; 26:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med 2015; 29:869–884. [DOI] [PubMed] [Google Scholar]
- 19▪.Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017; 53:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description and outcomes of a Delphi technique to develop a consensus definition for advance care planning. The definition can be used to guide clinical, research and policy initiatives.
- 20.Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manage 2015; 49:1070–1080. [DOI] [PubMed] [Google Scholar]
- 21.Sudore RL, Fried TR. Redefining the ‘Planning’ in advance care planning: preparing for end-of-life decision making. Ann Intern Med 2010; 153:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012; 125:1928–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmons MJ, MacIver J, Alba AC, et al. Using heart failure instruments to determine when to refer heart failure patients to palliative care. J Palliat Care 2013; 29:217. [PubMed] [Google Scholar]
- 24▪.MacIver J, Wentlandt K, Ross HJ. Measuring quality of life in advanced heart failure. Curr Opin Support Palliat Care 2017; 11:12–16. [DOI] [PubMed] [Google Scholar]; An article that discusses routine measurement of symptom severity and quality of life in Stage D heart failure.
- 25.Ezekowitz JA, Thai V, Hodnefield TS, et al. The correlation of standard heart failure assessment and palliative care questionnaires in a multidisciplinary heart failure clinic. J Pain Symptom Manage 2011; 42:379–387. [DOI] [PubMed] [Google Scholar]
- 26.Opasich C, Gualco A, De Feo S, et al. Physical and emotional symptom burden of patients with end-stage heart failure: what to measure, how and why. J Cardiovasc Med 2008; 9:1104–1108. [DOI] [PubMed] [Google Scholar]
- 27.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150:707–715. [DOI] [PubMed] [Google Scholar]
- 28.Sidebottom AC, Jorgenson A, Richards H, et al. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med 2015; 18:134–142. [DOI] [PubMed] [Google Scholar]
- 29.Evangelista LS, Lombardo D, Malik S, et al. Examining the effects of an outpatient palliative care consultation on symptom burden, depression, and quality of life in patients with symptomatic heart failure. J Card Fail 2012; 18:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪▪.Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol 2017; 70:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]; A randomized clinical trial that provides evidence that an integrated approach to heart failure palliative care improves the quality of life for patients at heart failure end of life.
- 31.O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: The ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol 2010; 55:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauptman PJ, Mikolajczak P, George A, et al. Chronic inotropic therapy in end-stage heart failure. Am Heart J 2006; 152:1096.e1–1096e.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershberger RE, Nauman D, Walker TL, et al. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail 2003; 9:180–187. [DOI] [PubMed] [Google Scholar]
- 34.Gorodeski EZ, Chu EC, Reese JR, et al. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324. [DOI] [PubMed] [Google Scholar]
- 35.Hashim T, Sanam K, Revilla-Martinez M, et al. Clinical characteristics and outcomes of intravenous inotropic therapy in advanced heart failure. Circ Heart Fail 2015; 8:880–886. [DOI] [PubMed] [Google Scholar]


