Supplemental Digital Content is available in the text.
Abstract
Background:
It has been proposed that negative pressure wound therapy (NPWT) applied prophylactically to a closed incision may decrease the incidence of wound complications. Patients undergoing reduction mammaplasty are at risk of wound complications such as delayed healing, infection, and dehiscence, and the bilateral nature of the surgery allows for a within-patient randomized study to evaluate incisional NPWT’s effect in reducing healing complications.
Methods:
In this multicenter trial, 200 patients undergoing bilateral reduction mammaplasty were treated with PICO Single-Use NPWT System or standard wound-care dressings randomized to right or left breast for up to 14 days to enable within-patient comparison. Follow-up assessments were conducted to evaluate the difference in incision healing complications up to 21 days postsurgery. Healing complications (for the primary endpoint) were defined as delayed healing (incision not 100% closed by 7 days) and occurrence of dehiscence or infection within 21 days. Individual healing complications were assessed separately as secondary endpoints.
Results:
Significantly fewer healing complications (primary endpoint) were noted in NPWT-treated breasts [113 (56.8%)] versus standard care [123 (61.8%)]. The difference of 10 (5.0%) patients with fewer healing complications using NPWT was statistically significant (P = 0.004). NPWT also resulted in a significantly lower incidence of dehiscence (secondary endpoint) compared with standard care [32 patients (16.2%) versus 52 patients (26.4%)] by day 21, a relative reduction of 38% (P < 0.001).
Conclusions:
This is the first major prospective, within-patient, randomized, controlled, multicenter study to provide evidence for an incisional NPWT strategy to reduce healing complications.
INTRODUCTION
Breast reduction or “reduction mammaplasty” is one of the most common procedures carried out by plastic surgeons, with more than 103,077 women in the United States alone undergoing the procedure in 2015 for significant breast hypertrophy, and a further 148,967 for breast-lift surgery.1 Patients with symptomatic macromastia experience both physical and psychosocial issues including back, neck, and shoulder pain, and low self-esteem. Several studies have demonstrated breast reduction surgery to be a safe and effective procedure offering long-term relief for most patients and improving quality of life.2–4 Despite women undergoing breast-reduction surgery being generally young and healthy, postoperative complications are relatively common, with reported overall complication rates after breast-reduction surgery as high as 53%.2,5 Most complications are minor, requiring minimal intervention, but some patients require hospital readmission, endure discomfort from nonhealing wounds with scar formation, and may require surgical revision.
Negative-pressure wound therapy (NPWT) is most widely known for managing complex open wounds, where it is deployed to progress nonhealing wounds to a point of being ready for closure, either surgically or through secondary intention.6–8 However, growing evidence suggests NPWT may also offer significant benefits to closed incisions.9–15 The present study aimed to investigate the potential of a single-use NPWT device in preventing composite wound morbidity (infection, dehiscence, and delayed wound healing) compared with standard care (SC) in patients undergoing bilateral reduction mammaplasty. The bilateral nature of the surgery offers optimal clinical conditions to undertake a direct comparison of the 2 treatment arms on the same patient. The effects of NPWT on the medium-term aesthetic appearance and quality of the resultant scar compared with SC were also assessed and will be reported elsewhere.
PATIENTS AND METHODS
Bilateral Randomization
A multicenter study was conducted across 6 sites: United States (n = 3), France (n = 1), South Africa (n = 1), and The Netherlands (n = 1). Two hundred eleven patients (n = 211) who were undergoing elective bilateral breast reduction were recruited from the population routinely seen by the investigators between June 1, 2012, and April 9, 2014. Ethics approval was first obtained at the institution of the principal investigator (R.D.G.), institutional review board at Northwestern University, Chicago, (STU00062369 - 5/22/2012), and at each of the other sites. Before entry into the study, all patients signed informed consent forms. Treatment randomization was within-patient (i.e., right or left breast) via a central Web site, www.SealedEnvelope.com. NPWT covered the horizontal portion and junction of the inverted “T” incision. Surgeons were free to choose the pedicle technique appropriate for their patient.
Sample Size
Sample size calculations showed that 197 patients would be required to detect an absolute difference of 10% in the complication rate between bilateral breasts treated either with NPWT or SC dressings, assuming 20% of wounds treated with SC dressings and 10% of wounds treated with NPWT develop a healing complication (a 50% reduction) and that there were 26% discordant pairs. This is on the basis of a 2-sided McNemar’s test at the α = 5% level of significance and 80% power. The sample size was rounded up to 200.
Inclusion and Exclusions
Women aged > 18 years who had undergone elective surgery for bilateral reduction mammaplasty and having postsurgical incisions of similar length on each breast were included in the study. Presurgical exclusion criteria included pregnancy or lactation, using steroids or other immune modulators known to affect healing, history of radiation of the breast, tattoos in the area of the incision, skin conditions such as cutis laxa that would result in poor healing or widened scars, patients with a known significant history of scar problems (i.e., hypertrophic scarring or keloids), and known allergies to product components. Postsurgical exclusion criteria were incisions still actively bleeding and incisions > 12 inches (30 cm) maximum linear dimension.
NPWT Intervention and SC
The NPWT device was PICO (Smith & Nephew Medical Limited, Hull, United Kingdom), a portable, single-use (disposable after 7 days) NPWT system delivering −80 mm Hg (nominal) negative pressure to the wound surface.16,17 Figure 1 shows the PICO device and a patient with the NPWT system in place (Fig. 1). Treatment commenced on day 0 and lasted up to 14 days. The pump has a 7-day lifespan, and the associated PICO NPWT dressing is left in place up to 7 days. Each PICO kit comes with 2 NPWT dressings, so, according to the needs of the individual patient and the level of exudate, dressing changes were permitted before 7 days at the investigator’s clinical judgment. Participating physicians were advised to discontinue treatment on day 14 and return patients to SC (see below) if the incision was still not closed at this time point. The recommended comparator treatment (SC) was 3M STERI-Strip (3M Health Care, St. Paul, Minn.). STERI-Strips were placed along the entire axis of the incision and covered with a dry gauze dressing or nonadherent dressing. Alternatively, investigators could use a nonadherent dry dressing if STERI-Strips were not deemed appropriate by the principal investigator at that site. The majority of patients received prophylactic antibiotics [n = 188 (94.5%)]. Sutures were used to close wounds in the majority of patients [n = 168 (84.4%)], or a combination of sutures and staples [n = 28 (14.1%)]. Wound assessments were carried out at dressing application (day 0), first dressing change (3–7 days), and any subsequent dressing changes, and at the day 21, 42, and 90 follow-up assessments.
Fig. 1.
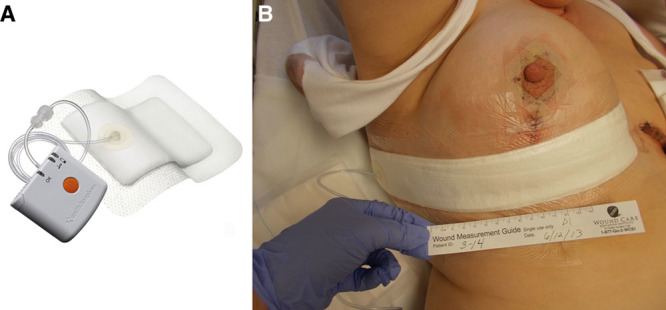
A, The PICO NPWT pump is lightweight (80 g) and measures 64 mm × 68 mm × 21 mm. It delivers −80 mm Hg and lasts for 7 days. There is no exudate canister and it is battery-powered. Exudate evaporates from the upper surface of the multilayer NPWT dressing, which transmits negative pressure across the surface of the wound. B, A patient from the trial randomized to receive PICO to the right breast following the reduction procedure. The dressing covers the entire lower incision, the T-junction, and much of the vertical incision except the nipple.
In some centers, 1 surgeon performed both procedure and the closure; in other centers, attending physicians were involved either with closure or with surgery and closure with 1 breast. The maximum level of experience (including attending surgeon, assistant surgeon, and resident) was similar in both treatment and SC groups (median 11 years, mean 13.2 NPWT, and 12.0 SC). Use of drains was according to individual practice and uniform across both breasts: actual data = NPWT 80.9% and SC 80.4% breasts received a drain.
Study Endpoints
The primary objective was to assess whether an incision developed healing complications within 21 days of surgery for both incisional NPWT and the comparator dressing. A healing complication was defined as presence of at least one of the following conditions: infection (superficial or deep), dehiscence (partial, superficial, or deep), or delayed healing (incision not 100% closed within 7 days of the first surgical procedure). Secondary objectives were to assess the number and type of these complications individually including other postsurgical complications: skin necrosis, nipple and areola necrosis, cellulitis, abscess, suture abscess, or hematoma occurring within 21, 42, and 90 days postoperatively. Aesthetic appearance and scar quality were also assessed at 42 and 90 days and, for a subset of patients from a single center, scar quality was analyzed up to 1 year. The scar results will be reported separately elsewhere.
RESULTS
Patient Demographics
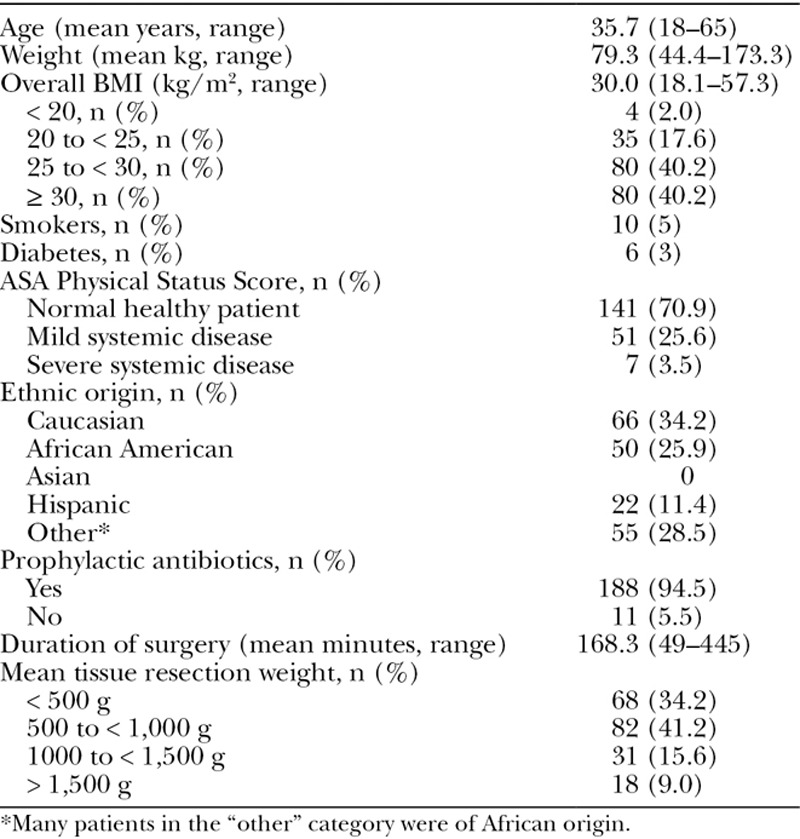
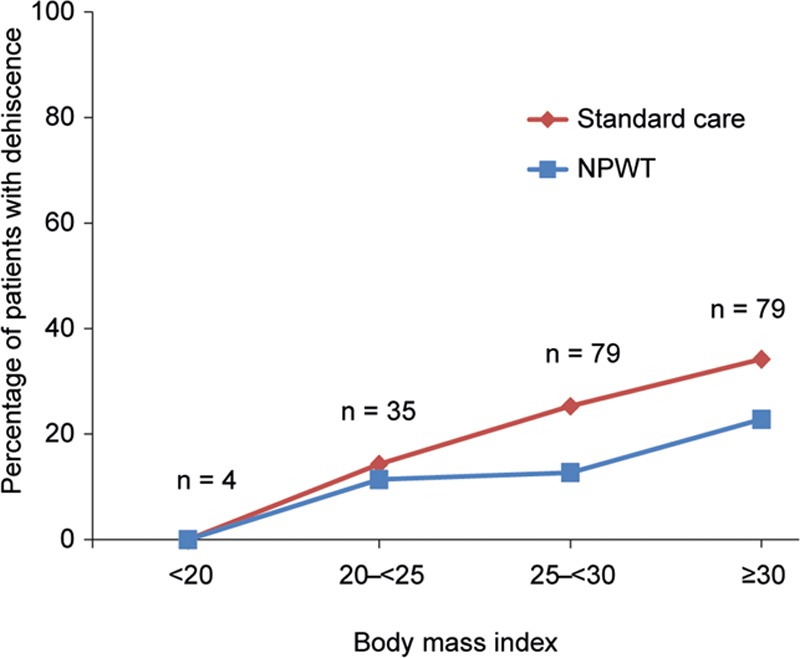
Two hundred patients were recruited into the study. Figure 2 shows participant flows. One patient withdrew before the first assessment. In total, 185 patients (92.5%) completed the day 21 assessment, and 178 (89%) completed the full 90-day study. Table 1 shows patient demographics. Overall, the patient population was relatively young (mean age, 35.7 years), and their general health was good. Of the 199 patients in the full analysis set, only 10 (5.0%) declared being smokers and 6 (3.0%) suffered from diabetes (all type 2). Most patients (70.9%; n = 141) were classed as “a normal healthy patient” by the American Society of Anesthesiology Physical Status Score. Slightly fewer than half of patients had a body mass index (BMI) > 30 kg/m2. Incision lengths were similar between treatment groups and centers (horizontal: NPWT mean 24.1 cm, SC mean 23.9 cm; vertical: NPWT mean 7.3 cm, SC mean 7.2 cm). Ease of application, comfort, and acceptability during wear were also assessed; SC and NPWT were very similar (data not shown).
Fig. 2.
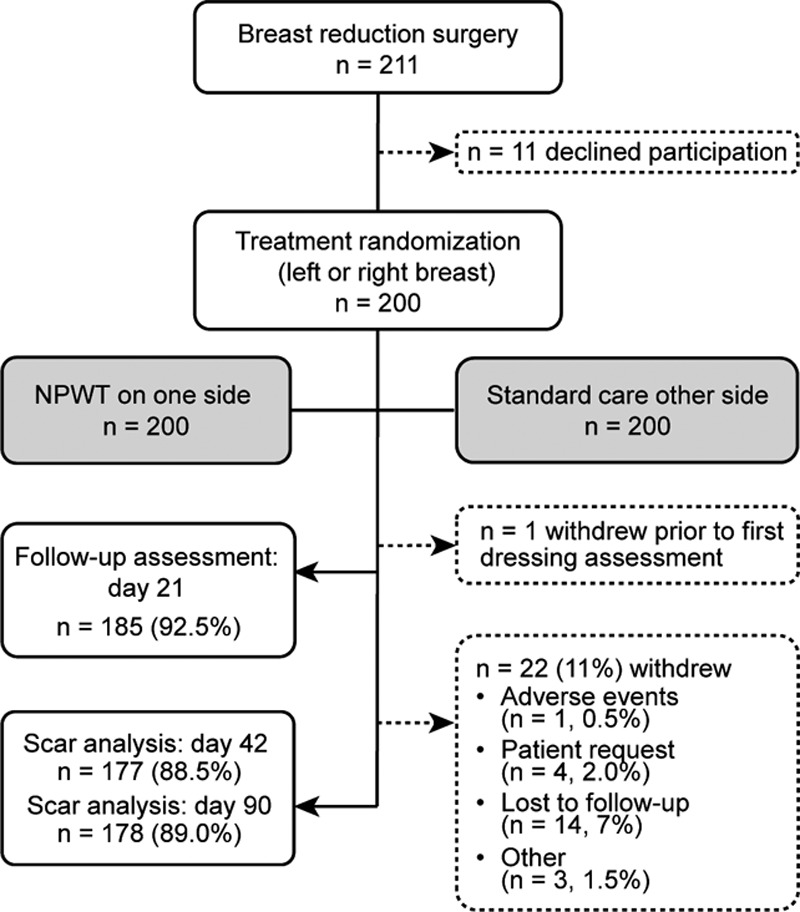
Patient disposition. Safety analysis set, n = 200 patients; full analysis set, n = 199 patients. One hundred seventy-eight patients (89.0%) completed the 90-day study; 22 patients (11%) were withdrawn: reasons were given as adverse event, n = 1 (0.5%); patient’s own request, n = 4 (2%); lost to follow-up, n = 14 (7%); and other, n = 3 (1.5%).
Table 1.
Patient Demographics (n = 199)
The Effect of NPWT on Wound-Healing Complications
Differences between breast incisions treated with NPWT and SC for both primary and secondary endpoints are compared in Figure 3. Use of NPWT on closed incisions resulted in a significant reduction in wound-healing complications within 21 days of surgery: 56.8% of NPWT-treated incisions (n = 113) experienced wound complications compared with 61.8% (n = 123) treated with SC (P = 0.004). The overall level of healing complications for SC (61.8%) seems high when compared with figures reported in the literature.2,5 In this study, delayed healing, which contributed to the primary endpoint of wound complications within 21 days, was defined as a wound not closed with 100% epithelialization within 7 days of surgery. In practice, however, an incision could not be assumed to be closed if a patient failed to attend an assessment within 7 days of surgery. Therefore, any incision not assessed within 7 days was defined as having delayed healing and classed as a healing complication, even if the wound may, in fact, have been 100% closed. A sensitivity analysis aiming to address this issue was added to the analysis protocol so the time point for the delayed healing component was changed from 7 days to 10 days after surgery. By this point, all patients should have returned for at least 1 visit and have complete information regarding wound closure. The sensitivity analysis supports the theory that the frequency of delayed wound healing was overestimated. Using the sensitivity analysis definition of delayed wound healing within 10 days (all other complications within 21 days), the total number of complications fell from 123 (61.8%) to 89 (44.7%) in SC-treated incisions, and compares more closely to complication rates reported elsewhere.18 The treatment effect was maintained in the sensitivity analysis, with NPWT resulting in a 5% absolute reduction or 10 fewer patients (39.7%, n = 79) experiencing a wound complication within 21 days (P = 0.033).
Fig. 3.
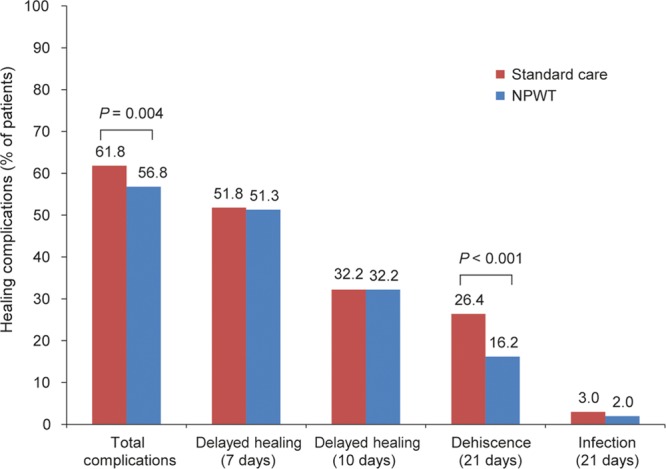
Frequency of complications during the postsurgical period in 199 patients treated with NPWT and SC. For the sensitivity analysis, the definition of delayed healing was changed from 7 to 10 days after surgery, with the aim that all patients should have returned for at least 1 visit at this point and we would therefore have complete information regarding closure. Using this cutoff, the results remained statistically significant [n = 79, 39.7% versus 89, 44.7% for NPWT and SC, respectively; treatment difference 5% (95% CI, 0.4–10.1); P = 0.033].
The Effect of NPWT on Wound Dehiscence
In addition to the primary variable, the effects of NPWT were assessed on a range of specific complication secondary endpoints. Figure 3 shows that NPWT significantly reduced the incidence of wound dehiscence within 21 days of surgery (Fig. 3). In total, 16.2% of NPWT-treated incisions (n = 32) experienced dehiscence (partial, superficial, or deep) compared with 26.4% (n = 52) treated with SC [P < 0.001, 95% confidence interval (CI) of the difference, 5.1–15.9]. The difference of 20 fewer patients (10.2%) represents a relative reduction in dehiscence of 38% as a result of incisional NPWT. There were 4 (2.0%) NPWT-treated incisions that developed infection (superficial or deep) compared with 6 (3.0%) treated with SC within 21 days of surgery. The difference of 2 patients (1.0%) with fewer infections on NPWT treatment was not statistically significant (P = 0.532; 95% CI, −1.9 to 4.3) and, naturally, the numbers were low. It is clear that, in this study, the major complication in breast reduction driving the significant difference in the primary endpoint is dehiscence (rather than infection) and that the application of incisional NPWT was successful in reducing the frequency of this complication.
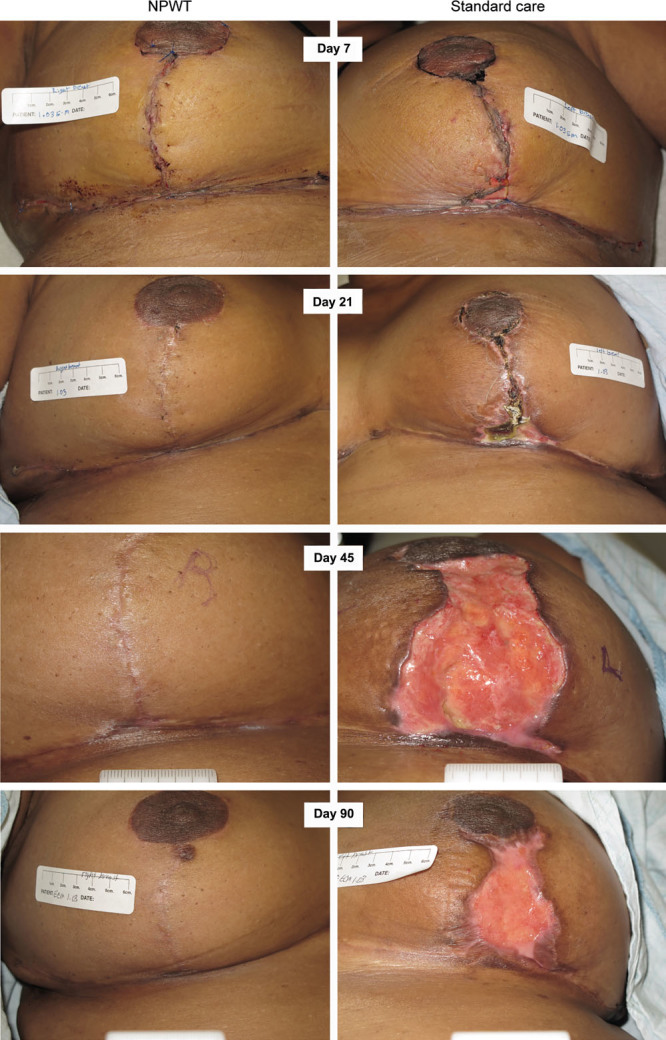
Superficial dehiscence was the most common form of dehiscence observed in the SC group (n = 35; 17.8%), followed by partial (n = 19; 9.6%), then deep dehiscence (n = 6; 3%). NPWT resulted in a significant reduction in both superficial dehiscence (n = 22; 11.2%) and partial dehiscence (n = 7; 3.6%). The rate of deep dehiscence in the NPWT-treated group was 3% (n = 3), compared with 6% in SC, but did not reach significance with such low numbers. The effects of NPWT on dehiscence rates could be observed as far out as 42 days after surgery. Figure 4 shows the proportion of dehisced wounds at 21, 42, and 90 days (Fig. 4). Figure 5 shows one of the patients who suffered deep dehiscence on the SC side but not on the NPWT-treated breast (Fig. 5). The dehisced wound remained unhealed at day 90.
Fig. 4.

Proportion of patients reporting a dehiscence at each individual assessment.
Fig. 5.
Effect of body mass index on wound dehiscence.
Patient Risk Factors for Wound Complications
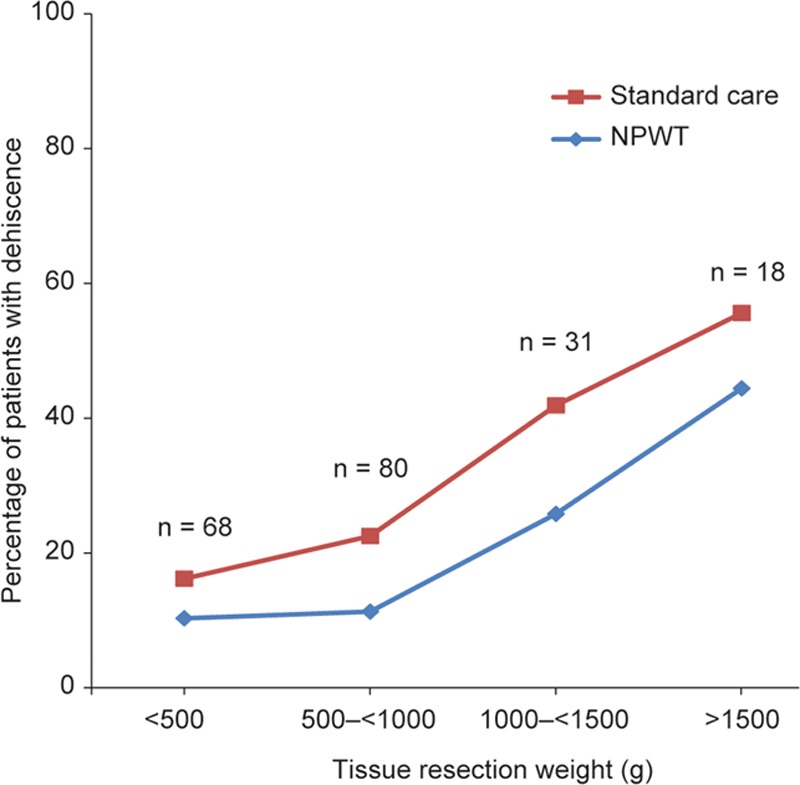
It has been noted by many authors that a greater mass of tissue resected in reduction mammaplasty is associated with an increased incidence of wound complications.18–21 Data were captured for some of the common risk factors associated with reduction mammaplasty in the present study, including smoking, diabetes, high BMI, and high tissue resection weight. The numbers of patients reported to be diabetic (n = 6) or smokers (n = 10) were too few to make any associations with outcomes. Figure 6 shows that NPWT appeared to have the greatest effect on dehiscence in the higher BMI categories (Fig. 6). Figure 7 shows that, although there is an increasing likelihood of a dehiscence in higher weights of tissue resection, the effects of NPWT were similar across all resection categories (Fig. 7). The effect of NPWT on preventing skin necrosis was noteworthy, despite it occurring infrequently in the study: 9/197 patients (4.6%) suffered skin necrosis within 21 days postsurgery, 7 (3.6%) instances of which occurred only in the incisions treated with SC, whereas 2 (1.0%) patients developed skin necrosis on both incisions (NPWT and SC; P = 0.008). Six of the 9 patients developing skin necrosis were found to be in the highest BMI category (≥ 30 kg/m2), and 4/9 were in the highest tissue resection weight category (≥ 1,500 g). Data from the analysis of nipple and areola necrosis, hematoma, seroma, abscesses, suture abscesses, or extrusions, and details of serious adverse events are in Supplemental Digital Contents 1–3. (see document, Supplemental Digital Content 1, which shows data from the analysis of nipple and areola necrosis, hematoma, seroma, abscesses, suture abscesses, or extrusions, http://links.lww.com/PRSGO/A600; see table, Supplemental Digital Content 2, which shows a detailed description of serious adverse events localized to a treatment for 2 patients in the NPWT group and 7 patients in the SC group, http://links.lww.com/PRSGO/A601; see figure, Supplemental Digital Content 3, which shows center effects for incidence of dehiscence, http://links.lww.com/PRSGO/A602).
Fig. 6.
Example of a patient with deep dehiscence on breast treated with SC and good healing on breast treated with NPWT.
Fig. 7.
Effect of tissue resection weight on dehiscence.
Adverse Events
The number of patients reporting a serious adverse event was n = 7 (3.5%) in the SC-treated incisions compared with n = 2 (1.0%) in NPWT-treated incisions. No adverse events were found to have a relationship to the device (NPWT or SC).
DISCUSSION
The aim of the study was to investigate the potential of single-use incisional NPWT as a treatment modality for prevention of wound complications (delayed wound healing, dehiscence, and infection) following reduction mammaplasty. The relatively common bilateral procedure, with a high rate of non–life-threatening complications, provides an opportunity to conduct a within-patient randomized study. The total number of complications within 21 days (including delayed healing up to 10 days as per the sensitivity analysis) fell from 44.7% in incisions treated with SC to 39.7% in incisions treated with NPWT (P = 0.033). Clinically more significant were those women experiencing dehiscence that could be of substantial impact, as the example in Figure 5 shows. NPWT significantly reduced the incidence of wound dehiscence within 21 days of surgery: 16.2% of NPWT-treated incisions (n = 32) experienced dehiscence (partial, superficial, or deep) compared with 26.4% (n = 52) treated with SC (P < 0.001), resulting in a difference of 20 fewer patients (10.2% or 38% relative reduction) sustaining a wound dehiscence as a result of NPWT treatment. The effects of NPWT on dehiscence rates could be observed as far out as 42 days after surgery [19.3% (n = 38) versus 29.4% (n = 58), P < 0.001].
Avoiding tension at the T-junction between horizontal and vertical incisions remains a basic goal in the design and closure of breast incisions. It is thought that one of the key mechanisms of action of NPWT over closed incisions is to minimize lateral tension. Reductions in lateral tension with incisional NPWT have been demonstrated using computer modeling and in vitro measurements in both the PICO22 and other devices.23 Animal studies have demonstrated increased breaking strength of wounds following the application of NPWT to closed incisions.24,25 Other potential mechanisms to explain this vulnerary effect are the ability of incisional NPWT to enhance tissue perfusion, which can be impaired at the T-junction site and flap edges in breast-reduction procedures, and the reduction of incisional edema, which is common to all surgical procedures and can further impede perfusion at the edges of the incision. It is notable that NPWT appeared to provide particular benefit in preventing wound complications in the higher tissue resection weight categories and in patients with higher BMI. There is, therefore, support for the concept that perhaps a combination of edema reduction, improved tissue perfusion, and minimizing lateral tension is key to NPWT’s mechanism of action in preventing complications when larger amounts of tissue are resected. Based on the present study, it would appear that there are certainly grounds for considering such devices in mammaplasty where BMI is above 25 (Fig. 6) or resection weight is above 500 g (Fig. 7).
The ideal duration to apply incisional NPWT is presently unknown. Many studies have used between 3 and 7 days.10–12 In the present study, sites could use up to 14 days. At most sites, overall duration of NPWT treatment was a median of 7 days (mean, 10.9 days). However, at site 5, a further NPWT treatment was nearly always applied even if the incision was closed or almost closed, so, in these patients, NPWT treatment duration (closed wounds) was median 14 days (mean, 14.3 days). A sensitivity analysis was performed to see if the primary endpoint was different with or without data from site 5. The size of the overall treatment difference was slightly reduced if the overall data excluded site 5 but the result remained significant. Thus, there was no indication of a difference between 7 and 14 days’ use, but this remains a topic for further research (analysis shown in the Supplemental Digital Content 1).
One limitation of the current study is that treatment could not be blinded. The use of a sham device would have been a possibility, but it would still have been obvious to the patient and physician which device was in operation. With these limitations in mind, the achievement of a statistically significant reduction in healing complications, in particular dehiscence, translates to significant clinical benefits when placed in context of the large number of breast-reduction procedures performed each year and provides high-level evidence supporting utilization of incisional NPWT in susceptible patients.
CONCLUSIONS
These findings support the growing body of evidence for the ability of incisional NPWT to reduce wound complications.10–12 This is the first major prospective, within-patient, randomized, controlled, multicenter study to provide evidence for a therapeutic strategy to reduce healing complications. Conducting a bilateral study allows for the most rigorous direct comparison of 2 treatment arms on the same patient and completely avoids subject-to-subject variability as a confounding factor when comparing 2 treatment types. Further studies within plastic surgery would be informative.
ACKNOWLEDGMENTS
The authors thank Alan Rossington and Robin Martin (Smith & Nephew Scientific & Medical Affairs) for their help with the article. The authors also thank Gary Patronek and Helen Marshall at Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, for writing and editorial assistance, funded by Smith & Nephew Wound Management, Inc.
Supplementary Material
Footnotes
Present address: Dartmouth Hitchcock Medical Center/Giesel School of Medicine at Dartmouth College, N.H.
Preliminary results from this study have been presented as posters at the following meetings: 12th ESPRAS Congress (Edinburgh, United Kingdom, July 2014), The British Association of Aesthetic Plastic Surgeons (BAAP’s) 30th Annual Scientific Meeting (London, United Kingdom, September 2014), and the 6th Annual Symposium on Advanced Wound Care Fall (SAWC Fall) meeting (Las Vegas, Nev., September 2015).
Supported by Smith & Nephew Wound Management, Inc. The following products, devices, and drugs were used in the study:
• PICO (Smith & Nephew Medical Limited, Hull, United Kingdom) is a single-use negative pressure wound therapy system consisting of a small portable pump, 2 lithium batteries, 2 dressings, and 10 fixation strips.
• 3M STERI-Strip Adhesive Skin Closures (Reinforced) was taken off the shelf from the local product inventory of the hospital/physician office.
• No-Sting Skin-Prep Protective Wipes (Smith & Nephew, Inc.) were supplied by the sponsor to each center.
Trial Registration: This study is registered under the name “A prospective, randomized, intra-patient, comparative, open, multi-centre study to evaluate the efficacy of a single-use negative pressure wound therapy (NPWT) System on the prevention of postsurgical incision healing complications in patients undergoing reduction mammaplasty,” ClinicalTrials.gov identification number NCT01640366 (http://clinicaltrials.gov/show/NCT1640366). Start date June 1, 2012, completion date April 9, 2014.
Disclosure: Robert D. Galiano, Donald Hudson, René van der Hulst, Franck Duteille, and Risal Djohan have acted as speakers for Smith & Nephew. John Cockwill, Sarah Megginson, and Elizabeth Huddleston are employees of Smith & Nephew Wound Management. Volkan Tanaydin and J. Shin have no potential conflicts of interest to report. The article processing charge was paid for by Watermeadow Medical, on behalf of Smith & Nephew.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.The American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank: Statistics. Aesthetic Surg J. 2016;36:1–29.. [DOI] [PubMed] [Google Scholar]
- 2.Davis GM, Ringler SL, Short K, et al. Reduction mammaplasty: long-term efficacy, morbidity, and patient satisfaction. Plast Reconstr Surg. 1995;96:1106–1110.. [PubMed] [Google Scholar]
- 3.Makki AS, Ghanem AA. Long-term results and patient satisfaction with reduction mammaplasty. Ann Plast Surg. 1998;41:370–377.. [DOI] [PubMed] [Google Scholar]
- 4.Thoma A, Sprague S, Veltri K, et al. A prospective study of patients undergoing breast reduction surgery: health-related quality of life and clinical outcomes. Plast Reconstr Surg. 2007;120:13–26.. [DOI] [PubMed] [Google Scholar]
- 5.Setälä L, Papp A, Joukainen S, et al. Obesity and complications in breast reduction surgery: are restrictions justified? J Plast Reconstr Aesthet Surg. 2009;62:195–199.. [DOI] [PubMed] [Google Scholar]
- 6.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562.. [DOI] [PubMed] [Google Scholar]
- 7.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576.; discussion 577. [PubMed] [Google Scholar]
- 8.Krug E, Berg L, Lee C, et al. Evidence-based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury. 2011;42:S1–S12.. [DOI] [PubMed] [Google Scholar]
- 9.Stannard JP, Volgas DA, McGwin G, 3rd, et al. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012;26:37–42.. [DOI] [PubMed] [Google Scholar]
- 10.Semsarzadeh NN, Tadisina KK, Maddox J, et al. Closed incision negative-pressure therapy is associated with decreased surgical-site infections: a meta-analysis. Plast Reconstr Surg. 2015;136:592–602.. [DOI] [PubMed] [Google Scholar]
- 11.Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103:477–486.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vries FEE, Wallert ED, Solomkin JS, et al. A systematic review and meta-analysis including GRADE qualification of the risk of surgical site infections after prophylactic negative pressure wound therapy compared with conventional dressings in clean and contaminated surgery. Medicine (Baltimore). 2016;95:e4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condé-Green A, Chung TL, Holton LH, 3rd, et al. Incisional negative-pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg. 2013;71:394–397.. [DOI] [PubMed] [Google Scholar]
- 14.Karlakki SL, Hamad AK, Whittall C, et al. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: a randomised controlled trial. Bone Joint Res. 2016;5:328–337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witt-Majchrzak A, Żelazny P, Snarska J. Preliminary outcome of treatment of postoperative primarily closed sternotomy wounds treated using negative pressure wound therapy. Pol Przegl Chir. 2015;86:456–465.. [DOI] [PubMed] [Google Scholar]
- 16.Hudson DA, Adams KG, Van Huyssteen A, et al. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no-canister system. Int Wound J. 2015;12:195–201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmsjö M, Huddleston E, Martin R. Biological effects of a disposable, canisterless negative pressure wound therapy system. Eplasty. 2014;14:e15. [PMC free article] [PubMed] [Google Scholar]
- 18.Shah R, Al-Ajam Y, Stott D, et al. Obesity in mammaplasty: a study of complications following breast reduction. J Plast Reconstr Aesthet Surg. 2011;64:508–514.. [DOI] [PubMed] [Google Scholar]
- 19.Lewin R, Göransson M, Elander A, et al. Risk factors for complications after breast reduction surgery. J Plast Surg Hand Surg. 2014;48:10–14.. [DOI] [PubMed] [Google Scholar]
- 20.Henry SL, Crawford JL, Puckett CL. Risk factors and complications in reduction mammaplasty: novel associations and preoperative assessment. Plast Reconstr Surg. 2009;124:1040–1046.. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham BL, Gear AJL, Kerrigan CL, et al. Analysis of breast reduction complications derived from the BRAVO study. Plast Reconstr Surg. 2005;115:1597–1604.. [DOI] [PubMed] [Google Scholar]
- 22.Loveluck J, Copeland T, Hill J, et al. Biomechanical modeling of the forces applied to closed incisions during single-use negative pressure wound therapy. Eplasty. 2016;16:e20. [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkes RP, Kilpad DV, Zhao Y, et al. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov. 2012;19:67–75.. [DOI] [PubMed] [Google Scholar]
- 24.Glaser DA, Farnsworth CL, Varley ES, et al. Negative pressure therapy for closed spine incisions: a pilot study. Wounds. 2012;24:308–316.. [PubMed] [Google Scholar]
- 25.Meeker J, Weinhold P, Dahners L. Negative pressure therapy on primarily closed wounds improves wound healing parameters at 3 days in a porcine model. J Orthop Trauma. 2011;25:756–761.. [DOI] [PubMed] [Google Scholar]


