Abstract
Background:
Adipose-derived stem cell (ADSC)–based treatments have the potential to treat numerous soft-tissue pathologies. It would be beneficial to develop an efficient and reliable intraoperative, nonenzymatic method of isolating ADSCs for clinical use. This study aims to determine the (1) viability and proliferative capacity of ADSCs after exposure to vibrational energies and (2) efficacy of vibrational energy as a method of ADSC isolation from surgically harvested infrapatellar fat pad (IFP).
Methods:
Cultured ADSCs were exposed to 15 minutes of vibration (60 Hz) with displacements ranging from 0 to 2.5 mm to assess cell viability and proliferation. Then, arthroscopically harvested adipose tissue (IFP; n = 5 patients) was filtered and centrifuged to separate the stromal vascular fraction, which was exposed to 15 minutes of vibration (60 Hz; 1.3 mm or 2.5 mm displacement). A viability analysis was then performed along with proliferation and apoptosis assays.
Results:
Vibration treatment at all displacements had no effect on the viability or proliferation of the cultured ADSCs compared with controls. There was an increased apoptosis rate between the 2.5 mm displacement group (7.53%) and controls (5.17%; P < 0.05) at day 1, but no difference at days 2, 3, and 14. ADSCs were not isolated from the IFP tissue after vibration treatment.
Conclusions:
ADSCs maintained viability and proliferative capacity after 15 minutes of vibration at 60 Hz and 2.5 mm displacement. ADSCs were not isolated harvested IFP tissue after the application of vibrational energy.
INTRODUCTION
Mesenchymal stem cells (MSCs) have interested clinicians for the reparative and regenerative potential as well as the capability to differentiate into many different musculoskeletal lineages. Adipose-derived stem cells (ADSCs) are particularly attractive to orthopedic and plastic surgeons for the ease and reliability of obtaining tissue while maintaining their multipotent differentiation.1–3 Although ADSCs can be isolated from any source of adipose tissue, the most common harvest sites are subcutaneous fat and the infrapatellar fat pad (IFP).3–7 ADSCs can be isolated from subcutaneous fat with liposuction and have been used for reconstructive grafts in plastic surgery and other subspecialties.7–10 ADSCs have also been proposed for use in the treatment of numerous orthopedic conditions such as cartilage defects, osteoarthritis, tendinopathy, and soft-tissue healing.11–15 The IFP is structurally similar to subcutaneous adipose tissue and has been shown to be a dependable source of ADSCs.4,16–19 The IFP-derived ADSCs can differentiate into both chondrogenic and osteogenic lineages.1,20 The IFP is an ideal source of ADSCs for orthopedic procedures since it is readily accessible during standard knee arthroscopy.
In previous work from our laboratory, we demonstrated that the tissue from the posterior border of the IFP is a rich source of both ADSCs and synovial-derived stem cells.4 However, the ADSCs and synovial-derived stem cells must be isolated from the adipocytes and extracellular tissues before clinic use. The stromal vascular fracture (SVF) is believed to contain the majority of progenitor cells, but has a heterogeneous mesenchymal population including adipose stromal and progenitor cells, endothelial cells, pericytes, and hematopoietic stem cells.4,15,21 ADSCs can then be isolated and cultured from the SVF using one of the several techniques.3,5,8,22,23 The most reliable and well-established method of ADSC isolation from the SVF is enzymatic digestion using collagenase, serum, and animal-derived medium.3,22,23 This enzymatic digestion technique is permissible in many countries and used to treat both osteoarthritis and cartilage defects.11,24–26 In the United States, however, this enzymatic method is considered to be more than “minimal manipulation” by the Food and Drug Administration and is not currently approved for patient care. Therefore, there is a need for alternative, nonenzymatic techniques of isolating ADSCs from adipose tissue. Ideally, this alternative isolation method would be rapid and could be performed in the operating room allowing for immediate ADSC availability.
A promising nonenzymatic ADSC isolation technique has been described using vibration to mechanically disrupt the extracellular fibrous tissue.8,23 Italian researchers have reported on the successful isolation of ADSCs from subcutaneous adipose tissue utilizing vibrational energy. The harvested adipose tissue was placed on a rotatory shaker for 6 minutes at 6,000 vibrations per minute (100 Hz) immediately followed by 6 minutes of centrifuge (1,600 rpm). However, the effect of different vibration frequencies or displacements was not investigated.
The purposes of this study were to (1) determine the viability and proliferative capacity of ADSCs after exposure to different vibrational energies and (2) investigate the efficacy of vibrational energy as an effective method of ADSC isolation.
METHODS
This study was approved by our University’s Institutional Review Board.
Experiment 1: Determine the Viability and Proliferative Capacity of Cultured ADSCs after Exposure to Different Vibrational Energies
In total, 5 × 103 cells of cultured ADSCs were placed into each Eppendorf tube. There were 2 days of testing, each with 6 experimental groups of different vibration displacement settings. Six Eppendorf tubes were used for each experimental group. A control group was assigned to every 2 experimental groups.
Vibration Machine
The vibration machine used was a Sieve Shaker (Octagon D200, Endecotts Ltd, London, United Kingdom), which allowed for an adjustable oscillating displacement (amplitude) and had a fixed frequency of 3,600 vibrations per minute (60 Hz). There were 9 different displacement settings, which were measured according to the manufacturer’s recommended method (Table 1). A custom styrofoam block with pockets for the Eppendorf tubes was fitted to the sieve container.
Table 1.
Results of Sieve Shaker Displacement Measurement

Vibration Procedure
Six tubes were securely placed into the Styrofoam pockets in the sieve container. The machine was turned on for 15 minutes of continuous vibration at the selected displacement. The tubes from the control groups were placed upright on the table next to the vibration machine for 15 minutes. Immediately after vibration treatment, all tubes were transported to the laboratory and were spun at 5,000 rpm (2,000 g) for 6 minutes at room temperature. The cells were carefully collected from the cell pellet by inserting the pipette tip to the bottom of the tube along its wall to avoid disturbing the layer above the pellet.
On the first experimental day, the ADSCs were exposed to vibration with displacements 0, 0.2, 0.5, 1.3, 1.6, and 2.0 mm. Based on the results of this first day of testing, larger displacements were used on the second experimental day (0.2, 1.3, 1.8, 2.0, 2.3, 2.5 mm).
Experiment 2: Determine Cell Number, Viability, and Proliferative Capacity of ADSCs Isolated from Infrapatellar Fat Pad after Vibration Treatment
IFP Harvest
The posterior border of the IFP was arthroscopically harvested from 5 patients (ages 16–43) during an anterior cruciate ligament reconstruction performed by the senior author. Tissues adjacent to the IFP, such as the posterior synovial lining, were harvested using a standard arthroscopic shaver. The harvested fat was collected into a lipoaspirate filtration system (AquaVage, M.D. Resource, Livermore, Calif.) that separates fluid from the adipose tissue. The adipose tissue was then fractionated using syringe emulsification, which consisted of passing the tissue from one 20 mL syringe into another through a Leur lock adapter 25 times. The harvested adipose tissue was further processed with a commercially available centrifuge system (Adiprep, Harvest Technologies Corporation, Plymouth, Mass.), separating the stromal vascular fraction from excess fluid and lipids. The tubes from the centrifuge system were immediately transported on ice to the laboratory for vibration treatment.
Vibration Procedure
The IFP samples were separated into 6 Eppendorf tubes and securely placed into the styrofoam pockets in the sieve. The machine was then turned on for 15 minutes of continuous vibration at either 1.3 mm or 2.5 mm (maximum) displacement. The control tubes were placed upright and next to the vibration machine for 15 minutes. Immediately after vibration treatment, the tubes were removed and transported to the laboratory for testing.
Viability Analysis
Immediately after the vibration treatment, each sample was tested for cell viability using the live/dead Viability/Cytotoxicity Kit for mammalian cells (Invitrogen Corporation, Carlsbad, Calif.), which is a 2-color fluorescence assay. Cellular viability was based on the simultaneous determination of live and dead cells with probes that recognize parameters of cell viability, such as plasma membrane integrity and intracellular esterase activity. The probes calcein-AM and ethidium homodimer (EthD-1) were used. Twenty microliters of 2 mM EthD-1 was added to 10 mL D-PBS while vortexing. The reagents were then combined with 5 μL of 4 mM calcein-AM. The final concentration for EthD-1 was 4 μM, and for calcein-AM was 2 μM.
The samples were collected by centrifugation. After spinning, the supernatant was carefully removed to ensure that the cell pellets were not disturbed. The cell pellets were then resuspended in 20 μL of staining solution. The cells were stained at room temperature for 30 minutes in the dark. The ratio of dead to live cells was assessed by fluorescent microscopy. For cultured ADSCs, microscopic analysis was completed immediately after incubation using a Zeiss Observer Z1 (Carl Zeiss Inc, Thornwood, N.Y.) fluorescent microscope. Five microliters of cell suspension was placed onto a clean slide, and the cells were allowed to settle for 30 seconds.
Images were obtained using an AxioCam MRm camera (Carl Zeiss, Jena, Germany). Two frames were taken using the GFP and DsRED2 filters, wavelengths 510 ± 10 and 590 ± 14, respectively, and were overlaid to produce a single image. Four random high-powered fields (100×) were acquired for each sample resulting in 12 high-powered fields per experimental testing condition. Each image was adjusted to maximize image quality and was flat equalized. The Live to Total Cell ratio was determined by manually counting live and dead cell numbers in each image.
In the IFP samples, the stained cells were analyzed by flow cytometry (Accuri C6, BD Biosciences, San Jose, Calif.). We used 488 nm excitation and measured green fluorescence emission for calcein (ie, 530/30 bandpass/FL-1) and red fluorescence emission for ethidium homodimer-1 (ie, 610/20 bandpass/FL-3). The results were analyzed by CFlow (BD Biosciences, San Jose, Calif.) following the manufacturer’s instructions. The entire solution was counted by a flow cytometer to calculate the total cell number of the sample.
Proliferation Assay
Each sample was tested for cell proliferation with the Alamar Blue cell viability reagent assay (Invitrogen, Life Technologies, Grand Valley, N.Y.). Cells collected from each Eppendorf tube were cultured in 48-well plates for 2 weeks. The cell proliferation was determined by supplementing 10% (v/v) Alamar Blue reagent and incubating for another 3 hours before measurement on days 1, 3, 6, 9, 12, and 14. One hundred microliters of supernatant were read at 570/585 nm in a SpectraMax/M2 microplate reader (Molecular Devices, Sunnyvale, Calif.). Cell numbers were determined using the standard curve generated from cultured ADSCs (2, 5, 10, 20, 39, 78, 156, 312, 625, 1,250, 2,500, and 5,000 cells per well).
Apoptosis Assay
Cellular apoptosis assays were performed after 14 days of cell proliferation by Annexin V-FITC/PI from BioLegend (San Diego, Calif.). Cells were collected from each well by trypsin treatment. After wash, cells were resuspended in 17 μl of staining buffer with 1 μL of FITC-Annexin V and 2 μL of propidium iodide solution. The cells were then incubated for 15 minutes at room temperature in the dark. After incubation, flow cytometry analysis was performed using 488 nm excitation to measure green fluorescence emission for Annexin V (ie, 530/30 bandpass/FL-1) and red fluorescence emission for propidium iodide (ie, 610/20 bandpass/FL-3). For the positive controls, ADSC cells were cultured in Dulbecco's Modified Eagle Medium/10% fetal bovine serum with ascorbic acid at 10 μg/ml until 70–80% confluency. UVC (254 nm/50 J/m2) was used to stimulate apoptosis in cultured ADSC with a Stratalinker 1,800 UV crosslinker (Stratagene, La Jolla, Calif.) and then incubated for 24 and 48 hours. All cells were collected for Annexin/PI staining, followed by Fluorescence-activated cell sorting analysis.
Statistics
Viability Analysis and Apoptosis Assay.
All experimental groups had continuous variables and were compared with controls using a Student’s t test. When comparing between experimental groups, the values relative to controls were analyzed using an analysis of variance test.
ADSC Proliferation.
For each assay, a standard curve was generated from proliferating ADSCs at a fixed density. The reading was then plotted for each experimental and control groups onto the curve to obtain the cell number for each group. To compare all experimental groups, the controls for all assays were averaged to 1 control. The values relative to the control were then analyzed using an analysis of variance test.
RESULTS
Experiment 1: Cultured Adipose-Derived Stem Cells
Viability Analysis
At each displacement value, the absolute viability of the cultured ADSCs was greater than 96% (Fig. 1). All samples had a viability greater than 98.7% relative to controls.
Fig. 1.

Results of the viability analysis of cultured ADSCs after vibration treatment (15 minutes) at different displacements.
ADSC Proliferation
Fourteen days after the vibration treatment, there were no differences in proliferation of the cultured ADSCs between any of the experimental (vibration treatment) groups and the control groups (Fig. 2). There were no differences in proliferation between the different experimental groups.
Fig. 2.
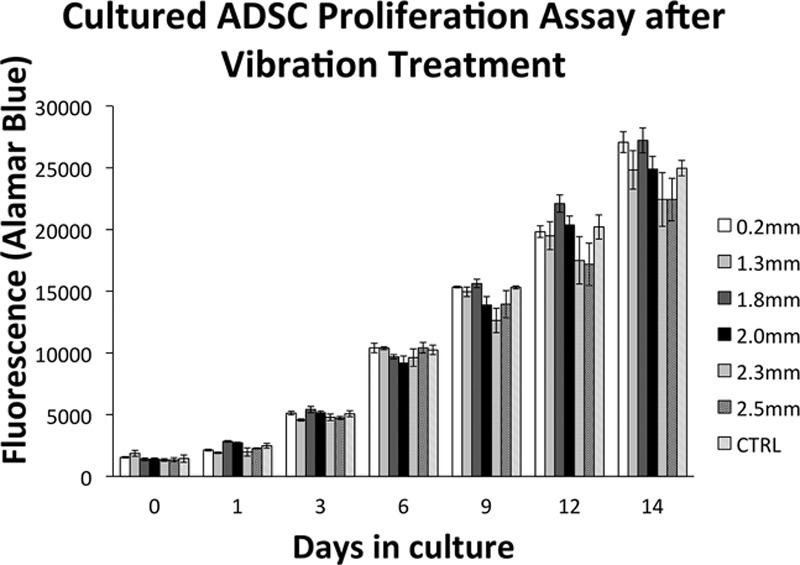
Proliferation assay results for both the cultured ADSCs and control groups.
Apoptosis Assay: 2.5 mm Displacement Versus Controls
On day 1, there was a significant difference in the apoptosis rate between the 2.5 mm displacement group (mean, 7.53%) and controls (5.17%; P < 0.05) (Fig. 3). However, there were no differences on day 2 and day 3.
Fig. 3.
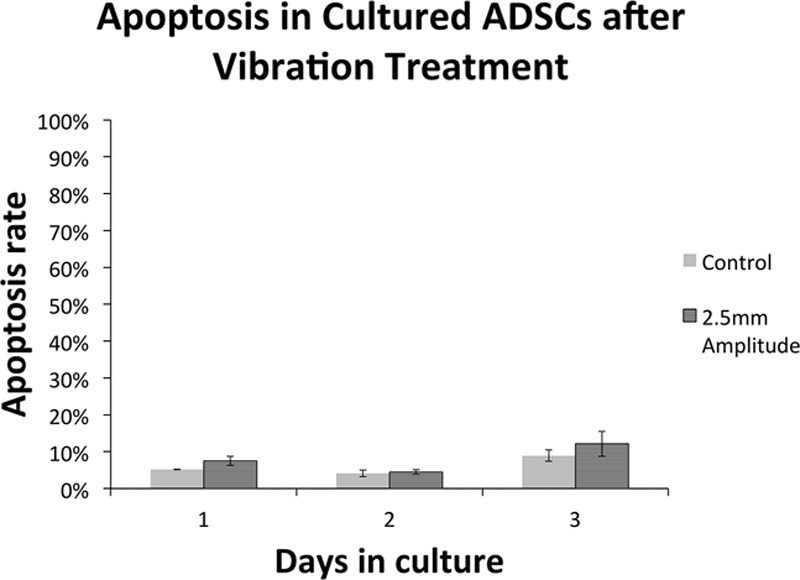
Apoptosis assay results comparing the maximum displacement (2.5 mm) experimental group of ADSCs and control at days 1, 2, and 3.
Day 14 Results
On day 14, there were no differences between the control, 0, 0.2, 0.5, 1.3, 1.6, and 2.0 mm displacement groups (Fig. 4). There was no experimental group that had a higher mean apoptosis rate than controls (33.7%).
Fig. 4.
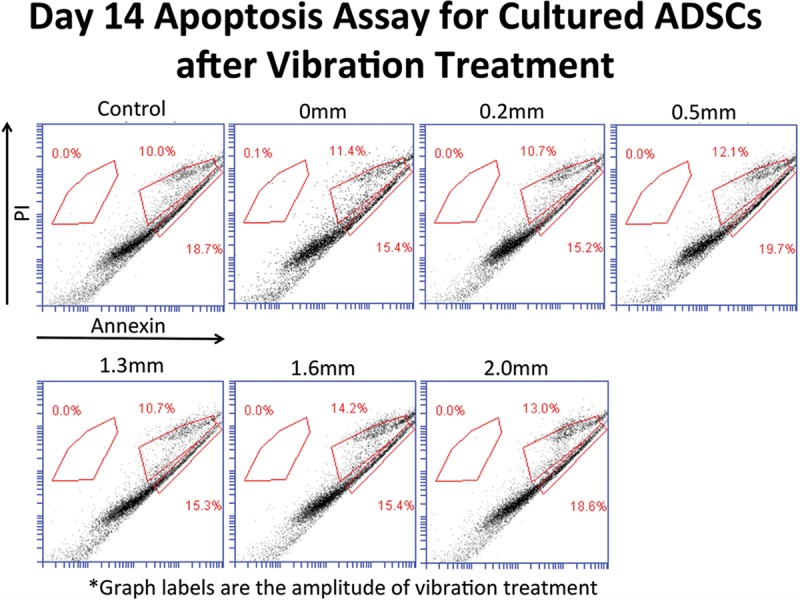
Apoptosis assay results between the experimental groups at different displacements and controls at day 14.
Part 2: Surgical Adipose Samples
Patients received either 1.3 mm (n = 1) or 2.5 mm (n = 4) displacement vibrational treatment.
Viability Analysis
The vibration treatment did not isolate ADSCs from the IFP tissue. The number of isolated ADSC cells ranged from 0 to 7 (n = 5). There were 17,733 cells in the ADSC control group.
ADSC Proliferation
Since the ADSCs were not isolated from the IFP tissue in the experimental groups, there was no proliferation (Fig. 5). There were no significant differences between experimental and control groups for each patient sample.
Fig. 5.
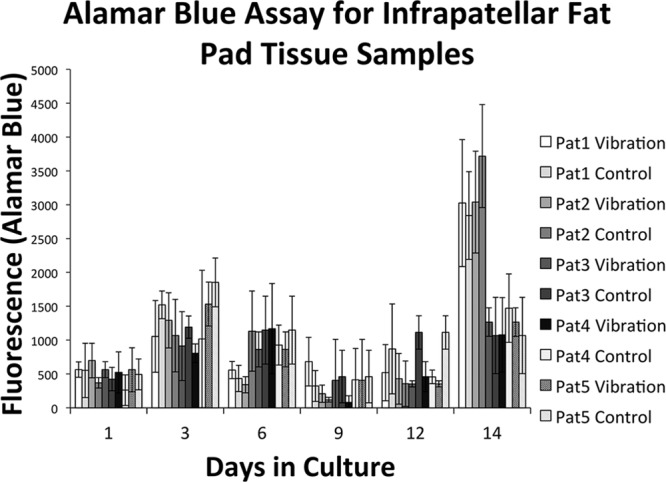
The Alamar Blue Assay for the IFP tissue samples.
Apoptosis Assay
The apoptosis assay was not performed due to the low number of available cells and failure of significant proliferation.
DISCUSSION
We demonstrated that cultured ADSCs maintained viability and proliferation capability throughout all our experimental vibration conditions. These data are consistent with other reports in the literature demonstrating that MSCs can be treated with vibration and maintain a high cell viability.27,28 Nikolaev et al.28 studied the viability of human mesenchymal stem cells in response to varying conditions of temperature and 24 hours of vibration. The authors investigated different frequency and displacement experimental conditions and found that the human mesenchymal stem cells had markedly decreased viability to vibration at 25 Hz, moderately decreased at 50 Hz, and no effect at 10 Hz. However, viability correlated best with the total vibrational energy, which is directly proportional to (Frequency × Displacement)^2. The MSCs that were exposed to the highest energy condition (25 Hz, 5.7 mm, 20.3 mJ/kg) had a final total cell number that was only 4% of the control and a 28% viability compared with controls. The next highest tested energy condition (50 Hz, 1.4 mm, 4.9 mJ/kg) maintained 95% of the total cell number compared with controls. The total cell numbers and cell viability were not significantly decreased compared with controls. Any vibrational condition tested at or below 2.6 mJ/kg maintained a high MSC cell count and viability (no significant difference compared with controls) despite frequencies as high as 5,000 Hz.
Based on these data reported by Nikolaev et al.28, we reasoned that the viability of MSCs decreased with vibration treatment between the energies of 4.9 mJ/kg and 20.3 mJ/kg. Therefore, we selected a vibration machine that was capable of producing 9 different energy levels ranging from 0.1 to 22.5 mJ/kg (Table 1). In our study, the application of vibrational force with the 22.5 mJ/kg of energy resulted in no difference in cell viability compared with controls. In contrast, Nikolaev et al.28 found that the application of vibrational force with the energy of 20.3 mJ/kg resulted in only 28% viability compared with controls. One possible explanation for the discrepancy between these 2 results is that we only applied 15 minutes of vibration compared with 24 hours in the study by Nikolaev et al.28 It is also possible that frequency (vibrations per minute) has an effect on cell viability independent to the total energy. Nikolaev et al.28 used a custom vibration system that had both adjustable frequency and displacement. However, a goal of our study was to use a vibration machine that could be easily transported in and out of the operating room. A second aim was to test different vibrational energy levels, and our sieve vibration machine was able to accomplish this goal by changing the displacement (amplitude) at a constant frequency. We extensively researched all available laboratory shakers, which used either a rotational force (not axial) or had fixed (nonadjustable) displacement and frequency.
Multiple groups have previously studied nonenzymatic methods of isolating ADSCs from human lipoaspirates.3,5,8,23,29,30 The majority of reported methods involve the use of centrifugation to concentrate the SVF. A method of vigorous washing of the floating lipoaspirate has also been reported and resulted in a 19-fold yield reduction and a 2.5-fold increase in culture time compared with collagenase digestion.5 Ghorbani et al.29 also described a nonenzymatic method of explanting small pieces of adipose tissue into a culture flask with fetal bovine serum, overnight incubation, and irrigation. However, this method required several days of proliferation. Although these alternative, nonenzymatic methods are promising, they are currently not appropriate for intraoperative ADSC isolation.
Raposio et al.8,23 have recently described a rapid, intraoperative technique using vibration to mechanically isolate ADSCs from lipoaspirate. The authors compared 2 methods of ADSC isolation, 1 based on a mechanical + enzymatic procedure and the second an exclusively mechanical isolation technique.23 They found that the mechanical + enzymatic procedure isolated a greater number of ADSCs (25.9%) compared with the mechanical procedure (5%).
We attempted to isolate ADSCs from IFP using a similar approach with vibrational energy. We tested different amplitudes to identify the ideal vibrational energy that would result in the highest ADSC yield while maintaining viability. Unfortunately, our efforts to isolate ADSCs from the IFP were not successful. Since in experiment 1 we proved that the maximum vibrational treatment had a minimal effect on ADSC viability and proliferation, our explanation for the paucity of live cells in experiment 2 is that the vibration treatment was not effective in isolating the ADSCs from the extracellular matrix and surrounding fibrous tissue. There are several possible explanations for our findings. Although we were able to deliver high vibrational energies, it is possible that greater energies are required to break up the extracellular matrix and fibrous tissue of the IFP tissue. Since the highest energy level did not affect ADSC viability or proliferation, the ADSCs may be able to tolerate higher energies while still maintaining viability and proliferative capacity. Second, we chose 15 minutes of vibration as a reasonable intraoperative treatment time, but a longer duration of vibration may be required to isolate ADSCs using this technique. Different frequencies may have been more efficacious in targeting the extracellular tissues independent of the total vibrational energy. Although we could not isolate ADSCs from the IFP, the use of subcutaneous adipose tissue may have been more successful. The IFP may have a different extracellular composition and architecture than subcutaneous adipose tissue, which could explain our inability to replicate the findings of Raposio et al.8,23 Finally, while we reasoned that axial displacement would be most effective at disrupting the extracellular matrix, it is possible that the rotatory vibration used by Raposio et al.8,23 is more effective.
In conclusion, we demonstrated that ADSCs maintained viability and proliferative capacity with 15 minutes of vibration at 22.5 mJ/kg (60 Hz; 2.5 mm displacement). We were not able to successfully isolate ADSCs from IFP tissue utilizing vibrational energy. Future studies are needed to investigate whether higher vibrational energies, longer vibration times, different frequencies, or using alternative adipose tissue sources would lead to different results.
Footnotes
No funding was received for this article, but the Adipreps kit was donated by Terumo.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Dragoo JL, Samimi B, Zhu M, et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740–747.. [PubMed] [Google Scholar]
- 2.Murray IR, Corselli M, Petrigliano FA, et al. Recent insights into the identity of mesenchymal stem cells: implications for orthopaedic applications. Bone Joint J. 2014;96-B:291–298.. [DOI] [PubMed] [Google Scholar]
- 3.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dragoo JL, Chang W. Arthroscopic harvest of adipose-derived mesenchymal stem cells from the infrapatellar fat pad. Am J Sports Med. 2017:363546517719454; 10.1177/0363546517719454. [DOI] [PubMed] [Google Scholar]
- 5.Shah FS, Li J, Dietrich M, et al. Comparison of stromal/stem cells isolated from human omental and subcutaneous adipose depots: differentiation and immunophenotypic characterization. Cells Tissues Organs. 2014;200:204–211.. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76.. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273.. [DOI] [PubMed] [Google Scholar]
- 8.Raposio E, Caruana G, Bonomini S, et al. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133:1406–1409.. [DOI] [PubMed] [Google Scholar]
- 9.Chung MT, Zimmermann AS, Paik KJ, et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl Med. 2013;2:808–817.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209.. [DOI] [PubMed] [Google Scholar]
- 11.Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755.. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Wu J, Zhu Y, Han J. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin Exp Med. 2014;14:13–24.; 10.1007/s10238-012-0218-1. [DOI] [PubMed] [Google Scholar]
- 13.Murrell WD, Anz AW, Badsha H, et al. Regenerative treatments to enhance orthopedic surgical outcome. PM R. 2015;7(4 Suppl):S41–S52.. [DOI] [PubMed] [Google Scholar]
- 14.Qi Y, Feng G, Yan W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012;39:5683–5689.. [DOI] [PubMed] [Google Scholar]
- 15.Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615–1621.. [DOI] [PubMed] [Google Scholar]
- 16.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902–907.. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Buckley CT, Almeida HV, et al. Infrapatellar fat pad-derived stem cells maintain their chondrogenic capacity in disease and can be used to engineer cartilaginous grafts of clinically relevant dimensions. Tissue Eng Part A. 2014;20(21–22):3050–3062.; 10.1089/ten.TEA.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pires de Carvalho P, Hamel KM, Duarte R, et al. Comparison of infrapatellar and subcutaneous adipose tissue stromal vascular fraction and stromal/stem cells in osteoarthritic subjects. J Tissue Eng Regen Med. 2014;8:757–762.; 10.1002/term.1565. [DOI] [PubMed] [Google Scholar]
- 19.Tangchitphisut P, Srikaew N, Numhom S, et al. Infrapatellar fat pad: an alternative source of adipose-derived mesenchymal stem cells. Arthritis. 2016;2016:4019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dragoo JL, Lieberman JR, Lee RS, et al. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665–1673.. [DOI] [PubMed] [Google Scholar]
- 21.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641–648.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228.. [DOI] [PubMed] [Google Scholar]
- 23.Raposio E, Simonacci F, Perrotta RE. Adipose-derived stem cells: comparison between two methods of isolation for clinical applications. Ann Med Surg (Lond). 2017;20:87–91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh YG, Kwon OR, Kim YS, et al. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32:97–109.. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Kwon OR, Choi YJ, et al. Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am J Sports Med. 2015;43:2738–2746.. [DOI] [PubMed] [Google Scholar]
- 26.Koh YG, Choi YJ, Kwon OR, et al. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42:1628–1637.. [DOI] [PubMed] [Google Scholar]
- 27.Gaston J, Quinchia Rios B, Bartlett R, et al. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS One. 2012;7:e30965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolaev NI, Liu Y, Hussein H, et al. The sensitivity of human mesenchymal stem cells to vibration and cold storage conditions representative of cold transportation. J R Soc Interface. 2012;9:2503–2515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghorbani A, Jalali SA, Varedi M. Isolation of adipose tissue mesenchymal stem cells without tissue destruction: a non-enzymatic method. Tissue Cell. 2014;46:54–58.. [DOI] [PubMed] [Google Scholar]
- 30.Oberbauer E, Steffenhagen C, Wurzer C, et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen (Lond). 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]


