Supplemental Digital Content is available in the text.
Abstract
Background:
Acellular dermal matrix was introduced in breast reconstruction in 2001 and is gradually becoming a standard component for immediate breast reconstruction and nipple-sparing mastectomy. The reconstructive technique allows for improved aesthetic outcomes. However, there seems to be uncertainty regarding complication rates. The aim of this review was to systematically evaluate complication rates related to this method.
Methods:
This systematic review was conducted according to the recommendations outlined in the Cochrane Handbook for reviews and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Relevant databases were searched for in the literature concerning the use of acellular dermal matrix in implant-based nipple-sparing mastectomy and immediate breast reconstruction. All studies underwent detailed quality assessment. Summarized outcome rates were computed using meta-analysis.
Results:
Nine of 1,039 studies were eligible for inclusion yielding 778 procedures. The quality was acceptable for all included studies. The meta-analysis found the rate of skin necrosis to be 11%, nipple necrosis 5%, infection in 12%, hematoma in 1%, treated seroma in 5%, explantation 4%, and unplanned return to the operating room in 9%.
Conclusion:
The use of acellular dermal matrix in nipple-sparing mastectomy and implant-based breast reconstruction can be done with acceptable complication rates in selected patients. We recommend future studies to include specific definitions when reporting complication rates. Furthermore, future studies should elaborate on demographic characteristics of the included study samples and include predictor analysis to enhance knowledge of high risk patients.
INTRODUCTION
The number of women undergoing nipple-sparing mastectomy (NSM) is increasing worldwide. Complication rates related to NSM appear to be comparable with those of skin-sparing mastectomy (SSM) and the ability to preserve the nipple areola complex (NAC) has led to a high level of patient satisfaction.1,2 Since the introduction of acellular dermal matrix (ADM) in breast reconstruction in 20013,4 the combination of NSM and immediate breast reconstruction (IBR) with ADM and submuscular placement of the implant has increased significantly for both therapeutic and risk reducing mastectomies.5 ADM is a durable, nonimmunogenic, and elastic material derived from human or porcine skin tissue and serves as a reinforcement in the reconstruction. The implant is thereby supported and covered at the lower pole, which may relieve tension on the mastectomy skin flaps.3 The technique may allow for larger volume reconstructions with good quality aesthetic outcomes in either 1- or 2-staged procedures. Most of the existing reviews on this topic have been focusing on the reliability of NSM and have included NSM with and without ADM.6–9
The impression from the current literature is that ADM is associated with an increased seroma rate and may be associated with an increased rate of infection.10 The general perspective accepted among plastic surgeons is that complications following NSM and IBR are technique dependent, particularly nipple ischemia and loss. This includes location of the incision, implant size, initial expander volume (in 2-stage reconstructions), the technical skill of the surgeon, and the quality of the mastectomy skin flaps.9,11 However, we do not know if the use of ADM affects the complication rate following NSM and IBR and thereby its outcome. The aims of this study were to systematically review the current literature concerning complications related to the use of ADM in NSM with IBR with assessment of the quality of the literature as well as summarizing the reported complication rates through meta-analysis.
METHODS
This systematic review was conducted according to the recommendations outlined in the Cochrane Handbook for reviews and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.12,13
For this review, we did not distinguish between NSM and total skin-sparing mastectomy (TSSM).
Sources and Study Selection
A literature search was conducted in the electronic databases PubMed and Embase using the following search terms: “total skin sparing” OR “total skin-sparing” OR “nipple sparing” OR “nipple-sparing.” Data lock point was November 2016. Only studies that provide original data specifically on NSM and IBR using ADM were included. Case reports and studies with less than 20 procedures were excluded. The search was limited to the English language. The references of included studies were examined for relevant studies not identified in the literature search (Fig. 1).
Fig. 1.
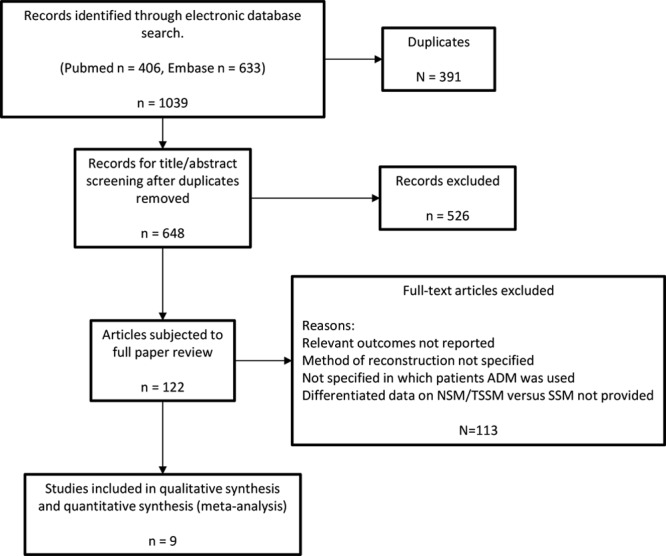
Flow diagram describing the systematic literature search.
The quality of the included studies was rated using checklists as recommended by the Cochrane group in a recently published review on methodological quality assessment tools.14 The Newcastle-Ottawa Scale15,16 was used for assessing nonrandomized cohort studies that included controls and the Institute of Health Economics Quality Appraisal tool (IHE QA)17,18 was used for assessing cohort studies without controls (case series studies). As recommended by the developers, the IHE QA was adapted to suit the studies included in this review (3 questions were omitted due to lack of relevance).17 The quality of the included studies was evaluated by 2 authors.
Statistical Analysis
We conducted a meta-analysis for the following complication outcomes regardless of severity, overall necrosis or ischemia including epidermolysis and wound dehiscence (any necrosis/ischemia), nipple necrosis including epidermolysis, infection, seroma, hematoma, and explantation as well as unplanned return to the operating room. We calculated proportions with a 95% CI based on a random-effects model due to the heterogeneous nature of the studies.19 The heterogeneity was investigated using chi-square and the I2 statistic. Chi-square values give information regarding the significance of heterogeneity, and I2 describes the percentage of total variation across studies which is due to heterogeneity rather than chance.20 Hence, a higher I2 value indicates larger heterogeneity. All statistical analyses were conducted using Stata/IC 14.0 (StataCorp LP).
RESULTS
Nine studies (Table 1) met the inclusion criteria; 2 prospective cohort studies with controls21,22 and 7 case series.11,23–28 The studies included a total of 778 cases of NSM reconstructed with the use of ADM (mean, 86; range, 32–281).
Table 1.
Characteristics of the Included Studies
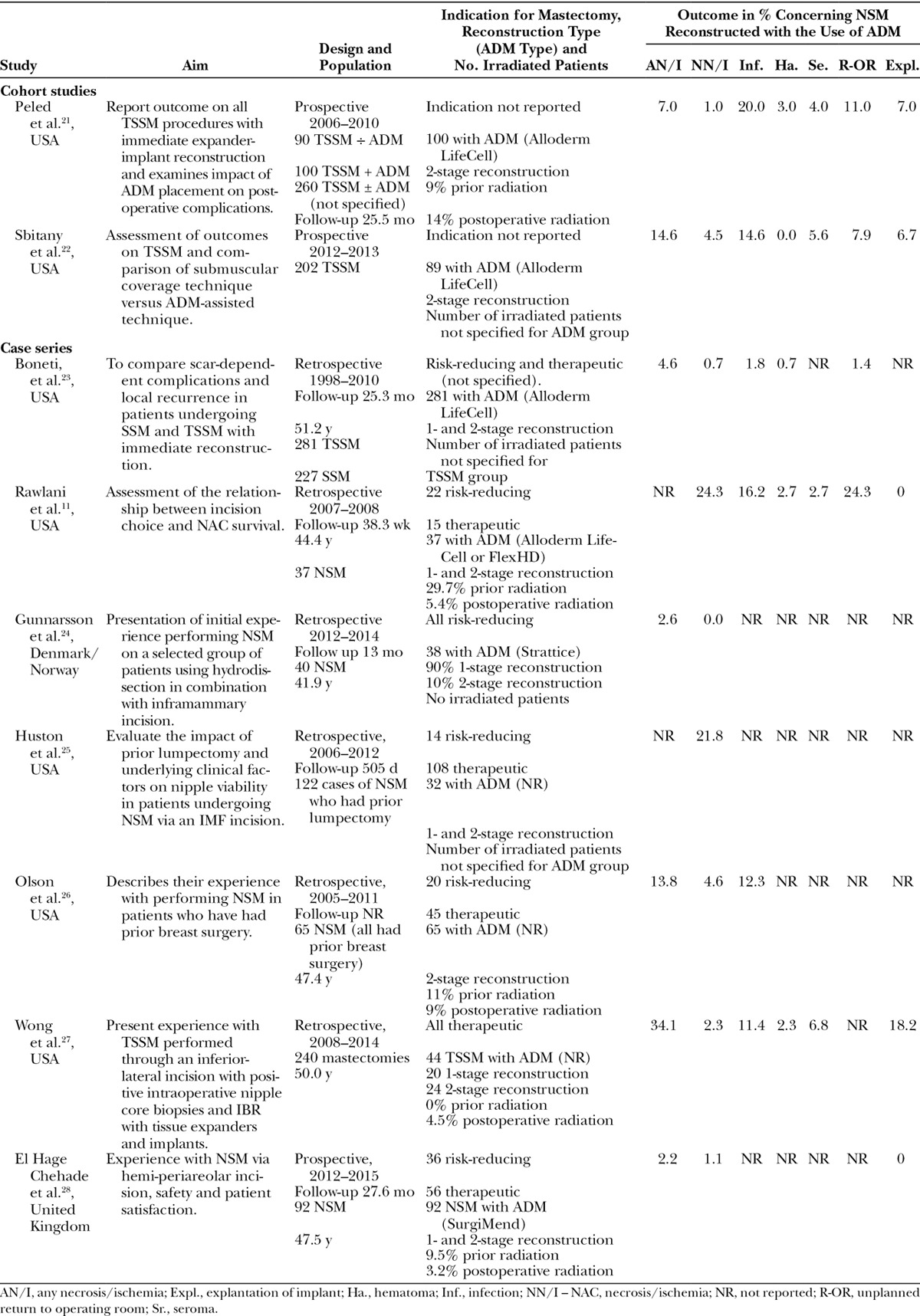
Cohort Studies with Controls
Data from a total of 189 cases of NSM reconstructed with use of ADM were included from 2 studies. Peled et al.21 included 450 patients in 3 consecutive cohorts. All patients underwent NSM and immediate expander implant placement. The first cohort included 90 patients reconstructed without ADM. The second cohort included 100 patients treated consecutively with ADM, and the third cohort consisted of the 260 patients in which ADM was used selectively. Data from the second cohort was included in this review.21 Sbitany et al.22 included 202 cases with NSM and immediate expander implant placement. ADM was used in 89 cases, and 113 cases were reconstructed with submuscular cover without ADM.22
Case Series
The 7 studies included a total of 589 cases. Rawlani et al.11 assessed the relation between choice of incision and NAC survival when performing 37 cases of NSM. All NSM were followed by acellular dermis-assisted tissue expander breast reconstruction. Periareolar incision placement resulted in significantly more cases of nipple necrosis compared with the other incision methods. Boneti et al.23 compared scar-dependent complications and patient satisfaction in 281 cases of TSSM and 227 cases of SSM. All 281 cases were eligible for inclusion. We found all cases of NSM in the study by Gunnarsson et al.24 eligible for this review and enrolled 38 cases of risk-reducing NSM reconstructed with the use of ADM. From the study by Wong et al.,27 we included 44 cases of TSSM performed through an inferior-lateral incision. Olson et al.26 included 65 cases of NSM with ADM. All patients had prior breast surgery. Huston et al.25 evaluated the impact of prior lumpectomy undergoing NSM. They evaluated on a group of 122 cases from which we found 32 cases eligible. El Hage Chehade et al.28 included 92 cases all reconstructed with the use of ADM. This study also evaluated patient-related outcomes.
Quality Assessment
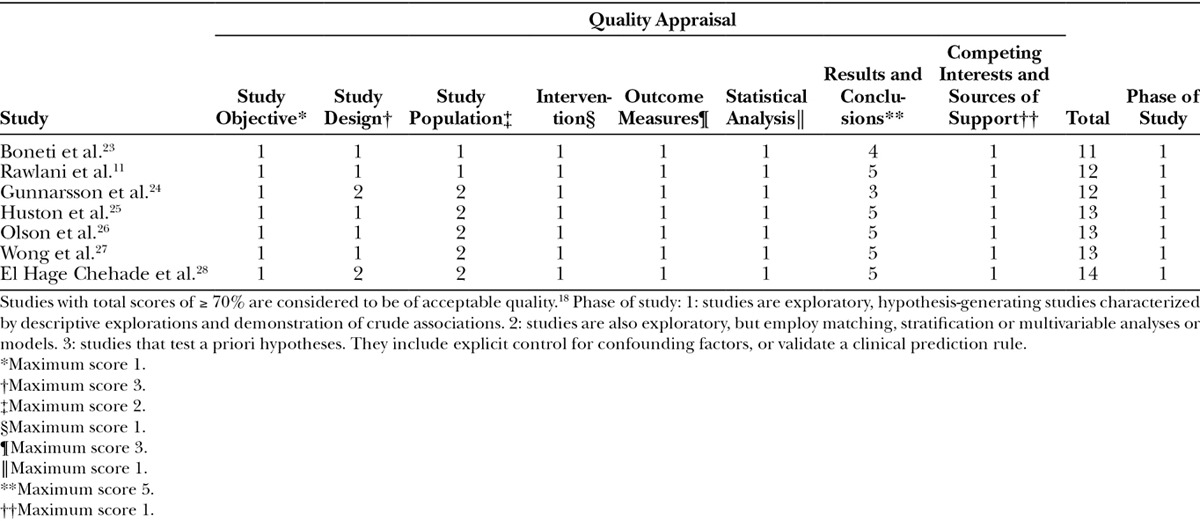
Both cohort studies were high-quality studies according to the NOS scoring system. Peled et al.21 scored the maximum score of 8 stars. Because of uncertainty regarding follow-up Sbitany et al.22 scored 7 stars (Table 2). The quality of the included case series studies varied. None of the studies achieved the maximum score of 15 stars when quality assessment investigated using the IHE QA tool. In general, studies did not achieve the highest scores due to study design, reporting of outcome measures, and study population (Table 3).
Table 2.
Critical Appraisal of Included Prospective Cohort Studies using the Newcastle-Ottawa Scale
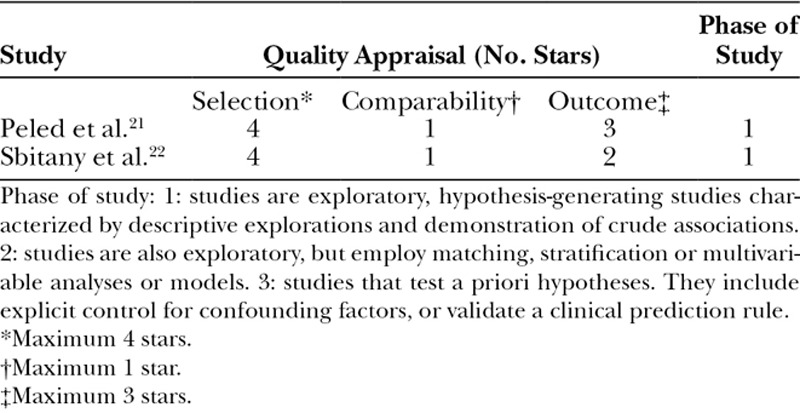
Table 3.
Critical Appraisal of Included Case Series Studies using the Institute of Health Economics Quality Appraisal Tool
Outcomes and Meta-analysis
Relevant data from all studies were included in the meta-analysis. Seven studies reported on any necrosis or ischemia, 9 studies reported on NAC necrosis or ischemia, 6 studies reported on infection, 5 studies reported on hematoma, 4 studies reported on seroma, 4 studies reported on unplanned return to OR, and 5 studies reported on implant explantation (Table 1). Results from the meta-analysis were 9% for any necrosis or ischemia, 4% for NAC necrosis or ischemia, 12% for infection, 1% for hematoma, 5% for seroma, 4% experienced explantation, and 9% experienced unplanned return to OR. I2 statistic was above 80% in most analyses. The results and Forrest plots are shown in Supplemental Digital Content 1 (see pdf, Supplemental Digital Content 1, which displays the meta-analysis of complication rates related to nipple-sparing mastectomy with acellular dermal matrix presented individually, http://links.lww.com/PRSGO/A650).
DISCUSSION
The use of ADM has rapidly become a well-established part of breast reconstruction with NSM.6,29 In many institutions it has become an integrated part of the reconstructive procedure. However, the literature regarding benefits and complications from this method seems sparse. To our knowledge, this systematic review provides the first summarized data on complications specifically related to NSM and IBR with ADM. The results of our meta-analysis indicate that approximately 4% (9 studies, 778 procedures) of patients experienced nipple necrosis or ischemia when using ADM for NSM and IBR. Furthermore, our meta-analysis computed a 12% infection rate (6 studies, 616 procedures), a seroma rate of 5% (4 studies, 270 procedures), and a hematoma rate of 1% (5 studies, 551 procedures). Because of a large heterogeneity, it is difficult to compare these findings. Although 1 study reported a significant decrease in the rate of infection in patients reconstructed with ADM compared with those reconstructed without ADM,21 it is not possible to conclude whether or not the use of ADM significantly affects the risk of these complications. The infection rate remains 1 of the controversial issues related to the use of ADM and more studies specifically addressing this issue are warranted. Five of the included studies reported on explantation (362 procedures). Peled et al.21 found a decrease of explantation from 17.8% to 7% when ADM was introduced. Sbitany et al.22 reported a small decrease of approximately 1% in the rate of explantation. None of the studies elaborated on the cause of explantation.
The identification of possible risk factors as selection criteria is mandatory for successful reconstruction. Olson et al.26 reported smoking as a significant risk factor for postoperative infection and that women later scheduled for postoperative chemotherapy had significantly higher risk of postoperative necrosis. Radiotherapy is a known risk factor for postoperative complications in breast reconstructive surgery, which was supported by findings in several of the included studies. Rawlani et al. demonstrated a trend toward increased nipple necrosis and soft-tissue infection in mainly women receiving neoadjuvant radiotherapy.11 This was in concordance with findings by Olson et al.26 who reported increased risk of necrosis in women receiving premastectomy radiation. In addition, Boneti et al.23 reported increased rate of capsular contracture in irradiated breasts but did not distinguish between TSSM and SSM. Peled et al.21 is the only study that reports complication rates specifically in the setting of postoperative radiotherapy. They found significantly decreased rates of infection, expander-implant loss, and unplanned return to the operating room in women who consecutively received ADM compared with women who did not receive ADM. This point toward a protective effect of ADM in these patients.21 Gunnarsson et al.24 only included nonsmoking women treated with risk-reducing mastectomy and presented complication rates that were considerably lower than in all other studies included in this review. In contrast, Wong et al.27 only included cases treated therapeutically and presented considerably higher complication rates. This suggests that ADM use in NSM is a relatively safe procedure in healthy women. However, it also indicates that caution should be taken when considering ADM for women with significant risk factors even though ADM may have a protective effect in women scheduled for postmastectomy radiotherapy.
Choice of material may also influence complication rates. Higher complication rates when using the human-derived Alloderm in terms of seroma, infection, and necrosis have been reported in a recently published review.30 Some surgeons have argued that porcine-derived materials are safer to use in terms of complications compared with human-derived materials.31 However, it should be emphasized that Alloderm is by far the most thoroughly evaluated material and reporting bias preclude any firm conclusions.30 Due to few studies included in this review, we were not able to investigate differences between ADM materials.
To fully appreciate results of this review, some aspects needs to be addressed. Studies had to be excluded due to their vagueness regarding rates of complications in the respective subgroups. Although the studies did define the surgical methods and the proportion of patients reconstructed using ADM versus muscle coverage, they did not differentiate between subgroups. Several of the included studies lack clear definitions of the nature of complications. It is recommended that authors clearly define which signs determine the presence of infection to enhance comparability with other studies. Skin flap/nipple necrosis should be clearly described with regard to thickness, level, and appearance. In addition, the reporting of epidermolysis varies and some authors may have chosen not to report this as a complication. Therefore, the risk of observer bias could limit the certainty of the meta-analysis results. Only a few studies elaborate on patient characteristics such as radiotherapy, demographic factors, smoking, and BMI. Only 1 of the 9 included studies include predictor analysis for high risk patients.26 The heterogeneity of the included studies should also be considered when interpreting results of this review. As expected, the heterogeneity as expressed by the I2 statistic was high in most of the meta-analyses. Besides unclear definitions, other factors are likely to explain this. The populations vary from healthy relatively young women treated with risk-reducing mastectomy to older women with considerable comorbidities treated with therapeutic mastectomy, sometimes with a history of prior breast surgery. Moreover, some of the studies did not distinguish between 1- and 2-stage reconstructions in the reporting of data (Table 1). Surgeon experience will naturally influence complication rates as well. The quality of the skin flaps are important determining factors for reducing complication rates, especially NAC necrosis.32 Although it may be difficult to carry out in real life, it would be of great interest to document the quality of the skin flaps using objective measures.
Lastly, potential commercial bias is an important aspect that needs mentioning. Information on conflicts of interest was provided in all studies. Seven studies reported no conflicting interests among the authors.11,21,24–28 One author was a member of the speakers bureau for LifeCell Corporation but also states that he did not receive any compensation or financial support for the study.22 Another study reported 1 author receiving support from Fashion Footwear Association of New York.23
In summary, this review and meta-analysis regarding NSM and IBR using ADM revealed a skin necrosis rate of 9%, nipple necrosis in 4%, infection in 12%, hematoma in 1%, seroma in 5%, explantation in 4%, and 9% experienced unplanned return to operating room. Complication rates computed in this review do not seem to vary considerably from complication rates reported in reviews on NSM in general.1,7,9,33
High-level scientific evidence concerning complications following the use of ADM in NSM and IBR is surprisingly limited in existing literature. There is nothing in the data collected for this review and meta-analysis, suggesting that the use of ADM changes the outcome of NSM and IBR. The favorable outcome is still based on the good judgment and skills of the joint efforts of breast- and plastic surgeons. On the other hand, no data suggests either that the use of ADM significantly aggravates the outcomes of ADM-based NSM and IBR.
CONCLUSIONS
This article is the first to provide systematically summarized data on complications specifically related to NSM and IBR with ADM. The use of ADM in NSM and IBR can be done with acceptable complication rates in carefully selected patients. The use of ADM does not seem to change the complication rates following NSM and IBR. However, the current literature regarding this subject is still limited and standardized reporting is warranted. We recommend future studies to include specific definitions when reporting complication rate. Furthermore, future studies should elaborate on demographic characteristics of the included study samples and include predictor analysis to enhance knowledge of high risk patients.
Supplementary Material
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The article processing charge was paid for by the Research Unit at the Department of Plastic and Reconstructive Surgery, Odense University Hospital, Denmark.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Sisco M, Yao KA. Nipple-sparing mastectomy: a contemporary perspective. J Surg Oncol. 2016;113:883–890.. [DOI] [PubMed] [Google Scholar]
- 2.Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg. 2010;125:818–829.. [DOI] [PubMed] [Google Scholar]
- 3.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5.. [DOI] [PubMed] [Google Scholar]
- 4.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239.. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Amara D, Peled AW, et al. Negative genetic testing does not deter contralateral prophylactic mastectomy in younger patients with greater family histories of breast cancer. Ann Surg Oncol. 2015;22:3338–3345.. [DOI] [PubMed] [Google Scholar]
- 6.Frederick MJ, Lin AM, Neuman R, et al. Nipple-sparing mastectomy in patients with previous breast surgery: comparative analysis of 775 immediate breast reconstructions. Plast Reconstr Surg. 2015;135:954e–962e.. [DOI] [PubMed] [Google Scholar]
- 7.Choi M, Frey JD, Alperovich M, et al. “Breast in a Day”: examining single-stage immediate, permanent implant reconstruction in nipple-sparing mastectomy. Plast Reconstr Surg. 2016;138:184e–191e.. [DOI] [PubMed] [Google Scholar]
- 8.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127:514–524.. [DOI] [PubMed] [Google Scholar]
- 9.Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–1054.. [DOI] [PubMed] [Google Scholar]
- 10.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356.. [DOI] [PubMed] [Google Scholar]
- 11.Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg. 2011;19:129–133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at www.cochrane-handbook.org. Accessed March 25, 2016.
- 14.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10.. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 25, 2016.
- 16.Hootman JM, Driban JB, Sitler MR, et al. Reliability and validity of three quality rating instruments for systematic reviews of observational studies. Res Synth Methods. 2011;2:110–118.. [DOI] [PubMed] [Google Scholar]
- 17.Guo B, Moga C, Harstall C, et al. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. 2016;69:199–207.e2.. [DOI] [PubMed] [Google Scholar]
- 18.Moga C, Guo B, Schopflocher D, et al. Development of a quality apraisal tool for case series studies using a modified Delphi technique. Available at http://www.ihe.ca. Accessed March 10, 2016.
- 19.Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62:97–128.. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg. 2012;129:901e–908e.. [DOI] [PubMed] [Google Scholar]
- 22.Sbitany H, Wang F, Peled AW, et al. Tissue Expander Reconstruction After Total Skin-Sparing Mastectomy: Defining the Effects of Coverage Technique on Nipple/Areola Preservation. Ann Plast Surg. 2016;77:17–24.. [DOI] [PubMed] [Google Scholar]
- 23.Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212:686–693.; discussion 693. [DOI] [PubMed] [Google Scholar]
- 24.Gunnarsson GL, Børsen-Koch M, Wamberg P, et al. How to perform a NAC sparing mastectomy using an ADM and an implant. Gland Surg. 2014;3:252–257.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huston TL, Small K, Swistel AJ, et al. Nipple-sparing mastectomy via an inframammary fold incision for patients with scarring from prior lumpectomy. Ann Plast Surg. 2015;74:652–657.. [DOI] [PubMed] [Google Scholar]
- 26.Olson J, Anderson LA, Ying J, Zhang MM, Agarwal JP. Nipple Sparing Mastectomy in Patients With Prior Breast Scars: Is It Safe? Ann Plast Surg. 2017;77:22–27.. [DOI] [PubMed] [Google Scholar]
- 27.Wong L, Wilson RM, Snapp WK, et al. Nipple pathology in total skin-sparing mastectomy: implications for immediate reconstruction. Ann Plast Surg. 2016;76:S340–S343.. [DOI] [PubMed] [Google Scholar]
- 28.El Hage Chehade H, Headon H, Wazir U, et al. Nipple-sparing mastectomy using a hemi-periareolar incision with or without minimal medial-lateral extensions; clinical outcome and patient satisfaction: a single centre prospective observational study. Am J Surg. 2017;213:1116–1124.. [DOI] [PubMed] [Google Scholar]
- 29.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506.. [DOI] [PubMed] [Google Scholar]
- 30.Skovsted Yde S, Brunbjerg ME, Damsgaard TE. Acellular dermal matrices in breast reconstructions—a literature review. J Plast Surg Hand Surg. 2016;50:187–196.. [DOI] [PubMed] [Google Scholar]
- 31.Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg. 2012;129:1223–1233.. [DOI] [PubMed] [Google Scholar]
- 32.Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications. Breast. 2017;34:S82–S84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41.. [DOI] [PubMed] [Google Scholar]


