Supplemental Digital Content is available in the text.
Abstract
Background:
The aim of this study was to develop, implement, and evaluate a standardized perioperative enhanced recovery after surgery (ERAS) clinical care pathway in microsurgical abdominal-based breast reconstruction.
Methods:
Development of a clinical care pathway was informed by the latest ERAS guideline for breast reconstruction. Key features included shortened preoperative fasting, judicious fluids, multimodal analgesics, early oral nutrition, early Foley catheter removal, and early ambulation. There were 3 groups of women in this cohort study: (1) traditional historical control; (2) transition group with partial implementation; and (3) ERAS. Narcotic use, patient-reported pain scores, antiemetic use, time to regular diet, time to first walk, hospital length of stay, and 30-day postoperative complications were compared between the groups.
Results:
After implementation of the pathway, the use of parenteral narcotics was reduced by 88% (traditional, 112 mg; transition, 58 mg; ERAS, 13 mg; P < 0.0001), with no consequent increase in patient-reported pain. Patients in the ERAS cohort used less antiemetics (7.0, 5.3, 2.2 doses, P < 0.0001), returned to normal diet 19 hours earlier (46, 39, 27 hours, P < 0.0001), and walked 25 hours sooner (75, 70, 50 hours, P < 0.0001). Overall, hospital length of stay was reduced by 2 days in the ERAS cohort (6.6, 5.6, 4.8 days, P < 0.0001), without an increase in rates of major complications (9.5%, 10.1%, 8.3%, P = 0.9).
Conclusions:
A clinical care pathway in microsurgical breast reconstruction using the ERAS Society guideline promotes successful early recovery.
INTRODUCTION
Clinical care pathways standardize and limit variation in postoperative care of patients.1 These pathways are multidisciplinary team driven, with the goal to ensure important steps in care are not delayed or forgotten.1,2 The literature is limited on the use of clinical care pathways for plastic surgery procedures, and not all are evidence based.3–7 Hwang et al.3 developed a postoperative care plan for women undergoing transverse rectus abdominis myocutaneous (TRAM) flap reconstruction to reduce variation in the care of patients. Davidge et al.4 implemented a multidisciplinary patient care plan in a cohort of women undergoing pedicled TRAM flap breast reconstruction and identified multimodal pain management as a key modifiable factor in facilitating early discharge. Similarly, use of clinical care pathways in perioperative management of patients undergoing microvascular breast reconstruction correspond with lower postoperative opioid requirements and decreased hospital length of stay (LOS).5–7 Our group previously developed and implemented a clinical care pathway for head and neck cancer patients undergoing microvascular flap reconstruction.8 This pathway reduced unnecessary practice variation, resulting in patients having fewer pulmonary complications and shorter hospital LOS.9
In 2001, a group of academic surgeons including Ljungqvist10 and Kehlet11 formed the Enhanced Recovery After Surgery (ERAS) study group, with the goal to develop a perioperative care protocol for elective colonic surgery based on best available evidence. The ERAS study group subsequently established a nonprofit international society (ERAS Society, www.ERASsociety.com) to develop bundles of perioperative care elements based on evidence and improve patient recovery. An expanding evidence base of clinical trials supports effectiveness of ERAS protocols in improving outcome with patients returning to normal function faster across multiple surgical procedures.11–22 Our group recently published ERAS Society–endorsed guidelines for breast reconstruction.23 This guideline describes 18 care elements in the pre-, intra-, and postoperative periods, such as minimal fasting, carbohydrate loading, multimodal pain and nausea prophylaxis, judicious fluids, early refeeding, and early ambulation.23
The goal of the present study was to design, implement, and evaluate a standardized perioperative care pathway for patients undergoing microsurgical abdominal-based autologous breast reconstruction. The international breast reconstruction ERAS guideline was used as the evidence base to inform the clinical pathway. We prospectively evaluated a cohort of women undergoing microsurgical breast reconstruction with abdominal tissue using this ERAS-based clinical care pathway, and compared their outcomes with traditional care.
METHODOLOGY
Study Design and Subjects
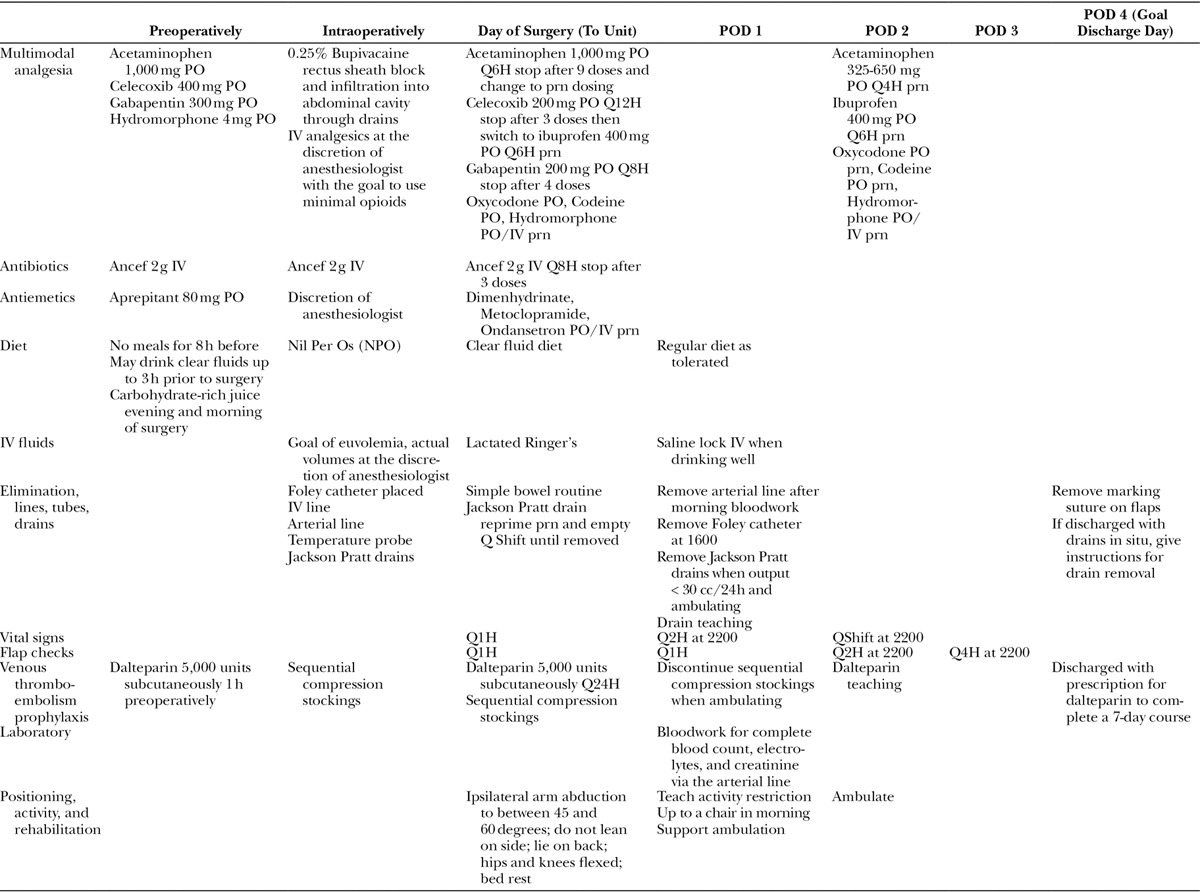
This cohort study was approved by the Alberta Research Ethics Community Consensus Initiative second panel review. Phase 1 of the study involved defining a microsurgical breast reconstruction perioperative care pathway (Table 1, see document, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A652, for further details on components of the ERAS care pathway). Elements of the pathway were established concurrently with the development of the ERAS Society guideline.23 Pathway drafts were circulated among team members, and feedback from a multidisciplinary team of plastic surgeons, anesthesiologists, nurses, and physiotherapist was incorporated. During this time frame, standardized order sets, patient logbooks, data collection sheets, and posters on the unit were developed to highlight changes in practice. This cohort study, included all consecutive women who underwent immediate or delayed microsurgical breast reconstruction using abdominal tissue between January 2009 and May 2016 at the Foothills Medical Center (Calgary, Alberta, Canada). Phase 2 involved a look-back at patients who had undergone traditional care, in a nonpathway approach. The historical control cohorts included the traditional group (January 2009 to December 2012), and the transition group with partial implementation of pathway elements (January 2013 to February 2015). Partial implementation in the transition group was secondary to differences in staff training, lack of a consistent order set, and inconsistent application by surgeons. The clinical outcome parameters for the control groups were collected retrospectively by reviewing electronic medical records and charts. Phase 3 began in March 2015 with full implementation of the ERAS care pathway and prospective data collection.
Table 1.
ERAS-Based Clinical Care Pathway
Outcome Measures
Narcotic use, patient-reported pain, antiemetic use, time to regular diet, time to first walk, hospital LOS, and 30-day postoperative complications and readmissions were used as outcome measures. Postoperatively, patients in the historical control cohort received opioids via patient-controlled analgesia (PCA). The ERAS protocol did not have PCA as part of the multimodal analgesia. ERAS patients had parenteral narcotics ordered on an as needed basis. The quantity of parenteral narcotics used during the first 3 postoperative days (POD 0–3) was calculated by conversion of all forms to intravenous (IV) morphine equivalents. Total narcotics used for the first 3 PODs was determined by conversion of all forms of oral and parenteral opioids taken into IV morphine equivalents. Patient-reported pain was scored on a scale from 0 to 10 (0 = no pain; 10 = worst pain) and collected as part of vital signs. The pain scores were averaged and reported for POD 0 and the first 3 PODs (POD 0–3). Total antiemetics used was based on the number of doses of all antiemetics ordered for the first 3 PODs. Time to regular diet, time to first walk, and hospital LOS were measured in hours from the time of admission in the morning of surgery to the time of discharge postoperatively. Major complications were collected up to 30 days postoperatively. These were defined as any related hospital readmission, any unexpected return to the operating room, and flap loss due to arterial or venous thrombosis.
Statistical Analysis
Characteristics were compared between the traditional, transition, and ERAS groups, and outcomes reported as means. Differences in proportions were analyzed using the chi-square test. Means for continuous variables for all groups were analyzed using Analysis of Variance (ANOVA). Tukey’s test was used to compare the mean between each group. A P value less than 0.05 was considered statistically significant.
RESULTS
There were 169 patients in the traditional (January 2009 to December 2012), 89 in the transition (January 2013 to February 2015), and 72 in the ERAS cohort (March 2015 to May 2016). Baseline characteristics of each cohort are shown in Table 2. Overall, the 3 populations demonstrated homogeneity in age (P = 0.06), Body Mass Index (BMI) (P = 0.09), American Society of Anesthesiologists (ASA) classification (P = 0.5), smoking status (P = 0.7), type of flap reconstruction (P = 0.9), and whether the reconstruction was unilateral or bilateral (P = 0.8). Compared with traditional patients, ERAS patients were older (P < 0.05) and had a higher BMI (P < 0.05) (Table 2). In addition, there was a higher proportion of patients in the ERAS group that underwent immediate than delayed reconstruction (P < 0.0001) (Table 2).
Table 2.
Baseline Characteristics

Postoperative outcomes are shown in Table 3. Total amount of IV narcotics required in the first 3 PODs was lower in the ERAS group (traditional, 112 mg; transition, 58 mg; ERAS, 13 mg; P < 0.0001). Similarly, patients in the ERAS group used less total narcotics in the first 3 PODs (131, 79, 44 mg, P < 0.0001) (Table 3). Despite using less narcotics, patients in the ERAS cohort did not report higher pain scores (Table 3). Rather, on POD 0 patients in the ERAS group had lower pain scores (2.8, 2.3, 1.7, P = 0.0002). Similarly, ERAS patients had lower pain scores averaging the first 3 PODs (POD 0–3) (3.0, 2.5, 2.3, P = 0.01).
Table 3.
Postoperative Outcomes
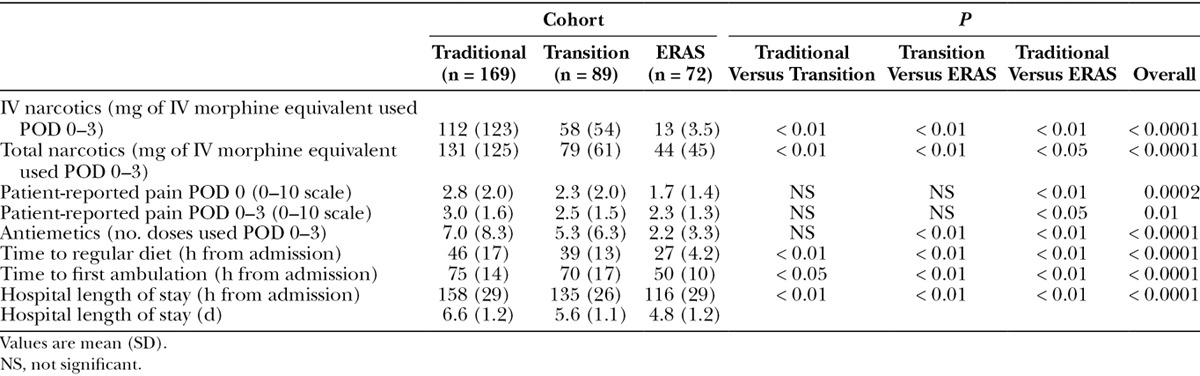
Antiemetics were available on an as needed basis postoperatively. Less antiemetics were used by patients in the ERAS cohort (7.0, 5.3, 2.2 doses, P < 0.0001) (Table 3).
Traditional patients had restricted oral intake for 46 hours from the time they arrived for surgery, whereas ERAS patients resumed a regular diet 27 hours after hospital arrival, 19 hours earlier than traditional patients (P < 0.01) (Table 3).
Patients in the traditional group walked 75 hours after hospital arrival, whereas ERAS patients walked 50 hours after admission, 25 hours earlier compared with traditional patients (P < 0.01) (Table 3).
Criteria for discharge were absence of early complications, return to normal diet, ability to void, independent mobilization and ambulation, and adequate pain control with oral analgesics. Hospital LOS was reduced by 42 hours in the ERAS group (158, 135, 116 hours, P < 0.0001) (Table 3). This corresponds to a 2-day earlier discharge (6.6, 5.6, 4.8 days, P < 0.0001).
Thirty-three of 330 patients had major complications, giving a 10% overall rate in all cohorts. Despite ERAS patients being discharged earlier, 30-day complication rates were similar between groups (9.5%, 10.1%, 8.3%, P = 0.9) (Table 4). Specific major postoperative complications included hematoma requiring evacuation (2.4%, 2.2%, 4.2%, P = 0.7), vascular compromise requiring operative exploration with or without anastomotic repair (5.9%, 5.6%, 2.8%, P = 0.6), flap failure (0%, 1.1%, 0%, P = 0.3), occurrence of deep vein thrombosis or pulmonary embolism (0.6%, 0%, 0%, P = 0.6), and major infection requiring readmission and IV antibiotic treatment (0.6%, 1.1%, 1.4%, P = 0.8). There was no increase in any of these complications with the implementation of the care pathway. To note, there was only 1 flap failure of 330 cases (0.3%) overall, and this was in the transition group. In addition, there was no increase in the number of readmissions to hospital within 30 days from surgery between the groups (1.2%, 1.1%, 1.4%, P = 0.99).
Table 4.
Thirty-day Postoperative Complications

DISCUSSION
A care pathway is an ideal approach to organize complex patient care, with multimodal perioperative care shown to decrease hospital LOS without a consequent increase in morbidity across multiple disciplines.11–22 There have been few published studies looking at the use of clinical care pathways for patients undergoing microvascular breast reconstruction.5–7 Batdorf et al.5 published on the use of a clinical care pathway for microsurgical breast reconstruction in a cohort of 49 patients and demonstrated lower postoperative opioid usage and a decreased hospital LOS from 5.5 to 3.9 days. However, there was heterogeneity between the cohorts with pathway patients having lower BMI, less chronic pain diagnosis, and higher proportion of deep inferior epigastric artery perforator (DIEP) flap reconstruction compared with controls.5 Obesity in the setting of autologous breast reconstruction has been associated with increased hospital LOS, whereas DIEP relative to TRAM reconstruction has been associated with lower patient morbidity.24–26 These differences could certainly be confounding and contribute to the observed lower opioid requirements and improved LOS in the care pathway cohort. Bonde et al.27 published on a care pathway that decreased hospital LOS from 7.4 days to 6.2 days. They subsequently refined their pathway that included patient counseling during initial consultation, preparing them mentally for discharge on POD 3.6 Their study included a limited number of patients (n = 16) of only delayed unilateral autologous breast reconstruction with an average hospital LOS of 3.1 days.6 Results by Afonso et al.7 also confirmed that use of a clinical care pathway decreased hospital LOS after microvascular breast reconstruction. They reported a reduction in LOS from 5 to 4 days in a cohort of 42 patients. In addition, they indicated that use of multimodal analgesia including transversus abdominis plane block with long acting liposomal bupivacaine decreases IV opioid requirements by 49%.7
Although the above-mentioned studies utilize a clinical care pathway in the management of patients undergoing microvascular breast reconstruction, the components of the pathways are variable. One goal of the present study was to utilize the 18-element ERAS Society guideline, deemed important for improving patient recovery, to develop a standardized clinical care pathway for microvascular autologous breast reconstruction.23 Using this ERAS-based clinical care pathway, we demonstrated that patients had less pain and nausea, walked and ate sooner, and were discharged from hospital earlier without an increase in complications.
It is difficult to ascertain which components of our ERAS-based care pathway are most responsible for improvement in patient outcomes. It is likely an interplay of all the elements including minimal fasting, carbohydrate loading, multimodal pain and nausea prophylaxis, judicious fluids, early refeeding, early Foley catheter removal, and early ambulation. As part of a multimodal analgesic regimen, we used rectus sheath blocks, as several others have found significant beneficial effects of local anesthetic blocks in abdominal-based autologous breast reconstruction.28–30 Both Batdorf et al.5 and Afonso et al.7 used liposomal bupivacaine and concluded a resulting decrease in postoperative opioid requirements. Liposomal bupivacaine does not have approval in our facilities and is similarly unavailable in many other centers performing autologous breast reconstruction. We therefore used standard bupivacaine directly injected into rectus sheath. Despite lack of liposomal bupivacaine, we showed a decrease in the use of IV narcotics. Although Afonso et al.7 demonstrated a decrease in the use of PCA from 98% to 21%, none of our ERAS patients required PCA. They showed a 49% decrease in postoperative IV opioids from 71 to 46 mg morphine equivalents, whereas our data indicate an 88% reduction from 112 to 13 mg IV morphine equivalents. Our data demonstrate that even without the use of liposomal bupivacaine, there is significant decrease in parenteral opioid requirements in the ERAS group, and that patients can still be successfully discharged earlier without high reported pain scores. Multimodal analgesic regimens, with use of celecoxib and gabapentin have been shown to be effective for controlling pain postoperatively.23,30–33 Overall, our ERAS patients had lower pain scores over the course of their hospital stay despite reduction in the use of opioid analgesics.
Certainly, limiting use of narcotics has been shown to decrease postoperative nausea and vomiting.34 We found that our patients on the ERAS pathway required less antiemetics, suggesting that they were less nauseated. Further, our results indicate that patients in the ERAS group returned to a regular diet 19 hours earlier compared with the traditional group. Return to normal diet may reflect lack of nausea and feeling better; however, it is important to note that this outcome is somewhat artificial as we implemented earlier feeding as part of the care pathway. Traditionally, patients took nothing per mouth for a minimum of 24 hours postoperatively in case there was a need to return to the operating room, whereas, the ERAS group started on clear fluids immediately postoperatively and then a regular diet as tolerated starting the morning after surgery. This combined with the early removal of the Foley catheter likely played a role in earlier ambulation and faster recovery of our ERAS patients leading to shorter hospital LOS.
In terms of patient characteristics, the traditional and ERAS patients were similar across ASA classification, smoking status, type of flap reconstruction, and whether the reconstruction was unilateral or bilateral. However, ERAS patients were older, had higher BMI, and underwent more immediate breast reconstruction compared with the traditional control patients. Older women undergoing autologous breast reconstruction have been shown to require longer hospitalization.35 Women with higher BMI have higher overall donor- and recipient-site complications after autologous breast reconstruction.24–26 These studies further strengthen our finding of the impact of the ERAS pathway. Whether hospital LOS is longer for immediate than delayed breast reconstruction is unresearched, but given that women with immediate breast reconstruction undergo concurrent mastectomy and possibly sentinel lymph node biopsy, it would be reasonable to assume that the recovery period for them would be longer. Despite a higher proportion of patients who were older, had higher BMI, and underwent immediate breast reconstruction in the ERAS cohort, complications were stable and patients were ready for discharge sooner.
To date, our study is the largest prospective cohort with 72 patients who underwent microvascular breast reconstruction using a perioperative ERAS-based clinical care pathway. We demonstrated that on average the ERAS cohort were discharged on POD 4. Afonso et al.7 similarly reported hospital LOS of 4 days in a cohort of 42 patients. In comparison, our patient population had higher percentage of bilateral breast reconstruction (63% versus 50%), less DIEP (56% versus 67%), but lower percentage of patients undergoing immediate breast reconstruction (44% versus 71%).
Decreased hospital LOS is a surrogate for patients doing better and meeting discharge criteria earlier. In our study, we demonstrated that even though patients are discharged earlier there was no associated increase in the rate of 30-day complications. Massenburg et al.24 recently reviewed the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and identified a 26% general complication rate for microvascular autologous breast reconstruction. Overall, the rate of complications at our center was 10%. The prevalence of flap failure for autologous breast reconstruction is reported to be 2.4%.24 The rate of flap failure in our series was 0.3% with a single case of failure. The majority of vessel thromboses that happen occur within 24–48 hours postoperatively. Late venous thrombosis occurring after 3 days postoperatively is a rare event.36 Therefore, keeping patients in the hospital longer to continue monitoring flaps is hard to support, especially given low overall rates of flap failure. In our study of 72 ERAS patients, there were 2 flaps that required anastomotic reexploration due to venous congestion, both of which occurred in the first 24-hour postoperative period. There were no anastomotic complications or flap failures as a result of earlier discharge. The only 30-day readmissions in the ERAS cohort was secondary to wound infection that required administration of IV antibiotics.
A standardized care plan highlights the priorities of the perioperative care to the collaborative team and to the patient. It has been shown that engagement strategies help patients feel empowered and have a sense of control over their recovery.37 Our perioperative care pathway for microsurgical autologous breast reconstruction shows success in early and safe recovery. As a follow-up study, we are assessing the patient-reported outcomes regarding the quality of the patient’s recovery on the ERAS pathway. If satisfaction is high, this will further strengthen our findings that not only are patients discharged earlier but that they recover faster and with more ease.
CONCLUSIONS
A standardized ERAS-based clinical care pathway in women undergoing microsurgical breast reconstruction successfully promotes early recovery. Patients in the ERAS group required less opioids without an increase in patient-reported pain. ERAS patients used less postoperative antiemetics, returned to normal diet earlier, and walked sooner. Finally, patients in the ERAS cohort had a shorter hospital LOS without an increase in the rate of major complications 30 days postoperatively.
Supplementary Material
Footnotes
Presented at the American Society for Reconstructive Microsurgery Annual Meeting in Jan 2017, Waikoloa, Hawaii; Canadian Society of Plastic Surgery Annual Meeting in June 2016, Ottawa, Ontario, Canada.
The study was approved by an institutional review committee, Alberta Research Ethics Community Consensus Initiative.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the University of Calgary Open Access Authors Fund.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Kinsman L, Rotter T, James E, et al. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen AY, Callender D, Mansyur C, et al. The impact of clinical pathways on the practice of head and neck oncologic surgery: the University of Texas M. D. Anderson Cancer Center Experience. Arch Otolaryngol Head Neck Surg. 2000;126:322–326.. [DOI] [PubMed] [Google Scholar]
- 3.Hwang TG, Wilkins EG, Lowery JC, et al. Implementation and evaluation of a clinical pathway for TRAM breast reconstruction. Plast Reconstr Surg. 2000;105:541–548.. [DOI] [PubMed] [Google Scholar]
- 4.Davidge KM, Brown M, Morgan P, et al. Processes of care in autogenous breast reconstruction with pedicled TRAM flaps: expediting postoperative discharge in an ambulatory setting. Plast Reconstr Surg. 2013;132:339e–344e.. [DOI] [PubMed] [Google Scholar]
- 5.Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:395–402.. [DOI] [PubMed] [Google Scholar]
- 6.Bonde CT, Khorasani H, Elberg J, et al. Perioperative optimization of autologous breast reconstruction. Plast Reconstr Surg. 2016;137:411–414.. [DOI] [PubMed] [Google Scholar]
- 7.Afonso A, Oskar S, Tan KS, et al. Is enhanced recovery the new standard of care in microsurgical breast reconstruction? Plast Reconstr Surg. 2017;139:1053–1061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dautremont JF, Rudmik LR, Yeung J, et al. Cost-effectiveness analysis of a postoperative clinical care pathway in head and neck surgery with microvascular reconstruction. J Otolaryngol Head Neck Surg. 2013;42:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung JK, Dautremont JF, Harrop AR, et al. Reduction of pulmonary complications and hospital length of stay with a clinical care pathway after head and neck reconstruction. Plast Reconstr Surg. 2014;133:1477–1484.. [DOI] [PubMed] [Google Scholar]
- 10.Ljungqvist O. ERAS—enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr. 2014;38:559–566.. [DOI] [PubMed] [Google Scholar]
- 11.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–641.. [DOI] [PubMed] [Google Scholar]
- 12.Lee L, Li C, Robert N, et al. Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg. 2013;100:1326–1334.. [DOI] [PubMed] [Google Scholar]
- 13.Lovely JK, Maxson PM, Jacob AK, et al. Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br J Surg. 2012;99:120–126.. [DOI] [PubMed] [Google Scholar]
- 14.Larson DW, Batdorf NJ, Touzios JG, et al. A fast-track recovery protocol improves outcomes in elective laparoscopic colectomy for diverticulitis. J Am Coll Surg. 2010;211:485–489.. [DOI] [PubMed] [Google Scholar]
- 15.Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;2:CD007635. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuishi W, Kohri T, Kodera K, et al. Usefulness of an enhanced recovery after surgery protocol for perioperative management following open repair of an abdominal aortic aneurysm. Surg Today. 2012;42:1195–1200.. [DOI] [PubMed] [Google Scholar]
- 17.Schultz NA, Larsen PN, Klarskov B, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg. 2013;100:138–143.. [DOI] [PubMed] [Google Scholar]
- 18.Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg. 2013;100:482–489.. [DOI] [PubMed] [Google Scholar]
- 19.Blom RL, van Heijl M, Bemelman WA, et al. Initial experiences of an enhanced recovery protocol in esophageal surgery. World J Surg. 2013;37:2372–2378.. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer AJ, Tarassoli P, Thomas W, et al. Enhanced recovery program in total hip arthroplasty. Indian J Orthop. 2012;46:407–412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malviya A, Martin K, Harper I, et al. Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop. 2011;82:577–581.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122:319–328.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temple-Oberle C, Shea-Budgell MA, Tan M, et al. ; ERAS Society. Consensus review of optimal perioperative care in breast reconstruction: Enhanced Recovery after Surgery (ERAS) Society recommendations. Plast Reconstr Surg. 2017;139:1056e–1071e.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massenburg BB, Sanati-Mehrizy P, Ingargiola MJ, et al. Flap failure and wound complications in autologous breast reconstruction: a national perspective. Aesthetic Plast Surg. 2015;39:902–909.. [DOI] [PubMed] [Google Scholar]
- 25.Schaverien MV, Mcculley SJ. Effect of obesity on outcomes of free autologous breast reconstruction: a meta-analysis. Microsurgery. 2014;34:484–497.. [DOI] [PubMed] [Google Scholar]
- 26.Lee KT, Mun GH. Effects of obesity on postoperative complications after breast reconstruction using free muscle-sparing transverse rectus abdominis myocutaneous, deep inferior epigastric perforator, and superficial inferior epigastric artery flap: a systematic review and meta-analysis. Ann Plast Surg. 2016;76:576–584.. [DOI] [PubMed] [Google Scholar]
- 27.Bonde C, Khorasani H, Eriksen K, et al. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: a case control study. J Plast Surg Hand Surg. 2015;49:367–371.. [DOI] [PubMed] [Google Scholar]
- 28.Dagtekin O, Hotz A, Kampe S, et al. Postoperative analgesia and flap perfusion after pedicled TRAM flap reconstruction—continuous wound instillation with ropivacaine 0.2%. A pilot study. J Plast Reconstr Aesthet Surg. 2009;62:618–625.. [DOI] [PubMed] [Google Scholar]
- 29.Heller L, Kowalski AM, Wei C, et al. Prospective, randomized, double-blind trial of local anesthetic infusion and intravenous narcotic patient-controlled anesthesia pump for pain management after free TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;122:1010–1018.. [DOI] [PubMed] [Google Scholar]
- 30.Tan KJ, Farrow H. Improving postoperative analgesia for transverse rectus abdominis myocutaneous flap breast reconstruction; the use of a local anaesthetic infusion catheter. J Plast Reconstr Aesthet Surg. 2009;62:206–210.. [DOI] [PubMed] [Google Scholar]
- 31.Beaussier M, Sciard D, Sautet A. New modalities of pain treatment after outpatient orthopaedic surgery. Orthop Traumatol Surg Res. 2016;102:S121–S124.. [DOI] [PubMed] [Google Scholar]
- 32.Gärtner R, Kroman N, Callesen T, et al. Multimodal prevention of pain, nausea and vomiting after breast cancer surgery. Minerva Anestesiol. 2010;76:805–813.. [PubMed] [Google Scholar]
- 33.Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults. Cochrane Database Syst Rev. 2010;12:CD008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts GW, Bekker TB, Carlsen HH, et al. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101:1343–1348.. [DOI] [PubMed] [Google Scholar]
- 35.Ludolph I, Horch RE, Harlander M, et al. Is there a rationale for autologous breast reconstruction in older patients? A retrospective single center analysis of quality of life, complications and comorbidities after DIEP or ms-TRAM flap using the BREAST-Q. Breast J. 2015;21:588–595.. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JA, Kim EM, Eftekhari K, et al. Late venous thrombosis in free flap breast reconstruction: strategies for salvage after this real entity. Plast Reconstr Surg. 2012;129:8e–15e.. [DOI] [PubMed] [Google Scholar]
- 37.Clarke LK. Pathways for head and neck surgery: a patient- education tool. Clin J Oncol Nurs. 2002;6:78–82.. [DOI] [PubMed] [Google Scholar]


