Abstract
Fatigue is the most common symptom of cancer at diagnosis, yet causes and effective treatments remain elusive. As tumors can be highly inflammatory, it is generally accepted that inflammation mediates cancer-related fatigue. However, evidence to support this assertion is mostly correlational. In this study, we directly tested the hypothesis that fatigue results from propagation of tumor-induced inflammation to the brain and activation of the central pro-inflammatory cytokine, interleukin-1 (IL-1). The heterotopic syngeneic murine head and neck cancer model (mEER) caused systemic inflammation and increased expression of Il1b in the brain while inducing fatigue-like behaviors characterized by decreased voluntary wheel running and exploratory activity. Expression of Il1b in the brain was not associated with any alterations in motivation, measured by responding in a progressive ratio schedule of food reinforcement, depressive-like behaviors, or energy balance. Decreased wheel running occurred prior to Il1b detection in the brain, when systemic inflammation was minimal. Further, mice null for two components of IL-1β signaling, the type 1 interleukin-1 receptor or the receptor adapter protein MyD88, were not protected from tumor-induced decreases in wheel running, despite attenuated cytokine action and expression. Behavioral and inflammatory analysis of 4 additional syngeneic tumor models revealed that tumors can induce fatigue regardless of their systemic or central nervous system inflammatory potential. Together our results show that brain IL-1 signaling is not necessary for tumor-related fatigue, dissociating this type of cancer sequela from systemic cytokine expression.
Keywords: fatigue, head and neck cancer, interleukin-1, cytokines, behavior
Introduction
Cancer-related fatigue is among the most common and distressing presenting symptoms of malignancy. Although nearly all patients report fatigue after treatment, various studies report the prevalence of fatigue prior to cancer treatment ranging from 25 to >50% of patients depending on sample and methodology (1). Fatigue strongly impacts quality of life (2) and physical functioning (3), and has been linked to poorer survival in colorectal cancer patients whose fatigue alters daytime activity patterns (4). The search for fatigue-directed treatments has been largely unsuccessful, yielding equivocal results with psychostimulants (5) and only modest benefit with exercise (6), a challenging intervention for severely fatigued patients. Multiple studies have linked cancer-related fatigue to psychosocial factors including pre-existing fatigue, depression, and childhood stress (7). However, fatigue remains prevalent in patients without these risk factors and is observed in animal models of cancer in the form of reduced motor activity (8,9), strongly supporting a conserved biological mechanism for cancer-related fatigue.
Multiple biological contributions to cancer-related fatigue have been investigated, including anemia, endocrine dysregulation, and altered metabolism (7). However, much of the attention has focused on the role of inflammatory cytokine signaling in the pathogenesis of fatigue. Studies carried out mainly in rodents injected with endotoxin demonstrate that peripheral inflammatory signals are propagated into the central nervous system, where cytokines act to cause fatigue, anorexia, and depressive-like behaviors (10). Intracerebroventricular administration of cytokines is sufficient to induce these behaviors (11), and blockade of cytokine signaling within the brain can abrogate fatigue in response to peripherally administered endotoxin (12). Cancer cells can both produce inflammatory cytokines, and induce potent local and systemic inflammatory responses in situ (13). Cancer treatment is also associated with an increase in circulating cytokine levels (14,15). Studies in both humans and rodents demonstrate enhanced cytokine expression in response to various stressors (16,17), mirroring the association between early life stress and fatigue seen in cancer patients. These converging data point to the possibility that fatigue and other behavioral changes caused by cancer are also mediated via propagation of peripheral inflammation into the brain (1,7,10,18).
Over the past 15 years, multiple researchers have investigated this connection, with the preponderance of studies focusing on the relationship between inflammation and fatigue during and after cancer therapy (7). Although associations between fatigue and circulating cytokines have been often reported in both the clinical and preclinical settings, data supporting the inflammatory hypothesis of fatigue have been entirely correlational in nature; few published accounts support a causative relationship between inflammatory cytokines and cancer-related fatigue, but they rely on murine models of cancer that quickly progress to cachexia (19,20). In the present study, we used a syngeneic murine model of human papillomavirus-positive head and neck cancer (mEER) (21,22), which reliably induces expression of interleukin-1 beta (Il1b), interleukin-6 (Il6) and tumor necrosis factor (Tnf) in the liver and Il1b in the brain, and reproducible fatigue-like behavior in the absence of anorexia and cachexia, to ask whether activation of IL-1 signaling is a required intermediary of cancer-related fatigue in the pre-treatment setting. Using voluntary wheel running and novel cage exploratory activity as behavioral measures of fatigue, we evaluated the time course of tumor-induced brain inflammation and tested mice null for the type 1 interleukin-1 receptor (IL-1R1−/−) and the inflammatory adaptor protein myeloid differentiation primary response gene 88 (MyD88−/−) to assess the possible relationship between fatigue-like behaviors and inflammatory signaling.
Methods and Materials
Animals
Male C57BL/6J wild type (WT), IL-1R1−/− (Stock# 003245), and MyD88−/− (Stock# 009088) mice were purchased from The Jackson Labs (Bar Harbor, ME, USA), and colonies were maintained in our animal facility via pairing of mice homozygous for knockout alleles. All mice were genotyped using standard protocols from The Jackson Labs. Experiments were conducted on 10-13 week old male mice individually housed in temperature and humidity controlled environments on 12 h light–dark cycles. Food and water were available ad libitum unless otherwise specified. All procedures described in this study were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees of the University of Texas MD Anderson Cancer Center.
Tumor Models
Adult male C57BL/6J mice (n=5-10/group) were injected with 100 ul of tumor cell suspension into the right flank subcutaneous space. The following heterotopic syngeneic tumor models and inoculation doses were used for these studies: HPV-related head and neck cancer model (mEER; 1×106 cells)(21); Lewis Lung Carcinoma (LLC; 5×105 cells)(23); ovarian ID8 (1×106cells)(8) and IG10 (1×106cells)(24); and an HPV-negative head and neck cancer model (shPTPBL/hRas; 1×106cells) (21). All tumor lines were maintained in exponential growth at 37°C in 95% O2/5% CO2 in a humidified incubator. The mEER, shPTPBL, and LLC cells were resuspended in sterile PBS for injection; ID8 and IG10 cells were resuspended in HBSS for injection. Control animals (CTL) received an identical volume of suspension buffer. The day tumors were injected is considered as “Day 0” for the purposes of daily recording. Tumor volume was determined from three mutually orthogonal tumor diameters (d1, d2, d3) measured weekly using Vernier calipers [volume = (π/6)(d1*d2*d3)](25). Body weight and food intake were assessed at weekly intervals. Mice were euthanized at pre-specified time points (generally 4 weeks after tumor injection), if pre-moribund, or when tumors reached IACUC-defined tumor burden criteria. Mice were euthanized by CO2 and blood was collected by percutaneous cardiac puncture. Mice were then transcardially perfused with ice cold PBS. Serum was collected from clotted blood, or plasma was collected from EDTA-treated whole blood and snap frozen in liquid nitrogen. Either whole brains or brain sections, livers, and tumor tissue were collected, snap frozen in liquid nitrogen, and stored at −80°C for RNA analysis.
Drugs and Administration
Lipopolysaccharide (LPS; serotype 012:B8, Sigma-Aldrich, St Louis, MO) was dissolved in phosphate-buffered saline (PBS) at a concentration of 100 μg/ml. Mice were injected intraperitoneally with 0.5 mg/kg LPS or PBS immediately following their baseline progressive ratio session (Zeitgeber Time 5, see Behavioral measures). This dose of LPS induces a robust inflammatory response in food restricted animals (26).
Behavioral measures
1/ Voluntary Wheel running
Mice were individually housed with an angled low-profile running wheel (15.5 cm diameter) paired to a wireless activity counter (Wheel Manager, Med Associates Inc.). Wheel running data (counts) were exported in one hour intervals and integrated across the 12 dark hours to determine nightly wheel running. Mice were acclimatized for 10 days with baseline wheel running defined as the mean overnight counts during the final three days of acclimatization. Mice were counterbalanced based on baseline running counts and body weights and assigned to experimental conditions. Animals retained access to running wheels without interruption throughout the duration of each study. Nightly activity was normalized to each mouse’s baseline activity. Days on which recording was interrupted or data were incomplete were dropped from the study.
2/ Exploratory Activity
During the light phase, mice were placed in a clean empty shoebox cage (18.4 × 29.2 cm) and their activity was video recorded for 5 min. Videos were analyzed using Ethovision software (Noldus) to determine the total distance traveled. Assessments were performed prior to tumor inoculation (baseline), then weekly beginning 2 weeks after tumor injection.
3/ Progressive ratio for food reinforcement
Progressive ratio training and testing were conducted in operant conditioning chambers equipped with a single nose-poke response unit and reward unit (Med Associates, St Albans, VT). Mice underwent food restriction to maintain their body weight 85-90% of baseline throughout training and testing. Chocolate-flavored Dustless Precision Pellets served as the reward (20 mg, BioServ, Frenchtown, NJ). Mice were initially trained on a fixed ratio (FR)-1 schedule for 8 days. They were then maintained on an FR5 schedule for 5 days until all mice were able to obtain at least 30 rewards in 60 minutes on 3 consecutive days. Mice were then advanced to a progressive ratio (PR)-3 schedule, in which they had to perform increasing numbers of nose-pokes to obtain a reward, according to the following schedule: PR=R*3, where R is equal to the number of food rewards already earned plus 1 to account for the next reinforcer. After three consecutive days of improving PR performance, mice were started on the experimental PR schedule, according to the following formula: PR = 5e(R*0.2) – 5 (27,28). PR sessions lasted a maximum of 45 minutes. Failure to nose-poke in any 5 minute period resulted in termination of the session. Breakpoint was defined as the final ratio completed. PR training was considered complete when the breakpoint varied by ≤ 10% for 3 consecutive days.
For the LPS experiment, mice were randomly assigned to receive either LPS (0.5 mg/kg; n =8) or vehicle (PBS; n=8) intraperitoneally immediately following the final PR training session. Food was removed from all cages at the time of injection to control for confounding effects of LPS-induced anorexia. Two hours after LPS injection, novel cage exploratory activity testing was performed to verify LPS effect. At 22 hours post-treatment, mice were again tested using the PR paradigm, which was followed by a 5 minute free-feeding task using 10 chocolate and 10 grain pellets (20 mg, BioServ, Frenchtown, NJ) to verify that all animals would consume freely available high- and low-reward food (29). Exploratory activity was again tested at 23 h to validate that the mice were no longer in the sickness phase following LPS. PR was again tested at 46 h post-treatment. Mice were then given ad libitum access to food for the following 7 days to allow for recovery of body mass. Mice were randomly assigned to receive mEER tumor (n=10) or vehicle injection (n=6), and, after 3 d, restricted feeding was resumed. These were the only mice to have been treated with LPS prior to tumor injection. Mice were tested in the PR task thrice weekly for three weeks, until the tumor began to exhibit log phase growth, at which time the test was performed 5 times weekly until the end of the study. Exploratory activity was measured prior to tumor injection (baseline), then weekly, beginning 2 weeks after tumor injection, as described above.
Quantitative Real-time PCR
Total brain, brain region, or liver RNA was extracted using the E.Z.N.A. Total RNA Kit II (Omega) according to the manufacturer’s instructions. cDNA was transcribed using TaqMan reverse transcription reagents and random primers according to the manufacturer’s instructions. PCR reactions were run on a CFX384 (BioRad), using Probe-Based qPCR master mix (Integrated DNA Technologies) with the following mouse PrimeTime gene expression assays: Gapd (Mm.PT.39a.1), Il1b (Mm.PT.58.41616450), Tnf (Mm.PT.58.12575861), Il6 (Mm.PT.58.13354106; Integrated DNA Technologies), and Itgam (Mm01271259_g1; Applied Biosystems). Gapd was used as the internal control. Relative expression was calculated using the ΔΔCt method and was normalized to PBS-injected control. Statistical analysis was performed on the normally distributed ΔCt values.
Serum ELISA
Serum IL-6 (Biolegend, San Diego, CA) and corticosterone (Enzo, Farmingdale, NY) levels were measured by ELISA according to the manufacturers’ instructions. IL-6 ELISA was sensitive to 2 pg/mL with 9.3% intra-assay coefficient of variation (CV) and 11.1% inter-assay CV. Corticosterone ELISA was sensitive to 27 pg/mL with 8.4% intra-assay CV and 8.2% inter-assay CV.
Statistical Analyses
Data were analyzed and graphed using Prism 6 (GraphPad) and SPSS (version 23, Chicago, IL). Descriptive statistics are presented as mean ± SEM. Repeated measures ANOVAs with post-hoc Bonferroni adjusted t-tests were used to analyze wheel running, exploratory activity, and progressive ratio data (mEER vs CTL, LPS vs PBS). For experiments comparing genotypes, pre-planned analyses utilizing repeated measures ANOVAs with post hoc Bonferroni adjusted t-tests were conducted separately to evaluate the effect of tumor (CTL/WT vs mEER/WT) and genotype (mEER/WT vs mEER/IL-1R1−/− or MyD88−/−). Cross-sectional data were analyzed using Student’s t-test (2 groups, normally distributed data) or one-way ANOVA with Bonferroni adjusted t-test (3 or more groups, normally distributed data), as appropriate. Differences between groups were considered significant when p < .05.
Results
Fatigue and inflammation induced by mEER tumor growth
We first examined the behavioral response to mEER tumor growth in mice using voluntary wheel running and novel cage exploratory activity. Because of its metabolic requirement voluntary wheel running is commonly used to assess fatigue, with reduced wheel running considered as a marker of fatigue (30). Tumors induced a rapid and persistent decrease in voluntary wheel running behavior compared to CTL animals (Tumor × time interaction, F(24, 192) =2.54, p<.001) with significant differences first observed 10 days after tumor injection (Figure 1A). We also assessed fatigue-like behavior using novel cage exploratory behavior (30,31). We observed a significant decrease in novel cage exploratory activity in mEER animals (Tumor × time interaction, F(3, 57) =3.12, p<.05), evident during the 4th week after tumor growth (Figure 1B). Tumor growth was consistent among the animals, with log-phase growth developing during the second week following injection (Figure 1C). Cytokine analysis at the time of sacrifice (day 27) demonstrated significant elevations in serum IL-6 in the mEER group vs CTL (t(8)=5.40, p<.001; Figure 1D). Serum IL-1β and TNF were not elevated, whereas TGF-β was decreased in tumor-bearing mice (Supplementary Fig 1A, B). Elevations in the mRNA levels of the inflammatory cytokines Il1b, Il6, and Tnf were observed in the livers of tumor-bearing animals, whereas only Il1b mRNA was elevated in the hippocampus (Figures 1E, F). A consistent induction of Il1b mRNA in the mEER mice was observed across brain regions, with region-specific elevations in Tnf observed in the frontal cortex and hypothalamus (Supplementary Figures 1C-G). Further mRNA analysis detected no increase in other pro-inflammatory cytokines in the hippocampus (Supplementary Figure 1H). Based on the reproducible upregulation of Il1b in the brain of tumor bearing mice and prior studies demonstrating that central IL-1β mediates behavioral symptoms of both inflammation-induced sickness (32) and depression (33), we investigated the role of this cytokine in mediating tumor-associated fatigue.
Figure 1.
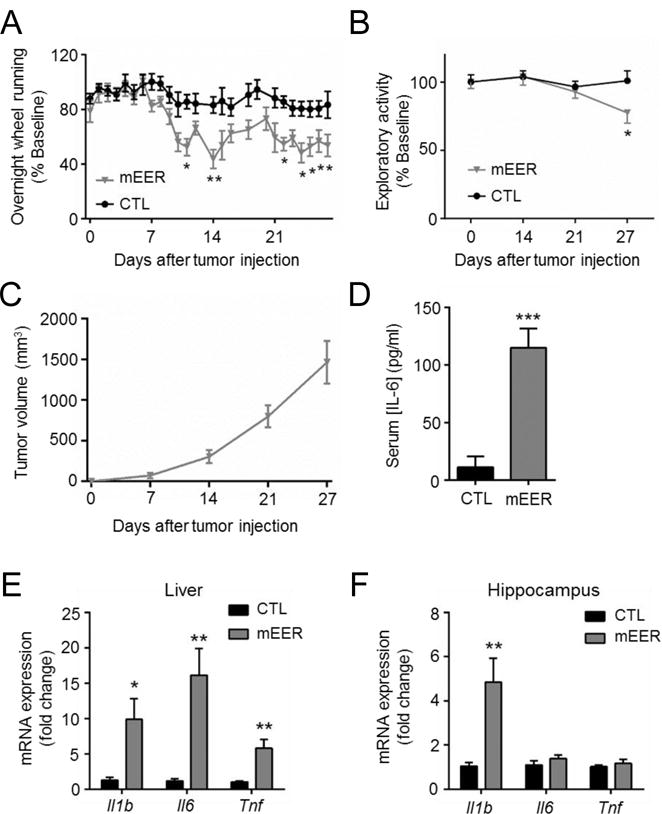
mEER tumors decrease wheel running and induce inflammation in blood, liver, and brain. A, nightly wheel running activity in mEER tumor-bearing (n=5) and control (n=5) mice. Tumor × time interaction effect (P=0.01) by repeated measures ANOVA. B, novel cage exploratory activity, expressed as % baseline. C, mEER tumor growth curve. Tumors increase D, serum IL-6 concentration, and inflammatory cytokine expression in E, liver, and F, hippocampus. *, P<0.05, **, P<0.01, ***, P<0.001, repeated measures ANOVA with post-hoc Bonferroni-corrected t-test (A); unpaired t-test (panels D, E, F).
Performance in the progressive ratio for food reinforcement intact in tumor bearing mice
Because cancer-related fatigue has a strong motivational component (7) we interrogated the possible involvement of IL-1 signaling in motivation by assessing performance of tumor-bearing mice in a PR operant conditioning task that is very sensitive to IL-1β (34,35). Once mice had developed steady performance in the PR task (Figure 2A), we confirmed the sensitivity of PR to inflammation by showing a decreased PR breakpoint 24 and 48 h following LPS treatment (Figure 2B), when acute sickness behavior had completely resolved as measured by motor activity in a novel cage (Supplementary Figure 2A). Body weight was maintained betweem 80-90% of baseline throughout training and testing to provide adequate task motivation (Supplementary Figure 2B). To confirm that this effect of LPS was not driven by persistent anorexia, mice were exposed to freely available chocolate and grain pellets after the PR task. No differences in latency to eat or time to finish were detected between treatment groups (Supplementary Figures 2C, D). After a recovery period, the same mice were randomly assigned to flank injections of mEER tumor or vehicle (CTL). Mice were food restricted and PR was monitored throughout tumor growth. Although nutrition was sufficient to allow for increased body weight in mEER mice (Supplementary Figure 2E), food restriction apparently slowed tumor growth (Supplementary Figure 2F). Exploratory activity was again decreased in mEER mice (Supplementary Figure 2G), however, PR performance remained unaffected by tumor growth (Figure 2C). Further studies showed no change in additional IL-1β sensitive behavioral domains, including ingestive (food intake, non-tumor body weight), anhedonic (sucrose preference), cognitive (novel object recognition), or anxiety (center time, eating in novel environment) behaviors in mEER mice (Supplementary Figures 2H-O).
Figure 2.
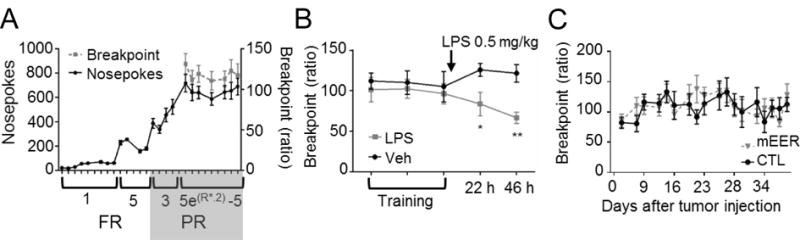
Motivation remains intact in tumor bearing mice. A, nosepoke and breakpoint performance during training for the PR task. B, PR performance is decreased 24 and 48 h after LPS (0.5 mg/kg) injection compared to PBS (n=8/group). C, tumor bearing mice (n=10) exhibit no decrease in PR performance compared to CTL (n=6). *, P<0.05, **, P<0.01, 2-way ANOVA with post-hoc Bonferroni-corrected t-test.
Wheel running deficits precede systemic inflammation in tumor-bearing mice
Because many of the behavioral changes associated with brain IL-1β are absent in tumor-bearing animals, we terminated tumor bearing animals at multiple time points (after 9, 16, and 27 days of growth) to determine whether fatigue-like behaviors are temporally associated with tumor-related inflammation. CTL mice were all terminated on day 27. Mice bearing mEER tumors again demonstrated a significant wheel running deficit, first apparent approximately 7 days after tumor injection (Figure 3A). The decrease in running reflected a progressive decline in both the rate and duration of wheel running throughout the dark cycle with no alteration in circadian pattern (Figure 3B). Tumors grew at a consistent rate with modest variability at each time point (Figures 3C, D). Although wheel running decreased among mEER mice across time, wheel running deficit did not correlate with tumor size at any time point (Supplementary Figures 3A-D). Il1b expression in the hippocampus was not elevated significantly until late in tumor growth (Figure 3E). Similarly, expression of Il1b, Il6, and Tnf in the liver were not significantly increased until day 27 (Figures 3F-H), a pattern which was repeated in plasma IL-6 protein levels (Figure 3I). A strong correlation between plasma IL-6 and wheel running decrement was observed. However, this was driven entirely by measures on day 27, with no observed correlation at earlier time points (Supplementary Figures 3E-G). Further examination of the hippocampus showed no elevation in IL-1β-response genes Nfkbia, Ptgs2 or the inflammasome-related genes Il18 and Nlrp3 at any time point, whereas in the liver, increased Il1b expression on day 27 was associated with increased expression of Nfkbia, Il18, and Nlrp3 (Supplementary Figures 3H, I). Thus, there was a temporal dissociation between the onset of fatigue and the cytokine response to the tumor in the liver and brain. Furthermore, no evidence of active brain IL-1β signaling was observed in the presence of increased brain Il1b expression.
Figure 3.
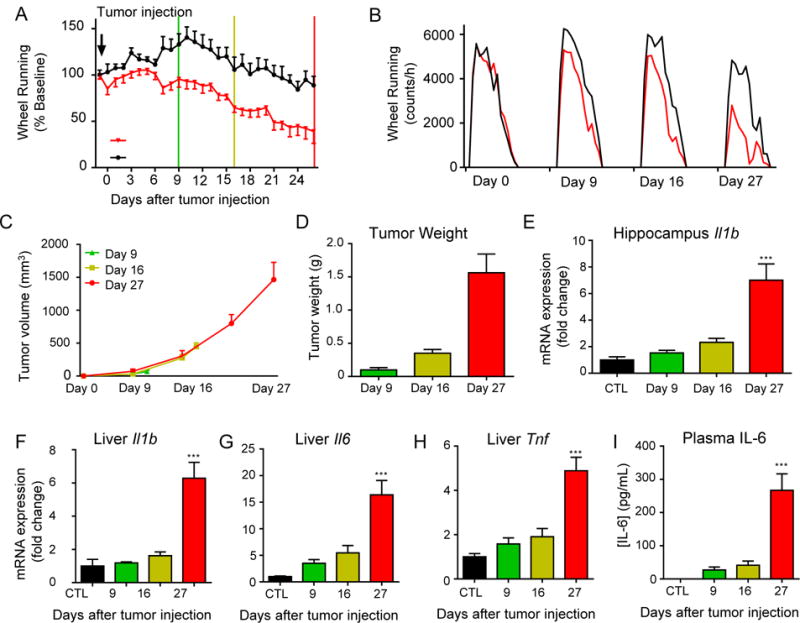
Tumor-associated wheel running decrement precedes inflammation in brain, liver, or serum. A, nightly wheel running time course in tumor bearing and control mice. Green line denotes first termination day (day 9), yellow line denotes second termination (day 16), and red line denotes final termination (day 27, n=6/group). B, average hourly wheel running in tumor bearing (red) and control (black) mice during days shown. Tumor bearing mice show a progressive loss in wheel running peak and duration throughout tumor growth. C, tumor volume, measured weekly, and, D, tumor weights from each group. E, Il1b expression time course in hippocampus. F, Il1b, G, Il6, and H, Tnf expression time course in liver. I, plasma IL-6 concentration. *, P<0.05, ***, P<0.001, 2-way ANOVA (A) or 1-way ANOVA (E, F, G, H, I) with post-hoc Bonferroni-corrected t-test.
Tumor induced fatigue is not mediated by IL-1R1
To further investigate the role of IL-1 signaling in fatigue development, we administered tumors to IL-1R1−/− mice, which lack the type 1 IL-1 receptor, exhibit normal vigor and acute phase response to LPS, but do not respond to IL-1 family ligands (36). Baseline wheel running and exploratory activity were not affected by IL-1R1 deletion. Wheel running and novel cage activity were decreased in tumor bearing WT mice, and deletion of IL-1R1 was unable to ameliorate these decrements (Figures 4A, B). Tumor growth was accelerated in IL-1R1−/− mice, so for biochemical analysis, WT mice were sacrificed (day 35) when their tumor volume approximated that of the knockouts (day 28) (Figure 4C). Tumors induced significant upregulation in circulating IL-6 and corticosterone in WT mice, which were attenuated in IL-1R1−/− mice (Figures 4D, E). The expression of Il1b in the brain in response to tumor was unaffected by IL-1R1 deletion, although Tnf mRNA was significantly elevated in the whole brains of knockouts, but not WT mice (Figure 4F). IL-1R1 deletion significantly attenuated cytokine expression in the livers of tumor bearing animals (Figure 4G). Because IL-1R1−/− mice may utilize compensatory inflammatory signaling pathways such as TNF-mediated signaling (37), we administered systemic IL-1 receptor antagonist (IL-1ra) to wild type MEER mice during the period that Il1b transcript levels are increased with no impact on tumor growth (Supplementary Figures 4 A-C). Prior to treatment, we observed a significant decrease in burrowing activity in tumor bearing mice, which was not improved following 5 days of treatment with IL-1ra (Supplementary Figure 4D). Therefore, intact IL-1 signaling is not required for the onset or propagation of tumor induced behavioral alterations.
Figure 4.
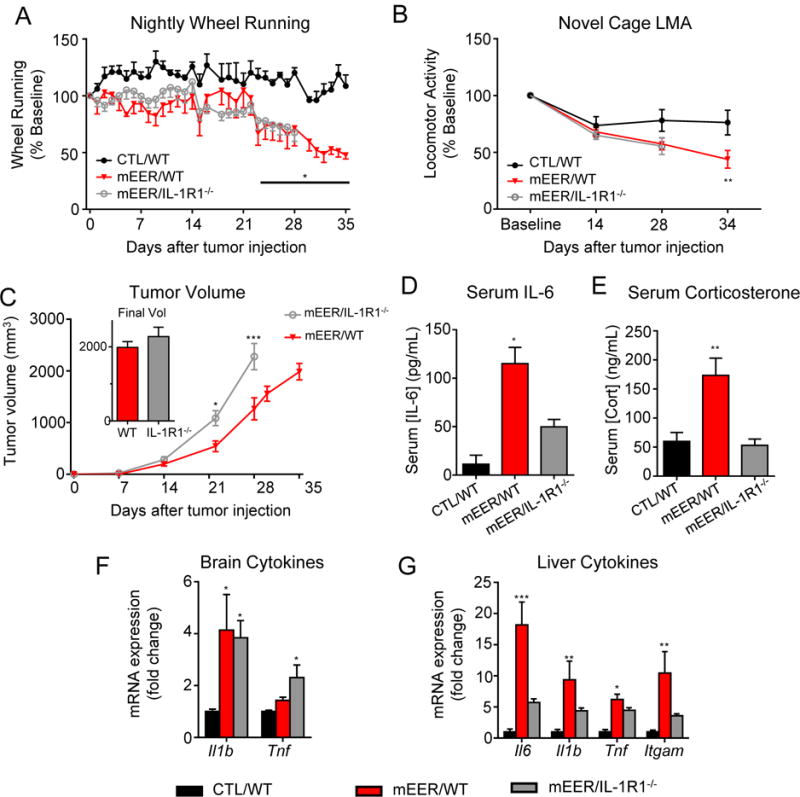
Global IL-1R1 deletion does not protect against tumor-associated fatigue. A, nightly wheel running in CTL/WT and tumor bearing WT and IL-1R1−/− mice. B, diminished novel cage exploration was not ameliorated by global IL-1R1 deletion. C, tumor growth in WT and IL-1R1−/− mice. Tumor growth was accelerated in IL-1R1−/− mice; WT mice were terminated when tumor size was equivalent for expression analyses (inset). D, Serum IL-6 concentration. E, Serum corticosterone concentration. F Brain inflammatory cytokine expression. G, Liver inflammatory cytokine expression. For panels A and C, in the absence of ideal control groups, repeated measures ANOVAs were performed to separately assess effect of genotype and tumor. *, P<0.05 vs CTL/WT, **, P<0.01 vs CTL/WT, ***, P<0.001 vs. CTL/WT and mEER/IL-1R1−/−, 1-way ANOVA with post-hoc Bonferroni-corrected t-test; ***, P<0.001, Repeated-measures 1-way ANOVA (A, C) or 1-way ANOVA (E, F, G, H) with post-hoc Bonferroni-corrected t-test. N=7-9/group.
Tumor-induced fatigue does not require MyD88 signaling
To test more broadly for inflammatory mechanisms of tumor-induced fatigue, we utilized transgenic mice deleted for myeloid differentiation factor 88 (MyD88−/−), a proximal adaptor protein common to toll-like receptors (TLRs), IL-1R1 and IL-18R that is central to initiation and propagation of innate immune responses (38). Intact MyD88 signaling is required for the suppressed locomotor activity and feeding that accompanies treatment with LPS or IL-1β (12,39,40). MyD88−/− mice exhibited normal wheel running and exploratory activity patterns that did not differ from WT mice at baseline. Following tumor injection MyD88−/− mice demonstrated decreased wheel running and novel cage exploratory behavior indistinguishable from WT animals (Figures 5A, B). Although no statistical differences in tumor growth were observed, MyD88−/− mice exhibited an apparent increase in tumor growth velocity during the final week of the study (Figure 5C). An increase in Il1b expression was found in the brains of both MyD88−/− and WT mice (Figure 5D), with liver cytokine expression less robustly elevated than in WT mice (Figure 5E).
Figure 5.
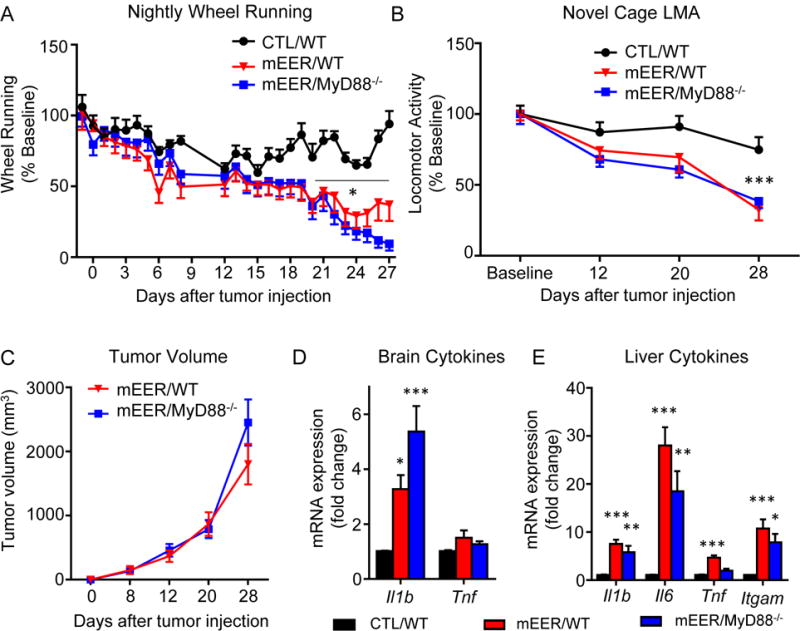
MyD88 deletion does not protect against tumor-associated fatigue. A, nightly wheel running in CTL/WT and tumor bearing WT and MyD88−/− mice. Time × tumor interaction F(25, 325)=3.82, P<.0001. Time × genotype interaction F(25, 325)=2.16, P=.001. B, exploratory activity in WT and MyD88−/− mice. Time × tumor interaction F(25, 325)=5.71, P=.002. Time × genotype interaction F(25, 325)=.69, P=.56. C, tumor growth in WT and MyD88−/− mice. D, Brain inflammatory cytokine expression. E, Liver inflammatory cytokine expression. For panels A and B, two repeated measures ANOVAs were performed to separately assess effect of tumor. *, P<0.05 vs CTL/WT, **, P<0.01 vs CTL/WT,; ***, P<0.001, Repeated-measures ANOVA (A, B, C) or 1-way ANOVA (D, E) with post-hoc Bonferroni-corrected t-test. N=7-8/group.
Fatigue in multiple syngeneic tumor models
To ensure that the asychrony between inflammation and fatigue is not an artifact of the mEER tumor model, we measured fatigue-like behavior and cytokine expression in mice bearing four other syngeneic tumors: two ovarian adenocarcinomas (ID8 and IG10), one lung adenocarinoma (LLC), and an HPV negative head and neck squamous cell tumor (shPTPBL). All four tumors induced significant decreases in wheel running, although the magnitude of this response varied (Figure 6A). Both the ID8 and IG10 ovarian tumors induced a fatigue phenotype similar to the mEER tumors, evident approximately 2 weeks after tumor injection, whereas both the LLC and shPTPBL lines induced a rapid and severe drop in running activity. Tumor growth was much faster in the LLC and shPTPBL tumors than either ovarian line, resulting in euthanasia by day 21 in adherence to institutional tumor burden policy (Figure 6B). For both ovarian tumors, local subcutaneous tumor burden remained low, but on necropsy nearly all mice were found to have peritoneal carcinomatosis. In the hippocampus of these mice, only the LLC line exhibited a significant increase in Il1b expression, while Tnf and Il6 expression remained unchanged in all groups (Figure 6C). Both the LLC and shPTPBL tumors elicited a significant increase in the expression of both Il1b and Il6, whereas only the LLC group showed an increase in Tnf expression (Figure 6D). Together, these tumor studies demonstrate that neither peripheral nor central expression of these cytokines is required for the development of fatigue-like behavior in response to tumor growth.
Figure 6.
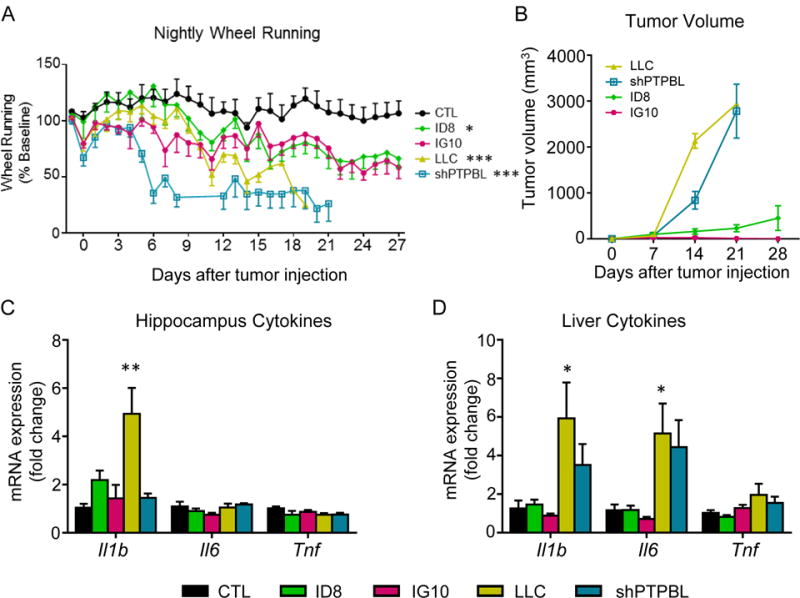
Multiple syngeneic tumors induce fatigue-like behavior irrespective of inflammatory cytokine expression in the brain. A, nightly wheel running in mice bearing one of 4 syngeneic tumors compared to CTL. B, tumor growth curves. C, Hippocampus inflammatory cytokine expression. D, Liver inflammatory cytokine expression. All tumors compared concurrently in single experiment. Mice were terminated at the earlier of 28 days or threshold tumor volume. For panel A, separate repeated measures ANOVAs were performed to compare each tumor to single CTL group. Time × tumor interactions: IG10 – F(28, 252) = 1.38, P=0.10; ID8 – F(28, 252) = 1.73, P=.01; LLC – F(16,144) = 2.72, P<.001; shPTPBL – F(19, 152) = 7.48, P<.001. *, P<0.05 vs CTL, **, P<0.01 vs CTL, ***, P<0.001 vs. CTL, Repeated-measures 1-way ANOVA (A) or 1-way ANOVA with post-hoc Bonferroni-corrected t-test (C, D). N=5-6/group.
Discussion
Fatigue is a common and debilitating co-morbidity of cancer that results from a combination of psychological and biological drivers. In this series of studies, we used a syngeneic murine HPV+ head and neck cancer tumor that induces systemic and central inflammatory cytokine expression (21,22) to investigate whether IL-1 signaling is the causal factor that mediates fatigue in the pre-treatment setting. Our systematic studies all converged on the evidence of a clear dissociation between IL-1 signaling and the development of fatigue-like behavior. Although the mEER tumor strongly induced Il1b expression in the liver and brain, the behavioral impact of this tumor was limited to reduction of the energetically demanding voluntary wheel running. We saw no evidence of negative energy balance, depression, or anxiety, all hallmarks of inflammatory signaling within the brain (10). Fatigue-like behaviors preceded the expression of inflammatory genes, demonstrating a temporal asynchrony between these processes. Furthermore, blockade of IL-1 signaling using mice deficient for IL-1R1 or MyD88 had no effect on tumor-induced wheel running decrement. These observations were supported in four additional syngeneic tumor lines, which exhibited degrees of decreased running wheel activity unrelated to brain or liver Il1b expression. Taken together, the data strongly indicate that IL-1 signaling is not required for cancer induced fatigue.
Universally, the mEER tumor bearing mice exhibited elevated Il1b expression throughout their brains, and brain IL-1β is known to mediate many of the behavioral effects elicited by systemic inflammation (10). However, neither genetic nor pharmacologic inhibition of IL-1 signaling yielded any discernible protection from tumor-induced fatigue behaviors. Because neither IL-1 response genes (Ptgs2, Nfkbia) nor inflammasome-related genes (Nlrp3, Il18) were elevated in the brains of tumor bearing animals, we posit that while Il1b gene expression was induced, active IL-1β was not released. This suggests that the inflammasome may not have been activated, which could account for the absence of other IL-1β related behaviors, such as decreased food intake, depression, and anxiety; the ineffectiveness of IL-1 blockade in reversing decreased wheel running; and the increased sensitivity to subthreshold doses of LPS, which is necessary to stimulate the inflammasome in the brains of tumor bearing mice (22). Tnf expression was elevated only in the hypothalamus of WT tumor-bearing mice, but was prominently induced in the brains of IL-1R1−/− mice. Although prior work has established that TNF can mediate LPS-induced sickness in IL-1R deficient mice (37), we observed no elevation in Tnf expression in tumor-bearing MyD88−/− mice, which are resistant to IL-1β-induced suppression of locomotor activity (12) yet exhibited no protection from tumor-induced fatigue. Furthermore, expression of cytokines within the brain was highly variable across the tumor lines tested with no evidence of central inflammation detected among mice exhibiting the most pronounced fatigue phenotype (shPTPBL). These mice exhibited very high expression of cytokines within the liver, clearly dissociating fatigue from propagation of peripheral inflammatory signals into the brain.
Ablation of IL-1 signaling, while having no effect on fatigue, did appear to accelerate the rate of tumor growth in both the IL-1R1−/− and MyD88−/− mice. Recent reports indicate that local and distant innate immune activation are important steps in advancing local invasion and metastasis, respectively (41). Our data indicate that IL-1 signaling may also play a local role in head and neck tumor control, as was recently reported in a murine mammary carcinoma model (42).
Although the clinical literature examining cancer-related fatigue in the pre-treatment setting is limited, several preclinical studies have tested the link between inflammation and fatigue. In each of the three studies, tumors induced fatigue-like behaviors associated with increased cytokine expression in the brain, and inflammation was modulated with pharmacologic interventions, using either the nonselective cyclooxygenase inhibitor ibuprofen or the anti-inflammatory antibiotic minocycline (19,20,22). These treatments improved inflammation markers and depression-like behaviors, but had no effect on tumor-induced decreases in wheel running. In the present study the correlation between circulating IL-6 and wheel running was strong at the end of the experiment, when IL-6 levels were highly elevated. However, there was no correlation at other time points, when IL-6 was lower. Ablation of IL-1 signaling suppressed systemic inflammation but had no impact on wheel running. These observations can be interpreted to indicate the presence of a non-linear threshold effect for inflammation on wheel running, serving to augment the fatigue phenotype at relatively high cytokine levels. Alternatively, because systemic inflammation was never completely abolished in any of the models presented here, very low levels of systemic inflammation may be a requisite permissive factor for fatigue induction. However, the relatively weak associations between peripheral inflammatory mediators and wheel running in all of these studies strongly implicate the collaboration of other, non-inflammatory mechanisms.
Whereas IL-1 signaling is not necessary for the onset of cancer-related fatigue, it is clear that inflammation and fatigue are closely related. We have previously shown an enhanced behavioral sensitivity to LPS in mEER tumor-bearing mice compared to controls, associated with increased brain cytokine expression (22). Similar findings were reported in female rats bearing mammary carcinomas, which showed increased neuroinflammation and weight loss following LPS (43). These data are analogous to the augmented cytokine release reported in LPS-stimulated monocytes from fatigued compared to non-fatigued breast cancer survivors (44,45). A conserved feature of cancer related fatigue may be an amplified immune response to inflammatory stimuli, which would then help explain both the consistent correlations between circulating cytokines and fatigue in cancer patients as well as the relationship between fatigue and depression in patients and rodents.
Our study does not identify IL-1 signaling as a common underlying mediator of cancer-related fatigue, yet certain features of the behavioral phenotype presented by tumor bearing mice offer important mechanistic insight. Activity onset was not impacted, however duration and intensity were diminished. Furthermore, tumors suppressed wheel running performance without any apparent influence on incentive motivation, reward, or cognition, suggesting that the influence on behavior is probably mediated peripherally, perhaps by altered bioenergetics in skeletal muscle. Tumors are known to both shift their own energy generation from oxidative phosphorylation to aerobic glycolysis (46) as well as metabolically reprogram surrounding cells to provide energy for the tumor (47). Together, these effects of tumors can have profound impacts on the resting energy expenditure of an animal. Decreased voluntary activity would be one strategy to maintain energy balance in the presence of increased energy consumption by the tumor. Although the relationship between food intake, basal metabolic rate, and energy balance is well described, the nature and importance of metabolic influences on voluntary activity remains largely unknown.
Several limitations of the present work warrant discussion. A potential confound for the use of wheel running and exploratory activity as endpoints is the effect of disability brought on by a locally enlarging tumor. For this reason we chose to heterotopically inject all tumors into the subcutaneous flank space of the mouse, where maximal tumor growth could occur with minimal disability. Our data indicate that local effects of tumor growth had minimal-to-no impact on wheel running or exploratory behavior, as evidenced by the absence of any clear correlation between tumor size and the magnitude of activity decrement at any given time point - either within a tumor cohort or across tumors. Another potential limitation is that heterotopic injection removes the cancer from its native local environment, which may impact both inflammation and behavior. Therefore, it is unclear how well this heterotopic paradigm models actual malignancy in situ. Nonetheless, the model allowed comparison across tumor lines and allowed for protracted behavioral studies unencumbered by the limitations of local tumor effects. Our interpretation of these data is predicated on the assumption that objective decreases in activity accurately model fatigue. We included three separate measures of decreased species specific behavior, as a safeguard against a single misleading measure. Based on prior clinical reports showing no correlation between actigraphy and patient reported fatigue, it remains unclear how well the behavior matches the clinical symptom (48). However, objective evaluation of fatigue separates the phenotype from confounding influences that may impact patient report.
In conclusion the present work challenges the hypothesis that propagation of inflammation to the brain underlies tumor-related fatigue. Despite robust inflammation late in tumor growth, fatigue onset preceded appreciable increases in circulating IL-6 and brain expression of Il1b and was not ameliorated by blockade of IL-1 signaling. Furthermore, tumor-bearing mice exhibited fatigue in the absence of other behavioral phenotypes characteristic of inflammation, such as reduced performance in the PR schedule of reinforcement. Thus, although increased brain expression of Il1b is sufficient to drive fatigue-like behaviors, our data favor non-inflammatory mechanisms for the development of fatigue. These findings reinforce the importance of examining causality when inflammation is detected by cross-sectional methods. Based on this work anti-inflammatory therapeutic approaches to cancer-related fatigue may be limited in efficacy, and further investigation into the non-inflammatory mechanisms of tumor-related fatigue are warranted.
Supplementary Material
Acknowledgments
The authors thank Dr. Steven Lin (MD Anderson Cancer Center) for providing the LLC cell line and Dr. Anil Sood (MD Anderson Cancer Center) for providing the ID8 and IG10 cell lines. This work was supported by the National Cancer Institute of the National Institutes of Health [R01 CA193522, R. Dantzer]. Additional support came from the University of Texas MD Anderson Cancer Center and the National Institutes of Health MD Anderson Cancer Center Support Grant [P30 CA016672]. The content is solely the responsibility of the authors and does not necessarily represent the official view of the funding sources.
Footnotes
Conflicts of interest: RD has received an honorarium from Danone Nutricia Research. The authors declare no other conflicts of interest.
References
- 1.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:971–82. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser MR, van Lanschot JJ, van der Velden J, Kloek JJ, Gouma DJ, Sprangers MA. Quality of life in newly diagnosed cancer patients waiting for surgery is seriously impaired. Journal of surgical oncology. 2006;93:571–7. doi: 10.1002/jso.20552. [DOI] [PubMed] [Google Scholar]
- 3.Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103:377–82. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- 4.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:3038–45. [PubMed] [Google Scholar]
- 5.Ruddy KJ, Barton D, Loprinzi CL. Laying to rest psychostimulants for cancer-related fatigue? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1865–7. doi: 10.1200/JCO.2014.55.8353. [DOI] [PubMed] [Google Scholar]
- 6.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:3830–42. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 7.Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nature reviews Clinical oncology. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamkin DM, Lutgendorf SK, Lubaroff D, Sood AK, Beltz TG, Johnson AK. Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain, behavior, and immunity. 2011;25:555–64. doi: 10.1016/j.bbi.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden DM, Devine R, Bicer S, Jing R, Reiser PJ, Wold LE, et al. Fluoxetine prevents the development of depressive-like behavior in a mouse model of cancer related fatigue. Physiology & behavior. 2015;140:230–5. doi: 10.1016/j.physbeh.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG, Jr, et al. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11376–86. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun TP, Grossberg AJ, Veleva-Rotse BO, Maxson JE, Szumowski M, Barnes AP, et al. Expression of myeloid differentiation factor 88 in neurons is not requisite for the induction of sickness behavior by interleukin-1beta. Journal of neuroinflammation. 2012;9:229. doi: 10.1186/1742-2094-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arpin D, Perol D, Blay JY, Falchero L, Claude L, Vuillermoz-Blas S, et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8748–56. doi: 10.1200/JCO.2005.01.7145. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5534–40. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, behavior, and immunity. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2617–23. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–25. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 19.Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, et al. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain, behavior, and immunity. 2015;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norden DM, McCarthy DO, Bicer S, Devine RD, Reiser PJ, Godbout JP, et al. Ibuprofen ameliorates fatigue- and depressive-like behavior in tumor-bearing mice. Life sciences. 2015;143:65–70. doi: 10.1016/j.lfs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Archives of otolaryngology–head & neck surgery. 2009;135:1137–46. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 22.Vichaya EG, Vermeer DW, Christian DL, Molkentine JM, Mason KA, Lee JH, et al. Neuroimmune mechanisms of behavioral alterations in a syngeneic murine model of human papilloma virus-related head and neck cancer. Psychoneuroendocrinology. 2017;79:59–66. doi: 10.1016/j.psyneuen.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippman MM, Laster WR, Abbott BJ, Venditti J, Baratta M. Antitumor activity of macromomycin B (NSC 170105) against murine leukemias, melanoma, and lung carcinoma. Cancer research. 1975;35:939–45. [PubMed] [Google Scholar]
- 24.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–91. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 25.Mason KA, Ariga H, Neal R, Valdecanas D, Hunter N, Krieg AM, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:361–9. [PubMed] [Google Scholar]
- 26.MacDonald L, Radler M, Paolini AG, Kent S. Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:R172–84. doi: 10.1152/ajpregu.00057.2011. [DOI] [PubMed] [Google Scholar]
- 27.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Hryhorczuk C, Fulton S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. Journal of visualized experiments : JoVE. 2012:e3754. doi: 10.3791/3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vichaya EG, Hunt SC, Dantzer R. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2884–90. doi: 10.1038/npp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comparative medicine. 2011;61:119–30. [PMC free article] [PubMed] [Google Scholar]
- 31.Bonsall DR, Kim H, Tocci C, Ndiaye A, Petronzio A, McKay-Corkum G, et al. Suppression of Locomotor Activity in Female C57Bl/6J Mice Treated with Interleukin-1beta: Investigating a Method for the Study of Fatigue in Laboratory Animals. PloS one. 2015;10:e0140678. doi: 10.1371/journal.pone.0140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluthe RM, Beaudu C, Kelley KW, Dantzer R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain research. 1995;677:171–6. doi: 10.1016/0006-8993(95)00194-u. [DOI] [PubMed] [Google Scholar]
- 33.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular psychiatry. 2008;13:717–28. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 34.Larson SJ, Romanoff RL, Dunn AJ, Glowa JR. Effects of interleukin-1beta on food-maintained behavior in the mouse. Brain, behavior, and immunity. 2002;16:398–410. doi: 10.1006/brbi.2001.0634. [DOI] [PubMed] [Google Scholar]
- 35.Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA, et al. Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/ chow feeding choice task. Psychopharmacology. 2014;231:727–36. doi: 10.1007/s00213-013-3285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. Journal of immunology (Baltimore, Md : 1950) 1997;159:3364–71. [PubMed] [Google Scholar]
- 37.Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. The European journal of neuroscience. 2000;12:4447–56. [PubMed] [Google Scholar]
- 38.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 39.Ogimoto K, Harris MK, Jr, Wisse BE. MyD88 is a key mediator of anorexia, but not weight loss, induced by lipopolysaccharide and interleukin-1 beta. Endocrinology. 2006;147:4445–53. doi: 10.1210/en.2006-0465. [DOI] [PubMed] [Google Scholar]
- 40.Wisse BE, Ogimoto K, Tang J, Harris MK, Jr, Raines EW, Schwartz MW. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology. 2007;148:5230–7. doi: 10.1210/en.2007-0394. [DOI] [PubMed] [Google Scholar]
- 41.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Dagenais M, Dupaul-Chicoine J, Douglas T, Champagne C, Morizot A, Saleh M. The Interleukin (IL)-1R1 pathway is a critical negative regulator of PyMT-mediated mammary tumorigenesis and pulmonary metastasis. Oncoimmunology. 2017;6:e1287247. doi: 10.1080/2162402X.2017.1287247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyter LM, El Mouatassim Bih S, Sattar H, Prendergast BJ. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain research. 2014;1552:55–63. doi: 10.1016/j.brainres.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:2759–66. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 45.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain, behavior, and immunity. 2007;21:251–8. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends in biochemical sciences. 2016;41:211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell MI, Engelbrecht AM. Metabolic hijacking: A survival strategy cancer cells exploit? Critical reviews in oncology/hematology. 2017;109:1–8. doi: 10.1016/j.critrevonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Yennurajalingam S, Tayjasanant S, Balachandran D, Padhye NS, Williams JL, Liu DD, et al. Association between Daytime Activity, Fatigue, Sleep, Anxiety, Depression, and Symptom Burden in Advanced Cancer Patients: A Preliminary Report. Journal of palliative medicine. 2016;19:849–56. doi: 10.1089/jpm.2015.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


