Abstract
Introduction
Coronary microvascular dysfunction (MVD) may contribute to the pathogenesis of heart failure with preserved ejection fraction (HFpEF). Speckle-tracking echocardiography is a feasible and reproducible method for identifying abnormalities in left ventricular (LV) and left atrial (LA) function among HFpEF patients. By assessing myocardial flow reserve (MFR), positron emission tomography (PET) imaging enables quantitative assessment of coronary MVD. We hypothesized that, in patients with risk factors for HFpEF and normal epicardial perfusion on cardiac PET, abnormal MFR is associated with LV diastolic dysfunction (DD) and reduced LV and LA strain.
Methods and Results
Retrospective study of patients without a history of heart failure who underwent clinically indicated rest/stress cardiac rubidium-82 PET imaging and transthoracic echocardiography within 90 days. Global MFR was calculated as the ratio of global stress to rest myocardial blood flow. Standard echocardiographic measures of diastolic function were recorded. Global longitudinal LA and LV strain were measured with a two-dimensional speckle-tracking technique. Relationships between MFR, LA strain, and LV strain were assessed with univariate and multivariate linear regression. The relationships of MFR with DD and estimated LV filling pressure were assessed with analysis of variance and test for trend.
Seventy-three patients (age 64 ± 11 years, 52% male) were identified with no epicardial perfusion defect on cardiac PET and an ejection fraction ≥ 50% (63.6 ± 4.6%). All patients had evaluable diastolic function on echocardiography, while 68 had imaging sufficient for strain analysis. Comorbidities were common [36% diabetes, 81% hypertension, 14% coronary artery disease (CAD), 34% chronic kidney disease (CKD), 22% paroxysmal atrial fibrillation, and 41% obesity (body mass index (BMI) ≥ 30 kg/m2)]. Decreased MFR was associated with LV DD (p = 0.02) and increased E/e′, an estimation of LV filling pressure [Low E/e′ (< 8) vs. High E/e′ (> 15), p<0.001]. MFR was associated with LA strain independent of age, gender, BMI, hypertension, CKD, diabetes, and CAD status (adjusted β = 2.6% per unit MFR, p = 0.046); however, MFR was only marginally related to LV strain.
Conclusions
In patients with normal ejection fraction, normal epicardial perfusion on PET imaging, and risk factors for HFpEF, decreased MFR was associated with DD, increased estimated LV filling pressure, and abnormal LA strain. These findings support the hypothesis that MVD contributes to cardiac functional alterations observed in HFpEF.
Keywords: Heart failure with preserved ejection fraction, cardiac positron emission tomography, myocardial flow reserve, diastolic dysfunction, left atrial strain, left ventricular strain
Introduction
Heart failure with preserved ejection fraction (HFpEF), the clinical syndrome associated with diastolic dysfunction (DD), is a growing epidemic resulting in significant morbidity and mortality (1). Traditionally, HFpEF has been defined as an ejection fraction ≥ 50% in the setting of heart failure symptoms. Recent studies have suggested that HFpEF is as prevalent as HFrEF (heart failure with reduced ejection fraction) (2, 3). Nonetheless, the pathophysiology of HFpEF is incompletely defined and treatment options remain limited (4, 5). Preclinical diastolic dysfunction (PDD) is defined as DD with normal ejection fraction without overt heart failure symptoms. PDD also is highly prevalent with rates exceeding 25% in the general population. In addition, PDD is a risk factor for HFpEF and all-cause mortality (2). However, PDD does not completely explain HFpEF as the majority of patients with DD do not display symptoms of heart failure (6).
The pathogenesis of HFpEF has traditionally been ascribed to DD in the setting of longstanding hypertension. A modern paradigm for HFpEF pathogenesis proposes that multiple comorbidities, including diabetes, obesity, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), anemia, and chronic kidney disease (CKD), contribute to a systemic pro-inflammatory state (7). This in turn leads to coronary microvascular endothelial inflammation resulting in increased production of reactive oxygen species and reduced nitric oxide bioavailability. Subsequent alterations in G-protein signaling lead to increased resting myocyte tension and promote cardiomyocyte hypertrophy. These changes, along with collagen deposition related to ongoing inflammation, result in structural changes to the myocardium. In this way, coronary microvascular dysfunction (MVD) may represent the presence of this inflammatory process that predisposes patients to DD and HFpEF (7–11). This paradigm may also explain the observed relationship between inflammatory markers and HFpEF (12).
Extensive literature has shown that HFpEF is more than simply DD, but is rather a syndrome involving multiple cardiac structural and functional alterations (4, 5, 13, 14). Speckle-tracking strain echocardiography is a clinically feasible and reproducible method for identifying abnormalities in left ventricular (LV) and left atrial (LA) function. When compared to normal controls and patients with hypertensive heart disease, HFpEF patients have significantly lower longitudinal and circumferential LV strain, even after adjusting for measures of diastolic function. Reduced LA strain is prognostically important in HFpEF independent of LV strain, and effectively separates HFpEF patients from hypertensive controls without heart failure (15–19). The role of MVD in the development of these abnormalities is poorly understood.
The vasodilatory capacity of the entire coronary circulation can be assessed non-invasively using cardiac positron emission tomography (PET). Abnormal PET measured myocardial flow reserve (MFR) is associated with cardiovascular outcomes including cardiac death, nonfatal myocardial infarction, revascularization, and heart failure hospitalization (20).
In this study, we sought to evaluate the interplay between MVD, as assessed by MFR, and LA and LV function. We hypothesized that, in patients with normal epicardial perfusion on cardiac PET and normal ejection fraction, the presence of MVD is associated with DD and reduced LA and LV strain.
Methods
Study Design and Patient Selection
This was a retrospective study of 73 subjects (age ≥ 18 years) who underwent clinically indicated rest/stress cardiac rubidium-82 PET imaging and transthoracic echocardiography within 90 days at the University of Michigan from May 2013 to November 2014. All subjects were required to have an EF ≥ 50% on echocardiography and PET imaging as well as evaluable diastolic function on echocardiography. Subjects were excluded if PET imaging showed any evidence of an epicardial coronary perfusion defect. Subjects also were excluded for a prior history of heart failure, infiltrative cardiomyopathy, cardiac transplantation, or moderate-severe valvular disease. While some subjects had a history of paroxysmal atrial fibrillation (AF), all subjects were in sinus rhythm at the time of echocardiography. Using these criteria, 80 patients were identified using data from PET imaging reports. However, further chart review identified that 7 of these patients had a history of heart failure prior to or at the time of PET imaging. Therefore, 73 subjects met criteria for analysis. Subjects were assessed for the presence of the following characteristics and comorbidities: age, gender, medications, body mass index (BMI), hypertension, CAD or history of myocardial infarction (MI), diabetes, obesity, history of AF, and CKD. Clinical data were previously collected as part of the standard history prior to all cardiac PET studies. Missing or incomplete data were supplemented by chart review.
PET Imaging
Patients were studied with a whole-body PET-computed tomography scanner (Siemens mCT, Siemens Medical Imaging, Knoxville, TN) after an overnight fast. Patients refrained from caffeine- and methylxanthine-containing substances and drugs for 24 hours before their scans. Myocardial blood flow was measured during rest and peak stress with rubidium-82 as a perfusion tracer, as described previously (21, 22). Briefly, after transmission imaging and beginning with the intravenous bolus administration of rubidium-82, list mode images were acquired for 7 minutes. Then, a standard intravenous infusion of regadenoson was given. At peak stress, a second dose of rubidium-82 was injected, and images were recorded in the same manner. Heart rate, blood pressure, and 12-lead ECG were recorded at baseline and every minute during and after pharmacologic stress.
Semiquantitative 17-segment visual interpretation of the gated myocardial perfusion images was performed by experienced observers using a standard 5-point scoring system (23). Summed rest and stress scores were calculated as the sum of individual segmental scores on the respective images, and their difference was recorded as a summed difference score. Based on these scores, all patients in this study had no evidence of an epicardial perfusion defect.
Absolute myocardial blood flow (MBF, mL/g/min) was computed from the dynamic rest and stress imaging series with commercially available software (Corridor4DM; Ann Arbor, MI) and previously validated methods (21, 22, 24). Automated factor analysis was used to generate blood pool (arterial input function) and tissue time-activity curves to a 2-compartment tracer kinetic model, as described previously (24). Per-patient global myocardial flow reserve (MFR) was calculated as the ratio of absolute MBF at stress over rest for the entire left ventricle. MVD was defined as a MFR less than 2.0 as this cutoff has been used in prior studies (25–27). Quantification of MBF was independently performed by 2 operators (M.K. and J.G.) on all studies; the average of the two measurements was used for analysis (Figure 1A).
Figure 1.
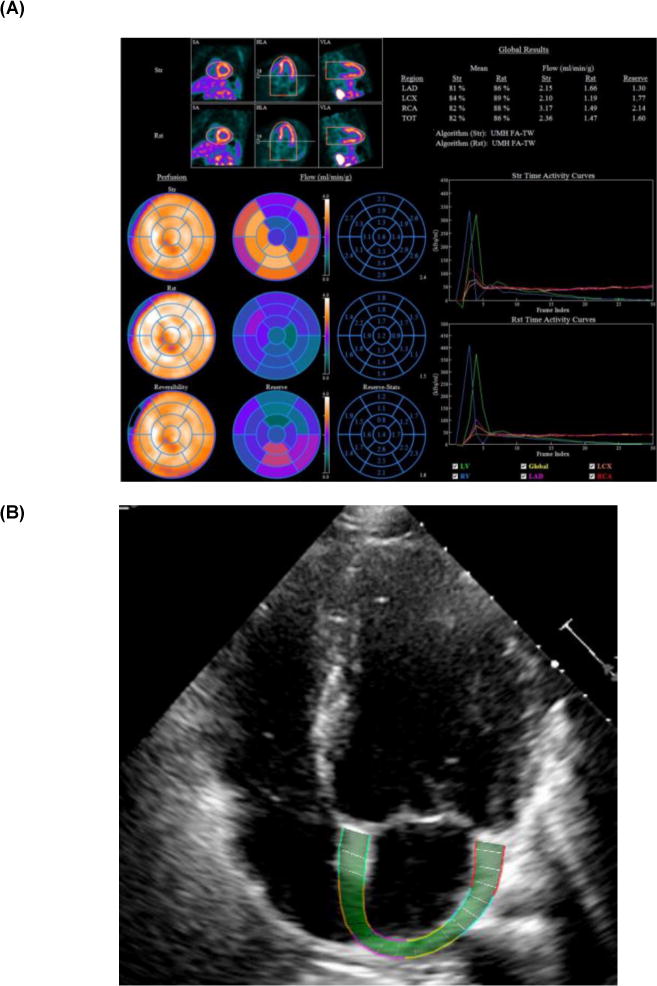
Examples of PET Myocardial Flow Reserve (A) and Left Atrial Strain (B) Imaging
Echocardiographic Analysis
Echocardiogram images for all 73 patients were reviewed by M.K. The following standard parameters of diastolic function were recorded: left atrial volume index, tricuspid regurgitation systolic jet velocity, mitral inflow peak E- and A-wave velocities as obtained by pulse wave Doppler (E/A ratio), tissue Doppler measurements of early diastolic mitral annular velocities, and estimated LV filling pressure (E/e′). Diastolic function was assessed for each patient in accordance with guidelines published by the American Society of Echocardiography (28). Briefly, DD was considered present if greater than two of the following were present: average E/e′ > 14, septal e′ velocity < 7 cm/s or lateral e′ velocity < 10 cm/s, tricuspid regurgitation systolic jet velocity > 2.8 m/s, and left atrial volume index > 34 ml/m2. If fewer than two of these parameters were present, diastolic function was considered normal. If exactly two of these abnormal findings were present, diastolic function was classified as indeterminate. Mitral inflow velocities were used along with these variables to assign DD grade in patients with DD (28). Using these four parameters, we also assigned a “DD score” based on each subject’s total number of abnormal parameters. DICOM files of echocardiogram images retrieved from the University of Michigan database archives were analyzed using vendor-independent speckle tracking software (EchoInsight, Epsilon Imaging Inc., Ann Arbor, MI, USA) (29). One cardiac cycle was selected for each of the apical 4- and 2-chamber views. The endocardial border was traced manually and the region of interest was adjusted to cover the thickness of the wall (Figure 1B). Appropriate tracking was verified visually, and the software then generated global longitudinal strain curves derived from six LA segments (for global LA longitudinal strain) and six LV segments (for global LV longitudinal strain) in each view using the QRS complex as the reference point. Global LA strain was measured at the positive peak of the strain curve at the end of systole and reflected the reservoir function of the LA. The global strain values from the 4-chamber and 2-chamber views were averaged to give a biplane global value, and this value was used in subsequent analyses. Strain values were expressed as an absolute value of the percentage of longitudinal deformation (lengthening for LA strain and shortening for LV strain). Adequate tracking quality was required in at least three of the segments per view for images to be considered sufficient for strain analysis. In order to assess inter-observer variability of strain results, 15 randomly selected examinations were analyzed by another observer (S.H.), who was blinded to patient clinical data.
Statistical Analysis
The relationships between clinical characteristics and MFR were evaluated with t-testing or chi-square testing as appropriate for continuous or categorical variables. Relationships between MFR, LA strain, and LV strain were assessed with univariate and multivariate regression, adjusting for known or potential demographic and comorbidity-related predictors. The relationships of MFR with DD and estimated LV filling pressure were assessed with analysis of variance and a non-parametric test for trend across categories. Inter-observer variability in strain measurements and MFR was assessed by calculating inter-observer intraclass correlations. Analyses were performed using STATA 10.0 (StataCorp, College Station, TX).
Results
Patient Characteristics
Seventy-three patients (age 64 ± 11 years, 52% male) were identified with no history of heart failure, no epicardial perfusion defects on cardiac PET, and an LVEF ≥ 50% (63.6 ± 4.6%). Comorbidities including diabetes, hypertension, CAD, CKD, obesity, and paroxysmal AF were common as well as the use of cardiac medications (Table 1). Subjects had at least one of the following clinical indications for PET stress evaluation (Table 1): chest discomfort (64%), dyspnea (15%), arrhythmia/palpitations/syncope (27%), preoperative risk assessment (11%), and other (7%). Most PET scans and echocardiograms were performed in close temporal proximity with 63% within 1 week and 77% within two weeks of each other.
Table 1.
Baseline Characteristics
| All Patients (n = 73) |
MFR ≥ 2.0 (n = 31) |
MFR < 2.0 (n = 42) |
|
|---|---|---|---|
| Demographics | |||
| Age, yrs | 64 ± 11 | 60 ± 11 | 68 ± 9* |
| Male, n (%) | 38 (52%) | 16 (52%) | 22 (52%) |
| Vital Signs | |||
| Resting heart rate, beats/min | 72 ± 13 | 66 ± 9 | 77 ± 14* |
| Resting systolic blood pressure, mm Hg | 143 ± 26 | 134 ± 23 | 151 ± 26* |
| Body mass index, kg/m2 | 30.1 ± 7.9 | 29.5 ± 6.1 | 30.5 ± 9.0 |
| PET Indications | |||
| Chest discomfort, n (%) | 47 (64%) | 27 (87%) | 20 (48%)* |
| Dyspnea, n (%) | 11 (15%) | 6 (19%) | 5 (12%) |
| Arrhythmia/palpitations/syncope, n (%) | 20 (27%) | 13 (42%) | 7 (17%) |
| Preoperative evaluation, n (%) | 8 (11%) | 1 (3%) | 7 (17%) |
| Other, n (%) | 5 (7%) | 0 (0%) | 5 (12%)* |
| Comorbidities | |||
| Coronary artery disease, n (%) | 10 (14%) | 3 (10%) | 7 (17%) |
| Hypertension, n (%) | 59 (81%) | 21 (68%) | 38 (90%)* |
| Diabetes mellitus, n (%) | 26 (36%) | 8 (26%) | 18 (43%) |
| Chronic kidney disease (GFR < 60 mL/min), n (%) | 25 (34%) | 4 (13%) | 21 (50%)* |
| Obesity (body mass index ≥ 30 kg/m2), n (%) | 30 (41%) | 12 (39%) | 18 (43%) |
| Paroxysmal atrial fibrillation, n (%) | 16 (22%) | 4 (13%) | 12 (28%) |
| Medications | |||
| ACE Inhibitor or Angiotensin Receptor Blocker, n (%) | 20 (27%) | 8 (26%) | 12 (28%) |
| Beta Blocker, n (%) | 33 (45%) | 16 (52%) | 17 (40%) |
| Calcium Channel Blocker, n (%) | 18 (25%) | 4 (13%) | 14 (33%)* |
| Aspirin, n (%) | 35 (48%) | 14 (45%) | 21 (50%) |
| Nitrate, n (%) | 3 (4%) | 1 (3%) | 2 (5%) |
| Statin, n (%) | 33 (45%) | 13 (42%) | 20 (48%) |
| Loop Diuretic, n (%) | 11 (15%) | 4 (13%) | 7 (17%) |
| Thiazide Diuretic, n (%) | 5 (7%) | 0 (0%) | 5 (12%)* |
| Echocardiography | |||
| Ejection fraction, % | 63.6 ± 4.6 | 63.1 ± 3.3 | 63.9 ± 5.4 |
| Left ventricular end-diastolic diameter, mm | 46.8 ± 5.2 | 47.1 ± 4.0 | 46.5 ± 6.0 |
| Left atrial end-systolic diameter, mm | 40.1 ± 6.0 | 38.9 ± 5.0 | 41.0 ± 6.7 |
| Left atrial end-systolic volume index, ml/m2 | 32.9 ± 11.3 | 28.9 ± 9.5 | 36.0 ± 11.8 |
| Relative wall thickness | 0.46 ± 0.1 | 0.41 ± 0.08 | 0.46 ± 0.12 |
| LV mass index, g/m2 | 91.6 ± 25.0 | 82.5 ± 25.4 | 98.3 ± 22.7 |
| E wave velocity, cm/s | 80.8 ± 27.0 | 74.0 ± 18.2 | 85.9 ± 31.4 |
| A wave velocity, cm/s | 83.1 ± 26.3 | 73.9 ± 19.1 | 89.8 ± 28.9 |
| Septal e′ velocity, cm/s | 7.2 ± 2.8 | 7.8 ± 2.8 | 6.8 ± 2.8 |
| Lateral e′ velocity, cm/s | 9.0 ± 3.4 | 10.0 ± 3.4 | 8.3 ± 3.3 |
| Average E/e′ | 11.2 ± 5.3 | 9.1 ± 2.7 | 12.8 ± 6.1* |
| TR velocity > 2.8 m/s | 14 (19%) | 3 (10%) | 11 (26%) |
| Normal diastolic function | 34 (46%) | 19 (61%) | 15 (36%)* |
| Indeterminate diastolic function | 26 (36%) | 9 (29%) | 17 (40%) |
| Diastolic dysfunction | 13 (18%) | 3 (10%) | 10 (24%)* |
| Global left atrial longitudinal strain, % | 28 ± 7 | 32 ± 7 | 25 ± 6* |
| Global left ventricular longitudinal strain, % | 16 ± 3 | 17 ± 2 | 16 ± 4 |
| Positron Emission Tomography | |||
| Resting myocardial blood flow, mL/min/g | 1.72 ± 0.56 | 1.38 ± 0.51 | 1.98 ± 0.46* |
| Stress myocardial blood flow, mL/min/g | 3.23 ± 0.90 | 3.53 ± 0.97 | 3.01 ± 0.80* |
| Myocardial flow reserve, stress/rest ratio | 2.02 ± 0.72 | 2.66 ± 0.60 | 1.55 ± 0.32* |
Continuous values expressed as mean ± one unit standard deviation.
Strain expressed as absolute value of percentage of longitudinal deformation.
Abbreviations: ACE: angiotensin-converting enzyme; MFR: myocardial flow reserve; PET: positron emission tomography.
p < 0.05 for comparison between MFR < 2.0 and ≥ 2.0.
Myocardial Flow Reserve and Diastolic Dysfunction
All patients had evaluable diastolic function on echocardiography. Thirty-four patients (46%) had normal diastolic function, 26 patients (36%) had indeterminate diastolic function, and 13 patients (18%) had DD (grade 2, n=12; indeterminate grade, n=1, Tables 1 and 2). There were no patients with grade 1 or grade 3 DD. Decreased MFR was associated with the presence of DD (p = 0.02, p = 0.008 for trend across categories, Tables 1 and 2, Figure 2A). Independent of a subject’s diastolic function classification, when considering the four parameters used to determine diastolic function, lower MFR values were associated with subjects having an increased number of abnormal measures [p = 0.04 for trend across categories (DD score), Figure 3]. Decreased MFR also was associated with increased E/e′, an estimation of LV filling pressure [Low E/e′ (< 8) vs. High E/e′ (> 15), p<0.001; p < 0.001 for trend across categories, Figure 2B]. The mean MFR was non-significantly lower in patients with pulmonary hypertension (defined as tricuspid velocity > 2.8 m/s; 2.1 vs. 1.9, p = 0.19) and left atrial enlargement (defined as left atrial volume index > 34 ml/m2; 2.1 vs. 1.8, p = 0.30).
Table 2.
PET and Echocardiographic Measurements by Diastolic Function
| Characteristic | Normal (n = 34) |
Indeterminate (n = 26) |
Abnormal (n = 13) |
|---|---|---|---|
| Positron Emission Tomography | |||
| Total resting myocardial blood flow, mL/min/g | 1.66 ± 0.55 | 1.62 ± 0.55 | 2.11 ± 0.48* |
| Total stress myocardial blood flow, mL/min/g | 3.48 ± 0.90 | 2.86 ± 0.82 | 3.32 ± 0.88* |
| Myocardial flow reserve, stress/rest ratio | 2.26 ± 0.81 | 1.91 ± 0.56 | 1.63 ± 0.49* |
| Echocardiography | |||
| Ejection fraction, % | 62.5 ± 4.81 | 64.0 ± 3.75 | 65.4 ± 5.19 |
| Left ventricular end-diastolic diameter, mm | 46.0 ± 4.06 | 48.2 ± 5.91 | 45.8 ± 6.33 |
| Left atrial end-systolic diameter, mm | 37.7 ± 4.49 | 41.4 ± 5.41 | 43.7 ± 8.27* |
| Left atrial end-systolic volume index, ml/m2 | 25.0 ± 6.00 | 38.6 ± 10.9 | 42.5 ± 8.52* |
| Relative wall thickness | 0.44 ± 0.10 | 0.44 ± 0.10 | 0.54 ± 0.09* |
| LV mass index, g/m2 | 82.3 ± 19.4 | 96.1 ± 26.0 | 107.0 ± 27.5* |
| E wave velocity, cm/s | 75.9 ± 21.2 | 74.2 ± 23.7 | 107.1 ± 32.8* |
| A wave velocity, cm/s | 73.2 ± 20.7 | 84.7 ± 27.5 | 84.8 ± 28.4* |
| Septal e′ velocity, cm/s | 8.18 ± 2.70 | 6.76 ± 3.24 | 5.87 ± 1.35* |
| Lateral e′ velocity, cm/s | 10.6 ± 3.17 | 8.13 ± 3.44 | 6.57 ± 1.86* |
| Average E/e′ | 8.70 ± 2.55 | 10.9 ± 3.04 | 18.4 ± 7.39* |
| TR velocity > 2.8 m/s | 0 | 8 (31%) | 6 (46%)* |
| Global left ventricular longitudinal strain, % | 17 ± 2 | 16 ± 4 | 15 ± 3 |
| Global left atrial longitudinal strain, % | 31 ± 7 | 25 ± 7 | 24 ± 4* |
Continuous values expressed as mean ± one unit standard deviation.
Strain expressed as absolute value of percentage of longitudinal deformation. Abbreviations: LV: left ventricle; MFR: myocardial flow reserve; TR: tricuspid regurgitation.
p < 0.05 for analysis of variance across diastolic function grades.
Figure 2. Relationships between Myocardial Flow Reserve, Diastolic Function (A), and Estimated Left Ventricular Pressure (B).
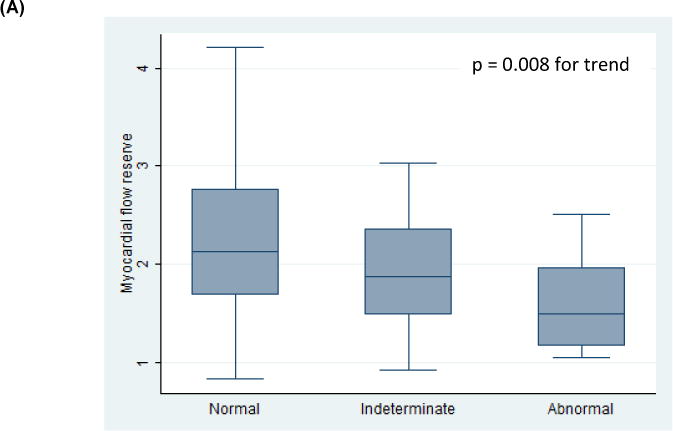
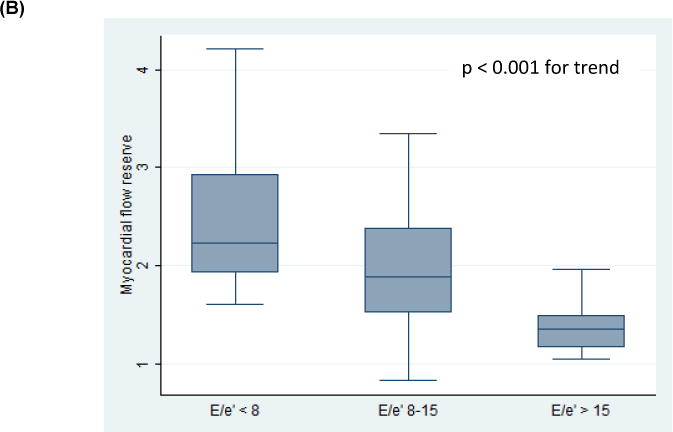
box: interquartile range; line: mean values; whiskers: upper and lower adjacent values.
Figure 3. Relationship between Myocardial Flow Reserve and Total Number of Abnormal Diastolic Function Parameters.
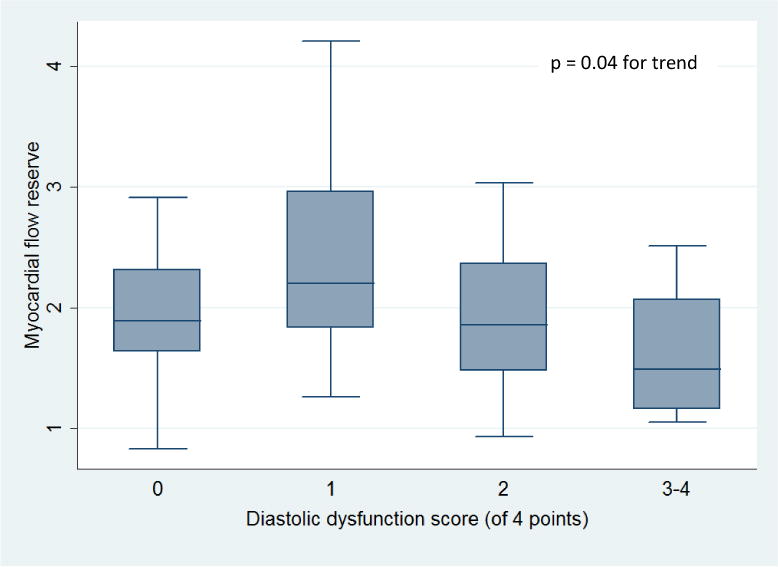
box: Diastolic dysfunction score based on presence of the following four factors: average E/e′ > 14, septal e′ velocity < 7 cm/s or lateral e′ velocity < 10 cm/s, tricuspid regurgitation systolic jet velocity > 2.8 m/s, and left atrial volume index > 34 ml/m2.
Left Atrial and Left Ventricular Strain and Diastolic Dysfunction
Decreased LA strain was associated with both the presence of DD (n = 66, p = 0.009, Figure 4A) and indeterminate diastolic function (p = 0.008, p = 0.001 for trend across categories, Figure 4A). Similarly, increased left atrial volume index (n = 71) was associated with both the presence of DD (p < 0.001, Figure 4B) and indeterminate diastolic function (p < 0.001, p < 0.001 for trend across categories, Figure 4B). LA strain was not significantly correlated with LA volume index (r = −0.18). LV strain was not associated with either abnormal diastolic function (n = 68, p = 0.34) or indeterminate diastolic function (p = 0.44).
Figure 4. Relationships between Left Atrial Strain (A), Left Atrial Volume Index (B), and Diastolic Function.
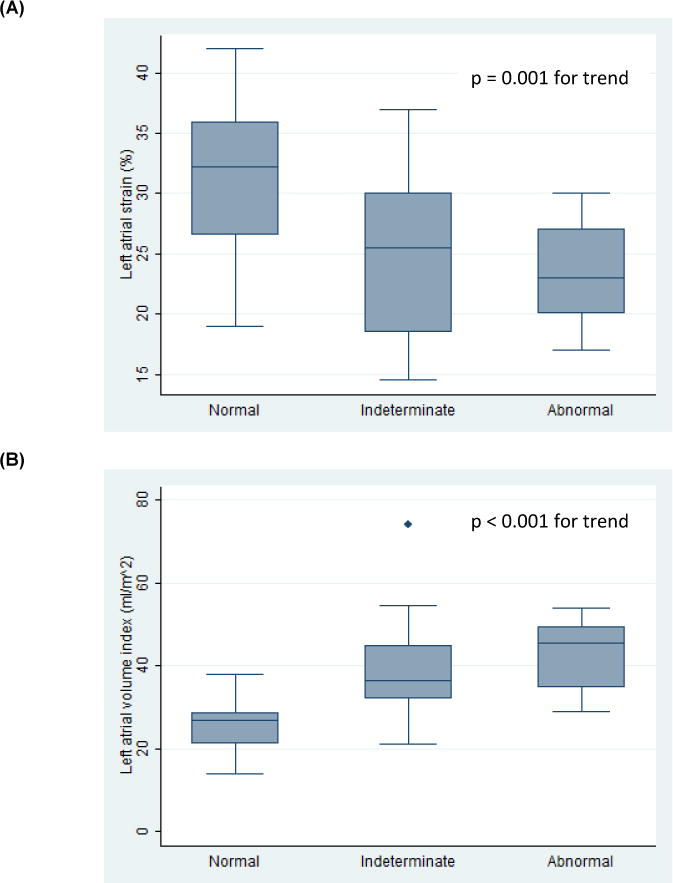
box: interquartile range; line: mean values; whiskers: upper and lower adjacent values; dots: outlier values.
Myocardial Flow Reserve and Left Atrial and Ventricular Strain
Sixty-eight patients had imaging sufficient for strain analysis. MFR correlated moderately well with LA strain (n = 66, r = 0.50, p < 0.001, Figure 5A) and was associated with LA strain independent of age, gender, BMI, hypertension, CKD, diabetes, paroxysmal AF, and CAD status (n = 66, unadjusted β = 5% per unit MFR, p < 0.001; adjusted β = 2.6% per unit MFR, p = 0.046). MFR was only marginally related to LV strain (n = 68, r = 0.20, p = 0.104, Figure 5B) and failed to predict LV strain on univariate regression (unadjusted β = 0.9% per unit MFR, p = 0.104). The inter-observer intra-class correlations for LA and LV strain were 0.99 (95% CI 0.96–1.00) and 0.69 (95% CI −0.15–0.90), respectively. The inter-observer intra-class correlation for MFR was 0.99 (95% CI 0.98–0.99).
Figure 5. Relationships between Myocardial Flow Reserve and Left Atrial (A) and Left Ventricular (B) Strain.
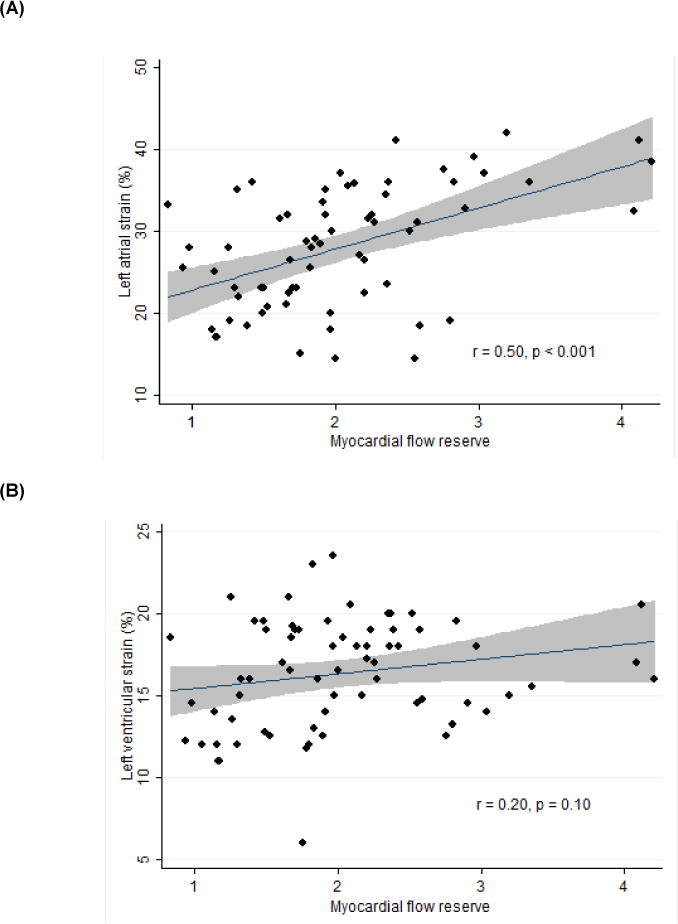
line: best fit; gray area: 95% confidence interval.
Myocardial Flow Reserve and HFpEF-Related Comorbidity Burden
A patient’s burden of HFpEF-related comorbidities (obesity, hypertension, CAD/MI, diabetes, paroxysmal AF, and CKD) was significantly associated with decreased MFR (p < 0.001 for trend across categories, Figure 6).
Figure 6. Myocardial Flow Reserve by Burden of HFpEF-Related Comorbidities.
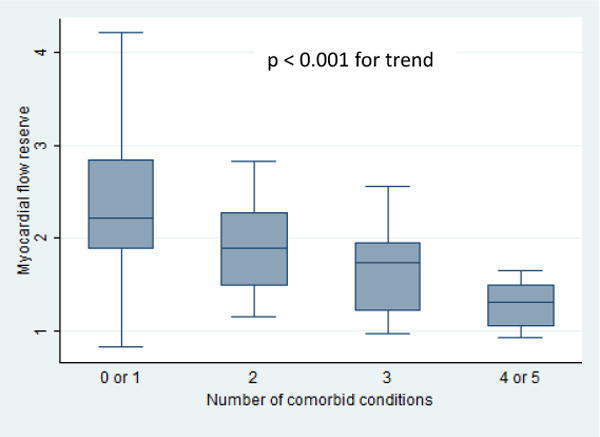
Abbreviations: HFpEF: heart failure with preserved ejection fraction.
box: interquartile range; line: mean values; whiskers: upper and lower adjacent values.
Discussion
The pathophysiology of HFpEF is complex involving multiple cardiac structural and functional alterations (4, 5, 13–16). Microvascular endothelial inflammation and dysfunction, secondary to HFpEF risk factors, are hypothesized to be critical factors in the development of HFpEF (7–11). This study found HFpEF risk factor burden to be inversely related to MFR, further implicating these risk factors in MVD. Previous imaging studies have associated reduced MFR with HFpEF. Kato et al. (2016) found that MFR as measured by MRI was significantly lower in HFpEF patients compared to patients with LVH and controls (30). Over 75% of HFpEF patients had an abnormal MFR (MFR<2.5) and abnormal MFR was also associated with elevated levels of brain natriuretic peptide (30). Similar findings have also been demonstrated with PET (31). Finally, invasive measures of MVD are also altered in HFpEF (32).
In this study of patients with risk factors for HFpEF but without heart failure, abnormal MFR was associated with DD, increased estimated LV filling pressure, and abnormal LA strain. MVD may signify the presence of ongoing microvascular inflammation that promotes increased myocyte tension, myocyte hypertrophy, and interstitial fibrosis as previously hypothesized (7, 8). These changes could contribute to increasing myocardial stiffness and subsequent deterioration of LA function. Microvascular inflammation may also contribute to left ventricular DD and increased left ventricular filling pressure which also promote LA remodeling and dysfunction.
PDD is a known risk factor for the development of HFpEF (2). However, the relationship between PDD and HFpEF is not fully understood as not all patients with PDD go on to develop HFpEF and not all patients with HFpEF meet criteria for DD (2, 33, 34). The observed relationship between MFR and DD in this study suggests that MVD may contribute to DD. A recent post-mortem study found that myocardium from HFpEF patients had significantly lower microvascular density and significantly increased myocardial fibrosis (9), supporting a mechanistic connection between MVD and DD. (7, 9). Of note, MFR was also inversely related to E/e′, which has been correlated with invasively measured LV end-diastolic pressure and pulmonary capillary wedge pressure in HFpEF patients (35). Consequently, MVD may be connected to increased LV filling pressure as a mechanism for its contribution to HFpEF symptoms.
Abnormal LA function has also been implicated in the development of HFpEF (13, 15, 34, 36). Specifically, LA strain is significantly lower in patients with HFpEF compared to those with PDD; it has been shown to provide independent and incremental diagnostic value over clinical and conventional echocardiographic parameters when distinguishing HFpEF patients from hypertensive controls (15, 37). While our study excluded HFpEF patients, we have demonstrated an association between abnormal MFR and this important alteration of LA function associated with HFpEF. Further research is necessary to determine whether MVD affects LA function directly or through its effect on left ventricular diastolic function. The relationship between MVD and LA strain may help explain recent data demonstrating that LA reservoir strain is associated with pulmonary vascular resistance, cardiac output, and exercise tolerance as well as cardiovascular outcomes in HFpEF patients (38).
Interestingly, there was no association between LV strain and MFR in our study. The relationship between LV strain and PDD has not been well described. Because microvascular inflammation is hypothesized to lead to LV DD, MVD may not be associated with this parameter of LV systolic function. It is possible that abnormal LV systolic function occurs further along in the disease pathway as LV strain has been shown, using several different modalities, to distinguish patients with HFpEF from those with PDD and normal controls (15, 16, 37, 39–41). Our study was unable to evaluate this as patients with a history of heart failure were excluded.
Noninvasive ischemia evaluations are commonly obtained for patients at risk for or with HFpEF. Abnormal MFR may serve as a means of identifying patients with PDD at greater risk for developing HFpEF. In addition, MFR could be used in the phenotyping of HFpEF patients which may facilitate the discovery of targeted therapies for the condition (5, 42–46). Given the association of MFR with brain natriuretic peptide in one study (30) and E/e′ in our study, MFR may have prognostic significance in PDD and HFpEF similar to these known prognostic markers (35, 47). It is not yet clear whether MFR would predict clinical outcomes in patients with PDD and HFpEF; therefore, prospective, outcome-based studies should be pursued. As these questions are addressed, it will be imperative to understand which comorbid conditions contribute most to MVD, and whether aggressively treating these comorbidities could potentially slow or even reverse the disease process (48). We recognize that both PET MFR and especially strain imaging may not be widely available to become part of routine clinical care for these patients. However, these technologies may improve our understanding of HFpEF pathophysiology.
Limitations to this study include the fact that this was a small, retrospective, cross-sectional single-center study. This study should not be viewed as an evaluation of HFpEF patients as we evaluated only patients with PDD and risk factors for HFpEF. Echocardiographic measures were only obtained at rest; therefore, we were unable to assess for changes in these measures with stress. Lastly, we are not yet able to determine to what degree abnormal MFR is secondary to primary changes in the microvasculature or instead a reflection of increased left-ventricular filling pressures at rest and/or with exercise.
Conclusions
In patients with an LV ejection fraction ≥ 50%, no significant valvular disease, and no perfusion defects attributable to epicardial stenotic disease on cardiac PET imaging, MFR was inversely related to DD and was independently associated with LA strain. These observations support the hypothesis that MVD contributes to the pathophysiology of HFpEF.
Manuscript Highlights.
Coronary microvascular inflammation may contribute to the development of HFpEF
Myocardial flow reserve, measured by cardiac PET, assesses microvascular function
Decreased myocardial flow reserve is associated with diastolic dysfunction
Decreased myocardial flow reserve is associated with left atrial dysfunction
Myocardial flow reserve is related to comorbidity burden in those at risk for HFpEF
Acknowledgments
Dr. Hummel was supported by K23HL109176 from the National Heart Lung and Blood Institute and an R21 AG047939 from the National Institute of Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126(1):65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 2.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. Journal of the American College of Cardiology. 2014;63(5):407–16. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. Jama. 2006;296(18):2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, et al. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart failure. 2014;2(2):97–112. doi: 10.1016/j.jchf.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. European heart journal. 2014;35(40):2797–815. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013;62(4):263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 8.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart failure. 2016;4(4):312–24. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131(6):550–9. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomberg-Maitland M, Shah SJ, Guazzi M. Inflammation in Heart Failure With Preserved Ejection Fraction: Time to Put Out the Fire. JACC Heart failure. 2016;4(4):325–8. doi: 10.1016/j.jchf.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Marechaux S, Samson R, van Belle E, Breyne J, de Monte J, Dedrie C, et al. Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. Journal of cardiac failure. 2016;22(1):3–11. doi: 10.1016/j.cardfail.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. Journal of the American College of Cardiology. 2010;55(19):2129–37. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nature reviews Cardiology. 2014;11(9):507–15. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 14.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. Journal of cardiac failure. 2005;11(3):177–87. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovascular imaging. 2013;6(7):749–58. doi: 10.1016/j.jcmg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2014;63(5):447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraiva RM, Demirkol S, Buakhamsri A, Greenberg N, Popovic ZB, Thomas JD, et al. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23(2):172–80. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, et al. Impaired left atrial function in heart failure with preserved ejection fraction. European journal of heart failure. 2014;16(10):1096–103. doi: 10.1002/ejhf.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132(5):402–14. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46(8):1264–71. [PubMed] [Google Scholar]
- 22.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 24.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50(7):1062–71. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. Journal of the American College of Cardiology. 2009;54(2):150–6. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 26.Camici PG, Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356(8):830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 27.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. Journal of the American College of Cardiology. 2011;58(7):740–8. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2016 doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 29.Kolias TJ, Hagan PG, Chetcuti SJ, Eberhart DL, Kline NM, Lucas SD, et al. New universal strain software accurately assesses cardiac systolic and diastolic function using speckle tracking echocardiography. Echocardiography. 2014;31(8):947–55. doi: 10.1111/echo.12512. [DOI] [PubMed] [Google Scholar]
- 30.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, et al. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association. 2016;5(2) doi: 10.1161/JAHA.115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, et al. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction. Circulation Heart failure. 2016;9(7) doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 32.Sucato V, Evola S, Novo G, Sansone A, Quagliana A, Andolina G, et al. Angiographic Evaluation of Coronary Microvascular Dysfunction in Patients with Heart Failure and Preserved Ejection Fraction. Microcirculation. 2015;22(7):528–33. doi: 10.1111/micc.12223. [DOI] [PubMed] [Google Scholar]
- 33.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, et al. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. Journal of the American College of Cardiology. 2007;49(6):687–94. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 34.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. Journal of the American College of Cardiology. 2007;49(2):198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 35.Bruch C, Grude M, Muller J, Breithardt G, Wichter T. Usefulness of tissue Doppler imaging for estimation of left ventricular filling pressures in patients with systolic and diastolic heart failure. The American journal of cardiology. 2005;95(7):892–5. doi: 10.1016/j.amjcard.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Tan YT, Wenzelburger F, Lee E, Nightingale P, Heatlie G, Leyva F, et al. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Heart. 2010;96(13):1017–23. doi: 10.1136/hrt.2009.189118. [DOI] [PubMed] [Google Scholar]
- 37.Kosmala W, Rojek A, Przewlocka-Kosmala M, Mysiak A, Karolko B, Marwick TH. Contributions of Nondiastolic Factors to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology. 2016;67(6):659–70. doi: 10.1016/j.jacc.2015.10.096. [DOI] [PubMed] [Google Scholar]
- 38.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circulation Cardiovascular imaging. 2016;9(3) doi: 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87(2):121–5. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart. 2002;87(1):29–31. doi: 10.1136/heart.87.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2009;54(5):410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circulation Heart failure. 2010;3(5):588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2010;56(11):845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. Journal of the American College of Cardiology. 2013;62(15):1339–42. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–79. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. European journal of heart failure. 2015;17(9):925–35. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circulation Heart failure. 2011;4(5):569–77. doi: 10.1161/CIRCHEARTFAILURE.111.962654. [DOI] [PubMed] [Google Scholar]
- 48.Fu M, Zhou J, Thunstrom E, Almgren T, Grote L, Bollano E, et al. Optimizing the Management of Heart Failure With Preserved Ejection Fraction in the Elderly by Targeting Comorbidities (OPTIMIZE-HFPEF) Journal of cardiac failure. 2016;22(7):539–44. doi: 10.1016/j.cardfail.2016.01.011. [DOI] [PubMed] [Google Scholar]


