Abstract
Executive control functions are associated with frontal, parietal, cingulate, and insular brain regions that interact through distributed large-scale networks. Here, we discuss how fMRI functional connectivity can shed light on the organization of control networks and how they interact with other parts of the brain. In the first section of our review, we present convergent evidence from fMRI functional connectivity, activation, and lesion studies that there are multiple dissociable control networks in the brain with distinct functional properties. In the second section, we discuss how graph theoretical concepts can help illuminate the mechanisms by which control networks interact with other brain regions to carry out goal-directed functions, focusing on the role of specialized hub regions for mediating cross-network interactions. Again, we use a combination of functional connectivity, lesion, and task activation studies to bolster this claim. We conclude that a large-scale network perspective provides important neurobiological constraints on the neural underpinnings of executive control, which will guide future basic and translational research into executive function and its disruption in disease.
Keywords: executive control, fMRI, functional connectivity, networks, cognitive control
1. Introduction
Executive control includes the set of processes that allow humans to flexibly adapt their behavior, deftly guiding neural processing to achieve goals at multiple timescales. Executive control (hereafter referred to more simply as control) encompasses processes involved in attentional enhancement of task-relevant inputs and guided processing of these inputs towards appropriate behavioral responses, as well as suppression of task-irrelevant distractors and inhibition of goal-inappropriate prepotent responses. In the past, two different approaches have provided useful insight into how the brain maintains and executes control-related processing: one is built on understanding the specialized processing within individual brain regions, especially in the prefrontal cortex, while the other is focused on distributed processing across different brain areas that are organized into large-scale networks (also called systems). More recently, the latter approach has evolved to adopt a complex systems view that has been especially fruitful in exploring the interactions among sets of control-related regions. Hence, in the current review, we present recent evidence that suggest that distinct control processes localize not only to different brain areas, but to functionally dissociable brain networks. Furthermore, we emphasize the contribution of specialized hub regions to executive control processes, via their role in mediating interactions between control networks and other networks involved in basic processing that are critical for accomplishing complex tasks.
Specifically, in the first section of our review, we will present evidence from studies of fMRI functional connectivity, brain lesions, and fMRI task activations that argue for the existence of (at least) two distinct control networks with dissociable roles in control. These findings suggest that regions within each network carry out related sets of processes, distinct from regions in other networks. In the second section we exploit newer concepts from complex systems science to examine how control networks interact with each other and with relevant processing regions – focusing on the role of specialized hub locations in these interactions. Again, we will use a combination of functional connectivity, brain lesions, and task activation results to support our findings. By integrating information about the functional specialization of regions with an understanding of large-scale network organization, we provide new insights into how common sets of control functions are organized and implemented in the brain.
Throughout the review we will focus on evidence for control network organization and hubs from our own work; however, related proposals have been made by other groups (e.g., see (Badre & D’Esposito, 2007; Duncan & Owen, 2000; Miller & Cohen, 2001; Sadaghiani, et al., 2010; Seeley, et al., 2007; Shenhav, Botvinick, & Cohen, 2013)). We discuss a portion of these briefly in relevant contexts of this manuscript, and refer the interested reader to these publications for a more in-depth treatment.
Given the nature of slow fluctuations in functional MRI, where most of the signal is below 0.1 Hz., the overall organization of these networks is quite stable across different behavioral contexts and states (Cole, Bassett, Power, Braver, & Petersen, 2014; Gratton, Laumann, Gordon, Adeyemo, & Petersen, 2016; Greicius, et al., 2008; Larson-Prior, et al., 2009; Laumann, et al., 2016). However, recent studies have shown evidence of subtle and systematic alterations in network interactions under goal-relevant task states (Cole, Bassett, Power, Braver, & Petersen, 2014; Gratton, Laumann, Gordon, Adeyemo, & Petersen, 2016; Krienen, Yeo, & Buckner, 2014), and we discuss these findings in detail. Thus, these measures provide a window into the stable and slowly-fluctuating organization of human brain networks, recapitulating known functional and neurobiological systems (e.g., (Biswal, Yetkin, Haughton, & Hyde, 1995; Power, et al., 2011; Vincent, et al., 2007)) at both the group and individual level (Braga & Buckner, 2017; Gordon, et al., 2017; Power, et al., 2011; Yeo, et al., 2011) that may be relevant for clinical practice.
Notably, it is also likely that additional levels of complexity and network interactions exist at faster time-scales. While fMRI functional connectivity is not ideally suited to measuring cognitively-relevant interactions at fast time-scales (see (Laumann, et al., 2016; Liegeois, Laumann, Snyder, Zhou, & Yeo, 2017) on issues with measuring faster dynamics in resting-state FC), exciting future work using other techniques such as ERP, EEG, and fast optical imaging may shed light on faster dynamics within these networks. In this domain, we refer the reader to other works in this special issue on “Cognitive Control” ((Gratton, 2018; Gratton, Cooper, Fabiani, Carter, & Karayanidis, 2018), see also (Baniqued, Low, Fletcher, Gratton, & Fabiani, 2017; Barcelo & Cooper, 2017; Boudewyn & Carter, 2017; Coleman, Watson, & Strayer, 2017; Wessel, 2017).
2. Evidence for multiple control networks: the cinguloopercular and frontoparietal networks
Numerous studies have suggested that regions across frontal and parietal cortex are important for executive control (e.g., (Corbetta & Shulman, 2002; D’Esposito, et al., 1995; Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Duncan & Owen, 2000; Hopfinger, Buonocore, & Mangun, 2000; Miller & Cohen, 2001; Moore, 2006)). These areas are among the most commonly engaged across different tasks (Nelson, et al., 2010), especially tasks that require goal-directed processing (Dosenbach, et al., 2006; Duncan & Owen, 2000). Indeed, these “control” regions are especially active during periods of tasks that are tightly associated with task control initiation, maintenance, and modification (Dosenbach, et al., 2006), such as when task instructions are cued, sustained across task execution periods, and when errors are made signaling the need for behavioral adjustments. Although some research has treated these areas as a singular network - e.g., the “multiple demand” or “executive control” network of the brain ((Duncan & Owen, 2000); see also (Miller & Cohen, 2001)) – evidence from resting state functional connectivity (rs-FC) studies suggest that these control regions are decomposable into (at least) two relatively separate networks: the cinguloopercular (CO) and frontoparietal (FP) networks ((Dosenbach, et al., 2007); although we do no focus on them here, in addition to the CO and FP networks, the salience (Seeley, et al., 2007), and dorsal and ventral attention networks (Corbetta & Shulman, 2002) may be considered additional members of this group of control networks; see Box 1 for a description of network nomenclature).
Box 1. Control network nomenclature.
There is substantial confusion in the literature regarding the nomenclature used to refer to different control networks, with groups using disparate terminology to refer to what may be the same underlying networks (and the same terminology to refer to slightly different networks). This issue is partly historical (e.g., see CO vs. Salience below) and partly due to the multi-scale nature of brain networks (e.g., 7 vs. 17 networks in (Yeo, et al., 2011)). In our work, we place the strongest emphasis on networks that (1) are fairly consistent across different scales (i.e, graph densities), (2) are consistent across datasets, and (3) can be supported by convergent evidence (e.g., activation, lesion, or electrophysiology studies). Below is a brief discussion of the relationship of the CO and FP networks to other putative control systems: salience, executive function, and DAN.
Dosenbach and colleagues originally presented evidence for the FP and CO networks (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach, et al., 2007; Dosenbach, et al., 2006) (which we discuss in great detail in this review; note that the ‘cinguloopercular’ network is also sometimes referred to as the ‘cingulo-insular’ network (Sadaghiani, et al., 2010), reflecting the relatively better alignment of the network with the anterior insula than the frontal operculum). Shortly thereafter, Seeley and colleagues (Seeley, et al., 2007) presented evidence for a similar network distinction between an executive control network (composed of regions in dorsolateral frontal and parietal cortex) and a salience network (composed of the dACC, ventral anterior insula, as well as subcortical and limbic areas). The salience network name was applied because of a connection between this network and levels of pre-scan anxiety.
Notably, these two sets of network definitions are quite similar to one another, with the executive control network essentially similar to the FP network (see note below, however), and the CO network equivalent, perhaps, to the salience network. Indeed, if one searches through the literature on studies tied to the “cinguloopercular” or “salience” networks, there is a large correspondence in their functional loci, with naming applied as a function of tradition rather than in an attempt to distinguish the two networks. However, data-driven approaches to whole-brain network definitions do sometimes identify two separate networks: e.g., a more dorsal “CO” network and a more ventral/rostral “salience” network in the anterior insula and medial frontal cortex ((Power, et al., 2011); see Figure 2A). Indeed, a detailed examination of the functional connectivity of the anterior insula reveals that the region may be a convergence zone for multiple different networks with different patterns of functional connectivity and task activations (Nelson, et al., 2010). Furthermore, histology of different anatomical divisions of the insula may also support a division between the CO and salience networks, as more ventral regions of the anterior insula and medial frontal cortex contain von Economo neurons, neurons with large, fast conducting axons that are enriched in hominids and have been linked to self-awareness and affective functions (Seeley, et al., 2012).
Perhaps this division could also help to explain inconsistencies in the descriptions for the CO/salience network, which has been tied to various operations ranging from homeostatic regulation, pain, tracking uncertainty, and tonic alertness to task control (Dosenbach, et al., 2006; Lieberman & Eisenberger, 2015; Neta, Schlaggar, & Petersen, 2014; Sadaghiani & D’Esposito, 2015; Seeley, et al., 2012). However, the vast majority of studies examining the functional properties of either the CO or the salience networks (including many of our own) have not carefully distinguished whether activations aligned better with one network than the other. Thus, a very interesting avenue for future study will be to delineate the relative roles of these two networks, by adopting precise anatomical and network definitions for distinguishing them, perhaps even on the level of individual subjects. In the meantime, however, we suggest that it is safest to assume that “cinguloopercular” or “salience” definitions are somewhat ambiguous in the literature unless a special effort has been made to distinguish the two.
The second source of confusion in network nomenclature surrounds the FP and dorsal attention networks. These two networks are at times grouped into a single larger “executive control” network (e.g., (Liang, Zou, He, & Yang, 2016; Sridharan, Levitin, & Menon, 2008)); indeed, the label of executive/frontoparietal control network is particularly slippery, as it sometimes also refers to a conjunction of the FP and CO networks (e.g., (Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013)). Once again, data-driven clustering of networks across the brain may help to outline the differences between these networks. In our hands and that of others (Power, et al., 2011; Yeo, et al., 2011), the FP and dorsal attention networks clearly divide: the FP is composed of regions along lateral prefrontal cortex bilaterally, and regions in the intraparietal sulcus, whereas the dorsal attention network includes regions along a dorsal premotor strip (including, perhaps, the frontal eye fields) and dorsal parietal cortex spanning between the somatomotor and visual networks along the superior parietal lobule (Power, et al., 2011; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). However, as with the cinguloopercular and salience distinction, we find that the boundary between these two networks is variable across individuals (Gordon, et al., 2017) - and that, indeed, they sometimes join together at coarser “scales”, when more permissive thresholds are used (Gordon, et al., 2017). Thus, they may have related properties.
While FP & DAN (and CO & Salience) show a great deal of similarity, note that, in contrast, the CO and the FP networks themselves are clearly distinct. They have been identified as dissociable systems in two large independent samples with different data-driven network approaches (Figure 1A; (Power, et al., 2011; Yeo, et al., 2011)), and are uniformly identified as two separate networks in highly-sampled individual subjects (Gordon, et al., 2017). Furthermore, the two networks show low – and even slightly negative – inter-correlations (Figure 3A, left), underscoring their distinct nature. Together with the lesion and functional evidence reviewed in the first half of this paper, these findings emphasize that the CO and FP networks are two clearly dissociable systems. Thus, we primarily discuss the two system (CO/FP) division in this work, given the dissociations between the CO and FP systems and their strong relationships to generalizable control processes. However, it is likely that other large-scale systems contribute to aspects of control, including the DAN, Salience, VAN, and possible also DMN (Figure 2A).
Functional connectivity evidence for multiple networks
In contrast to task-based functional Magnetic Resonance Imaging (fMRI), which measures Blood-Oxygen-Level-Dependent (BOLD) activation to experimentally time-locked events, rs-FC is an fMRI technique that measures synchronous fluctuations in the BOLD signal across brain areas while participants are asked to lie quietly in the scanner, in the absence of task instructions. Regions that are part of the same network - for example, motor cortical and subcortical regions (Biswal, Yetkin, Haughton, & Hyde, 1995) – have highly correlated resting-state BOLD timecourses; thus, correlations in spontaneous BOLD signals can be used to identify functional brain networks (in addition, other related techniques, such as coherence or ICA can be used to detect synchronicity among brain regions and generally produce similar decompositions (Bassett, Meyer-Lindenberg, Achard, Duke, & Bullmore, 2006; Smith, et al., 2009; Sun, Miller, & D’Esposito, 2004)).
Rs-FC measures are related to anatomical connectivity (Honey, et al., 2009) but notably measure both direct and indirect connections as well as state-dependent correlations in the slow fluctuations of underlying neural activity (Al-Aidroos, Said, & Turk-Browne, 2012; Damoiseaux & Greicius, 2009; Greicius, Supekar, Menon, & Dougherty, 2009; Vincent, et al., 2007). The bulk of rs-FC measures are thought to reflect a stable history of functional co-activations between regions that shape the networks, possibly through Hebbian mechanisms (Harmelech, Preminger, Wertman, & Malach, 2013; Wilf, et al., 2017). This connectivity approach has the advantage of providing a powerful data-driven way to examine the systems-level organization of the brain in a living human at an unprecedented level of detail. Furthermore, this approach allows one to measure the baseline intrinsic organization of regions into brain networks, without the need to probe for selective task activations in individual networks.
When the BOLD signal of control related regions is measured at rest, rs-FC demonstrates that the regions segregate into two networks, the CO network and FP network (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach, et al., 2007). The CO and FP networks have high rs-FC among regions of their own network, but low (or negative) correlations between the two networks. The core of the CO network encompasses regions of the dorsal anterior cingulate and bilateral anterior insula, while the FP network is primarily composed of regions in bilateral dorsolateral frontal cortex and the intraparietal sulcus.
Interestingly, the same networks emerge when data-driven community-detection techniques are used to define large-scale networks throughout the entire brain, as with the more focused region-of-interest based examinations of the CO and FP networks described above; this finding has been replicated in two large datasets with different subject groups, different scanning sites, and different network definition methods (Figure 1A; (Power, et al., 2011; Yeo, et al., 2011); using, respectively, an information-based community detection algorithm (Rosvall & Bergstrom, 2008) and a non-euclidean similarity based clustering algorithm (Lashkari, Vul, Kanwisher, & Golland, 2010)). Similar divisions can also be identified in highly-sampled individual subjects (Gordon, et al., 2017). These findings emphasize the robust and segregated nature of the CO and FP networks. These whole-brain approaches also demonstrate that the CO and FP networks extend into other areas that are less commonly discussed or detected in fMRI task-activation studies of executive control, such as the posterior insula, rostral frontal cortex (CO), and the lateral occipital lobe (FP). One interesting question for future research will be to determine how these areas relate to the core regions within each network, and how the CO and FP systems relate to other putative control systems (such as the Salience and Dorsal Attention network, see Box 1).
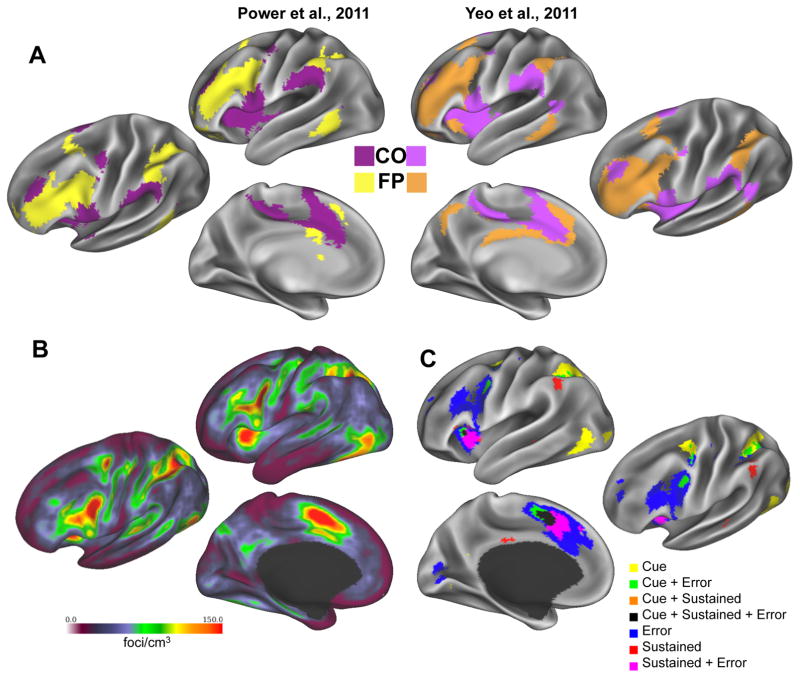
Figure 1. The cinguloopercular (CO) and frontoparietal (FP) networks.
(A) Control related regions segregate at rest into two different networks comprised of regions in the anterior insula and dorsal anterior cingulate (CO) and dorsolateral frontal and parietal cortex (FP). The CO and FP networks have been identified from resting state data using a number of different data-driven approaches (e.g., (Power, et al., 2011) definition on the left, (Yeo, et al., 2011) definition on the right) in large groups of individuals, suggesting the robustness of their separation.
(B) Furthermore, CO and FP regions are among the most common sites of activations across different tasks. This figure (adapted from (Nelson, et al., 2010)) shows a spatial overlap of activation loci from over 1000 different studies.
(C) Finally, CO and FP regions are especially common sites of control related activations in different tasks. This conjunction map of task-set signals was derived from three meta-analyses (combining, respectively, 3 (Sun, et al., under review), 12 (Neta, et al., 2015), and 13 studies (Dubis, Siegel, Neta, Visscher, & Petersen, 2016)) to show common activations for trial-level cues, errors, and sustained activity, respectively. Each individual meta-analysis map of trial-level cue, error, and sustained activity was thresholded to show the top 1% of activations and then combined to form the final conjunction map of task-set signals.
Lesion evidence for multiple networks
Effects of lesions on control network organization have also bolstered the distinction between CO and FP networks. Specifically, Nomura and colleagues measured rs-FC in patients who had chronic lesions (> 5 months post incident) to regions of either the CO or FP networks (Nomura, et al., 2010). A double dissociation was seen between the patterns of damage and the functional connectivity within each network: patients with increasing amounts of damage to regions in the CO network had progressively decreased correlations among CO, but not FP regions, and patients who had increasing amounts of damage to the FP network had decreased correlations among FP, but not CO regions. This double dissociation provides strong evidence for the independence of the CO and FP networks at rest, by showing that long-term brain damage leads to largely separable effects to the two networks. Interestingly, a subsequent project on acute brain disruptions from transcranial magnetic stimulation (TMS) demonstrated a substantially different pattern of effects, with an increase in widespread cross-network interactions after TMS (Gratton, Lee, Nomura, & D’Esposito, 2013). This finding hints that the CO and FP networks may interact dynamically in some circumstances, and is discussed more in the second half of the review.
Functional evidence for multiple networks involved in executive control
The final line of research on the differences between the CO and FP networks has focused on evidence for their distinct functional roles, as measured with activation estimates in fMRI. Resting state and lesion evidence provide strong support for the presence of two separable networks associated with FP and CO regions; however, early investigations into the functional properties of these regions highlighted the common, and ubiquitous, role of both the CO and FP networks in task activations, especially during control periods of a task ((Dosenbach, et al., 2006; Duncan & Owen, 2000); Figure 1B,C). Thus, the important question left to address is: what is the purpose of having two distinct networks for task control? How do they differ in the sets of processes that they carry out?
In order to investigate this question, we have largely focused on the contributions of the CO and FP network to three different types of control signals: signals related to cueing at the start of a task, signals related to errors made during a task, and signals sustained throughout a task. These three types of signals were selected because they highlight aspects of control related to maintaining an up-to-date task set (i.e., task-level cues to initialize task set parameters, errors to signal necessary updates to those parameters, and sustained activity to maintain those parameters across a task period), as well as more adaptive moment-to-moment forms of control (i.e., task- or trial- level cues to signal how to respond to stimuli in the subsequent period, and responses to errors to increase next-trial control and performance). This way of conceptualizing different types of control is partly driven by previous computational models of control (Logan & Gordon, 2001), which suggest that parameters related to task-set and to moment-to-moment aspects of control may be maintained and updated through distinct (but dynamically interacting) systems.
In an early study, we conducted a large-scale meta-analysis to investigate the role of FP and CO regions in these three different types of control signals (Dosenbach, et al., 2006). While regions in both the CO and FP networks were both activated in many of these control contexts, a careful assessment of these findings suggested that core CO network regions (bilateral anterior insula, dorsal anterior cingulate) were the most commonly and consistently activated in all three task-control signals. FP network regions, instead, were more closely tied with cueing and error activations. Additionally, FP network regions had previously been linked to top-down trial-specific signals related to selective attention and working memory maintenance functions (e.g., (Curtis & D’Esposito, 2003; D’Esposito, Postle, & Rypma, 2000; Gazzaley, et al., 2007)). These findings suggest that the CO network is closely tied to functions related to maintaining task sets, whereas the FP network may be more closely tied to moment-to-moment adaptive control needed for implementing specific configurations of the task.
A practical example may help to illustrate the hypothesized distinctions between these two networks. In daily life, we frequently call on different forms of top-down control, including during our engagement in recreational sports. We suggest that within this context, the CO network is important for maintaining a sustained representation of which activity one is playing (e.g., baseball or soccer) and the relevant parameters for that activity (e.g., use your hands to throw the ball vs. use your feet to kick the ball). The FP network, instead, would help to enact processes that are relevant to particular portions of the game (e.g., while batting, it is important to respond to whether the pitcher is throwing fast or curve balls in order to improve your batting percentage, and while playing soccer it is important to detect whether a goalie is shifting left or right when shooting a penalty kick to improve chances of scoring). Thus, the start of the game will be associated with both networks (the CO to maintain the relevant game parameters, the FP to coordinate the specific plays that occur at the start of the game), as well as error signals (the CO to update task representations to improve performance, e.g., if your understanding of one of the game rules or their enforcement by the referee was incorrect; the FP to adjust subsequent actions in response to the consequences of the error). The CO network will more uniquely, however, exhibit sustained activity across the entire game necessary for representing the task-state at hand.
Refinements to the model of CO and FP functions
Recent work from our laboratory has helped to bolster and refine this account, by teasing apart the specific cognitive and timecourse parameters associated with these types of control signals. For example, we have found that sustained signals are present in the CO network only in cognitively demanding (i.e., “resource-limited”), not perceptually demanding, tasks (Dubis, Siegel, Neta, Visscher, & Petersen, 2016) and that the CO network shows several different forms of performance reporting signals (e.g., responses related to reaction time and ambiguity, in addition to errors (Neta, Schlaggar, & Petersen, 2014)).
The story for activations in the FP network is a bit more mixed, largely driven by asymmetries between activations in the left and right hemispheres. The FP network is quite large and encompasses much of the lateral frontal lobe as well as pieces of the intraparietal sulcus and middle frontal gyrus. Past evidence has suggested that this large network may have distinct subunits, perhaps reflecting a finer-scaled network subdivision: lesions to the left and right frontal cortex produce distinct behavioral deficits (Stuss, 2011; Stuss & Alexander, 2007) and resting state investigations show that the FP network is among the most lateralized in the brain (Wang, Buckner, & Liu, 2014). Indeed, a number of activation studies have alluded to hemispheric asymmetries in functional activations of the frontal lobe (e.g., (D’Esposito, et al., 1998; Dobbins, Simons, & Schacter, 2004; Habib, Nyberg, & Tulving, 2003; Henson, Rugg, Shallice, & Dolan, 2000; Kelley, et al., 1998; Nee, Wager, & Jonides, 2007)) although most of these findings did not specifically place their findings in the context of the FP network.
Our recent work also demonstrates that the left (L) and right (R) divisions of the FP network have different roles in control. In one study conducting a meta-analysis of error-related responses across the brain, error-related activations were seen in both the L and R FP network, but had different characteristics (Neta, et al., 2015). The L FP network had fast and transient errors, whereas regions in the R FP network had prolonged error responses. A second meta-analysis focused on trial-level cueing and target activations (Sun, et al., under review). In this study, we found that the left FP regions had early cue responses and strong target activations, whereas the right FP network had delayed cued responses with moderate target activations. These studies indicate that the left and right FP network may be associated with distinct processes.
Although these previous findings have provided evidence for distinct functional roles in the CO and FP networks, they did so primarily by contrasting their functions in different task or trial-types. A recent set of studies from our laboratory adopted a specialized “slow reveal” paradigm to investigate whether these networks also had distinct roles, but within a decision-making trial (Gratton, et al., 2017; Ploran, et al., 2007; Wheeler, et al., 2006). In this paradigm, a stimulus is gradually unveiled from behind a noise mask. Participants are asked to respond when they have identified the item (or, in other cases, made a decision about whether they have seen the item before). By examining activation timecourses from trials in which participants responded early or late, we can separate trial-level responses with different characteristics. Interestingly, we found that regions in the CO, L FP, and R FP segregated from one another using a data-driven hierarchical clustering approach (Gratton, et al., 2017). This provides evidence that the CO, as well as the lateralized pieces of the FP network have distinct functional roles within trials. Furthermore, an analysis of the timecourses from the CO and FP networks suggested that these functions are associated with distinct aspects of decision-making: L FP regions had early onsets with gradually increasing responses that peaked around the moment of decision, much like in evidence accumulator models (Gold & Shadlen, 2007), CO regions had transient responses tightly linked to the decision, as would be expected with a performance report measure, and R FP regions had delayed and prolonged responses, primarily occurring after the response, suggesting that they were associated with post-response processing like response re-evaluation and adjustment (Gratton, et al., 2017). A related study from Sestieri and colleagues (Sestieri, Corbetta, Spadone, Romani, & Shulman, 2014) also found the FP and CO had distinct roles in long trials for perceptual and memory tasks, dissociating from one another both by the timecourses of their activity as well as their functional connectivity profiles.
Jointly, these findings are important because they provide evidence for distinct functional roles for the CO and FP networks – and for fine-tuned functional distinctions within the FP network that relate the left hemisphere more to early, stimulus or bottom-up processing functions (evidence accumulation, early cueing, strong target activations) and the right FP network to late, post-response processing that may be more tightly associated with subsequent top-down control (prolonged error responses, post-decision responses, and delayed cueing activations). Notably, in many experiments from our laboratory, these distinctions have emerged from data-driven approaches (Gratton, et al., 2017; Neta, et al., 2015; Sun, et al., under review): that is regions within the same network clustered together based on their functional activation properties alone, providing convergent evidence to the resting state findings for the presence of at least two distinct control networks in the brain.
Alternative models for the cinguloopercular and frontoparietal regions
Unitary vs. multi-system models of executive control
An early and influential model of control functions of the human brain emphasized their common activation across many different task contexts that required control (Duncan & Owen, 2000). Evidence of commonalities in the patterns of frontal activity associated with many different cognitive demands were shown in a systematic review conducted by Duncan & Owen (2000). Specifically, Duncan and Owen conducted a large-scale meta-analysis combining 20 studies in the literature that had selectively manipulated one of five cognitive demands in the context of an otherwise identical task. The demands ranged from suppression of strong but inappropriate response tendencies, to increasing working memory load, to varying perceptual difficulty. All five demands showed a similar pattern of co-recruitment in common control regions, e.g. the dorsal anterior cingulate, the inferior frontal sulcus, and the frontal operculum. Hence, one interpretation of the result argues for the presence of a unitary model of control in these “multiple demand” regions, which collectively adjust their function to match the requirements of the particular task at hand. Indeed, as we’ve already noted, these regions are among the most commonly engaged in different tasks and are difficult to distinguish based on functional activation (see previous section on the relatively fine-tuned differences in temporal properties that are only evident in analyses comparing the timecourses of activation profiles). Further support for flexible task-related adjustments in these multiple-demand regions comes from analyzing multi-voxel patterns of activity in these regions, which can discriminate a range of task-relevant information including stimuli, task rules, and participant responses (Woolgar, Afshar, Williams, & Rich, 2015; Woolgar, Hampshire, Thompson, & Duncan, 2011; Woolgar, Thompson, Bor, & Duncan, 2011). However, a recent study from Crittenden and colleagues (Crittenden, Mitchell, & Duncan, 2016) demonstrated that the CO and FP networks can also be distinguished in the information that they carry about task rules. This suggests that the unitary characterization of the multi-demand “network” may also contain evidence for two distinct control networks within it. In this study, FP regions contained significantly more specific task rule information than CO regions. As the rules changed from trial to trial, this may be consistent with an adaptive control function of the FP network.
Another prominent model from Botvinick and colleagues (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Kerns, et al., 2004; MacDonald, Cohen, Stenger, & Carter, 2000), proposes that the dACC and lateral prefrontal cortex are central to cognitive control, with the dACC serving as a “conflict monitor”, which, when conflict is detected, signals lateral prefrontal cortex to implement needed adjustments in control. This model resembles our findings in proposing a central role for the dACC and lateral prefrontal cortex in control. However, we differ in proposing that these regions are actually part of two separate networks with different sets of functions and largely independent responses. By placing these results within the context of the cinguloopercular and frontoparietal networks, we add precision to the anatomical description of these regions (e.g., Figure 1 and 2A, which show that many distinct networks overlap in lateral and medial prefrontal cortex) and bring to prominence the similarity of function among regions within each network (i.e., between the dACC and the anterior insula, and between the FP portions of dorsal frontal and parietal cortex– regions that are not typically included in the Botvinick model). Moreover, we differ in the specific interpretations of functional roles for these regions (e.g., we suggest that the CO, including both the dACC and aI/fO, is involved in task set maintenance, rather than conflict monitoring, due in part to its pronounced cueing responses, sustained representations during tasks, and transient activations at decision points of trials).
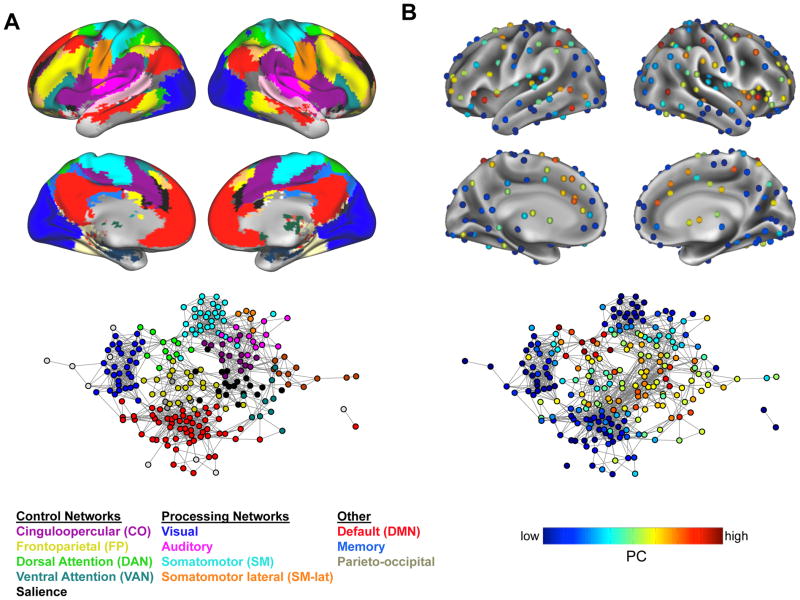
Figure 2. The CO and FP networks in the context of whole-brain interactions.
(A) In addition to the CO and FP networks, a number of other networks are found in healthy individuals that have specialized roles in control (e.g., DAN, VAN, Salience), basic processing (visual, auditory, somatomotor, and somatomotor-lateral), or other functions (e.g., default mode, parietal memory, and the parieto-occipital network). Large-scale networks can be displayed either on a brain, emphasizing their anatomical locations (top), or in a graph to emphasize their topological properties (bottom). In the graph model, individual brain regions are shown as nodes, and connections between brain regions are shown as lines or edges; regions have been arranged using a spring-embedding algorithm, such that nodes with stronger connections are placed more closely together. This graph display highlights the relationships between regions in each network, and between networks, showing that network nodes cluster closely together, with relatively few connections between networks. Control networks (CO, FP, DAN, salience) tend to lie between other networks and have many nodes that are centrally positioned in the graph.
(B) Graph measures can also be used to better understand cross-network interactions within the context of multiple networks throughout the brain. Within this framework, certain brain regions (connector hubs) serve as sites for mediating cross-network interactions. These regions can be identified using the participation coefficient (PC) metric, which measures the distribution of a node’s connections across different networks. As is shown, high PC regions are found largely, but not uniquely, in control networks, and may serve as integral sites for modulating basic processing based on top-down goals.
Non-control based models for the functions of the CO and FP networks
Although we have focused on a “control”-based interpretation for the CO and FP networks, related distinctions between these networks have also been proposed by other groups, which emphasize functions not as directly related to top-down control. For example, one early study identified two networks that segregated at rest with very similar profiles to the CO and FP networks, but suggested that the CO network was related to “salience” rather than task control, in part due to its connections with measures of pre-scan anxiety (Seeley, et al., 2007). Thus, one interpretation of this network is that it may be involved in more generic arousal, rather than task control per-se (note also, as discussed in Box 1, that the salience network may represent a functionally distinct, but anatomically adjacent, network from the CO).
A related set of studies has focused on the connection between the CO and FP networks and ongoing fluctuations in activity, leading Sadaghiani and colleagues to connect the CO network to generalized tonic alertness (rather than task-control), and the FP network with phasic inhibition (Sadaghiani, et al., 2012; Sadaghiani, et al., 2010). Sadaghiani and colleagues have examined the roles for the CO and FP networks using fMRI and combined fMRI-EEG studies. In their initial findings, they observed that pre-trial activity in the CO (also called the cingulo-insular-thalamic, see Box 1) network was associated with an increased chance that participants would observe a near-threshold stimulus in a prolonged sustained attention task (pretrial activity in the FP and DAN, instead, were associated with misses; (Sadaghiani, Hesselmann, & Kleinschmidt, 2009)). This suggested to the authors that the network might be connected to aspects of tonic alertness; and indeed, a second study using a combination of fMRI and EEG recordings found that fluctuations in endogenous alpha power – a signature of sustained or tonic alertness – were selectively correlated with the CO network (Sadaghiani, et al., 2010). The FP network, instead, was more related to alpha phase synchrony (Sadaghiani, et al., 2012), suggesting that it may be indexing phasic components of alertness and inhibition.
This interpretation is connected to our proposed models of the CO and FP network; both models suggest that the CO network is related to a more sustained signal, whereas the FP network is more closely related to a moment-to-moment adaptive signal, although they differ in whether sustained activations are interpreted in terms of a generalized alertness signal or a more specialized task-set maintenance signal. Indeed, in a later study, Sadaghiani and colleagues (Sadaghiani & D’Esposito, 2015) manipulated task alertness by comparing activations to near-threshold stimuli in a jittered or regular presentation. They found that this manipulation selectively modulated activity in the CO, rather than the FP or dorsal attention networks (which were, instead, affected by the difficulty of a selective attention comparison between pitch intervals). Again, these results could be interpreted as consistent with a tonic alertness role for the CO network, but could alternatively be interpreted as consistent with a task set role in which task set is modified by stimulus jittering. However, other findings that were previously discussed may be harder to reconcile with a pure alertness model for the CO network – including (a) the presence of multiple specific task set and control signals in these regions (Dosenbach, et al., 2006; Duncan & Owen, 2000; Woolgar, Afshar, Williams, & Rich, 2015; Woolgar, Hampshire, Thompson, & Duncan, 2011; Woolgar, Thompson, Bor, & Duncan, 2011), (b) the separable activation of CO regions to multiple performance-related variables such as ambiguity and errors in addition to reaction time (Neta, Schlaggar, & Petersen, 2014), and (c) the selectivity of sustained CO activations to resource- rather than perceptually-limited tasks (Dubis, Siegel, Neta, Visscher, & Petersen, 2016). It is possible that the CO network carries out processes related to both alertness and top-down control; alternatively, the tonic-alertness processes ascribed to the CO network may be better related to nearby regions within the salience network (perhaps consistent with previous models associating the salience network with arousal).
Summary
In summary, in this first section of our paper we have provided convergent evidence for the presence of two distinct control networks of the brain, with different network interactions, dissociable responses to chronic lesions, and distinct functions in a task – even in single trials of a task. The presence of these two networks places constraints on the mechanisms that underlie goal-directed behavior, arguing that there are likely two separate sets of executive control functions associated, respectively, with task-set maintenance (for the CO network) and adaptive control (for the FP network). Furthermore, recent evidence from our laboratory suggests that the FP network may have separate subcomponents of adaptive control associated with the left and right hemispheres. Future research will be needed to understand the specialized roles of each of the regions within these two networks, and their association with specific processes involved in top-down control. In the next section we tackle another important question – how the CO and FP networks interact with each other and with other parts of the brain to help us carry out goal-directed behavior.
3. How control networks interact: the role of hubs in control
In the previous section, we presented convergent evidence for the existence of at least two distinct control networks in the human brain based on functional connectivity, lesion, and activation studies. However, given the presence of multiple specialized control systems, the question arises of how these systems interact with one another in order to coordinate control signals. Furthermore, a critical function of control networks is to modify the processing within other networks (Figure 2A) based on one’s current goals. Thus, in this second half of the review we will examine evidence for changes in interactions among networks throughout the brain during complex tasks, and the role of specialized hub regions in mediating these changes.
Control network interactions
First, we present evidence that control network interactions are malleable and vary in their interactions across different contexts. As we discussed in the first half of this review, over long time-scales (i.e., > 5 months after a lesion) control networks respond independently to brain damage localized within each network (Nomura, et al., 2010). However, acute disruptions from continuous theta-burst transcranial magnetic stimulation (TMS), which has been demonstrated to transiently inhibit underlying tissue (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005), elicited a different pattern of responses, with widespread, cross-network interactions after TMS to control networks that were not present with TMS to somatosensory processing regions (Gratton, Lee, Nomura, & D’Esposito, 2013). While the mechanisms of TMS differ in a number of ways from the effects of a chronic brain lesion, these findings may suggest that control networks interact dynamically in certain contexts – perhaps in situations where a recent perturbation of the system (e.g., from acute disruptions or demanding task goals) require increased coordination across control networks.
In support of this idea, network interactions are subtly but systematically modified while participants are engaged in complex tasks compared with rest (Figure 3A; (Cole, Bassett, Power, Braver, & Petersen, 2014; Gratton, Laumann, Gordon, Adeyemo, & Petersen, 2016). Furthermore, these studies have demonstrated that, during tasks, control networks show especially altered interactions with processing networks (e.g., FP to visual, motor networks). Convergent evidence from cortical cooling of control regions in macaques and TMS to control regions in humans also indicates that these changes in interactions between control and processing networks may be important for properly redirecting basic processing based on task-goals. In these studies, disruptions in control related regions decreased the tuning of extrastriate visual cortex responses and caused decrements in task performance (Lee & D’Esposito, 2012; Miller & D’Esposito, 2005; Moore & Armstrong, 2003; Ruff, et al., 2008; Ruff, et al., 2006; Zanto, Rubens, Thangavel, & Gazzaley, 2011). Jointly, these findings indicate that control networks alter their interactions with processing networks during task contexts to modify processing based on task goals.
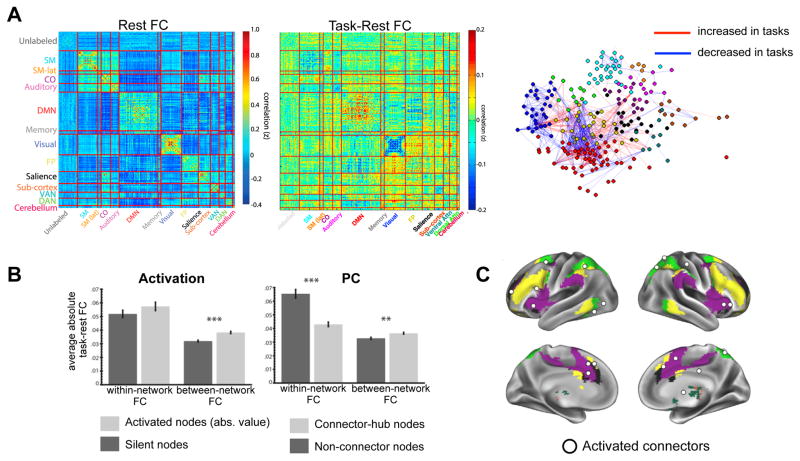
Figure 3. Network organization is systematically altered in different task contexts, especially for activated regions and hubs.
(A) Network organization at rest is shown in the form of a correlation matrix (left). At rest, network organization is dominated by high-correlations within each network (on diagonal), and low correlations between different networks (off-diagonal). During tasks, FC changes systematically both within and between networks, as shown in both the difference correlation matrix (center) and spring embedded plot (right). Especially prominent changes are seen within processing networks (visual, somatomotor, and DMN), between control and processing networks (e.g., FP to visual), and among control networks (e.g., FP to DAN and CO/Salience).
(B, Left) Task-activated regions had high changes in functional connectivity during tasks, especially between networks (B, Right) Connector hubs showed more fine-tuned modulations during tasks – like activated regions, connector hubs showed highly altered functional connectivity between networks, but additionally showed relatively stable within network functional connectivity.
(C) Regions that were both activated and connector hubs (“activated connectors”; shown as white spheres) had a constellation of attributes that tightly linked them to task-control, including their localization primarily to control-networks of the brain (color underlay; yellow = FP, purple = CO, green = DAN, black = salience).
These figures were modified from Gratton et al. (2016).
In addition to changes between control and processing networks, control networks also become more integrated with one another (e.g., FP with CO) during complex tasks (Figure 3A, (Gratton, Laumann, Gordon, Adeyemo, & Petersen, 2016)). This effect may be augmented even further with increases in the working-memory load of tasks (Cohen, Gallen, Jacobs, Lee, & D’Esposito, 2014). One possibility is that these increases are necessary in complex tasks to allow control networks to coordinate, update, and maintain relevant parameters for completing the tasks. Sridharan and colleagues (Sridharan, Levitin, & Menon, 2008) also found changes in control network interactions during tasks, and suggest that the CO network (or salience, in their study) may have a top-level role in directing changes in functional connectivity between the FP and default mode network, and, potentially, more generally between networks throughout the brain (Menon & Uddin, 2010). These findings support the idea that control network interactions may be altered dynamically, although subtly, in different goal-directed contexts.
Finally, a number of studies have shown that control network interactions can also be modified systematically with age. Spreng et. al. (2012) show that control networks can flexibly interact with the default mode network, depending on the demands of a task; however this ability is lost in older subjects, whose control networks more constantly interact with the default mode. Similar reports of de-differentiation of networks have been reported in aging across many networks, but are especially linked with the loss of modularity in control networks (Chan, Park, Savalia, Petersen, & Wig, 2014). These findings indicate that interactions between networks, especially control-related networks, may be integral to healthy brain function and cognition.
Role of hubs in network interactions
We therefore turn to the question of how different networks interact – and whether certain regions are particularly critical for these interactions. In order to examine this idea, we utilize concepts borrowed from complex systems science, and in particular the idea of “hubs” (Power, Schlaggar, Lessov-Schlaggar, & Petersen, 2013). The term “hub” has a colloquial, intuitive appeal as a centrally important unit in a large system, whether it is an affable person in a social network of friendships, a popular website in a network of internet traffic, or a busy airport in a network of flights across the world. However, there are many ways to quantify importance. To take the example of the air transportation network (Guimera, Mossa, Turtschi, & Amaral, 2005), some airports may be important because of the high amount of incoming domestic flights. Others may be important because they are the international transferring sites between one country (e.g., the US) and another (e.g., the UK). The consequences of a disruption to each of these airports may differ in the magnitude and extent of impact.
Similarly, brain networks may be made up both of hubs that are important for the function within a particular brain network and hubs that may be important for the transfer of information between networks. Graph theory can help us quantify each of these properties and identify such hubs. Graph theoretical methods are based on modeling the brain as a graph, in which each brain region is represented by a node on the graph and connections (i.e., correlations from resting state functional connectivity) are represented as edges between the nodes (Sporns, 2011). Different types of hubs can be measured using these techniques. “Within-module degree” is a normalized measure of the number of connections a region has to its own network, and thus measures within-network hubs. “Participation coefficient” is a measure of the dispersal of a region’s connections across different networks, and can thus be used to measure cross-network connector hubs (Guimera & Nunes Amaral, 2005). For technical reasons, defining hubs based on their total number of connections (i.e., degree) with functional connectivity is problematic (this has to do with the use of correlation metrics, which allow for shared common variance among signals that are in the same network, and thus conflate degree-based measures with network size (Power, Schlaggar, Lessov-Schlaggar, & Petersen, 2013)). Connector hubs are frequently found in the anterior insula, middle frontal cortex, and dorsolateral frontal and parietal cortex, whereas within-network hubs are found in regions such as the posterior and rostral anterior cingulate (Figure 2B). Notably, other approaches to measuring cross-network hubs, using link-communities (Sun, Gratton, & Petersen, under review) and system density (Power, Schlaggar, Lessov-Schlaggar, & Petersen, 2013) converge on identifying similar hub locations as participation coefficient.
Because control functions are tightly linked with coordinating the interactions between control and processing networks, we might expect that connector hubs will be especially important for brain function, especially in completing complex tasks. In a recent study, we put this hypothesis to the test (Gratton, Laumann, Gordon, Adeyemo, & Petersen, 2016). Specifically, we examined how functional connectivity was modified across three diverse complex tasks, requiring semantic processing, visual coherence judgments, or mental rotation of objects (Figure 3A). We then examined whether functional connectivity changes were especially likely to be mediated by connector hubs. We found support for a unique role for connector hubs: connectors were sites of high changes in functional connectivity between different networks, but exhibited relatively stable functional connectivity within their own network (Figure 3B). This result is also related to findings from the literature showing that hubs are sites of particularly flexible activations across many different cognitive processes (Bertolero, Yeo, & D’Esposito, 2015; Yeo, et al., 2015), and with suggestions that hubs may make important contributions to many different task contexts (Cole, et al., 2013).
Interestingly, we found that task-activated regions (i.e., regions specialized for the task at hand) also showed high changes in functional connectivity, but independent from the changes seen with connectors (Figure 3B). This suggests that these two properties – hub specialization within a complex system, and functional specialization based on a region’s processing characteristics – index two distinct factors that allow our brain networks to flexibly adapt to different task contexts. Of note, regions that were both connectors and activated (see locations in Figure 3C, top 25% of both properties) had a unique combination of attributes that tied them strongly to task control: (a) they were composed of core regions in the FP, CO, and dorsal attention networks, (b) they showed the highest levels of between-network functional connectivity changes, relative to regions that were only activated or only connectors, (c) these changes included increases in functional connectivity with relevant processing networks as well as other control systems, and (d) activated connectors had especially high flexibility in the pattern of functional connectivity changes across different task contexts (i.e., their functional connectivity varied more from task to task than simple activated regions; see (Gratton, Laumann, Gordon, Adeyemo, & Petersen, 2016) for more details on these measures). These attributes argue that activated connectors may help to mediate task control.
A final pair of lesion studies provides convergent evidence that connectors are indeed critical to brain network organization and the performance of complex tasks (Figure 4), providing physical evidence to corroborate previous simulation studies that suggested that connector hub removal might be especially damaging to brain networks (He, et al., 2009; Honey & Sporns, 2008). In these studies, patients with brain lesions to connector hub locations were compared with patients who had damage to non-connector (e.g., within network-hub) locations. In an initial study, we (Gratton, Nomura, Perez, & D’Esposito, 2012) examined rs-FC network organization in patients and found that damage to connector hubs was associated with widespread network disruption of the brain, while the same was not true of damage to network-hubs (Figure 4A). A second study (Warren, et al., 2014) found that damage to connector hubs was also associated with widespread disruption in behavioral performance, across many different neuropsychological domains, which was not seen with damage to regions associated with only high numbers of connections (Figure 4B). Together, these studies suggest that connector hubs may be critical locations of the brain, responsible for maintaining healthy network organization and behavioral performance.
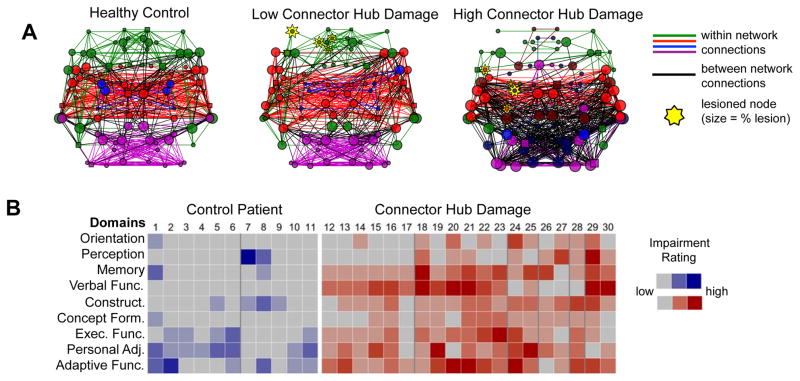
Figure 4. Impact of damage to connector hubs.
(A) We examined how damage to connector hubs impacted brain network organization by looking at rs-FC in patients with high or low connector hub damage. Each column shows an example brain network from health control patients (left), a patient with low amounts of connector hub damage (middle), and a patient with high amounts of connector hub damage (right; the two patients had approximately equal total amounts of damage). The black lines mark between-network connections and high numbers of black lines indicate poor division of the brain into different networks. Damage to connector hubs dramatically alters brain network organization, decreasing the integrity of networks throughout the brain. Modified from Gratton et al. (2012).
(B) Damage to connector hubs is also associated with impaired behavioral performance. Here, each column represents data from a single patient with damage to either a control location (blue, left side) or to a connector hub (red, right side). Different rows represent different Lezak behavioral domains. Patients were rated according to their impairment in each behavioral domain by trained neuropsychologists. Damage to connector hubs was associated with more widespread and profound impairment across many behavioral domains, than was damage to control locations. Modified from Warren et al. (2014).
This line of research has novel implications for how we think about brain damage and disease – it suggests that understanding the network organization of the brain, and how control is implemented through these complex systems interactions, can provide unique information about severity and symptomology after brain damage, and may guide us to new understandings for how to treat patients in the future. Already, one study has found that information about network organization can predict which patients with traumatic brain injury are most likely to improve after a course of intensive cognitive training (Arnemann, et al., 2015). Further exciting work along this line of research is being conducted in animal models using DREADDS (designer drugs targeted at specialized genetically inserted receptors) to specifically deactivate network components (in this case, the amygdala) and monitor for changes in distributed large-scale network properties (Grayson, et al., 2016).
Summary
In this section we discuss convergent evidence that control network interactions may underlie many goal-directed functions in the brain, and that hubs may be especially critical sites for these between-network interactions. This view is supported by data from functional connectivity at rest and during tasks, as well as the impact of brain lesions to hubs on network organization and performance. These findings are important because they argue that top-down control functions in the brain are (a) linked to cross-network interactions and (b) constrained by aspects of the intrinsic network architecture of the brain. Furthermore, they suggest that hub regions are specialized and especially critical regions of the brain that may help to mediate various goal-directed functions.
4. Looking forward: summary and future directions
Across the course of this review, we have demonstrated that the network organization of the brain has consequences for cognition and, especially, goal-directed functions. The first section indicates that at least two dissociable classes of top-down control processes are present and organized into separate brain systems. In the second section, we show that using novel network analysis techniques to identify hubs highlights regions that are important for network maintenance and engaging in a variety of different complex tasks with variable control and processing demands. This research demonstrates that a network view of control functions can illuminate new aspects of the neurobiological underpinnings of top-down control.
The next task will be to describe more clearly what mechanisms are employed by hubs to maintain and change brain networks, and how they interact with the functional specializations within each region. For example, one question is whether hubs are general purpose, or if the use of specific hubs varies depending on the task context. An important, related, question is how regions within the CO (or FP) network differ from one another in their relative functional specializations (i.e., what distinct functions does the aI/fO compute relative to the dACC, given their similar activation profile?). A combination of process-level investigations, computational models, and experiments in lesion and animal models may shed new light on these issues.
Another issue of interest will be to investigate the clinical impact of these findings. Recent work is beginning to demonstrate that control network localization varies somewhat across individuals, although these networks are often quite stable within an individual (Gordon, Laumann, Adeyemo, & Petersen, 2017; Laumann, et al., 2015; Mueller, et al., 2013). Future work will need to investigate the implications of this individual variability for our understanding of control networks and hub functions; furthermore, these observations argue for moving toward a detailed analysis of data from individual subjects in order to make observations that will be relevant for personalized medicine (Gordon, et al., 2017).
Finally, as we noted at the start, functional connectivity measures are best suited to measure stable intrinsic network properties or very slow temporal dynamics associated with shifts in different states (e.g., tasks, sleep). An interesting avenue for the future will be to combine these detailed spatial observations regarding brain network organization in fMRI with other methodologies with faster temporal resolutions (e.g., EEG, MEG, optical imaging, or ECOG) that may measure the faster temporal dynamics within each of these networks. Together, these techniques may further serve to illuminate the mechanisms that underlie top-down control, at multiple spatial and temporal levels.
Acknowledgments
We thank Steve Nelson for his help with creating Figure 1B, and G. Gratton and M. Fabiani for their feedback on the manuscript. We would also like to acknowledge our funding sources (McDonnell Foundation Collaborative Activity Award, NIH R01NS32979, and NIH R01NS06424 to S.E.P., an NIH F32NS092290 to C.G. and NIH T32 HS).
References
- Al-Aidroos N, Said CP, Turk-Browne NB. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proc Natl Acad Sci U S A. 2012;109:14675–14680. doi: 10.1073/pnas.1202095109. https://doi.orig/10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnemann KL, Chen AJ, Novakovic-Agopian T, Gratton C, Nomura EM, D’Esposito M. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology. 2015;84:1568–1574. doi: 10.1212/WNL.0000000000001476. https://doi.orig/10.1212/WNL.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. https://doi.orig/10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Baniqued PL, Low KA, Fletcher MA, Gratton G, Fabiani M. Shedding light on gray(ing) areas: Connectivity and task switching dynamics in aging. Psychophysiology. 2017 doi: 10.1111/psyp.12818. https://doi.orig/10.1111/psyp.12818. [DOI] [PubMed]
- Barcelo F, Cooper PS. An information theory account of late frontoparietal ERP positivities in cognitive control. Psychophysiology. 2017 doi: 10.1111/psyp.12814. https://doi.orig/10.1111/psyp.12814. [DOI] [PubMed]
- Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci U S A. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. https://doi.orig/10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BT, D’Esposito M. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A. 2015;112:E6798–6807. doi: 10.1073/pnas.1510619112. https://doi.orig/10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. https://doi.orig/10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Boudewyn MA, Carter CS. Electrophysiological correlates of adaptive control and attentional engagement in patients with first episode schizophrenia and healthy young adults. Psychophysiology. 2017 doi: 10.1111/psyp.12820. https://doi.orig/10.1111/psyp.12820. [DOI] [PMC free article] [PubMed]
- Braga RM, Buckner RL. Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron. 2017;95:457–471. e455. doi: 10.1016/j.neuron.2017.06.038. https://doi.orig/10.1016/j.neuron.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111:E4997–5006. doi: 10.1073/pnas.1415122111. https://doi.orig/10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Gallen CL, Jacobs EG, Lee TG, D’Esposito M. Quantifying the reconfiguration of intrinsic networks during working memory. PLoS One. 2014;9:e106636. doi: 10.1371/journal.pone.0106636. https://doi.orig/10.1371/journal.pone.0106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. https://doi.orig/10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. https://doi.orig/10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Watson JM, Strayer DL. Working memory capacity and task goals modulate error-related ERPs. Psychophysiology. 2017 doi: 10.1111/psyp.12805. https://doi.orig/10.1111/psyp.12805. [DOI] [PubMed]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. https://doi.orig/10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crittenden BM, Mitchell DJ, Duncan J. Task Encoding across the Multiple Demand Cortex Is Consistent with a Frontoparietal and Cingulo-Opercular Dual Networks Distinction. J Neurosci. 2016;36:6147–6155. doi: 10.1523/JNEUROSCI.4590-15.2016. https://doi.orig/10.1523/JNEUROSCI.4590-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. https://doi.orig/10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. https://doi.orig/10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 2004;16:908–920. doi: 10.1162/0898929041502751. https://doi.orig/10.1162/0898929041502751. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. https://doi.orig/10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. https://doi.orig/10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. https://doi.orig/10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubis JW, Siegel JS, Neta M, Visscher KM, Petersen SE. Tasks Driven by Perceptual Information Do Not Recruit Sustained BOLD Activity in Cingulo-Opercular Regions. Cereb Cortex. 2016;26:192–201. doi: 10.1093/cercor/bhu187. https://doi.orig/10.1093/cercor/bhu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D’Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–135. doi: 10.1093/cercor/bhm113. https://doi.orig/10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. https://doi.orig/10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Petersen SE. Individual Variability of the System-Level Organization of the Human Brain. Cereb Cortex. 2017;27:386–399. doi: 10.1093/cercor/bhv239. https://doi.orig/10.1093/cercor/bhv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NUF. Precision Functional Mapping of Individual Human Brains. Neuron. 2017;95:791–807. e797. doi: 10.1016/j.neuron.2017.07.011. https://doi.orig/10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Gordon EM, Adeyemo B, Petersen SE. Evidence for Two Independent Factors that Modify Brain Networks to Meet Task Goals. Cell Rep. 2016;17:1276–1288. doi: 10.1016/j.celrep.2016.10.002. https://doi.orig/10.1016/j.celrep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Lee TG, Nomura EM, D’Esposito M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci. 2013;7:124. doi: 10.3389/fnsys.2013.00124. https://doi.orig/10.3389/fnsys.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Neta M, Sun H, Ploran EJ, Schlaggar BL, Wheeler ME, Petersen SE, Nelson SM. Distinct Stages of Moment-to-Moment Processing in the Cinguloopercular and Frontoparietal Networks. Cereb Cortex. 2017;27:2403–2417. doi: 10.1093/cercor/bhw092. https://doi.orig/10.1093/cercor/bhw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Nomura EM, Perez F, D’Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J Cogn Neurosci. 2012;24:1275–1285. doi: 10.1162/jocn_a_00222. https://doi.orig/10.1162/jocn_a_00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G. Brain Reflections: A circuit-based framework for understanding information processing and cognitive control. Psychophysiology. 2018 doi: 10.1111/psyp.13038. [DOI] [PubMed] [Google Scholar]
- Gratton G, Cooper P, Fabiani M, Carter CS, Karayanidis F. Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology. 2018 doi: 10.1111/psyp.13016. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, Fair DA, Amaral DG. The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron. 2016;91:453–466. doi: 10.1016/j.neuron.2016.06.005. https://doi.orig/10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. https://doi.orig/10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. https://doi.orig/10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Mossa S, Turtschi A, Amaral LA. The worldwide air transportation network: Anomalous centrality, community structure, and cities’ global roles. Proc Natl Acad Sci U S A. 2005;102:7794–7799. doi: 10.1073/pnas.0407994102. https://doi.orig/10.1073/pnas.0407994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. https://doi.orig/10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci. 2003;7:241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Harmelech T, Preminger S, Wertman E, Malach R. The day-after effect: long term, Hebbian-like restructuring of resting-state fMRI patterns induced by a single epoch of cortical activation. J Neurosci. 2013;33:9488–9497. doi: 10.1523/JNEUROSCI.5911-12.2013. https://doi.orig/10.1523/JNEUROSCI.5911-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. https://doi.orig/10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp. 2008;29:802–809. doi: 10.1002/hbm.20579. https://doi.orig/10.1002/hbm.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. https://doi.orig/10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. https://doi.orig/10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. https://doi.orig/10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. https://doi.orig/10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Yeo BT, Buckner RL. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philos Trans R Soc Lond B Biol Sci. 2014:369. doi: 10.1098/rstb.2013.0526. https://doi.orig/10.1098/rstb.2013.0526. [DOI] [PMC free article] [PubMed]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. https://doi.orig/10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashkari D, Vul E, Kanwisher N, Golland P. Discovering structure in the space of fMRI selectivity profiles. Neuroimage. 2010;50:1085–1098. doi: 10.1016/j.neuroimage.2009.12.106. https://doi.orig/10.1016/j.neuroimage.2009.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, Schlaggar BL, Mumford JA, Poldrack RA, Petersen SE. Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron. 2015;87:657–670. doi: 10.1016/j.neuron.2015.06.037. https://doi.orig/10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A, Gordon EM, Gratton C, Adeyemo B, Gilmore AW, Nelson SM, Berg JJ, Greene DJ, McCarthy JE, Tagliazucchi E, Laufs H, Schlaggar BL, Dosenbach NU, Petersen SE. On the Stability of BOLD fMRI Correlations. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw265. https://doi.orig/10.1093/cercor/bhw265. [DOI] [PMC free article] [PubMed]
- Lee TG, D’Esposito M. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J Neurosci. 2012;32:15458–15466. doi: 10.1523/JNEUROSCI.0627-12.2012. https://doi.orig/10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. Topologically Reorganized Connectivity Architecture of Default-Mode, Executive-Control, and Salience Networks across Working Memory Task Loads. Cereb Cortex. 2016;26:1501–1511. doi: 10.1093/cercor/bhu316. https://doi.orig/10.1093/cercor/bhu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci U S A. 2015;112:15250–15255. doi: 10.1073/pnas.1515083112. https://doi.orig/10.1073/pnas.1515083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois R, Laumann TO, Snyder AZ, Zhou J, Yeo BTT. Interpreting temporal fluctuations in resting-state functional connectivity MRI. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.09.012. https://doi.orig/10.1016/j.neuroimage.2017.09.012. [DOI] [PubMed]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. https://doi.orig/10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. https://doi.orig/10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. https://doi.orig/10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. https://doi.orig/10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. https://doi.orig/10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–595. doi: 10.1016/j.neuron.2012.12.028. https://doi.orig/10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. https://doi.orig/10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Miezin FM, Nelson SM, Dubis JW, Dosenbach NU, Schlaggar BL, Petersen SE. Spatial and temporal characteristics of error-related activity in the human brain. J Neurosci. 2015;35:253–266. doi: 10.1523/JNEUROSCI.1313-14.2015. https://doi.orig/10.1523/JNEUROSCI.1313-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Schlaggar BL, Petersen SE. Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. Neuroimage. 2014;99:59–68. doi: 10.1016/j.neuroimage.2014.05.053. https://doi.orig/10.1016/j.neuroimage.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D’Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci U S A. 2010;107:12017–12022. doi: 10.1073/pnas.1002431107. https://doi.orig/10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME. Evidence accumulation and the moment of recognition: Dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27:11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. https://doi.orig/27/44/11912 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. https://doi.orig/10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. https://doi.orig/10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci U S A. 2008;105:1118–1123. doi: 10.1073/pnas.0706851105. https://doi.orig/10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. https://doi.orig/10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. https://doi.orig/10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, D’Esposito M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cereb Cortex. 2015;25:2763–2773. doi: 10.1093/cercor/bhu072. https://doi.orig/10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. https://doi.orig/10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, D’Esposito M, Kleinschmidt A. alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J Neurosci. 2012;32:14305–14310. doi: 10.1523/JNEUROSCI.1358-12.2012. https://doi.orig/10.1523/JNEUROSCI.1358-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. https://doi.orig/10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. https://doi.orig/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Merkle FT, Gaus SE, Craig AD, Allman JM, Hof PR. Distinctive neurons of the anterior cingulate and frontoinsular cortex: a historical perspective. Cereb Cortex. 2012;22:245–250. doi: 10.1093/cercor/bhr005. https://doi.orig/10.1093/cercor/bhr005. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 2014;26:551–568. doi: 10.1162/jocn_a_00504. https://doi.orig/10.1162/jocn_a_00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. https://doi.orig/10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. https://doi.orig/10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Networks of the brain. Cambridge, Mass: MIT Press; 2011. [Google Scholar]
- Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. https://doi.orig/10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. https://doi.orig/10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. https://doi.orig/10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17:759–765. doi: 10.1017/S1355617711000695. https://doi.orig/10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. https://doi.orig/10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Miller LM, D’Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004;21:647–658. doi: 10.1016/j.neuroimage.2003.09.056. https://doi.orig/10.1016/j.neuroimage.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Sun H, Gratton C, Petersen SE. Overlapping functional systems organization identifies inter-community relationships under review. [Google Scholar]
- Sun H, Neta M, Gratton C, Church JA, Dubis JW, Schlaggar BL, Wheeler ME, Petersen SE. Distinct response patterns in functional systems during goal-directed tasks under review. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. https://doi.orig/10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. https://doi.orig/10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Liu H. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J Neurosci. 2014;34:12341–12352. doi: 10.1523/JNEUROSCI.0787-14.2014. https://doi.orig/10.1523/JNEUROSCI.0787-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, Sun H, Petersen SE, Tranel D. Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci U S A. 2014;111:14247–14252. doi: 10.1073/pnas.1322173111. https://doi.orig/10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR. Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology. 2017 doi: 10.1111/psyp.12871. https://doi.orig/10.1111/psyp.12871. [DOI] [PubMed]
- Wheeler ME, Shulman GL, Buckner RL, Miezin FM, Velanova K, Petersen SE. Evidence for separate perceptual reactivation and search processes during remembering. Cereb Cortex. 2006;16:949–959. doi: 10.1093/cercor/bhj037. https://doi.orig/10.1093/cercor/bhj037. [DOI] [PubMed] [Google Scholar]
- Wilf M, Strappini F, Golan T, Hahamy A, Harel M, Malach R. Spontaneously Emerging Patterns in Human Visual Cortex Reflect Responses to Naturalistic Sensory Stimuli. Cereb Cortex. 2017;27:750–763. doi: 10.1093/cercor/bhv275. https://doi.orig/10.1093/cercor/bhv275. [DOI] [PubMed] [Google Scholar]
- Woolgar A, Afshar S, Williams MA, Rich AN. Flexible Coding of Task Rules in Frontoparietal Cortex: An Adaptive System for Flexible Cognitive Control. J Cogn Neurosci. 2015;27:1895–1911. doi: 10.1162/jocn_a_00827. https://doi.orig/10.1162/jocn_a_00827. [DOI] [PubMed] [Google Scholar]
- Woolgar A, Hampshire A, Thompson R, Duncan J. Adaptive coding of task-relevant information in human frontoparietal cortex. J Neurosci. 2011;31:14592–14599. doi: 10.1523/JNEUROSCI.2616-11.2011. https://doi.orig/10.1523/JNEUROSCI.2616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolgar A, Thompson R, Bor D, Duncan J. Multi-voxel coding of stimuli, rules, and responses in human frontoparietal cortex. Neuroimage. 2011;56:744–752. doi: 10.1016/j.neuroimage.2010.04.035. https://doi.orig/10.1016/j.neuroimage.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MW. Functional Specialization and Flexibility in Human Association Cortex. Cereb Cortex. 2015;25:3654–3672. doi: 10.1093/cercor/bhu217. https://doi.orig/10.1093/cercor/bhu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. https://doi.orig/10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. https://doi.orig/10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]