Abstract
Dengue virus (DENV) is a member of the Flaviviridae family, which is transmitted to mammalian species through arthropods, and causes dengue fever or severe dengue fever in humans. The DENV genome encodes for multiple nonstructural (NS) proteins including NS1. NS1 plays an essential role in replication by interacting with other viral proteins including NS4B, however how these interactions are regulated during virus infection is not known. By using bioinformatics, mass spectrometry analysis, and co-immunoprecipitation assays, here we show that DENV-NS1 is ubiquitinated on multiples lysine residues during DENV infection, including K189, a lysine residue previously shown to be important for efficient DENV replication. Data from in vitro and cell culture experiments indicate that dengue NS1 undergoes modification with K48-linked polyubiquitin chains, which usually target proteins to the proteasome for degradation. Furthermore, ubiquitinated NS1 was detected in lysates as well as in supernatants of human and mosquito infected cells. Ubiquitin deconjugation of NS1 using the deubiquitinase OTU resulted in increased interaction with the viral protein NS4B suggesting that ubiquitinated NS1 has reduced affinity for NS4B. In support of these data, a K189R mutation on NS1, which abrogates ubiquitination on amino acid residue 189 of NS1, also increased NS1-NS4B interactions. Our work describes a new mechanism of regulation of NS1-NS4B interactions and suggests that ubiquitination of NS1 may affect DENV replication.
Keywords: Dengue virus, NS1, NS4B, Ubiquitination, K48-linked polyubiquitin, Deubiquitinase, Proteasome
1. Introduction
Dengue is a disease caused by the dengue virus (DENV) and it is transmitted to humans by the vector Aedes, mainly Aedes aegypti and Aedes albopictus (Gubler, 1998). Four serotypes are distinguished: DENV-1, DENV-2, DENV-3, and DENV-4. A recent study estimates that approximately 390 million dengue infections occur annually worldwide (Bhatt et al., 2013). The clinical presentation of dengue infections may range from asymptomatic to a broad spectrum of clinical symptoms including the more sever form of the disease, characterized by vascular leakage and shock syndrome (WHO, 2009). DENV is an enveloped, nonsegmented, positive-sense single-stranded RNA virus from the genus Flavivirus (family Flaviviridae). DENV is closely related to other flaviviruses that cause important human disease including zika virus (ZIKV), West Nile virus (WNV), and yellow fever virus (YFV) (Holbrook, 2017). Translation of the viral positive-sense genomic RNA produces a polyprotein that is co- and post-translationally cleaved by host and viral proteases. Structural proteins C, prM, and E are located in the polyprotein amino terminus, followed by nonstructural (NS) proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (Welsch et al., 2009; Yi et al., 2012). After viral entry and release of the RNA into the cytoplasm, expressed NS proteins induce intracellular membrane modification and formation of virus-specific membrane structures, which house the viral replication complex (RC) (Paul and Bartenschlager, 2013; Welsch et al., 2009). Nucleocapsid formation, thought to occur in close proximity to replication sites, is likely accompanied by acquisition of a lipid envelope via budding into endoplasmic reticulum (ER) membranes enriched in the envelope protein E and prM (Welsch et al., 2009), through as yet undefined mechanisms. Assembled virions, stored within the ER and stacked in highly ordered arrays, are then released from the cell via the conventional secretory pathway, where cleavage of the prM protein by furin, a protease residing in the trans-Golgi network (TGN), renders the viral particles infectious (Scaturro et al., 2015).
NS1 is a multifunctional 42–48 kDa glycoprotein that is translocated into the ER lumen co-translationally. Within the ER, NS1 promptly dimerizes upon addition of high-mannose carbohydrates (Edeling et al., 2014), and is targeted to three destinations: the viral replication sites, the plasma membrane, and the extracellular compartment. Most of the secreted NS1 is a soluble proteolipid particle, which forms an open-barrel hexameric shell with a central channel occupied by lipids (Gutsche et al., 2011). Secreted NS1 as well as NS1 residing on the plasma membrane and within cells, play important roles in immune evasion via binding to complement proteins (Muller and Young, 2013; Watterson et al., 2016) and modifying or antagonizing their functions. In addition to its immune evasive functions, NS1 modulates early events in viral RNA replication. Intracellular NS1 co-localizes with double-stranded RNA (dsRNA) and other components of the viral replication complex and may play a role in replication (Junjhon et al., 2014; Mackenzie et al., 1996; Muller and Young, 2013). Mutagenesis studies and trans-complementation experiments have shown that flaviviral NS1 performs a crucial function early in replication and potentially contributes to the formation of the RC (Khromykh et al., 2000; Muylaert et al., 1996). Since NS1 is physically separate from the remaining components of the RC, it has been suggested that it is likely to be involved in RNA replication by providing an anchor mechanism to hold the RC to the ER (Muller and Young, 2013).
Using WNV as a model system, it was previously shown that NS4B interaction with NS1 modulates viral replication, and residues 10 and 11, within the β-roll domain of WNV NS1 are critical for this function (Youn et al., 2012). Furthermore, co-immunoprecipitation (coIP) and mass spectrometry analysis identified a physical interaction between NS1 and NS4B (Youn et al., 2012). However, how this interaction is regulated during virus infection is not known. In addition, whether NS1 undergoes other types of post-translational modifications in addition to glycosylation, and how these modifications may regulate NS1 functions, is currently unclear.
The ubiquitin-proteasome system plays a key role in performing fundamental cellular functions ensuring the quality control of proteins and maintaining a critical level of important regulatory proteins. Ubiquitin (Ub) is known to play important roles in cell cycle, development and cell differentiation, endocytosis, signal transduction, DNA repair, kinase activation, immunity, and in the regulation of transcription, among other functions (Ebner et al., 2017; Husnjak and Dikic, 2012; Luo, 2016; Yau and Rape, 2016). Ub has seven lysines (K) each of which can be conjugated with another Ub molecule to form poly-ubiquitin (poly-Ub) chains. The function of polyubiquitinated proteins depends on the type of poly-Ub linkage, for example, proteins covalently modified with K48-linked poly-Ub chains are targeted for degradation by the proteasome. In contrast, K63-linked poly-ubiquitinated proteins can have immune signaling functions (Ebner et al., 2017; Husnjak and Dikic, 2012; Suryadinata et al., 2014; Yau and Rape, 2016). Unanchored poly-Ub chains, which are not covalently attached to any protein, have also been shown to have functions in innate immune signaling and virus replication (Rajsbaum et al., 2014a; Rajsbaum et al., 2014b). Importantly, viruses have the ability to manipulate this cellular machinery to promote viral propagation and evade the immune response. The Ub system is involved in host defense mechanisms by eliminating viral components, or by regulating immune signaling and production of antiviral cytokines (Ebner et al., 2017; van Tol et al., 2017). However, viruses have evolved to antagonize Ub-mediated antiviral responses by degrading or inactivating cellular proteins that limit viral growth (Bharaj et al., 2016; Calistri et al., 2014; Rajsbaum and Garcia-Sastre, 2013; van Tol et al., 2017). In addition, viruses can also hijack components of the Ub system, and ubiquitination of viral proteins can result in enhanced virus replication (Bharaj et al., 2017; Byk et al., 2016; Rajsbaum and Garcia-Sastre, 2014; van Tol et al., 2017). For example, DENV suppress the signaling of the antiviral type-I interferon (IFN-I) cytokine (Ashour et al., 2009; Ashour et al., 2010; Munoz-Jordan et al., 2005). In particular, the NS5 protein mediates the degradation of the transcription factor STAT2 during DENV replication. The E3-Ub ligase UBR4 was identified as a host protein that is specifically used by the virus to inhibit the IFN-I signaling pathway by promoting degradation of STAT2 (Morrison et al., 2013). Furthermore, recent studies have shown that cells infected with DENV have decreased production of infectious virions when cells are treated with specific proteasome inhibitors (Fink et al., 2007; Kanlaya et al., 2010; Padilla et al., 2014), suggesting that the Ub-proteasome system is important for virus replication. However, which viral proteins are ubiquitinated during virus replication and how the ubiquitination process may affect virus replication is still unclear.
Here, we show that the DENV-NS1 protein is ubiquitinated with K48-linked polyubiquitin chains on multiple lysine residues, including K189, a lysine residue located on the NS1 β-ladder domain that has been recently shown to be important for DENV replication (Scaturro et al., 2015). We further show that NS1 ubiquitination reduces the ability of NS1 to interact with its viral partner NS4B, supporting the hypothesis that NS1 ubiquitination may affect virus replication.
2. Material Methods
2.1. Cells and Viruses
HEK293T and Huh7 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM L-glutamine and 1% penicillin-streptomycin (Gibco-BRL) and 10% fetal bovine serum (FBS). The cells were maintained in a humidified 5% CO2 incubator at 37°C. Dengue virus serotype-2 (New Guinea DENV-2) was a generous gift of Dr. Mariano Garcia-Blanco and Dr. Shelton Bradrick (UTMB, USA). High-titer DENV stocks were obtained by passage in C6/36 mosquito cells that were cultured at 28 °C in Minimum Essential Medium 119 (MEM, Gibco, USA) supplemented with nonessential amino acids and 10% FBS in a 5%-CO2 incubator. The cells were pre-seeded in cell culture flasks and infected by DENV (MOI=1) when they have reached 90% confluence. After incubation for 4–7 days, the cell culture supernatant was collected for viral titration and preparation of viral stocks and was stored at −80°C until used. The pXJ-HA-NS4B plasmid was provided generously by Dr. Pei-Yong Shi (UTMB).
2.2. Reagents, plasmids, and purification of GST-NS1
Cloning and purification of GST– NS1 fusion protein. The NS1 gene was amplified from cDNA Dengue Virus by PCR using the following primers: 5′NS1 Bam HI 5-GGATCCGAGAYASTGGTTGYRTTGTGAGC 3′ and 5′NS1 Sal I 5-GTCGACGCTGTGACCARGGARTTRACC 3′.and ligated between the EcoRI and Sal I restriction sites of pGEX-5X (Pharmacia). The plasmid was cloned into Escherichia coli BL21 for optimum expression. GST– NS1 protein was expressed by induction with 0.1 mM IPTG for 24 h at 37°C. After centrifugation, the bacterial cells were resuspended in buffer (100 mM Tris HCl, 100 mM Na2HPO4, 10 mM 2-β-mercaptoethanol and 8M Urea), lysed by sonication and the lysate was clarified by centrifugation at 6000 g. for 30 min. Expressed GST–NS1 protein was bound to anti-glutathione antibody–Protein A beads (life technologies), and eluted with 3.6 M MgCl2 and 0.05M Tris pH 6.6. Finally, the elution was dialyzed overnight in PBS 1X. Protein was collected and analyzed by SDS-PAGE and used in ubiquitination in vitro assays.
The NS1 was cloned also into the pCAGG plasmid using the primers: 5′NS1-EcoRI 5-AATTGAATTCATGGATAGTGGTTGCGTT3′; 5′NS1-KpnI 5-GTACGGTACCTGTGACCAAGGAGTTG-3′, and Ubiquitin expression constructs HA-tagged ubiquitin (HA-Ub), HA-Ub-K48only, HA-Ub-K48R HA-Ub-K63R, Flag-OTU and Flag-OTU-2A have been described before (Rajsbaum et al., 2014b). All sequences were confirmed by sequencing analysis at the UTMB molecular genomics core facility.
The following antibodies were used for immunblotting or immunofluorescence: mouse anti-NS1 antibody were from Gentex (GTX GTX630556), mouse rabbit anti-HA antibody, anti-FLAG antibodies, rabbit anti-β-actin antibodies were from Sigma. Rabbit monoclonal anti-ubiquitin Lysine 48 (K48, clone Apu2) was purchased from Millipore. Rabbit anti-GST antibody (OTI4G1) was from Bethyl Laboratories. Fluorescently labeled secondary antibodies for imaging: Alexa Fluor 488 goat anti-mouse, Alexa Fluor 488 donkey anti-rabbit, Alexa Fluor 555 goat anti-mouse, Alexa Fluor 555 donkey anti-rabbit, and DAPI were purchased from ThermoScientific (Life Technologies).
2.3. In vitro ubiquitination assay
All in vitro ubiquitination assays were performed using the HeLa S100 kit (Boston Biochem), following the manufacturer recommendations. In brief, 15 μl (0.7 mg) of purified GST-NS1 were mixed with 10 μg fraction Hela S100, 5 μM MG132, 0.5 μg Ubiquitin aldehyde solution, 2 μg Ub-WT, Ub-K48only, Ub-K48R or Ub-K63R, and regeneration energy solution (1x). The mixtures were incubated at 37 °C for 2h and analyzed by SDS-PAGE and immunoblot.
2.4. Transient transfection and infection
The Huh7 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and cultured at 37 °C in a humidified atmosphere with 5% CO2. When cells reached approximately 90% confluent in six well cell culture plates, approximately 400,000 cells were transfected with 3.2 μg of Ub plasmids and lipofectamine-2000 ratio 1:2 (Invitrogen) in 1 ml DMEM medium without antibiotics. After 24 h, the cells were trypsinized and replated to perform viral infection experiments. The culture medium was replaced by serum-free DMEM, and then DENV-2 was added to the cultured cells at MOI of 1. After 1h, the medium was removed and fresh medium added to the cells for 48 h before harvesting.
For transfection only experiments, HEK293T cells (ATCC) were transfected with 300 ng of Ub plasmids (HA-WT-Ub, HA-UbK48only, HA-Ub-K48R HA-Ub-K63R), 700 ng NS1 plasmid, and TransIT-LT1 (Mirus) ratio 1:3 as recommended by the manufacturer. After 30 h of transfection, cells were harvested for further analysis. For MG132 treatments, 24 h post-transfection, cells were treated with either DMSO as control or 20 μM MG132 (Tocris), for 6 h.
2.5. Co-immunoprecipitation and immunoblotting
Transfected 293T cells were harvested in RIPA lysis buffer (250 μl) containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% [v/v] NP40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail [Roche], and supplemented with 5 mM N-ethylmaleimide (NEM) and Iodoacetamide as deubiquitinase inhibitors. Cell lysate was clarified by microcentrifugation at 15,000 rpmi for 20 min at 4°C. 25 μl from clarified lysate was mixed with 2x Laemmli buffer containing β-mercaptoethanol, boiled for 8 minutes and stored at −20 °C for immunoblots (whole cell extracts, [WCE], 5% of IP was loaded to the gels). To the rest of the lysate, mouse anti-HA antibody cross-linked to agarose beads (EZ View Red Anti-HA Affinity Gel Sigma) were added and rotated on a benchtop shaker overnight at 4°C. Beads were extensively washed, and the bound proteins were eluted by boiling for 10 min in Laemmli loading buffer. For immunoblotting, proteins were resolved by SDS-polyacrylamide gel electrophoresis (7.5% or 4–15% SDS-PAGE, BioRad) and transferred onto a PVDF membrane (Immobilon BioRad Laboratories). The membrane was blocked with 5% nonfat milk in TBS-tween (TBS-T) solution and incubated with primary antibodies overnight at 4 °C. The following primary antibodies were used: anti-Ub-K48 (1:1,000) (Millipore), anti-HA (1:5,000) (Sigma), anti-GST (1:2,000) (Bethyl Laboratories), and anti-β-actin (1:5,000). Then, the membrane was washed with TBS-T for three times and incubated with secondary antibodies: ECL anti-rabbit IgG horseradish peroxidase conjugated whole antibody from donkey, and ECL anti-mouse IgG horseradish peroxidase conjugated whole antibody from sheep (GE Healthcare; Buckinghamshire, England) at room temperature for 1 h. After washed four times with TBS, the protein was visualized by SuperSignal West Chemiluminescent Substrate (Thermo Scientific) in a developer (Konica SRX-201A).
2.6. Immunofluorescence assay
HeLa cells were seeded into Lab-Tek II 8-well chamber slides (CC2 Glass slide, Nunc; Rochester, NY). After 16 h, 200ng of HA-UB and NS1 plasmids were transfected with Lipofectamine 2000 for 30 h, followed by treatment with MG-132 (20 mM) (or DMSO as control) for 6 h. The cells were washed with DPBS 1X, fixed with 4% paraformaldehyde, permeabilized with 0.5% NP-40 (v/v) in DPBS 1X, and blocked with 0.3% BSA, 0.2% fish gelatin in DPBS 1X for 1 h (blocking solution). Staining was performed with anti-HA and anti-NS1 antibodies (1:200) (prepared in blocking solution) overnight at 4°C. The next day, cells were washed three times with DPBS 1X and incubated with the secondary antibodies donkey anti-rabbit Alexa-fluor 488 and anti-mouse 555 (Invitrogen) diluted in blocking buffer along with DAPI (1:2000). GFP and RFP fluorescence-positive cells were imaged by microscopy under a 60X objective lens on a Cytation 5 Imaging reader (Biotek Instruments).
2.7. Site-directed mutagenesis
With the information obtained from MS analysis, we generated Lysine–to-arginine (K-to-R) mutants of NS1 protein by site-directed mutagenesis of K182 and K189 residues. K-to-R mutations have the advantage of losing ubiquitination while conserving the positive charge of the specific residues, which reduces the risk of causing structural changes. We generated expression vectors containing WT and the identified NS1 mutants. The pCAGG-NS1 plasmid, was used as the template for PCR- based site- directed mutagenesis. The reaction mixture (50 μl) consisted of 50ng of template DNA, 2.5 U of high fidelity AccuPrime Taq DNA Polymerase (Invitrogen) and 0.2 μM of forward and reverse primers used before in NS1 cloning and primers with mutations (The red underline represent the codon changes).
NS1K182R:(F)TTTTGTGACTCAAGACTCATGTCAGCGGCCATTAAAGACAA,
NS1K182R:(R)GCTGACATGAGTCTTGAGTCACAAAATACATCCTGTTT,
NS1K189R:(F)TCAGCGGCCATTCGAGACAACAGAGCCGTTCATGCCGATAT,
NS1K189R:(R)GGCTCTGTTGTCTCGAATGGCCGCTGACATGAGCTTTGAG.
Followed by 35 runs of the thermal cycling program (95°C for 30 s, 56 °C for 30 s, and 72 °C for 30 s). Overlapping PCR was performed using two amplified products of NS1 PCR fragments as templates after purification by QIAquick™ PCR purification kit (Qiagen, Germany). To provide equal numbers of DNA molecules of each fragment in the overlapping PCR, 18 ng of NS1 first fragment and 15 ng of NS1 second fragment PCR products were used. The amplified products were digested with EcoRI and KpnI restriction enzymes and directly cloned into vector pCAGG. The ligation product was transformed into E. coli XL Gold supercompetent cells, and single colonies were picked up from the ampicillin LB plates and the mutations were verified by DNA sequencing and used in overexpression assay.
3. Results
3.1. Dengue NS1 is ubiquitinated
Previous data indicate that proteasome inhibitors can block DENV replication (Fink et al., 2007; Kanlaya et al., 2010; Padilla et al., 2014), suggesting that viral proteins may undergo ubiquitination during virus infection. To determine whether DENV proteins are ubiquitinated, we first used bioinformatics analysis to predict potential sites of ubiquitination on the polyprotein sequence of DENV-2. To this end, a prediction algorithm called UbiPred (Tung and Ho, 2008) was used. These analyses revealed potential ubiquitination sites on NS1, NS4B, and NS5 (Figure 1A and 1B, scores above 0.5 indicate predicted ubiquitinated sites). Next, to verify that ubiquitination occurs on DENV proteins during infection, we performed mass spectrometry (MS) analysis from DENV-infected Huh7 samples enriched for ubiquitinated proteins by immunoprecipitation (IP) of ectopically expressed HA-tagged-Ub, followed by trypsin digestion. This MS analysis identified peptides of NS1 containing lysines K48, K78, K182, K189, K214, and K221, which were linked to diglycine residues that are usually found on ubiquitinated peptides after trypsin digestion (Figure 1C). The MS analysis showed that K48, K85, K182, K189, K214, and K221 are present within the peptides identified by both UbiPred and MS (Figure 1A, residues highlighted in blue). Of these, K85 and K214 were the only ubiquitin acceptor sites identified by MS that were not present in the ubiquitination prediction (Figure 1A–1C). We also found potentially ubiquitinated peptides corresponding to M, NS3, and NS5 (data not shown). Since we found multiple ubiquitination sites on NS1 by bioinformatics that were corroborated by MS, and no significant ubiquitination overlap was found using both techniques on other viral proteins, we focused our next experiments on further characterization of NS1 ubiquitination.
Figure 1. Identification of ubiquitination sites on NS1 by bioinformatics and mass spectrometry analysis.
A) Full sequence NS1 protein showing ubiquitinated lysines obtained by UbiPred (K shown in red) and mass spectrometry (K shown in blue). Identified ubiquitinated lysines by both methods are highlighted in dark blue. B) Prediction of ubiquitination sites on NS1 using UbiPred. The NS1 protein sequence of the dengue virus was analyzed using the UbiPred bioinformatics tool. Potential ubiquitinated sites have a prediction score above 0.5 (K in Red). C) HEK293T cells were transfected with NS1 and HA-Ub. After 30 h cells were harvested and whole cell extracts (WCE) were used for HA immunoprecipitation (IP) using anti-HA beads. Ubiquitinated proteins were eluted with HA peptide and analyzed by mass spectrometry. Peptides corresponding to NS1 containing diglycine residues (GlyGly), which typically indicate ubiquitinated sites, are shown in blue.
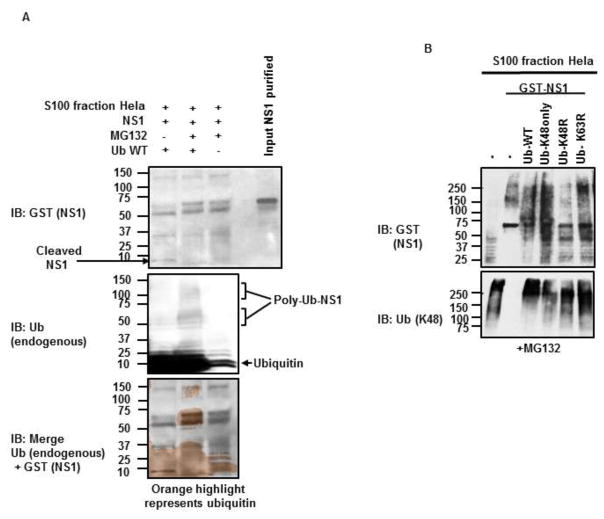
3.2. DENV-NS1 is ubiquitinated in vitro
To confirm that DENV-NS1 is ubiquitinated, we reconstituted the ubiquitination reaction in vitro in the presence of purified DENV-NS1. First, we performed an in vitro ubiquitination assay with purified recombinant glutathione S-transferase (GST)-tagged DENV-NS1 and the S100 fraction of HeLa cells, which contains all the required factors for the ubiquitination reaction, including an active proteasome. To increase the efficiency of the reaction and the detection of potentially ubiquitinated NS1, we added purified recombinant Ub to the reaction, in the presence or absence of the proteasome inhibitor MG132. In the presence of proteasome inhibitor, we detected poly-Ub migrating bands at a higher molecular weight than the one predicted for non-ubiquitinated GST-NS1 (Figure 2A, middle panel). These ubiquitin bands overlapped with migrating bands of GST-NS1 (Figure 2A, top panel. Digital overlap of Ub and GST-NS1 is shown in Figure 2A, bottom panel), and this occurred only when Ub was exogenously added to the reaction (Figure 2A, middle panel, compare lane 2 to lane 3). Furthermore, GST-NS1 appeared to be cleaved in the absence of the proteasome inhibitor MG132 (Figure 2A, top panel, compare lane 1 and 2), indicating that NS1 is ubiquitinated and partly degraded by the proteasome. Since proteins that are degraded by the proteasome are usually covalently modified with K48-linked poly-Ub chains, we next tested whether these type of poly-Ub chains are attached to NS1. We performed similar experiments as described above, but this time in the presence of WT-Ub or Ub mutants that are unable to form K48-linked (Ub-K48R) or K63-linked chains (Ub-K63R), or an ubiquitin mutant that can only form K48-linked poly-Ub chains (all K have been mutated to R, except for K48, “Ub-K48only”). As expected, slower migrating forms of GST-NS1 were detected by SDS-PAGE when WT or Ub-K48only mutant were added to the reaction (Figure 2B, top panel, lanes 3 and 4), which presumably correspond to ubiquitinated forms of NS1. Similar results were observed in the presence of Ub-K63R mutant, which does not form K63-linked poly-Ub chains but is still able to form K48-linked poly-Ub chains (Fig 2B, top panel, lane 6). In contrast, in the presence of the Ub-K48R mutant, which cannot form K48-linked chains, the GST-NS1 migrated at its predicted molecular weight (~ 67 kDa). Note that the S100 extract contains large amounts of K48-linked poly-Ub chains (Figure 2B, bottom panel, lane 1), however these are not enough to rescue the ubiquitination of NS1 when Ub-K48R is added to the reaction (Figure 2B, top panel, lane 5). Together these data suggest that NS1 is ubiquitinated with K48-linked poly-Ub chains, and that NS1 can be cleaved by the proteasome.
Figure 2. DENV-NS1 is ubiquitinated in vitro with K48-linked poly-Ub chains.
A–B) E. coli purified GST-NS1 was used in a ubiquitination in vitro assay in the presence or absence of different ubiquitin mutants (Ub-WT, Ub-K48only [all K mutated to R except for K48], Ub-K48R and Ub-K63R), and the proteasome inhibitor MG132. The indicated proteins were detected by immunoblot (IB). Representative of at least 2 experimental repeats are shown.
3.3. DENV-NS1 is ubiquitinated with K48–linked chains in cells and co-localizes with Ubiquitin in cytoplasmic structures
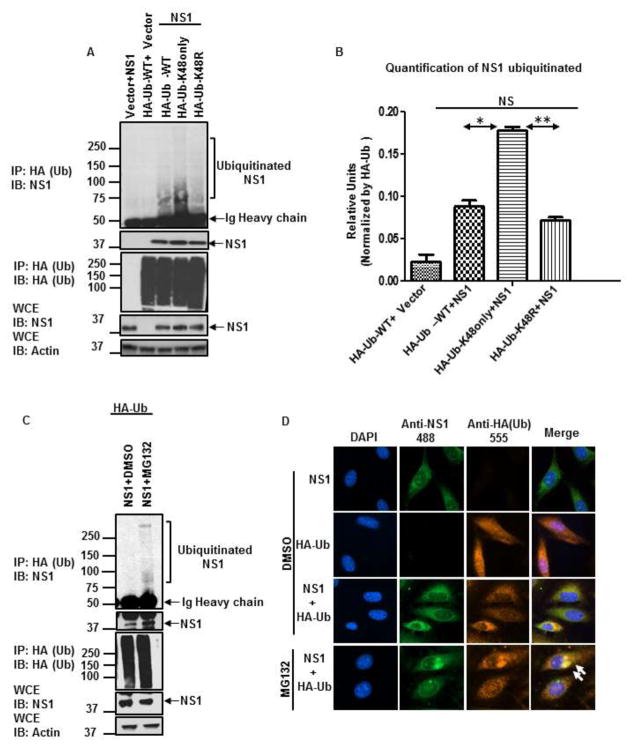
To confirm that NS1 is modified with K48-linked poly-Ub chains in cell culture, NS1 was ectopically expressed together with WT HA-tagged Ub or different Ub mutants followed by co-immunoprecipitation (coIP) assays, in the absence of proteasome inhibitors. In these conditions and in agreement with our in vitro data, very low levels of ubiquitinated NS1 were detected when WT-Ub was used (Figure 3A, lane 3). However, co-immunoprecipitation of NS1 with Ub-K48only chains was significantly increased as compared to WT-Ub (Figure 3A, compare lane 4 to lane 3, quantification shown in Figure 3B). In contrast, association of NS1 with Ub-K48R was reduced as compared to Ub-K48only (Figure 3A, lane 4 and 5), and although not statistically significant, association of NS1 with Ub-K48R was also reduced as compared to WT-Ub (Figure 3A, compare lane 5 to lane 3, and quantification shown in Figure 3B). In addition, treatment with proteasome inhibitor MG132 also enhanced co-immunoprecipitation of NS1 with WT poly-Ub chains (Figure 3C).
Figure 3. DENV-NS1 is ubiquitinated with K48 poly-Ub chains in cells and co-localizes with ubiquitin.
A–C) HEK293T cells were transfected with NS1 and HA-Ub-WT, HA-Ub-K48only, and HA-Ub-K48R. Twenty-four hours post-transfection, cells were treated with either DMSO (control, panel A) or 20 μM MG132 for an additional 6 h (panel C). Cells were harvested and whole cell extracts (WCE) were used for HA-Ub pull-down using HA-beads. B) Quantification of ubiquitinated NS1 was performed using ImageJ software. To normalize the efficiency of HA-Ub pulldown, the densitometry values obtained for the full smear of ubiquitinated NS1 (top blot, IP: HA, IB: NS1) were normalized by the levels of immunoprecipitated HA-Ub (IP: HA, IB: HA). The quantification was performed for two independent experiments and SEM is shown. Statistical significance was determined in Graphpad software. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant. D) Localization of NS1 with ubiquitin. HeLa cells were transfected with NS1 and HA –Ub for 24 h, followed by treatment with DMSO or MG132 for an additional 6 h. The cells were then fixed and stained with the indicated antibodies. The cells were visualized in a fluorescent microscope. The results shown are representative of 2 independent experiments.
Next, we examined whether NS1 localizes with Ub in the same cell compartments, by fluorescence microscopy. Ectopically expressed NS1 showed mostly a cytoplasmic granular localization (Figure 3B). In contrast, ectopically expressed HA-tagged Ub has both a nuclear and cytoplasmic localization, mostly diffused throughout the cell. Co-expression of NS1 and Ub resulted in localization of a portion of NS1 and Ub in apparent cytoplasmic granules. This localization of NS1 and Ub in similar cellular compartments and the size of the granules significantly increased when cells were treated with MG132 (Figure 3B bottom panel). Taken together these data strongly suggest that NS1 is ubiquitinated with K48-linked poly-Ub chains in cells, and that NS1 is targeted to the proteasome for degradation.
3.4. DENV-NS1 is ubiquitinated with K48-linked poly-Ub chains during infection
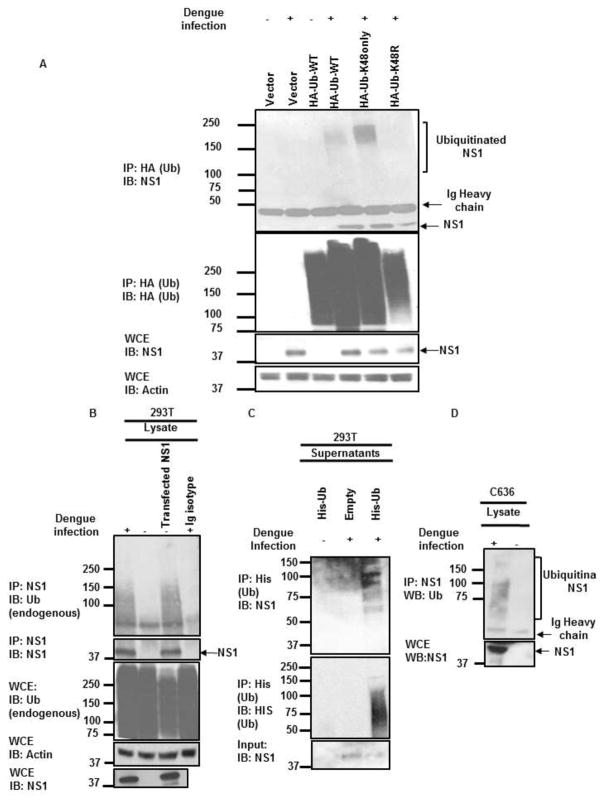
To examine whether NS1 is ubiquitinated during infection, permissible Huh7 cells were transfected with WT-Ub, Ub-K48only, and K48R followed by infection with DENV. NS1 ubiquitination was then assessed by coIP assays. Consistent with our in vitro and cell culture assays, NS1 co-immunoprecipitated with WT and K48only poly-Ub chains but not with K48R poly-Ub chains (Figure 4A), confirming that NS1 is ubiquitinated during virus infection. NS1 also associated with endogenous Ub during infection (Figure 4B), and ubiquitinated NS1 was also detected in the supernatants of infected cells ectopically expressing His-tagged-Ub (Figure 4C, IP: His). These data suggest that ubiquitination of NS1 does not interfere with release of NS1 to the extracellular compartment. Finally, ubiquitination of NS1 was also detected in infected C6/36 mosquito cells (Figure 4D), suggesting that NS1 ubiquitination is a conserved feature of DENV replication and is likely important for NS1 functions.
Figure 4. DENV-NS1 is ubiquitinated during infection.
A) HuH7 cells were transfected with HA-Ub-WT, HA-Ub-K48only, and HA-Ub-K48R for 24 h followed by infections with DENV (MOI 1) for 48 h. Cells were harvested and WCE were used for HA-Ub pull-down using anti-HA beads. B) HEK293T cells were infected with DENV at MOI of 1 for 48 h. Cells were then lysed and immunoprecipitation (IP) was performed using anti-NS1 antibody. An IgG isotype was used as control for the IP. For comparison, IP of transfected NS1 is shown. Ubiquitinated NS1 with endogenous ubiquitin was detected using anti-Ub antibodies by immunoblot (IB). C) To enrich for ubiquitinated NS1 from supernatants of infected HEK293T cells, supernatants from HEK293T cells transfected with His-Ub and infected with DENV, were used for IP with anti-His (Ub) antibody. D) Mosquito C6/36 cells were infected with DENV and NS1 immunoprecipitation was performed as described in B. Experiments shown are representative of at least 2 independent experiments.
3.5. Interaction of NS1 with NS4B is reduced in the presence of ubiquitinated NS1
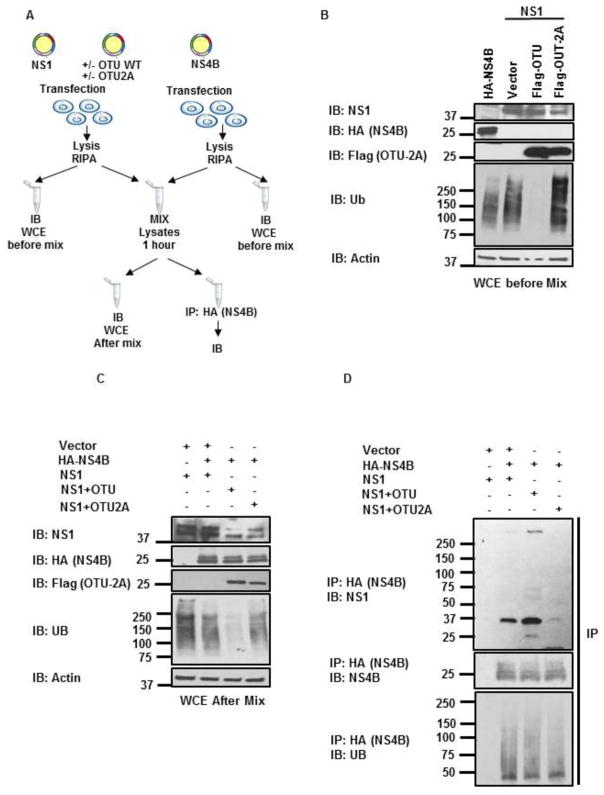
We have shown that DENV-NS1 is ubiquitinated in both overexpression assays and during virus infection. Since protein ubiquitination may serve as a signal for downstream events by recruiting binding partners, we asked whether NS1 ubiquitination alters binding with known virus interacting proteins. Previous data using WNV showed that NS1 interacts with NS4B, and that this interaction is important for virus replication (Rastogi et al., 2016; Youn et al., 2012). To determine whether DENV-NS1 ubiquitination affects the ability to bind to NS4B, we performed an experiment in which poly-Ub chains were removed from NS1 by co-expressing the ovarian tumor (OTU) domain of Crimean Congo hemorrhagic fever virus (CCHFV) large (L) protein, which is known to cleave covalently linked poly-Ub chains from ubiquitinated proteins (Frias-Staheli et al., 2007). Lysates containing this deubiquitinated NS1 were then used to test binding with NS4B (a diagram of the experimental approach is shown in Figure 5A). As shown in Figure 5B, lysates from cells co-expressing NS1 and OTU showed very low to undetectable levels of poly-ubiquitinated proteins. In contrast, cell lysates expressing NS1 alone or together with a catalytically inactive mutant of OTU (OTU-2A) showed large amounts of poly-Ub and poly-ubiquitinated proteins. Note that cell lysates from NS4B expression cells also showed detectable levels of poly-Ub chains. Importantly, to avoid cleavage of poly-Ub chains potentially associated with NS4B or other proteins, the OTU activity was inactivated by addition of N-ethylmaleimide (NEM) in the lysis buffer. After confirming the deubiquitination of NS1, the fractions containing deubiquitinated NS1 (NS1+OTU) or the control NS1 (NS1+OTU-2A) were mixed with the NS4B containing fraction (Figure 5C) and binding of deubiquitinated NS1 with NS4B was examined by coIP assay (Figure 5D). Unexpectedly, OTU-treated NS1 interacted more efficiently with NS4B as compared to OTU-2A treated or untreated NS1 (Figure 5D). These results indicate that deubiquitination of NS1 promotes interaction with NS4B and suggest that ubiquitination of NS1 interferes with its binding to NS4B.
Figure 5. Ubiquitinated NS1 has reduced affinity for NS4B.
A) Schematic representation of the experimental approach. B–D) HEK293T cells were transfected with HA-NS4B or NS1 with or without the deubiquitinase Flag-OTU or the catalytically inactive mutant Flag-OTU2A. Cells were lysed and whole cell extracts (WCE) were used for immunoblot (B). Then, the NS4B expressing WCE were mixed with WCE containing ubiquitinated or deubiquitinated NS1 (C) and immunoprecipitation (IP) of NS4B was performed using anti-HA beads (D). The indicated proteins were detected by immunoblots (IB). The experiment shown is a representative of 2 independent experiments.
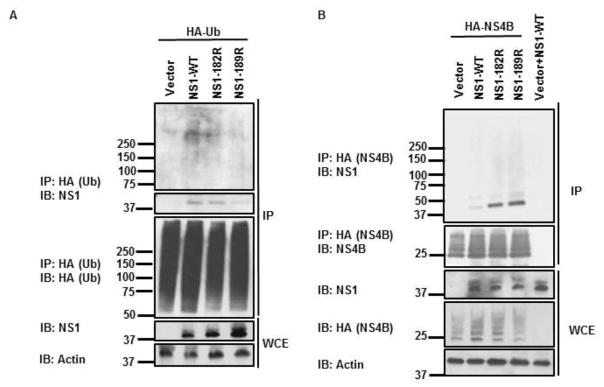
Finally, to corroborate that ubiquitination of NS1 affects its interaction with NS4B, and to identify the precise amino acid residues on NS1 that are involved in this function, we generated vectors expressing lysine-to-arginine mutants of NS1 lacking ubiquitination on residues 182 and 189 (NS1-K182R and NS1-K189R). We chose to test these residues based on our MS data (Figure 1) and a previous report indicating that a recombinant DENV encoding the NS1 mutant K189A is attenuated in cells (Scaturro et al., 2015). As expetced, CoIP experiments using ectopically expressed HA-Ub and NS1-WT and mutants showed reduced leves of ubiquitination on NS1-K182R and NS1-K189R as compared to NS-WT (Figure 6A), confirming that these residues on NS1 are ubiquitinated. Importanly, binding of both NS1-K182R and NS1-K189R to NS4B was significantly increased as compared to NS1-WT in coIP assays (Figure 6B), further confirming that NS1 ubiquitination interferes with its binidng to NS4B. In addition, the strongest interactions between NS1 and NS4B were observed with the NS1-K189R mutant, suggesting that ubiquitination on this resiudue and its ability to interfere with NS4B binding may indeed be functionally important and may explain the virus attenuation of the recombinant DENV-K189A oberved in previous studies (Scaturro et al., 2015).
Figure 6. Ubiquitination of NS1 on Lysines 182 and 189 confers reduced affinity for NS4B.
A) HEK293T cells were transfected with vectors expressing NS1-WT, NS1-K182R, or NS1-K189R and HA-Ub-WT. Thirty hours post-transfection cells were harvested and whole cell extracts (WCE) were used for IP with anti-HA beads. Immunoblots (IB) were performed with the indicated antibodies. B) HEK293T cells were transfected with vectors expressing HA-NS4B and NS1-WT, NS1-K182R or NS1-K189R. Cells were harvested and WCE were used for HA-NS4B IP using HA-beads. Immunoblots (IB) with respective antibodies are shown.
4. Discussion
In this study, we demonstrate that DENV-NS1 undergoes covalent ubiquitination with K48-linked poly-Ub chains on multiple lysine residues, and confirmed that residues K182 and K189 are indeed ubiquitinated. Our findings indicate that NS1 is ubiquitinated in vitro and during infection of human and mosquito cells. We further show that ubiquitinated NS1 can be detected in supernatants of cells infected with DENV. Our findings also suggest that poly-ubiquitination of NS1 on K182 and K189 interferes with binding to its viral partner NS4B.
The NS1 protein is a highly conserved 42–55 kDa glycoprotein containing 12 cysteine residues that form six intermolecular disulfide bonds and two glycosylation sites. The NS1 protein is translocated co-translationally into the lumen of the ER by the C-terminal transmembrane signal sequence of the E protein (Muller and Young, 2013). It also possesses twenty-six lysine residues from which bioinformatics analysis suggests ten of them could be a target of ubiquitination. The results obtained by mass spectrometry analysis suggest that four lysine residues on NS1 may undergo ubiquitination (K48, K182, K189 and K221) and confirmed the results obtained from the UbiPred bioinformatics tool. In this study, the bioinformatics tool predicted most of the ubiquitinated sites confirmed by mass spectrometric analysis (Figure 1). The K182 residue of NS1 protein is conserved in DENV-2 and WNV. The K189 residue, located in the β-ladder domain, is conserved in all flaviviruses and a recent study showed that a recombinant DENV encoding the NS1 mutant K189A is significantly attenuated (Scaturro et al., 2015), further supporting a role of ubiquitination of NS1 in promoting virus replication.
Our results suggest that the ubiquitination of residues K182 and K189 on NS1 may affect its oligomerization state, since dimeric NS1 has been shown to be formed through interaction of the β sheet domain in a head-to-head arrangement (Edeling et al., 2014). Almost all residues in this interface are conserved and more than half are hydrophobic. The combination of these highly conserved hydrophobic residues provides a dimer interface maintaining the structure of the protein (Edeling et al., 2014). In fact, we found that removal of Ub from NS1 using OTU results in increased levels of potentially dimeric and higher molecular weight NS1 complexes that are able to interact with NS4B (Figure 5D, top panel, bands at approx. 75 kDa and 250 kDa). Therefore, the presence of ubiquitin may affect oligomerization of NS1 and could affect NS1 cellular location as well as its different functions during viral infection. The multiple oligomeric forms of the NS1 protein are also known to affect viral pathogenicity. For example, hexameric NS1, which is the secreted form of NS1, induces endothelial hyperpermeability through the disruption of the endothelium (Modhiran et al., 2015; Puerta-Guardo et al., 2016). It should be noted that we cannot rule out the possibility that ubiquitination of NS1 could promote the formation of hexamers, resulting in reduced binding to NS4B. Future works will be required to better understand the kinetics of interaction between this and other important proteins in the early and late times of DENV infection.
The NS1 protein is important for the replication process of DENV since it participates in the replication complex (Fan et al., 2014; Muller and Young, 2013). Due to its location in the lumen of the ER, NS1 plays a structural supporting role by interacting with NS4A and NS4B (Akey et al., 2014), and these interactions are necessary for the early synthesis of viral RNA (Brinton, 2002; Muller and Young, 2013). Reports indicate that the NS1 and NS4B proteins in WNV interact in the early steps of viral RNA replication via two residues, R10 and Q11 in NS1, and F86 in NS4B (Youn et al., 2012). Although the ubiquitination sites identified in our study are found in a different region of NS1, it could be that by affecting the oligomerization state, ubiquitination of NS1 indirectly interferes with NS4B binding. Importantly, this previous study failed to detect ubiquitination of NS1 (Youn et al., 2012), probably because of the relatively low levels of ubiquitinated NS1 found in the absence of proteasome inhibitors. In contrast, here we used an approach to enrich for ubiquitinated proteins by ectopic expression of HA-tagged-Ub followed by coIP. This allowed us to identify NS1 ubiquitination, which was significantly enhanced in the presence of proteasome inhibitors.
Although the ubiquitin-proteasome system in DENV and other flaviviruses has been poorly studied, there is evidence it plays a role in promoting virus replication. The Ub ligase CBLL1 has been shown to be essential for WNV replication (Fernandez-Garcia et al., 2011), and NS3 and NS5 proteins of JEV have been shown to be ubiquitinated (Ye et al., 2013). Studies have shown that cells infected with DENV have decreased production of infectious virions when cells were treated with specific proteasome inhibitors (Fink et al., 2007; Kanlaya et al., 2010; Padilla et al., 2014), and an inhibitor of the E1 activating enzyme blocks viral genome release during infection (Byk et al., 2016). In addition, previous evidence suggests that ubiquitination of NS3 is necessary to interact with NS2B to form the protease complex (Liu et al., 2017), further supporting a role for Ub in regulating interactions between viral proteins. Since proteasome inhibitors increase ubiquitination of NS1, our study further supports a role of the ubiquitin-proteasome system in flavivirus replication. Further studies will be required to elucidate the precise function of the proteasome and/or degradation of NS1 in DENV replication.
In conclusion, we demonstrate that DENV NS1 is ubiquitinated with K48-linked poly-Ub chains both in vitro and within cells during virus infection and that ubiquitination on NS1 residues K182 and K189 interferes with NS1 binding to NS4B. Our work describes a new mechanism of regulation of NS1-NS4B interactions and suggests that ubiquitination of NS1 could potentially affect DENV replication. Our study provides new insights on the role of the host ubiquitin system during DENV replication, which we hope will contribute to future development of better treatments and diagnosis of dengue infections.
Highlights.
Bioinformatics and mass spectrometry analysis identified ubiquitination of multiple lysine residues on DENV-NS1.
Dengue NS1 undergoes modification by K48-linked polyubiquitin chains.
K189, a lysine residue previously shown to be important for efficient DENV replication, was identified as a ubiquitination site on DENV-NS1
Ubiquitin deconjugation of NS1 reduces NS1 interaction with the viral protein NS4B.
Acknowledgments
The authors wish to thank Dr. Mariano Garcia-Blanco, Shelton Bradrick, Ruben Soto and other members of their lab (UTMB), for their immense generosity in sharing reagents and advice. We also would like to thank Dr. Adolfo Garcia-Sastre for certain expression vectors. This work was supported by funding from the University of Quindío to JCCO. Dr Rajsbaum’s lab is supported by grants from the John Sealy Memorial Endowment Fund for Biomedical Research, a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH, from NIH ORWH and NICHD), and grants R21 AI132479-01 and R21 AI126012-01A1 from NIH/NIAID. We also thank Dr. Preeti Bharaj and Dr. Linsey Yeager for editing and critical reading of this manuscript.
Footnotes
Conflict of Interest:
All the authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Akey DL, Brown WC, Dutta S, Konwerski J, Jose J, Jurkiw TJ, DelProposto J, Ogata CM, Skiniotis G, Kuhn RJ, Smith JL. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science. 2014;343(6173):881–885. doi: 10.1126/science.1247749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour J, Laurent-Rolle M, Shi PY, Garcia-Sastre A. NS5 of dengue virus mediates STAT2 binding and degradation. J Virol. 2009;83(11):5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour J, Morrison J, Laurent-Rolle M, Belicha-Villanueva A, Plumlee CR, Bernal-Rubio D, Williams KL, Harris E, Fernandez-Sesma A, Schindler C, Garcia-Sastre A. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe. 2010;8(5):410–421. doi: 10.1016/j.chom.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharaj P, Atkins C, Luthra P, Giraldo MI, Dawes BE, Miorin L, Johnson JR, Krogan NJ, Basler CF, Freiberg AN, Rajsbaum R. The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication. J Virol. 2017;91(18) doi: 10.1128/JVI.00833-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharaj P, Wang YE, Dawes BE, Yun TE, Park A, Yen B, Basler CF, Freiberg AN, Lee B, Rajsbaum R. The Matrix Protein of Nipah Virus Targets the E3-Ubiquitin Ligase TRIM6 to Inhibit the IKKepsilon Kinase-Mediated Type-I IFN Antiviral Response. PLoS Pathog. 2016;12(9):e1005880. doi: 10.1371/journal.ppat.1005880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- Byk LA, Iglesias NG, De Maio FA, Gebhard LG, Rossi M, Gamarnik AV. Dengue Virus Genome Uncoating Requires Ubiquitination. MBio. 2016;7(3) doi: 10.1128/mBio.00804-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calistri A, Munegato D, Carli I, Parolin C, Palu G. The ubiquitin-conjugating system: multiple roles in viral replication and infection. Cells. 2014;3(2):386–417. doi: 10.3390/cells3020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner P, Versteeg GA, Ikeda F. Ubiquitin enzymes in the regulation of immune responses. Crit Rev Biochem Mol Biol. 2017;52(4):425–460. doi: 10.1080/10409238.2017.1325829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling MA, Diamond MS, Fremont DH. Structural basis of Flavivirus NS1 assembly and antibody recognition. Proc Natl Acad Sci U S A. 2014;111(11):4285–4290. doi: 10.1073/pnas.1322036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Liu Y, Yuan Z. Critical role of Dengue Virus NS1 protein in viral replication. Virol Sin. 2014;29(3):162–169. doi: 10.1007/s12250-014-3459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Garcia MD, Meertens L, Bonazzi M, Cossart P, Arenzana-Seisdedos F, Amara A. Appraising the roles of CBLL1 and the ubiquitin/proteasome system for flavivirus entry and replication. J Virol. 2011;85(6):2980–2989. doi: 10.1128/JVI.02483-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J, Gu F, Ling L, Tolfvenstam T, Olfat F, Chin KC, Aw P, George J, Kuznetsov VA, Schreiber M, Vasudevan SG, Hibberd ML. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl Trop Dis. 2007;1(2):e86. doi: 10.1371/journal.pntd.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Staheli N, Giannakopoulos NV, Kikkert M, Taylor SL, Bridgen A, Paragas J, Richt JA, Rowland RR, Schmaljohn CS, Lenschow DJ, Snijder EJ, Garcia-Sastre A, Virgin HWt. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe. 2007;2(6):404–416. doi: 10.1016/j.chom.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche I, Coulibaly F, Voss JE, Salmon J, d’Alayer J, Ermonval M, Larquet E, Charneau P, Krey T, Megret F, Guittet E, Rey FA, Flamand M. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci U S A. 2011;108(19):8003–8008. doi: 10.1073/pnas.1017338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook MR. Historical Perspectives on Flavivirus Research. Viruses. 2017;9(5) doi: 10.3390/v9050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnjak K, Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem. 2012;81:291–322. doi: 10.1146/annurev-biochem-051810-094654. [DOI] [PubMed] [Google Scholar]
- Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J Virol. 2014;88(9):4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. The ubiquitin-proteasome pathway is important for dengue virus infection in primary human endothelial cells. J Proteome Res. 2010;9(10):4960–4971. doi: 10.1021/pr100219y. [DOI] [PubMed] [Google Scholar]
- Khromykh AA, Sedlak PL, Westaway EG. cis- and trans-acting elements in flavivirus RNA replication. J Virol. 2000;74(7):3253–3263. doi: 10.1128/jvi.74.7.3253-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang L, Sun J, Chen W, Li S, Wang Q, Yu H, Xia Z, Jin X, Wang C. Endoplasmic Reticulum Protein SCAP Inhibits Dengue Virus NS2B3 Protease by Suppressing Its K27-Linked Polyubiquitylation. J Virol. 2017;91(9) doi: 10.1128/JVI.02234-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. Interplay between the virus and the ubiquitin-proteasome system: molecular mechanism of viral pathogenesis. Curr Opin Virol. 2016;17:1–10. doi: 10.1016/j.coviro.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JM, Jones MK, Young PR. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220(1):232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, Young PR. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7(304):304ra142. doi: 10.1126/scitranslmed.aaa3863. [DOI] [PubMed] [Google Scholar]
- Morrison J, Laurent-Rolle M, Maestre AM, Rajsbaum R, Pisanelli G, Simon V, Mulder LC, Fernandez-Sesma A, Garcia-Sastre A. Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS Pathog. 2013;9(3):e1003265. doi: 10.1371/journal.ppat.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013;98(2):192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79(13):8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muylaert IR, Chambers TJ, Galler R, Rice CM. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology. 1996;222(1):159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Rodriguez A, Gonzales MM, Gallego GJ, Castano OJ. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch Virol. 2014;159(3):573–579. doi: 10.1007/s00705-013-1849-6. [DOI] [PubMed] [Google Scholar]
- Paul D, Bartenschlager R. Architecture and biogenesis of plus-strand RNA virus replication factories. World J Virol. 2013;2(2):32–48. doi: 10.5501/wjv.v2.i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016;12(7):e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R, Garcia-Sastre A. Viral evasion mechanisms of early antiviral responses involving regulation of ubiquitin pathways. Trends Microbiol. 2013;21(8):421–429. doi: 10.1016/j.tim.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R, Garcia-Sastre A. Virology. Unanchored ubiquitin in virus uncoating. Science. 2014;346(6208):427–428. doi: 10.1126/science.1261509. [DOI] [PubMed] [Google Scholar]
- Rajsbaum R, Garcia-Sastre A, Versteeg GA. TRIMmunity: the roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. J Mol Biol. 2014a;426(6):1265–1284. doi: 10.1016/j.jmb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R, Versteeg GA, Schmid S, Maestre AM, Belicha-Villanueva A, Martinez-Romero C, Patel JR, Morrison J, Pisanelli G, Miorin L, Laurent-Rolle M, Moulton HM, Stein DA, Fernandez-Sesma A, tenOever BR, Garcia-Sastre A. Unanchored K48-linked polyubiquitin synthesized by the E3-ubiquitin ligase TRIM6 stimulates the interferon-IKKepsilon kinase-mediated antiviral response. Immunity. 2014b;40(6):880–895. doi: 10.1016/j.immuni.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M, Sharma N, Singh SK. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol J. 2016;13:131. doi: 10.1186/s12985-016-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaturro P, Cortese M, Chatel-Chaix L, Fischl W, Bartenschlager R. Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins. PLoS Pathog. 2015;11(11):e1005277. doi: 10.1371/journal.ppat.1005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadinata R, Roesley SN, Yang G, Sarcevic B. Mechanisms of generating polyubiquitin chains of different topology. Cells. 2014;3(3):674–689. doi: 10.3390/cells3030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung CW, Ho SY. Computational identification of ubiquitylation sites from protein sequences. BMC Bioinformatics. 2008;9:310. doi: 10.1186/1471-2105-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol S, Hage A, Giraldo MI, Bharaj P, Rajsbaum R. The TRIMendous Role of TRIMs in Virus-Host Interactions. Vaccines (Basel) 2017;5(3) doi: 10.3390/vaccines5030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson D, Modhiran N, Young PR. The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antiviral Res. 2016;130:7–18. doi: 10.1016/j.antiviral.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5(4):365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: 2009. [PubMed] [Google Scholar]
- Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18(6):579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- Ye J, Chen Z, Zhang B, Miao H, Zohaib A, Xu Q, Chen H, Cao S. Heat shock protein 70 is associated with replicase complex of Japanese encephalitis virus and positively regulates viral genome replication. PLoS One. 2013;8(9):e75188. doi: 10.1371/journal.pone.0075188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Yuan Z, Rice CM, MacDonald MR. Flavivirus replication complex assembly revealed by DNAJC14 functional mapping. J Virol. 2012;86(21):11815–11832. doi: 10.1128/JVI.01022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S, Li T, McCune BT, Edeling MA, Fremont DH, Cristea IM, Diamond MS. Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J Virol. 2012;86(13):7360–7371. doi: 10.1128/JVI.00157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]