Abstract
Purpose
To evaluate the echo dependence of three-dimensional ultrashort echo time quantitative susceptibility mapping (3D UTE-QSM) and R2* measurement in the setting of high concentrations of iron oxide nanoparticles (IONPs).
Methods
A phantom study with iron concentrations ranging from 2 to 22 mM was performed using a 3D UTE Cones sequence. Simultaneous QSM processing with Morphology Enabled Dipole Inversion (MEDI) and R2* single exponential fitting were conducted offline with the acquired 3D UTE data. The dependence of UTE-QSM and R2* on echo spacing (ΔTE) and the first echo time (TE1) was investigated.
Results
A linear relationship was observed between UTE-QSM measurement and iron concentration up to 22 mM only with the minimal TE1 of 0.032 ms and ΔTE of less than 0.1 ms. A linear relationship was observed between R2* and iron concentration up to 22 mM only when TE1 was less than 0.132 ms and ΔTE was less than 1.2 ms. UTE-QSM with MEDI processing showed strong dependence on ΔTE and TE1, especially at high iron concentrations.
Conclusion
UTE-QSM is more sensitive than R2* measurement to echo time selection. Both an ultrashort TE1 and a small ΔTE are needed in order to achieve accurate QSM for high iron concentrations.
Keywords: ultrashort echo time, cones, QSM, R2*, high iron concentration
Introduction
Iron oxide nanoparticles (IONPs) have been increasingly used to generate magnetic resonance imaging (MRI) contrast for molecular imaging applications (1,2). The ability to accurately and non-invasively quantify IONPs is desirable for many emerging applications, including drug delivery (3–6), cell labeling and tracking (7–9), and magnetic fluid hyperthermia (10–12). In addition, endogenous iron, an essential cofactor for proteins with functions including oxygen delivery, mitochondrial respiration and the inactivation of harmful oxygen radicals, can accumulate under pathological conditions (13–16). When systemic or local iron concentrations exceed the binding capacity of iron storage and transport proteins, the free iron will deposit into tissues. These iron deposits accelerate the production of free radicals, resulting in membrane lipid peroxidation, cellular injury, and ultimately organ dysfunction (17–20). For example, patients receiving frequent blood transfusions such as those with sickle cell anemia or thalassemia major can develop secondary hemochromatosis with resultant liver cirrhosis and heart failure (21,22), and patients with hemophilia can have repeated joint hemorrhage leading to focal iron deposition and subsequent joint deterioration (23). Noninvasively measuring endogenous iron deposits would be clinically useful in medical conditions associated with very high iron content, for instance to monitor response to chelation therapy so that iron burden can be reduced while minimizing the risk of over chelation. Consequently, there is a growing interest in quantitative in vivo estimation of both endogenous and exogenous iron accumulation.
Iron is a paramagnetic transition metal that causes shortened T1, T2, and T2* relaxation times as well as phase changes of nearby water protons by its magnetic susceptibility effect (24–26). Since there is a linear increase in susceptibility with iron concentration, quantitative susceptibility mapping (QSM) MRI methods have been developed to estimate iron accumulation in vivo (27–32). There are two widely used QSM processing algorithms: the Morphology Enabled Dipole Inversion (MEDI) (33,34) and iLSQR (subtracting the susceptibility artifacts from the initial susceptibility by the sparse linear equation and least-squares algorithm) methods (35,36). These two methods and their extensions have shown success in quantifying tissue susceptibility with applications in brain (37,38), cartilage (with STAR-QSM) and cortical bone (with Chemical Shift QSM) (39,40).
Most QSM methods calculate the tissue frequency shift using phase information at different echo times (TEs), and therefore are highly dependent on the accuracy of phase measurement. High iron concentrations can be particularly problematic because the high degree of T2* shortening leads to rapid signal decay with a low or no signal when using conventional clinical multi-echo gradient recalled echo (GRE) sequences. High iron concentrations also greatly increase the resonance frequency shift, which can cause severe phase wrapping beyond the capability of traditional phase unwrapping methods. As a result, QSM based on GRE sequences with longer TEs (e.g., TE > 2 ms) may fail when evaluating high iron concentrations.
Ultrashort echo time (UTE) sequences greatly reduce TE from the several milliseconds typically used in conventional clinical sequences down to tens of microseconds or less, allowing the direct detection of signals from short T2 tissues such as cortical bone (41). UTE sequences have been used to measure R2* (1/T2*) of high IONP concentrations, and may be used to detect the associated fast phase evolution. The improvement in signal detection and phase measurement with UTE suggests that its combination with QSM (UTE-QSM) may allow for more accurate estimation of susceptibility when the T2* is greatly reduced by high iron concentrations. However, although originally believed to be TE-independent, recent literature has shown that QSM measurements can be highly TE-dependent (42). Therefore, it is of critical importance to understand the TE-dependence of UTE-QSM type sequences at high iron concentrations.
In this study, we aimed to investigate the capability and limitations of the UTE-QSM technique in evaluating high iron concentrations. A phantom study using different iron concentrations was carried out with a three-dimensional (3D) UTE sequence combined with the MEDI method. The dependence of UTE-QSM and R2* measurement on echo time was also investigated by using different combinations of echo spacing (ΔTE) and the first echo time (TE1).
Methods
Phantom Preparation
Two sets of phantoms were prepared for this study. The first set was a gadolinium (Gd) phantom, with diluted gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, NJ, USA) in six 3 mL syringes in a cylindrical container (10 cm in diameter; height 30 cm) filled with agarose gel (0.9% by weight). The syringes contained six different concentrations of Magnevist: 1.5, 3, 4.5, 6, 7.5 and 9 mg/ml. The second set was an iron phantom which was composed of 3 mL syringes (1 cm diameter) filled with 2 mL of Feridex I.V. solution (ferumoxides injectable solution, Berlex Laboratories, Wayne, New Jersey, USA) at six different concentrations: 2, 6, 10, 14, 18 and 22 mM. The syringes were put in a cylindrical container (10 cm in diameter; height 30 cm) filled with agarose gel (0.9% by weight). During MRI, the longitudinal direction of the syringes was parallel to the B0 field to minimize susceptibility effects.
Pulse Sequences
MR imaging of the phantoms was performed on a 3T Signa HDxt scanner (GE Healthcare Technologies, Milwaukee, Wisconsin, USA) using a previously reported three-dimensional UTE Cones (3D UTE Cones) sequence (43–45). The basic 3D Cones sequence employed a short rectangular excitation pulse followed by 3D spiral trajectory k-space sampling with a conical view ordering. A transmit/receive quadrature coil (BC-10, Medspira, Minneapolis, Minnesota, USA) with a diameter of 22 cm was used for signal excitation and reception.
For the gadolinium (Gd) phantom study, imaging parameters included: matrix = 128×128×100, voxel size = 1×1×1 mm3, TR/TE = 30/3/4/5 ms, flip angle = 12°, bandwidth = 83.3 kHz, scan time = 5 minutes per scan.
For the iron phantom study, imaging parameters included: acquisition matrix = 200×200×60, voxel size = 0.4×0.4×0.5 mm3, repetition time (TR) = 11.8 ms, flip angle = 18°, bandwidth = 62.5 kHz, scan time = 4 minutes per scan. Initially, the linearity between R2* and iron concentration was examined using 12 TEs: 0.032, 0.132, 0.232, 0.332, 0.432, 0.632, 0.932, 1.232, 1.832, 2.432, 3.632, and 4.832 ms. Five sets of UTE acquisitions at evenly spaced TEs starting at TE1 were used for UTE-QSM and R2* analyses of the echo combination datasets. To study the effect of ΔTE on UTE-QSM analysis, five different echo spacings (ΔTE = 0.06, 0.1, 0.3, 0.6, 1.2 ms) were investigated with TE1 kept at a fixed value of 0.032 ms, leading to a total of 25 scans. To study the effect of TE1, six different TE1s (TE1 = 0.032, 0.132, 0.232, 0.332, 0.632, 0.932 ms) were investigated with ΔTE kept at 0.1 ms, leading to a total of 30 scans.
Quantitative Susceptibility Mapping
Each 3D UTE Cones acquisition was reconstructed into both magnitude and phase images using a re-gridding algorithm, which interpolates the measured signal from Cones spokes onto a Cartesian grid. Nominal TEs were used for QSM calculation. Because of the non-uniform sampling density of our spiral trajectory, density compensation was applied to the measured signal prior to re-gridding. To form an echo combination dataset with specified ΔTE and TE1, 5 single echo acquisitions with increasing TE were combined to form a 4D complex matrix.
The MEDI QSM reconstruction algorithm (33) was applied offline with the same complex matrix for measuring the susceptibilities from each of the different iron concentrations. The cylindrical phantom was masked and the B0 direction calculated from localization information. The first three echoes of each dataset were used for estimating frequency shift in an iterative fashion. A region growing based phase unwrapping algorithm (46) was implemented to obtain the global frequency shift. The Projection onto Dipole Fields (PDF) algorithm was used to obtain the background removed frequency shift and phase map. Dipole inversion of the local susceptibility distribution was achieved using an iterative Bayesian regulation method. For all datasets, the regularization parameter λ and radius for the spherical mean value operator were kept as 1000 and 5, respectively, for calculating magnetic susceptibility χ.
ROI Data Analysis and R2* Fitting
The relationship between QSM and R2* for different iron concentrations and different echo combinations was derived from user-defined regions of interest (ROIs). ROIs with fixed diameters of 1 cm were used to cover each tube. χ for each ROI was calculated using the MEDI algorithm. To study the stability of the QSM results for the different iron concentrations, averaged χ for each ROI of the different echo combinations were plotted. To study the linear relationship between the QSM results and different iron concentration, normalized QSM results (divided by χ for the 6 mM phantom, which was chosen arbitrarily) were plotted for each echo combination.
R2* values for each ROI were obtained using a Levenberg-Marquardt fitting algorithm developed in-house, based on Eq. 1. A constant term C was introduced to account for background noise and artifacts associated with UTE data acquisition and image reconstruction.
| [1] |
R2* and UTE-QSM analysis algorithms were written in Matlab (MathWorks, Natick, MA) and were executed offline on axial UTE images obtained with the protocols described above. The program allowed placement of ROIs on the first image of the series, which were then copied to the corresponding position on each of the subsequent images. The mean intensity within each of the ROIs was used for R2* curve fitting.
Results
The gadolinium phantom study demonstrated an excellent linear relationship between UTE-QSM measurements and Gd concentrations (Supporting Fig. S1). Linear regression shows a R2 of 0.9984, suggesting that the UTE-QSM sequence together with MEDI processing can reliably estimate susceptibility.
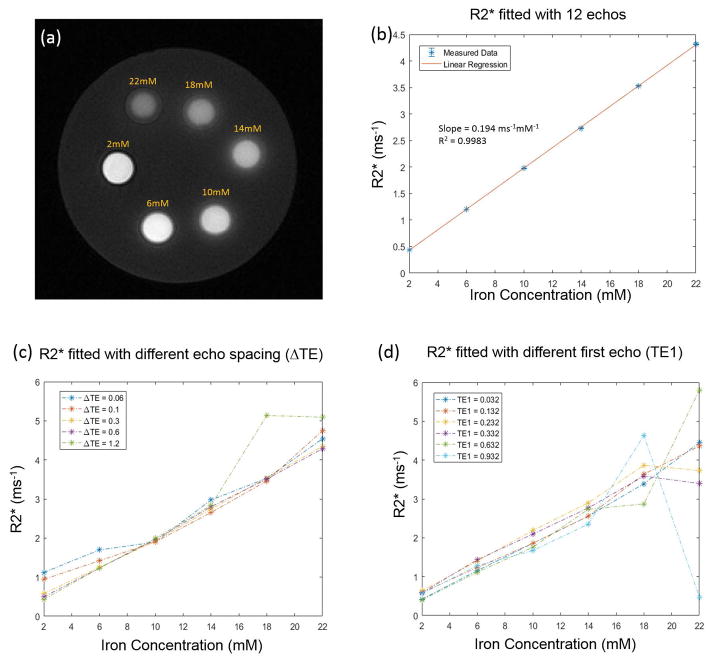
Figure 1 shows the R2* fitting results for the iron phantom study using UTE-QSM. The magnitude image of the phantom at the minimum echo time is shown in Fig 1(a), where the syringe with the lowest iron concentration demonstrates the highest signal intensity. Using 12 TEs ranging from 0.032 ms to 4.832 ms, a linear relationship was observed between R2* and iron concentration as shown in Fig 1(b), with a R2 of 0.9983 and a slope of 0.194 ms−1/mM. Fig 1(c) shows that iron concentrations below 18 mM are relatively linear over a range of ΔTE. However, the longest ΔTE at 1.2 ms overestimated the R2* at the highest iron concentrations of 18 and 22 mM. Interestingly, the R2*s of the lowest two iron concentrations were slightly overestimated with the shortest two ΔTEs of 0.06 and 0.1 ms. Fig 1(d) shows that R2* fitting is relatively linear for iron concentrations lower than 16 mM for all TE1, but at iron concentrations above 16 mM the linear relationship becomes worse as TE1 increases.
Figure 1.
(a) Phantoms with iron concentrations ranging from 2 to 22 mM are detected with high signal with the 3D UTE sequence using a TE of 0.032 ms. (b) A linear relation was observed between R2* and iron concentration using 12 TEs (0.032 to 4.832 ms). (c) Dependency of the apparent R2* of various iron concentrations on echo spacing (ΔTE) with TE1 fixed at 0.032. (d) Dependency of the apparent R2* of various iron concentrations on the first echo time (TE1) with ΔTE fixed at 0.1 ms.
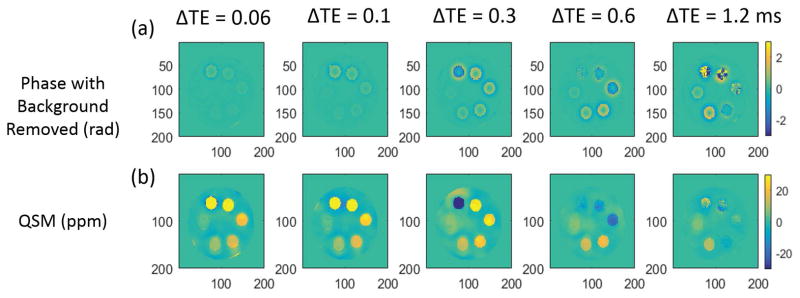
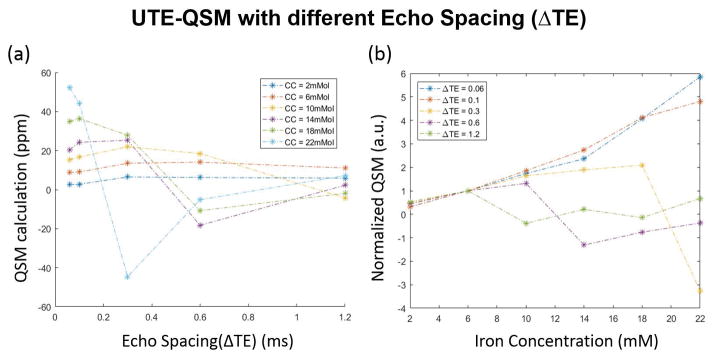
Figure 2 demonstrates how the QSM results depend on both iron concentration and echo spacing. Detailed results on UTE-QSM measurements for different IONP concentrations using different ΔTE and TE1 are shown in Supporting Table S1. Fig 2(a) and (b) are the calculated phases after background removal and QSM results from MEDI using the PDF algorithm. QSM results were successfully obtained from the phase information. An obvious signal decay, as well as phase wrapping, can be observed at higher iron concentrations. Additional phase images are available in Supporting Figure S2. Figure 3 demonstrates that the QSM of iron concentrations below 6 mM remain stable for all the echo combinations with MEDI processing. A linear relationship exists between QSM value and iron concentration for ΔTE = 0.06 ms. However, the calculated QSM value of higher iron concentrations is increasingly underestimated as echo spacing increases.
Figure 2.
QSM results of the iron phantom for different ΔTE with TE1 fixed at 0.032 ms. The (a) calculated phase (with background removed) and (b) QSM reconstructed with the MEDI algorithm are shown.
Figure 3.
QSM ROI analysis depends on both iron concentration and echo spacing (ΔTE). (a) MEDI derived QSM values change with ΔTE dependent on the iron concentration (CC). (b) MEDI derived QSM values at higher iron concentrations are more linear with short ΔTE. QSM values for each dataset were normalized by the QSM value at 6 mM iron.
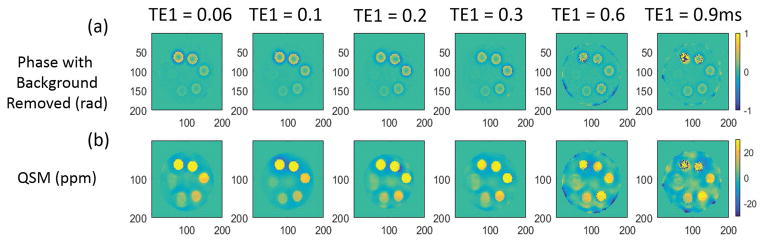
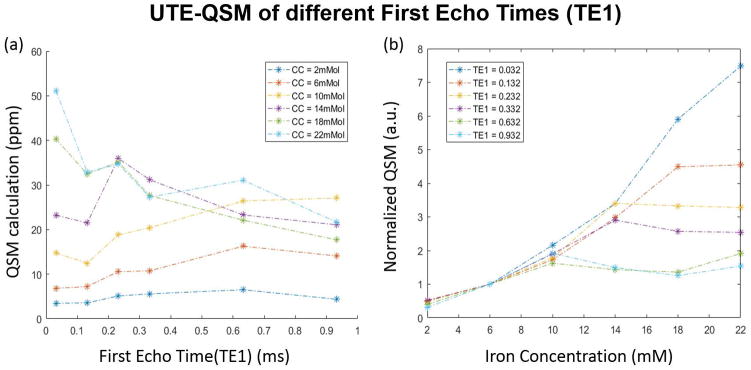
Figure 4 shows the QSM results for echo combinations with different TE1s. An obvious signal decay, as well as phase wrapping, can also be observed at higher iron concentrations in Fig 4(a) and (b). Additional phase images are available in Supporting Figure S3. Figure 5 demonstrates that the QSM of iron concentrations below 10 mM are approximately linear for all tested TE1s, however the QSM values appeared to plateau at higher iron concentrations in a manner dependent on TE1. Only the QSM values from the shortest TE1 at 0.032 ms were approximately linear over all tested iron concentrations.
Figure 4.
QSM results of the iron phantom for different TE1 with ΔTE fixed at 0.1 ms. The (a) calculated phase (with background removed) and (b) QSM reconstructed with the MEDI algorithm are shown.
Figure 5.
QSM ROI analysis depends on both iron concentration and first echo time (TE1). (a) MEDI-derived QSM values change with TE1 dependent on the iron concentration (CC). (b) QSM values are consistently underestimated by longer TE1 as the iron concentration increases. QSM values for each dataset were normalized by the QSM value at 6 mM iron.
Discussion
It is of great clinical significance to quantify endogenous and exogenous iron deposition in the human body. Clinical MRI sequences with conventional TE combinations are not able to accurately quantify iron overload (31). Sequences with much reduced TEs, such as UTE, zero echo time (ZTE) or sweep imaging with Fourier transformation (SWIFT) type sequences make it possible to accurately quantify iron overload (47–49). Nevertheless, these methods have limitations for accurate quantification in vivo, as paramagnetic and diamagnetic substances are unable to be separated due to the positive-real nature of R2* or R1 (32). By shortening the first echo time with 3D radial UTE, QSM has been successfully applied for the study of ultra-short T2* tissues such as cortical bone (50). In this study, simultaneous QSM and R2* measurements were carried out to systematically investigate the capability of UTE-QSM sequences to quantify iron over a large dynamic range. Our results demonstrated that high iron concentrations will significantly reduce the signal decay time and induce dramatic phase wrapping within conventional echo times, leading to inaccurate R2* and MEDI-based QSM measurements. We found that QSM measurements were only accurate over a large dynamic range of iron concentrations when the first echo time was greatly reduced and echo spacing was narrowed.
This study systematically investigated the dependency of MEDI-based QSM as well as R2* relaxometry on echo time by compiling single echo 3D UTE Cones acquisitions of a phantom containing six different IONP concentrations into echo combination datasets. The echo combination datasets varied either by the first echo time or by echo spacing interval. By comparing Fig 3(b) and Fig 5(b), it can be concluded that a linear relationship between QSM and iron concentration only exists when TE1 is reduced to 0.032 ms and ΔTE is less than 0.1 ms. The linear relationship gradually worsens for higher iron concentrations when TE1 or ΔTE are increased. As might be expected, the R2* analysis was more dependent on the first echo time than the echo spacing at high iron concentrations, reflecting the more severe reduction in initial signal magnitude. Our results are consistent with a recent study that showed that QSM of bone could only be successfully obtained with reduced TE1 and ΔTE (50). In contrast, another study using a TE1 of 3 ms and ΔTE of 2 ms was unable to calculate bone susceptibility because bone signal was not detected (39). For clinical studies on iron overload, UTE with minimal nominal TEs and short echo spacing will be necessary for accurate QSM measurement, particularly in zones with highly concentrated iron.
Comparisons of MEDI- and iLSQR-based QSM, as well as other QSM methods, were not carried out in this study(53). The iLSQR algorithm may show different dependence on TE1 and ΔTE than the MEDI algorithm, especially for higher iron concentrations. Sood et al. first reported that iLSQR-based QSM is dependent on echo time selection, especially for different tissue properties (42). After a more systemic study, Cronin et al. concluded that phase wrapping algorithms as well as tissue properties might be the main reasons for the TE dependence in iLSQR (51). In future studies, we will investigate the TE dependence in UTE-QSM with iLSQR processing, together with Laplacian unwrapping and other phase unwrapping algorithms.
A birdcage coil was used in this study for signal reception to avoid QSM reconstruction errors caused by phase combination. In practice, both the magnitude and phase images can be combined with an improved adaptive combined method when using multichannel coils (52).
This study has several limitations. First, IONPs of different concentrations were suspended homogeneously in our phantom, however IONPs would be expected to accumulate or aggregate within biologic tissues, causing nonhomogeneous susceptibility values in vivo. Second, the chemical shift effect was not considered in this study. UTE-QSM together with IDEAL techniques may help resolve potential issues. This study focuses on the measurable dynamic range changes with current QSM methods when combined with UTE sequences. The chemical shift effect is unlikely to affect the UTE-QSM results in this study. Third, the highest concentration of IONPs in this study was 22 mM, which is much lower when compared with 37.5 mM in a study of UTE T2* or T1 measurement (47), and 57.5 mM in a study using SWIFT (48,49). As one of the main findings in this study, the QSM dynamic range is highly dependent on both the first echo time and echo spacing. By further reducing echo spacing, even higher iron concentrations are expected to be accurately quantified at the cost of longer scan time. Parallel imaging or compressed sensing can be applied to reduce the scan time (54, 55). Fourth, multiple single echo 3D UTE Cones acquisitions were used for QSM study of high iron concentrations, which is more accurate but very time consuming and inappropriate for clinical applications. Interleaved multi-echo or echo-shifted 3D UTE Cones data acquisitions could be used for accurate QSM of both low and high iron concentrations while greatly reducing the total scan time (56). Fifth, UTE-QSM with iLSQR processing was not conducted in this study. Since iLSQR and iLSQR-based susceptibility tensor imaging have shown greater robustness for long T2 tissues, the TE1 and ΔTE dependence of iLSQR using UTE-QSM at high iron concentrations would be interesting and will be investigated in the future.
Conclusion
Simultaneous QSM and R2* measurements of high iron concentration up to 22 mM was carried out based on 3D UTE Cones sequences. The effects of the first echo time and echo spacing on the accuracy in QSM and R2* measurements were investigated on iron phantoms. QSM shows greater dependence on the first echo time and echo spacing than R2*. UTE-QSM with MEDI processing shows a strong dependence on both echo spacing and the first echo time, especially for high iron concentrations. Reasonable selection of echo spacing and the first echo time is important for future QSM study of iron overload diseases.
Supplementary Material
Supporting Table S1. UTE-QSM with MEDI processing for different IONPs concentrations using five different ΔTEs ranging from 0.06 to 1.2 ms and six different TE1s ranging from 0.032 to 0.932 ms.
Supporting Figure S1. Phantom experimental validation of the UTE-QSM sequence with MEDI processing: (a) susceptibility map in ppm of six different Gd concentrations: 1.5, 3, 4.5, 6, 7.5, and 9 mg/ml, and (b) linear regression plot of UTE-QSM measurement by Gd concentration.
Supporting Figure S2. Phase map (rad) of the iron phantom at each TE in the variable echo spacing dataset. The echo spacing (ΔTE) changes as shown by the row headings. The first TE was fixed at 0.032 ms.
Supporting Figure S3. Phase map (rad) of the iron phantom at each TE in the variable first echo time dataset. The first echo time (TE1) changes as shown by the row headings. Echo spacing was fixed at 0.1 ms.
Acknowledgments
The authors acknowledge grant funding from Bioverativ, Human Resource and Service Agency (HRSA) H30MC24045, the NIH (R01AR062581-01A1, 1R01 NS092650, and T32EB005970), VA Clinical Science R&D Service (Merit Award I01CX001388), National Natural Science Foundation of China (NSFC 51607169) and Chinese Scholarship Council Grant (CSC 201504910174).
References
- 1.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Thorek DLJ, Chen AK, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Annals of Biomedical Engineering. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 3.Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Molecular Pharmaceutics. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 5.Marcu A, Pop S, Dumitrache F, et al. Magnetic iron oxide nanoparticles as drug delivery system in breast cancer. Applied Surface Science. 2013;281:60–65. [Google Scholar]
- 6.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. International Journal of Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595–615. doi: 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrow M, Taylor A, Murray P, Rosseinsky MJ, Adams DJ. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem Soc Rev. 2015;44:6733–6748. doi: 10.1039/c5cs00331h. [DOI] [PubMed] [Google Scholar]
- 9.Rosen JE, Chan L, Shieh D-B, Gu FX. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:275–90. doi: 10.1016/j.nano.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Dutz S, Häfeli UO, Mahmoudi M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Advances in Colloid and Interface Science. 2011;166:8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano. 2016;10:2436–2446. doi: 10.1021/acsnano.5b07249. [DOI] [PubMed] [Google Scholar]
- 12.Blanco-Andujar C, Walter A, Cotin G, Bordeianu C, Mertz D, Felder-Flesch D, Begin-Colin S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine. 2016;11:1889–1910. doi: 10.2217/nnm-2016-5001. [DOI] [PubMed] [Google Scholar]
- 13.Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. Journal of Research in Medical Sciences. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 14.Sangani RG, Ghio AJ. Iron, human growth, and the global epidemic of obesity. Nutrients. 2013;5:4231–4249. doi: 10.3390/nu5104231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. International Journal of Hematology. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp PA. Intestinal iron absorption: Regulation by dietary & systemic factors. International Journal for Vitamin and Nutrition Research. 2010;80:231–242. doi: 10.1024/0300-9831/a000029. [DOI] [PubMed] [Google Scholar]
- 17.Fleming RE, Ponka P. Iron overload in human disease. The New England journal of medicine. 2012;366:348–59. doi: 10.1056/NEJMra1004967. [DOI] [PubMed] [Google Scholar]
- 18.Siddique A, Kowdley KV. Review article: The iron overload syndromes. Alimentary Pharmacology and Therapeutics. 2012;35:876–893. doi: 10.1111/j.1365-2036.2012.05051.x. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: Pathophysiology, diagnosis, and treatment. Journal of Cardiac Failure. 2010;16:888–900. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment, and monitoring. Hemoglobin. 2009;33(Suppl 1):S46–57. doi: 10.3109/03630260903346676. [DOI] [PubMed] [Google Scholar]
- 21.Brittenham GM, Cohen AR, McLaren CE, Martin MB, Griffith PM, Nienhuis AW, Young NS, Allen CJ, Farrell DE, Harris JW. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. American Journal of Hematology. 1993;42:81–85. doi: 10.1002/ajh.2830420116. [DOI] [PubMed] [Google Scholar]
- 22.Taher AT, Musallam KM, Inati A. Iron Overload: Consequences, Assessment, and Monitoring. Hemoglobin. 2009;33:S46–S57. doi: 10.3109/03630260903346676. [DOI] [PubMed] [Google Scholar]
- 23.Roosendaal G, Vianen ME, Wenting MJ, van Rinsum a C, van den Berg HM, Lafeber FP, Bijlsma JW. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. The Journal of bone and joint surgery British volume. 1998;80:540–545. doi: 10.1302/0301-620x.80b3.7807. [DOI] [PubMed] [Google Scholar]
- 24.Rad AM, Arbab AS, Iskander aSM, Jiang Q, Soltanian-Zadeh H. Quantification of superparamagnetic iron oxide (SPIO)-labeled cells using MRI. Journal of magnetic resonance imaging : JMRI. 2007;26:366–74. doi: 10.1002/jmri.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhlpeter R, Dahnke H, Matuszewski L, Persigehl T, von Wallbrunn A, Allkemper T, Heindel WL, Schaeffter T, Bremer C. R2 and R2* mapping for sensing cell-bound superparamagnetic nanoparticles: in vitro and murine in vivo testing. Radiology. 2007;245:449–457. doi: 10.1148/radiol.2451061345. [DOI] [PubMed] [Google Scholar]
- 26.Girard OM, Ramirez R, Mccarty S, Mattrey RF. Toward absolute quantification of iron oxide nanoparticles as well as cell internalized fraction using multiparametric MRI. Contrast Media and Molecular Imaging. 2012;7:411–417. doi: 10.1002/cmmi.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage. 2012;59:2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magnetic Resonance in Medicine. 2015;73:82–101. doi: 10.1002/mrm.25358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage. 2012;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan H, Liu T, Wu Y, et al. Evaluation of Iron Content in Human Cerebral Cavernous Malformation Using Quantitative Susceptibility Mapping. Investigative Radiology. 2014;49:498–504. doi: 10.1097/RLI.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma SD, Fischer R, Schoennagel BP, Nielsen P, Kooijman H, Yamamura J, Adam G, Bannas P, Hernando D, Reeder SB. MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: Comparison with SQUID-based biomagnetic liver susceptometry. Magnetic Resonance in Medicine. 2016 doi: 10.1002/mrm.26358. [DOI] [PMC free article] [PubMed]
- 32.Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, Nestor PJ, Düzel E. High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R 2 * measurements at 7 T. NeuroImage. 2016;138:43–63. doi: 10.1016/j.neuroimage.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 33.De Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, Wu J, Wang Y. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: Validation and application to brain imaging. Magnetic Resonance in Medicine. 2010;63:194–206. doi: 10.1002/mrm.22187. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Liu T, de Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. NeuroImage. 2012;59:2560–2568. doi: 10.1016/j.neuroimage.2011.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Avram AV, Wu B, Xiao X, Liu C. Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR in Biomedicine. 2014;27:219–227. doi: 10.1002/nbm.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Wang N, Yu F, Han H, Cao W, Romero R, Tantiwongkosi B, Duong TQ, Liu C. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. NeuroImage. 2015;108:111–122. doi: 10.1016/j.neuroimage.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langkammer C, Liu T, Khalil M, Enzinger C, Jehna M, Fuchs S, Fazekas F, Wang Y, Ropele S. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551–9. doi: 10.1148/radiol.12120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei H, Xie L, Dibb R, Li W, Decker K, Zhang Y, Johnson GA, Liu C. Imaging whole-brain cytoarchitecture of mouse with MRI-based quantitative susceptibility mapping. NeuroImage. 2016;137:107–115. doi: 10.1016/j.neuroimage.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei H, Dibb R, Decker K, Wang N, Zhang Y, Zong X, Lin W, Nissman DB, Liu C. Investigating magnetic susceptibility of human knee joint at 7 tesla. Magnetic Resonance in Medicine. 2017;0:1–11. doi: 10.1002/mrm.26596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dimov AV, Liu T, Spincemaille P, Ecanow JS, Tan H, Edelman RR, Wang Y. Joint estimation of chemical shift and quantitative susceptibility mapping (chemical QSM) Magnetic Resonance in Medicine. 2015;73:2100–2110. doi: 10.1002/mrm.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. Journal of Magnetic Resonance. 2010;207:304–311. doi: 10.1016/j.jmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Sood S, Urriola J, Reutens D, O’Brien K, Bollmann S, Barth M, Vegh V. Echo time-dependent quantitative susceptibility mapping contains information on tissue properties. Magnetic Resonance in Medicine. 2016 doi: 10.1002/mrm.26281. [DOI] [PubMed] [Google Scholar]
- 43.Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magnetic Resonance in Medicine. 2016;76:577–582. doi: 10.1002/mrm.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma YJ, Zhu Y, Lu X, Carl M, Chang EY, Du J. Short T2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR–UTE–Cones) sequence. Magnetic Resonance in Medicine. 2017 doi: 10.1002/mrm.26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magnetic Resonance in Medicine. 2006;55:575–582. doi: 10.1002/mrm.20796. [DOI] [PubMed] [Google Scholar]
- 46.Cusack R, Papadakis N. New Robust 3-D Phase Unwrapping Algorithms: Application to Magnetic Field Mapping and Undistorting Echoplanar Images. NeuroImage. 2002;16:754–764. doi: 10.1006/nimg.2002.1092. [DOI] [PubMed] [Google Scholar]
- 47.Hong W, He Q, Fan S, Carl M, Shao H, Chen J, Chang EY, Du J. Imaging and quantification of iron-oxide nanoparticles (IONP) using MP-RAGE and UTE based sequences. Magnetic Resonance in Medicine. 2016;0:19–24. doi: 10.1002/mrm.26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Tang W, Zhen Z, Chen H, Xie J, Zhao Q. Improving detection specificity of iron oxide nanoparticles (IONPs) using the SWIFT sequence with long T2 suppression. Magnetic Resonance Imaging. 2014;32:671–678. doi: 10.1016/j.mri.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Chamberlain R, Etheridge M, Idiyatullin D, Corum C, Bischof J, Garwood M. Quantifying iron-oxide nanoparticles at high concentration based on longitudinal relaxation using a three-dimensional SWIFT look-locker sequence. Magnetic Resonance in Medicine. 2014;71:1982–1988. doi: 10.1002/mrm.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimov AV, Liu Z, Spincemaille P, Prince MR, Du J, Wang Y. Bone quantitative susceptibility mapping using a chemical species-specific R2* signal model with ultrashort and conventional echo data. Magnetic Resonance in Medicine. 2017 doi: 10.1002/mrm.26648. [DOI] [PubMed] [Google Scholar]
- 51.Cronin MJ, Wang N, Decker KS, Wei H, Zhu W-Z, Liu C. Exploring the origins of TE-dependent QSM measurements in healthy tissue and cerebral microbleeds. NeuroImage. 2017;149:98–113. doi: 10.1016/j.neuroimage.2017.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y-J, Liu W, Zhao X, Tang W, Zhang Z, Tang X, Fan Y, Li H, Gao J-H. Improved adaptive reconstruction of multichannel MR images. Medical Physics. 2015;42:637–644. doi: 10.1118/1.4905163. [DOI] [PubMed] [Google Scholar]
- 53.Langkammer C, Schweser F, Shmueli K, Kames C, Li X, Guo L, Milovic C, Kim J, Wei H, Bredies K, Buch S, Guo Y, Liu Z, Meineke J, Rauscher A, Marques JP, Bilgic B. Quantitative susceptibility mapping: Report from the 2016 reconstruction challenge. Magn Reson Med. 2017 doi: 10.1002/mrm.26830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma YJ, Liu W, Tang X, Gao JH. Improved SENSE imaging using accurate coil sensitivity maps generated by a global magnitude–phase fitting method. Magnetic resonance in medicine. 2015;74(1):217–24. doi: 10.1002/mrm.25375. [DOI] [PubMed] [Google Scholar]
- 55.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic resonance in medicine. 2007;58(6):1182–95. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 56.Ma YJ, Liu W, Zhao X, Tang W, Li H, Fan Y, Tang X, Zhang Y, Gao JH. 3D interslab echo–shifted FLASH sequence for susceptibility weighted imaging. Magnetic resonance in medicine. 2016;76(1):222–8. doi: 10.1002/mrm.25872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table S1. UTE-QSM with MEDI processing for different IONPs concentrations using five different ΔTEs ranging from 0.06 to 1.2 ms and six different TE1s ranging from 0.032 to 0.932 ms.
Supporting Figure S1. Phantom experimental validation of the UTE-QSM sequence with MEDI processing: (a) susceptibility map in ppm of six different Gd concentrations: 1.5, 3, 4.5, 6, 7.5, and 9 mg/ml, and (b) linear regression plot of UTE-QSM measurement by Gd concentration.
Supporting Figure S2. Phase map (rad) of the iron phantom at each TE in the variable echo spacing dataset. The echo spacing (ΔTE) changes as shown by the row headings. The first TE was fixed at 0.032 ms.
Supporting Figure S3. Phase map (rad) of the iron phantom at each TE in the variable first echo time dataset. The first echo time (TE1) changes as shown by the row headings. Echo spacing was fixed at 0.1 ms.