Abstract
Background
Heart failure (HF) is common among skilled nursing facility (SNF) residents, yet patients with HF in the SNF setting have not been well described.
Methods
Using Minimum Data Set 3.0 cross-linked to Medicare data (2011–2012), we studied 150,959 HF patients admitted to 13,858 SNFs throughout the USA. ICD-9 codes were used to differentiate patients with HF with preserved ejection fraction (HFpEF), reduced ejection fraction (HFrEF), or unspecified HF.
Results
The median age of the study population was 82 years, 68% were women, 34% had HFpEF, and 27% had HFrEF. HFpEF patients were older than those with HFrEF. Moderate/severe physical limitations (82%) and cognitive impairment (37%) were common, regardless of HF type. The burden and pattern of common comorbidities, with the exception of coronary heart disease, were similar among all groups, with a median of 5 comorbidities. One half of patients with HF had been prescribed angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and 39% evidence-based β-blockers.
Conclusions
SNF residents with HF are old and suffer from significant physical limitations and cognitive impairment and a high degree of comorbidity. These patients differ substantially from HF patients enrolled in randomized clinical trials and that might explain divergence from treatment guidelines.
Keywords: Skilled nursing facility, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, Cross-sectional study
Introduction
Heart failure (HF) is the leading cause of hospitalization among Americans aged ≥65 years [1], and almost one-fourth of elderly Medicare beneficiaries are discharged to a skilled nursing facility (SNF) after being hospitalized for HF [2, 3]. The use of SNF care for patients with HF has steadily increased in recent decades [4]. In 2012,the Readmissions Reduction Program under the Affordable Care Act took effect, which imposes financial penalties on hospitals with excess 30-day readmissions for conditions such as HF [5]. In 2014, the Improving Medicare Post-Acute Care Transformation Act was passed, which intends to shift Medicare payments, including SNF payments, from volume to value [6]. As the population of elderly, high-risk, hospitalized patients with HF expands, and changes in Medicare payment policies gradually take effect, growth in the reliance on SNFs is expected [4].
Although SNFs are a center of transitional care from hospital to home with a focus on rehabilitation, HF is one of the leading causes for potentially preventable re-hospitalizations from SNFs [7, 8]. Furthermore, patients with HF discharged to SNFs have an increased risk of mortality compared with those discharged to home [3]. Although HF is common among SNF residents (20–37%), large randomized clinical trials of HF therapy usually exclude SNF residents [9], and no studies have characterized the clinical condition and psychosocial status of SNF patients with HF in sufficient detail to direct patient-focused interventions to reduce unnecessary hospitalizations and mortality [10].
In 2015, the American Heart Association and the Heart Failure Society of America issued the first scientific statement to guide HF management in SNFs, and acknowledged that the epidemiology of HF among SNF residents has not been well described [9]. Therefore, the objectives of this observational study were to describe the clinical and functional characteristics and use of various cardiac medications among SNF patients with HF, with further stratification according to HF type, using a nationwide dataset including all residents of SNFs in the USA.
Methods
Data sources
We used the Minimum Data Set (MDS) 3.0 cross-linked to the Medicare Beneficiary Summary Files and Medicare Parts A and D. The MDS 3.0 is a federally-mandated comprehensive clinical assessment of all nursing home residents in all Medicare/Medicaid certified facilities. It captures resident-level information on an extensive array of variables including demographics, diagnoses, and physical and psychosocial functioning on admission, quarterly, annually, or following a significant change in the resident’s status by trained nursing staff [11]. Extensive studies have confirmed the reliability and validity of common MDS 3.0 items including residents’ medical, cognitive, functional, and psychological status [11–16]. The summary files contain beneficiaries’ demographic and enrollment information. Medicare Part A contains uniform administrative and clinical elements obtained from discharge abstracts for acute hospital stays of all fee-for-service beneficiaries. Medicare Part D is a prescription drug insurance benefit intended to improve access to essential medications for Medicare beneficiaries. This study was approved by the Institutional Review Board at the University of Massachusetts Medical School.
Study population
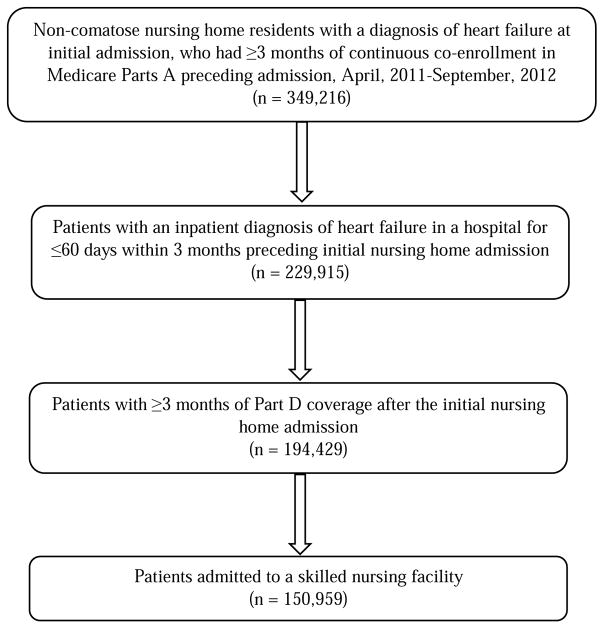
In this cross-sectional study, we identified 349,216 non-comatose nursing home residents with a diagnosis of HF at their initial admission MDS assessment, who had continuous co-enrollment in Medicare Part A for at least 3 months preceding admission between April, 2011 and September, 2012. Among these, 229,915 had been hospitalized for ≤60 days within the 3 months preceding nursing home admission with an inpatient diagnosis of HF [including primary or secondary diagnosis; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.x]. We subsequently further selected 194,429 residents who had ≥3 months of Part D coverage after the initial nursing home admission. Finally, we identified 150,959 patients with HF admitted to a SNF (not long-stay nursing homes) from 13,858 SNFs (Fig. 1). Individuals who died within the 90-day period after the initial admission were excluded because they did not have ≥3 months of Part D coverage.
Fig. 1.
Selection of patients with heart failure
Using the inpatient diagnosis of HF during the patient’s index hospitalization, namely the most recent hospital admission with a diagnosis of HF prior to the initial SNF admission, HF type was determined to be either HF with preserved ejection fraction (HFpEF; ICD-9-CM codes for diastolic HF: 428.3x), HF with reduced ejection fraction (HFrEF; ICD-9-CM codes for systolic HF: 428.2x or 428.4x), or unspecified HF (ICD-9-CM codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, or 428.9).
Primary diagnosis during the patient’s index hospitalization
Because we did not restrict the study population to those with a primary diagnosis of HF, we used the Clinical Classifications Software (CCS) for ICD-9-CM to identify the top 10 medical conditions for the patient’s index hospitalization using the primary diagnosis recorded. The CCS “is one in a family of databases and software tools developed as part of the Healthcare Cost and Utilization Project (HCUP), a Federal-State-Industry partnership sponsored by the Agency for Healthcare Research and Quality”, which is available to the public [17]. We used the single level CCS rankings for the aggregation of various medical conditions.
Patient characteristics, functional and health status, and comorbidities
We considered sociodemographic characteristics including age (18–64, 65–74, 75–84, and ≥85 years), gender, and race/ethnicity (Hispanics of any race, non-Hispanics who areWhite, African American, or a residual category of all others) as well as lifestyle risk factors, including body mass index (BMI; <18.5, 18.5–<25, 25–<30, and ≥30 kg/m2), and current smoking status. Physical function was assessed based on the activities of daily living (ADL) score [18], and categorized as either normal or minimal limitations (0–2), moderate limitations (3–4), or severe limitations/dependency (5–6); cognition was measured based on the Centers for Medicare & Medicaid Services definition integrating the self-reported Brief Interview for Mental Status (BIMS) or a staff-reported Cognitive Performance Scale (CPS), and categorized as normal or minimal impairment (BIMS 13-15 or CPS 0-2), moderate impairment (BIMS 8–12 or CPS 3–4), or severe impairment (BIMS 0–7 or CPS 5–6) [13, 19, 20]. The reliability and validity of the ADL, BIMS, or CPS scores have been demonstrated in comparison with other research instruments [12, 16]. We also considered other conditions usually related to aging including signs or symptoms of delirium (based on the Confusion Assessment Method items) [21, 22], urinary incontinence, falls in the previous 180 days, and pressure ulcers (stage 1 or above) [23, 24]. We considered self- or staff-reported symptoms of dyspnea, diagnosis with cardiovascular comorbidities [including hypertension, coronary heart disease (CHD), cerebrovascular disease, peripheral vasculardisease, atrial fibrillation], and non-cardiovascular comorbidities [including hyperlipidemia, diabetes, anemia, chronic obstructive pulmonary disease (COPD)/asthma, depression, renal impairment, dementia (vascular-type dementia or Alzheimer’s disease), arthritis, osteoporosis, thyroid disorder, and cancer]. All patient characteristics, functional and health status, and comorbidities, with the exception of atrial fibrillation,were based on information from the initial admission MDS assessment. Because atrial fibrillation was not assessed in MDS, it was based on a discharge diagnosis (ICD-9-CM code: 427.3x) claimed in Part A within 3 months preceding the initial SNF admission.
Receipt of pharmacotherapy
Medications administered during a SNF stay are bundled into the per diem cost of the SNF and are not billed to Part D. Therefore, we used part D claims within the 90 days after the SNF admission to define pharmacotherapy use assuming patients would continue their medications even after discharge.
Based on US clinical practice guidelines [25, 26], we identified several HF-related medications including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), evidence-based β-blockers (EBBBs, including bisoprolol, carvedilol, and metoprolol succinate extended release), aldosterone antagonists (including spironolactone and eplerenone), nitrates, hydralazine, loop diuretics, thiazides, potassium-sparing diuretics (with the exception of spironolactone and eplerenone), and digoxin. We also ascertained the prescribing of several other cardiac medications, including non-evidence-based β-blockers (all other β-blockers except those included in EBBBs), antiarrhythmic agents (class I and III), calcium channel blockers (including dihydropyridine, diltiazem, and verapamil), renin inhibitors, anticoagulants, and statins. Since aspirin and omega-3 fatty acid supplements are over the counter therapies, we did not have information available about the use of these medications and supplements.
We described all study variables (including primary diagnosis for the index hospitalization, patient characteristics, functional and health status, comorbidities, and pharmacotherapy use) overall and further stratified according to HF type. Missing data were <3% for all collected variables with the majority being <0.1%.
Results
The study population consisted of 150,959 SNF patients with HF. The median age of this population was 82 years, 68% were women, 34%, 27%, and 39% had HFpEF, HFrEF, and unspecified HF, respectively (Table 1). The proportion of the oldest patients (aged ≥85 years) was 42% in those with HFpEF and 36% in those with HFrEF. Women accounted for 76% and 58% of the patients with HFpEF or HFrEF, respectively. Approximately 40% of patients with HFpEF were obese, while 30% of those with HFrEF were obese. On average, 82% of patients with HF suffered from at least moderate physical limitations and 37% had cognitive impairment, regardless of HF type. Patients with unspecified HF had characteristics between those with HFpEF and HFrEF, and were slightly more likely to have symptoms or conditions related to aging including delirium, urinary incontinence, and a fall history compared to those with HFpEF or HFrEF. HF was the leading cause for the index hospitalization for all three groups accounting for 21.1% among those with HFpEF, 25.9% in those with HFrEF, and 9.9% in patients with unspecified HF. COPD and urinary tract infections were among the top 10 conditions for the index hospitalization for patients with HFpEF, but not for patients with HFrEF. Acute myocardial infarction was the second cause for the index hospitalization for patients with HFrEF, while it was not a top 10 condition for patients with HFpEF.
Table 1.
Sociodemographic and clinical characteristics of patients with heart failure (HF) admitted to a skilled nursing facility, stratified by type of HF
| HFpEF (n = 51,162) | HFrEF (n = 41,340) | Unspecified HF (n =58,457) | |
|---|---|---|---|
| Age, years (%) | |||
| 18–64 | 6.8 | 8.3 | 8.6 |
| 65–74 | 17.6 | 20.3 | 19.4 |
| 75–84 | 33.9 | 35.8 | 34.4 |
| 85+ | 41.7 | 35.6 | 37.6 |
| Women (%) | 76.2 | 58.1 | 68.5 |
| Race/ethnicity(%) | |||
| White | 82.1 | 80.5 | 80.0 |
| African American | 10.9 | 12.0 | 11.7 |
| Hispanic | 3.3 | 3.8 | 4.6 |
| Other | 1.6 | 1.6 | 1.7 |
| BMI, kg/m2 (%) | |||
| <18.5 | 4.2 | 5.6 | 4.7 |
| 18.5-<25 | 28.8 | 35.1 | 31.3 |
| 25-<30 | 25.0 | 27.1 | 26.3 |
| 30+ | 39.4 | 30.1 | 35.3 |
| Current smoking(%) | 2.6 | 3.4 | 3.6 |
| Physical function (%) | |||
| Moderate limitations | 61.8 | 60.7 | 60.8 |
| Severe limitations/dependency | 20.8 | 19.5 | 22.6 |
| Cognitive status (%) | |||
| Moderate impairment | 24.0 | 24.7 | 24.7 |
| Severe impairment | 11.7 | 12.4 | 14.3 |
| Delirium(%) | 3.0 | 3.1 | 3.5 |
| Urinary incontinence(%) | 26.8 | 24.4 | 28.3 |
| Fall (previous 180 days)(%) | 37.2 | 35.1 | 40.5 |
| Pressure ulcers (stage 1 or above)(%) | 16.5 | 18.0 | 18.2 |
| Dyspnea(%) | 37.1 | 34.5 | 30.5 |
| Top ten medical conditions for index hospitalization (%)* | |||
| HF | 21.1 | 25.9 | 9.9 |
| Septicemia | 6.3 | 6.3 | 5.3 |
| Pneumonia | 5.5 | 4.0 | 5.1 |
| Hip fracture | 4.3 | 3.7 | 5.1 |
| Renal failure | 3.8 | 3.6 | 3.5 |
| Acute myocardial infarction | 2.4† | 6.6 | 2.2‡ |
| Cardiac arrhythmias | 3.9 | 4.1 | 2.6 |
| COPD | 3.4 | 2.0§ | 3.2 |
| Respiratory failure | 3.5 | 2.6 | 2.2‡ |
| Urinary tract infections | 2.5 | 1.8§ | 3.5 |
HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Among all patientswith heart failure
Not a top 10 condition for index hospitalization among patients with HFpEF.
Not a top 10 condition for index hospitalization among patients with unspecified HF.
Not a top 10 condition for index hospitalization among patients with HFrEF.
The study patients had a median of five comorbidities (two cardiovascular and three non-cardiovascular conditions), and this was similar for all three HF types (Table 2). Hypertension was the most common condition present in all three groups. A history of CHD was prevalent with 52% in patients with HFrEF and 38% among those with HFpEF. Hyperlipidemia, atrial fibrillation, and diabetes were also common in all three HF types. A slightly higher proportion of patients with HFpEF had COPD/asthma and arthritis than those who had HFrEF.
Table 2.
Comorbid conditions of patients with heart failure (HF) recorded at admission to a skilled nursing facility, stratified by type of HF
| Condition | HFpEF (n = 51,162) | HFrEF (n = 41,340) | Unspecified HF (n = 58,457) |
|---|---|---|---|
| Cardiovascular comorbidities (%) | |||
| Hypertension | 83.1 | 80.7 | 82.6 |
| Atrial fibrillation | 45.8 | 48.5 | 41.1 |
| Coronary artery disease | 38.0 | 51.6 | 42.5 |
| Cerebrovascular disease | 11.0 | 11.8 | 13.3 |
| Peripheral vascular disease | 10.5 | 12.5 | 11.0 |
| Number of cardiovascular comorbidities, Median (25 - 75 percentile) | 2 (1–2) | 2 (1–3) | 2 (1–3) |
| Non-cardiovascular comorbidities (%) | |||
| Diabetes | 44.8 | 46.5 | 44.7 |
| Hyperlipidemia | 44.6 | 47.5 | 43.3 |
| COPD/asthma | 39.3 | 33.8 | 36.1 |
| Anemia | 37.3 | 33.9 | 34.6 |
| Depression | 32.4 | 29.2 | 33.3 |
| Arthritis | 28.8 | 23.9 | 29.1 |
| Thyroid disorder | 25.6 | 22.2 | 24.1 |
| Renal impairment | 26.0 | 27.7 | 23.1 |
| Dementia | 18.8 | 18.1 | 21.5 |
| Osteoporosis | 12.6 | 9.3 | 11.9 |
| Cancer | 5.8 | 5.9 | 6.5 |
| Number of non-cardiovascular comorbidities, Median (25 - 75 percentile) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; COPD, chronic obstructive pulmonary disease.
Loop diuretics were the most common medications prescribed in all three groups. One half of patients with HF had been prescribed ACEIs/ARBs: 47% of HFpEF patients and 56% of HFrEF patients; 39% used EBBBs: 33% of HFpEF patients and 53% of HFrEF patients (Table 3). Use of aldosterone antagonists and digoxin accounted for 11% and 13% of patients with HFpEF and 17% and 20% of patients with HFrEF, respectively. Nearly one third of HF patients used non-evidence-based β-blockers, 31% used calcium channel blockers (mainly dihydropyridine), and one half used statins.
Table 3.
Pharmacological management of patients with heart failure (HF) within 90 days after the initial skilled nursing facility admission, stratified by type of HF
| HFpEF (n = 51,162) | HFrEF (n = 41,340) | Unspecified HF (n = 58,457) | |
|---|---|---|---|
| HF-related medications (%) | |||
| ACEIs/ARBs* | 47.2 | 55.7 | 47.6 |
| ACEIs | 32.8 | 43.2 | 34.2 |
| ARBs | 16.3 | 14.7 | 15.2 |
| EBBBs† | 33.0 | 52.7 | 35.3 |
| Aldosterone antagonists | 10.6 | 17.4 | 11.0 |
| Nitrates | 19.8 | 24.3 | 18.2 |
| Hydralazine | 9.4 | 8.7 | 6.8 |
| Loop diuretics | 71.3 | 72.8 | 65.3 |
| Thiazide diuretics | 14.0 | 12.3 | 12.0 |
| Potassium-sparing diuretics | 2.1 | 1.5 | 1.7 |
| Digoxin | 12.9 | 20.4 | 15.5 |
| Other cardiac medications (%) | |||
| Non-evidence-based β-blockers | 38.1 | 28.2 | 31.9 |
| Antiarrhythmic agents | 9.0 | 14.1 | 9.3 |
| Calcium channel blockers | 39.1 | 22.8 | 30.7 |
| Dihydropyridine | 25.3 | 15.3 | 20.5 |
| Diltiazem | 12.1 | 6.7 | 8.9 |
| Verapamil | 1.7 | 0.8 | 1.3 |
| Renin inhibitors | 0.5 | 0.3 | 0.4 |
| Anticoagulants | 29.8 | 32.1 | 28.6 |
| Statins | 48.8 | 54.2 | 46.9 |
HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; EBBB, evidence-based β-blocker.
5% of patients had claims for both ACEIs and ARBs and were counted in both ACEI and ARB subcategories, so the sum of ACEI/ARB is not equal the sum of ACEI and ARB subcategories;
Includes bisoprolol, carvedilol, and metoprolol succinate extended release.
Discussion
Our study documented that SNF patients with HF in the USA are old, more often women, have a high degree of functional and cognitive impairment, and a high burden of various comorbidities. One half of patients with HF were prescribed ACEIs/ARBs, and 39% were prescribed EBBBs, with higher proportions in patients with HFrEF than those with HFpEF.
Patient characteristics
The importance of HFpEF has been increasingly recognized by clinicians and researchers in recent decades. Among patients with HF, HFpEF accounts for approximately one half of all cases, with estimates ranging between 40% and 70% in the published literature [27, 28]. Patients with HFpEF are older, more often women, and more likely to be obese than those with HFrEF [29–35]. Our study findings are consistent with previous reports, although both HFpEF and HFrEF patients in the SNF setting were old (median age >80 years). Regardless of HF type, SNF patients with HF had a high degree of functional and cognitive impairment which are consistent with previous reports [36, 37]. Further, patients with HFpEF were more likely to have non-cardiac conditions for their index hospitalizations compared to those with HFrEF. The characteristics of patients with unspecified HF exhibited demographic and clinical features between those with HFpEF and HFrEF, likely because these patients reflect non-specific ICD-9 coding, rather than a series with unmeasured ejection fraction.
Comorbidities
Many studies have reported that patients with HFpEF were more likely to have hypertension than those with HFrEF [29–35]. In our study, hypertension was highly prevalent in all three groups of patients with HF (>80%). Patients with HFpEF have also been shown to be more likely to have atrial fibrillation previously diagnosed compared to those with HFrEF [29–34, 38]. However, in our study patients with HFpEF were slightly less likely to have a medical history of atrial fibrillation compared to those with HFrEF. Silent (undiagnosed) atrial fibrillation is common, especially in older populations and in patients with HF [39]. Because we estimated atrial fibrillation using a claimed inpatient diagnosis in the 3-month look back period before admission to a SNF, it is possible to miss silent atrial fibrillation which may at least partially explain our finding. CHD has been reported to be less common in patients with HFpEF than in those with HFrEF [29–35], and our study reaffirms this finding. Overall, the burden and pattern of comorbidity varied only slightly between SNF patients with HFpEF and HFrEF.
Pharmacotherapy practices
Appropriate pharmacotherapy can prolong survival and reduce morbidity among patients with HFrEF, but not HFpEF [25, 26]. Hypertension, which is highly prevalent in patients with HFpEF, should be managed in accordance with current practice guidelines [9, 25, 26]. Consistent with these recommendations, the proportions of patients with HFpEF who had received disease-modifying medications (including ACEIs/ARBs, EBBBs, and aldosterone antagonists) are lower compared to those with HFrEF. ACEIs/ARBs are probably used to manage hypertension in patients with HFpEF, but the indications of the specific prescriptions are not available in Part D claims data. While the use of loop diuretics was similar between those with HFpEF and HFrEF, use of digoxin was more common in patients with HFrEF. Current guidelines recommend digoxin as adjunctive therapy to alleviate symptoms in HFrEF patients who fail to respond adequately to standard HF medications, including ACEIs/ARBs and EBBBs [26], which may at least in part explain this finding.
On the other hand, the proportions of patients with HFpEF who had been treated with non-evidence-based β-blockers, or calcium channel blockers are higher than those with HFrEF. Because atrial fibrillation is common in the study HF patients, non-evidence-based β-blockers might be used for rate control in these patients at least in part. Calcium channel blockers are not recommended as routine treatment for patients with HFrEF [26]. Because calcium channel blockers are effective in hypertension treatment as ACEIs/ARBs [40], these drugs are probably used to manage hypertension in patients with HFpEF. The main findings of HF-related pharmacotherapy use from our study were similar to those from the Get With The Guidelines (GWTG)-HF program at the time of hospital admission, but lower than that recorded at the time of hospital discharge [33]. Approximately 60% of the participants in the GWTG-HF program had been prescribed ACEIs (HFpEF: 51%; HFrEF: 75%) at the time of hospital discharge, 22% had been prescribed ARBs (HFpEF: 23%; HFrEF: 21%), and 85% β-blockers (HFpEF: 79%; HFrEF: 92%).
Study strengths and limitations
This study provides insights from a national perspective into an increasingly important healthcare setting with extensive information on the demographic and clinical characteristics of patients with HF. Despite this, there are several limitations. Our study was cross-sectional in nature, and was restricted to patients who survived 90 days after the initial SNF admission, perhaps selecting patients with milder forms of this clinical syndrome. We did not have direct measurements of an ejection fraction to validate the diagnosis of HFpEF and HFrEF, but instead based our classification on ICD-9-CM codes; 77% of Medicare beneficiaries with ICD-9-CM codes for systolic HF (428.2x or 428.4x) had an ejection fraction <45% based on medical record review [41]. Therefore, some patients with HFpEF might have been classified as having HFrEF or vice versa. Further, nearly 40% of patients with HF could not be classified as having either HFpEF or HFrEF on the basis of ICD-9-CM codes in the present study. We evaluated pharmacotherapy use within 3 months after the initial SNF admission assuming that patients would continue their medications even after discharge, which is an untested assumption. Lastly, we had no information on physician or other healthcare provider visits. However, the Centers for Medicare & Medicaid Services require an initial comprehensive physician visit in a SNF within 30 days of admission (and alternating with a licensed physician assistant, nurse practitioner, or clinical nurse specialist, thereafter).
Conclusions
Residents of a SNF with HF appear to differ substantially from HF patients enrolled in randomized clinical trials, who are relatively young and relatively free from comorbidities and functional limitations [26, 42]. With the increasing use of SNF care for patients with HF, studies to provide evidence-based data to guide patient-centered care for SNF patients with HF in the context of multimorbidity and limited functional status are urgently needed.
Highlights.
34% of heart failure (HF) patients in skilled nursing facility (SNFs) had HF with preserved ejection fraction (HFpEF), 27% had reduced ejection fraction (HFrEF), and 39% had non-specific International Classification of Diseases codes.
HF patients in SNFs suffer from multiple comorbidities and significant functional impairment.
HFpEF and HFrEF patients in SNFs had similar comorbidity burden and patterns.
50% of HF patients in SNFs used angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and 39% used evidence-based β-blockers.
Acknowledgments
Funding
Dr Lin Li has received funding from a National Institutes of Health Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant (5T32HL120823-02). Dr Robert Goldberg has received partial salary support provided by National Institutes of Health Grant 1U01HL105268–01 and R01 HL35434.
Footnotes
Disclosures
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National hospital discharge survey: 2007 summary. Natl Health Stat Report. 2010;29:1–20. 24. [PubMed] [Google Scholar]
- 2.Dolansky MA, Xu F, Zullo M, Shishehbor M, Moore SM, Rimm AA. Post-acute care services received by older adults following a cardiac event: a population-based analysis. J Cardiovasc Nurs. 2010;25:342–9. doi: 10.1097/JCN.0b013e3181c9fbca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen LA, Hernandez AF, Peterson ED, Curtis LH, Dai D, Masoudi FA, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300. doi: 10.1161/CIRCHEARTFAILURE.110.959171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr NM, Forman DE, De Matteis G, Gambassi G. Heart failure among older adults in skilled nursing facilities: more of a dilemma than many now realize. Curr Geriatr Rep. 2015;4:318–26. doi: 10.1007/s13670-015-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services. [accessed 17-02-15];Readmissions Reduction Program (HRRP) 2016 https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html.
- 6.Centers for Medicare & Medicaid Services. [accessed 17-02-15];SNF Quality Reporting Program (IMPACT Act 2014) https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Skilled-Nursing-Facility-Quality-Reporting-Program/SNF-Quality-Reporting-Program-IMPACT-Act-2014.html.
- 7.David S, Sheikh F, Mahajan D, Greenough W, Bellantoni M. Whom do we serve? Describing the target population for post-acute and long-term care, focusing on nursing facility settings, in the era of population health in the United States. J Am Med Dir Assoc. 2016;17:574–80. doi: 10.1016/j.jamda.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Kramer A, Lin M, Fish R, Min SJ. [accessed 17-02-15];Development of potentially avoidable readmission and functional outcome SNF quality measures. 2014 https://www.providigm.com/wp-content/uploads/2013/08/Mar14_2014_SNFQualityMeasures_CONTRACTOR.pdf.
- 9.Jurgens CY, Goodlin S, Dolansky M, Ahmed A, Fonarow GC, Boxer R, et al. Heart failure management in skilled nursing facilities: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circ Heart Fail. 2015;8:655–87. doi: 10.1161/HHF.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 10.Jung M, Yeh AY, Pressler SJ. Heart failure and skilled nursing facilities: review of the literature. J Card Fail. 2012;18:854–71. doi: 10.1016/j.cardfail.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services. [accessed 17-02-15];Long-term care facility resident assessment instrument 3.0 User's Manual. 2015 version 1.13; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Downloads/MDS-30-RAI-Manual-V113.pdf.
- 12.Chodosh J, Edelen MO, Buchanan JL, Yosef JA, Ouslander JG, Berlowitz DR, et al. Nursing home assessment of cognitive impairment: development and testing of a brief instrument of mental status. J Am Geriatr Soc. 2008;56:2069–75. doi: 10.1111/j.1532-5415.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 13.Saliba D, Buchanan J, Edelen MO, Streim J, Ouslander J, Berlowitz D, et al. MDS 3. 0: brief interview for mental status. J Am Med Dir Assoc. 2012;13:611–7. doi: 10.1016/j.jamda.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Rahman M, Tyler D, Acquah JK, Lima J, Mor V. Sensitivity and specificity of the Minimum Data Set 3. 0 discharge data relative to Medicare claims. J Am Med Dir Assoc. 2014;15:819–24. doi: 10.1016/j.jamda.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3. 0. J Am Med Dir Assoc. 2012;13:602–10. doi: 10.1016/j.jamda.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Saliba D, Buchanan J. Development & validation of a revised nursing home assessment tool: MDS 3.0. Santa Monica, CA: RAND Health Corporation; 2008. [Google Scholar]
- 17.HCUP Home. Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: Mar, 2017. [accessed 17-03-07]. www.hcup-us.ahrq.gov/home.jsp. [PubMed] [Google Scholar]
- 18.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–53. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 19.Morris JN, Fries BE, Mehr DR, Hawes C, Phillips C, Mor V, et al. MDS cognitive performance scale. J Gerontol. 1994;49:M174–82. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. [accessed 17.03.15];Nursing Home Data Compendium 2015 Edition. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf.
- 21.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK. The Short Confusion Assessment Method (Short CAM): Training manual and coding guide. Boston: Hospital Elder Life Program; 2014. [Google Scholar]
- 23.Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3. 0. J Am Med Dir Assoc. 2012;13:595–601. doi: 10.1016/j.jamda.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heart Failure Society of America. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–10. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–32. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 31.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RJ, Gurwitz JH, Saczynski JS, Hsu G, McManus DD, Magid DJ, et al. Comparison of medication practices in patients with heart failure and preserved versus those with reduced ejection fraction (from the Cardiovascular Research Network [CVRN]) Am J Cardiol. 2013;111:1324–9. doi: 10.1016/j.amjcard.2013.01.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–30. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, et al. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am J Med. 2016;129:635, e15–26. doi: 10.1016/j.amjmed.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Loop MS, Van Dyke MK, Chen L, Brown TM, Durant RW, Safford MM, et al. Comparison of length of stay, 30-day mortality, and 30-day readmission rates in Medicare patients with heart failure and with reduced versus preserved ejection fraction. Am J Cardiol. 2016;118:79–85. doi: 10.1016/j.amjcard.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gambassi G, Forman DE, Lapane KL, Mor V, Sgadari A, Lipsitz LA, et al. Management of heart failure among very old persons living in long-term care: Has the voice of trials spread? Am Heart J. 2000;139:85–93. doi: 10.1016/s0002-8703(00)90313-2. [DOI] [PubMed] [Google Scholar]
- 37.Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. 2014;11:316–28. doi: 10.11909/j.issn.1671-5411.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saczynski JS, Go AS, Magid DJ, Smith DH, McManus DD, Allen L, et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc. 2013;61:26–33. doi: 10.1111/jgs.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis RC, Hobbs FD, Kenkre JE, Roalfe AK, Iles R, Lip GY, et al. Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace. 2012;14:1553–9. doi: 10.1093/europace/eus087. [DOI] [PubMed] [Google Scholar]
- 40.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20:700–8. doi: 10.1002/pds.2146. [DOI] [PubMed] [Google Scholar]
- 42.Azad N, Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol. 2014;11:329–37. doi: 10.11909/j.issn.1671-5411.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]