Abstract
The cGMP signaling axis has been implicated in the suppression of intestinal cancers but the inhibitory mechanism and the extent to which this pathway can be targeted remains poorly understood. The present study has tested the effect of cGMP-elevating agents on tumorigenesis in the ApcMin/+ mouse model of intestinal cancer. Treatment of ApcMin/+ mice with the receptor guanylyl-cyclase C (GCC) agonist linaclotide, or the phosphodiesterase-5 (PDE5) inhibitor sildenafil, significantly reduced the number of polyps per mouse (67% and 50% respectively). Neither of the drugs affected mean polyp size, or the rates of apoptosis and proliferation. This was possibly due to increased PDE10 expression, since endogenous GCC ligands were not deficient in established polyps. These results indicated that the ability of these drugs to reduce polyp multiplicity was primarily due to an effect on non-neoplastic tissues. In support of this idea, ApcMin/+ mice exhibited reduced levels of endogenous GCC agonists in the non-neoplastic intestinal mucosa compared to wild type animals, and this was associated with crypt hyperplasia and a loss of goblet cells. Administration of either sildenafil or linaclotide suppressed proliferation, and increased both goblet cell numbers and luminal apoptosis in the intestinal mucosa. Taken together, the results demonstrate that targeting cGMP with either PDE5 inhibitors or GCC agonists alters epithelial homeostasis in a manner that reduces neoplasia, and suggests that this could be a viable chemoprevention strategy for patients at high-risk of developing colorectal cancer.
Keywords: cGMP, colon cancer, PDE-5 inhibitor, GC-C agonist, prevention
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in the United States with 1 in 20 being diagnosed each year (1). Early screening and treatment is the cornerstone of colorectal cancer prevention; but the high mortality rate associated with diagnosis at advanced stages makes it one of the leading causes of cancer related deaths. Chemoprevention has therefore emerged as a complementary strategy for patients that are predisposed to colon cancer because of hereditary disease, ulcerative colitis, or family history (2,3). The most widely investigated chemopreventative agents are non-steroidal anti-inflammatory drugs (NSAIDs). They have been shown to reduce the incidence of colorectal cancer when used long-term; but side effects in the average-risk population of predisposed patients limits their utility (4).
A derivative of the NSAID sulindac called Exisulind, lacks COX-inhibitory activity but was found to block growth in colon cancer cell lines by inhibiting phosphodiesterases (PDE) (5,6). The underlying mechanism was shown to involve inhibition of β-catenin signaling, leading to apoptosis (7,8). In clinical trials, Exisulind suppressed tumorigenesis in familial adenomatous polyposis (FAP) patients (9). Although Exisulind induced regression of rectal polyps in FAP patients it did not get FDA-approval due to harmful side effects reported in Phase III trials (10). Although Exisulind was ultimately not successful, it did highlight the therapeutic potential of cyclic guanosine monophosphate (cGMP) signaling.
The central source of cGMP in the intestinal epithelium arises from the activity of guanylyl cyclase C (GCC), which is activated by the endogenous peptide secretagogues uroguanylin and guanylin (11). The well-established role of cGMP in the intestine is ion channel regulation to promote fluid secretion (12), but a growing body of evidence has shown that it also controls epithelial homeostasis. Analysis of knockout mice suggests that the cGMP signaling axis suppresses proliferation, and apoptosis, while promoting differentiation in the intestinal epithelium (13–15). In addition to its role in regulation of epithelial homeostasis, growing evidence also suggests that activating cGMP signaling in the colon could have therapeutic potential for colon cancer. GCC knockout mice exhibit increased tumorigenesis in both AOM and ApcMin/+ mouse models of colon cancer (16,17). The endogenous GCC peptides uroguanylin and guanylin are commonly lost gene products in most human colon cancers suggesting that supplementing with exogenous hormone could be a potential treatment strategy (18,19). In support of this idea, administration of the exogenous uroguanylin reduced tumorigenesis in ApcMin/+ mice, but the protective mechanism was not addressed (20). More recent studies with the GCC agonist plecanatide (21), and the PDE5 inhibitor sildenafil demonstrate that cGMP elevation can promote barrier integrity, and protect from inflammation-driven colorectal cancer (22–24). However, the relative efficacy of the two approaches to cGMP elevation, and the role of suppressing colitis in the protective mechanism remain unclear. The present study sought to compare the tumor-prevention effects of orally administered sildenafil and linaclotide in a colitis-independent intestinal polyposis model.
Materials and Methods
Animals and Drug Administration
All procedures were approved by the Augusta University Committee on the Care and Use of Animals. For tumorigenesis studies, male C57/BL6J-ApcMin/+ mice and female C57/BL6J mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) at age 6 weeks for breeding. Mice were housed in the Augusta University animal facility in a temperature and humidity controlled room with free access to food and water. Pharmaceutical grade sildenafil citrate (Revatio™) was ground with a mortar and pestle, and stored in suspension with diH2O at −80°C until use. Stock sildenafil was diluted in drinking water to a concentration representing a dose of 5.7 mg/kg. Linaclotide was prepared by grinding the contents of Linzess® capsules in diH2O using a tissue homogenizer. Aliquots were stored at −80°C and were diluted fresh for each experiment. Mice were gavaged daily with 100µl of the linaclotide suspension at a concentration of 2.07 µg/ml.
Tumorigenesis studies
Mice were genotyped by PCR from tail-clip DNA at 3 weeks of age and drug treatment started at 4 weeks. Body weight was recorded weekly to monitor disease progression. At the end of 14 weeks, the animals were sacrificed and the entire gastrointestinal tract from stomach to rectum was removed and flushed with ice-cold PBS. The small intestine was then divided into three sections, opened longitudinally, and spread on a light box for the acquisition of high-resolution images. The images were subsequently enhanced and enlarged for identification and selection of polyps, which were quantitated and sized using ImageJ software (National Institutes of Health, Bethesda, Maryland). After capturing images, representative polyps were collected for RNA, protein, or histological analysis.
Histopathology
Intestinal tissues from age-matched mice were formalin-fixed, embedded in paraffin, and sectioned at 5µm thickness by the Augusta University Histology Core. Sections were processed as follows: deparaffinization with xylene, rehydration with decreasing concentrations of ethanol (100%-50%), antigen-retrieval by boiling in citrate buffer. Tissues were stained directly by the histology core with H&E and Alcian blue/periodic acid Schiff to visualize polyps and goblet cells. The tissues were probed using antibodies against Ki-67 (1:100; Dako Cytomation, Carpinteria, CA, USA) and cleaved caspase-3 (1:500; Cell Signaling, Danvers, MA, USA). Antibody visualization was done using an anti-rat/anti-rabbit ImmunoCruz ABC kit (Santa Cruz Biotechnology, Dallas,TX, USA) to enhance DAB staining. Mucus density and proliferative and apoptotic indices were quantitated using ImageJ software. Equivalent regions of intestine were compared between groups for all quantitative histological studies. Briefly, for surrounding tissue, 10 different sections containing approximately 8 crypts per section were analyzed for each mouse. For polyps, three sections from four different polyps per mouse were analyzed.
Analysis of gene expression
Polyps were removed with scissors and forceps, and the mucosa was scraped from the underlying connective tissues. Tissue was vortexed briefly in TRIzol reagent (Life Technologies) and flash-frozen. RNA was DNAse treated (TURBO DNA-free kit, Life Technologies) and converted to cDNA using M-MLV reverse transcriptase (Invitrogen™). Quantitative PCR (qRT-PCR) analysis of the cDNA was performed using SYBR Green PCR Master Mix (Applied Biosystems). Relative expression levels were calculated using the 2–ΔΔCT method with β-actin (ACTB) as a reference. Amplifications were performed in triplicate wells and melt curve analysis was done to confirm the specificity of the primers used. Primers were designed using Primer Blast Software (NCBI; Table S1)
Statistical analysis
All data are expressed as mean +/− SEM, unless stated otherwise. A One-Way ANOVA was used to test the significant differences between control mice and the two treatment groups, unless otherwise stated. Statistical significance was set at P< 0.05.
Results
cGMP-elevating agents reduce intestinal polyp multiplicity in ApcMin/+ mice
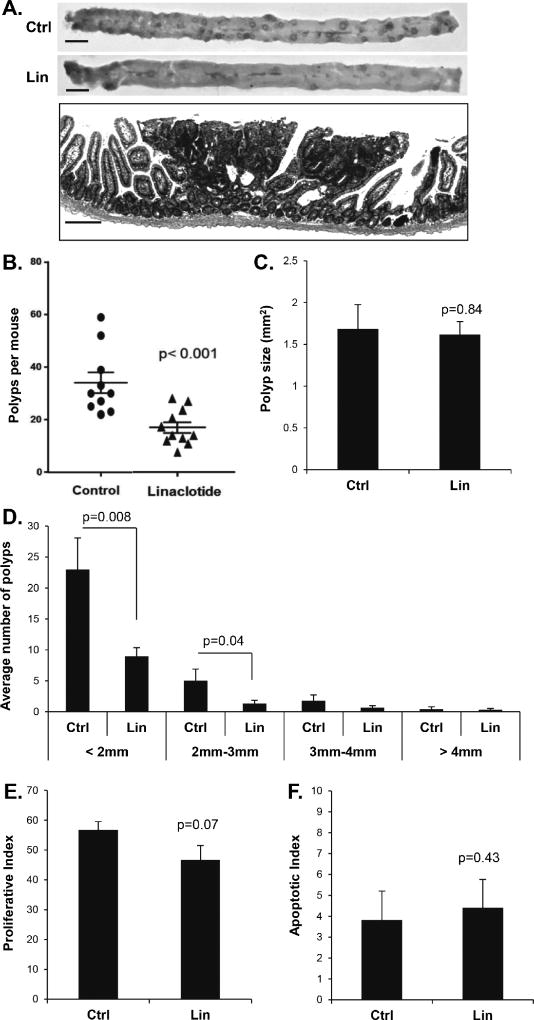
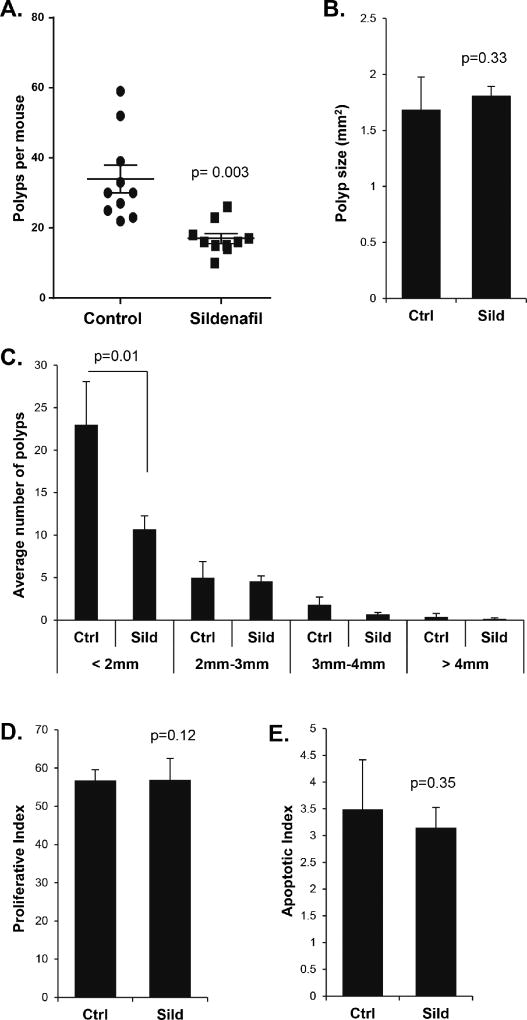
Treatment with the GCC agonist plecanatide or with the PDE5 inhibitor sildenafil has recently been shown to suppress polyp multiplicity in inflammation-driven models of colon cancer in mice (21,23). To better understand the inhibitory mechanism, the present study compared the two approaches to cGMP elevation in a non-inflammatory model of intestinal carcinogenesis. Loss of heterozygosity at the Apc locus causes ApcMin/+ mice to develop numerous adenomatous polyps primarily in the small intestine (Fig. 1A). Daily administration of linaclotide by gastric gavage reduced the number of intestinal polyps per mouse by 67% compared to controls given water alone (p<0.001, Fig. 1B). The suppressive effect of linaclotide on polyp number was more pronounced on smaller polyps (<2mm), but the mean polyp size was unaffected (Fig. 1C–D). Further analysis of polyp sections by IHC showed that linaclotide treatment of the host did not significantly affect proliferation (p= 0.07) or apoptosis (p= 0.43) of the polyps (Fig. 1E–F). Treatment of ApcMin/+ mice with sildenafil in the drinking water resulted in 56% fewer intestinal polyps compared to control mice on water alone (p=0.003, Fig. 2 A). As with the linaclotide-treated animals, the smaller polyps from sildenafil-treated mice were also suppressed while mean polyp size did not differ compared to controls (Fig. 2 B–C). Histological analysis showed that the polyps did not differ with respect to proliferative and apoptotic indices (Fig. 2 D–E).
Figure 1. Linaclotide treatment suppresses polyp multiplicity in the ApcMin/+ mouse intestine.
A, Upper panels show representative jejunal sections from the intestines of 14-week old ApcMin/+ mice, untreated (Ctrl) or treated for ten-weeks with linaclotide (Lin). Lower panel shows an H&E-stained section containing a representative intestinal polyp from a control animal. B, Plot shows the effect of linaclotide on median polyp number per animal. C, Mean polyp size in ApcMin/+ mice, untreated (Ctrl) or treated for ten-weeks with linaclotide (Lin). D, Average number of polyps in various size classes in ApcMin/+ mice, untreated (Ctrl) or treated for ten-weeks with linaclotide (Lin). E and F, show histological analysis of proliferative index (Ki-67), and apoptotic index (CC3) within polyps from control and linaclotide treated animals. Indices were calculated as staining nuclei (or cells for CC3) as a function of the total number of nuclei stained by hematoxylin. Error bars show SEM, and the p-values are from a two-tailed Student’s t-test. Scale bars represent upper panel 10 mm, lower panel 50 µm.
Figure 2. Sildenafil treatment reduces the number but not the phenotype of intestinal polyps from ApcMin/+ mice.
A, The effect of sildenafil on median polyp number per animal. B, Mean polyp size in ApcMin/+ mice, untreated (Ctrl) or treated for ten-weeks with sildenafil (sild). C, Average number of polyps in various size classes in ApcMin/+ mice, untreated (Ctrl) or treated for ten-weeks with sildenafil (Sild). D and E, show histological analysis of proliferative index (Ki-67), and apoptotic index (CC3) within polyps from control and sildenafil-treated animals. Indices were calculated as staining nuclei (or cells for CC3) as a function of the total number of nuclei stained by hematoxylin. Error bars show SEM, and the p-values are from a two-tailed Student’s t-test.
Sildenafil does not affect inflammation in the ApcMin/+ mouse intestine
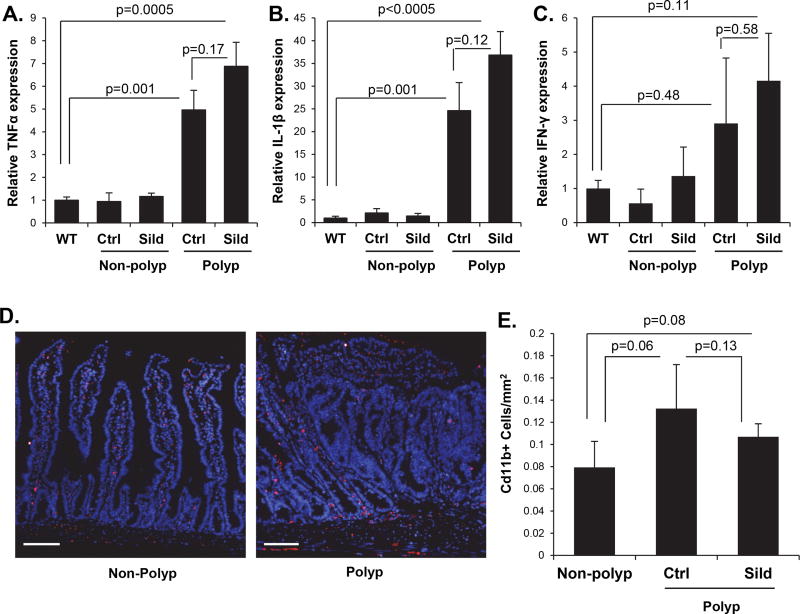
Sildenafil has been reported to suppress myeloid-derived suppressor cell (MDSC) function and reduce inflammation in colonic polyps (23,25,26). In order to determine whether this phenomenon played a role in the present study, RT-qPCR was used to measure inflammatory cytokine expression in the ApcMin/+ mouse intestinal tissues. These studies showed that the expression of IL-1β, TNFα, and IFNγ in the non-polyp tissue from the ApcMin/+ mouse intestine was comparable to the levels in wild-type mice (Fig. 3 A–C). In contrast, the intestinal polyps showed dramatic increases in the mRNA levels of these inflammatory cytokines. Furthermore, treatment of mice with sildenafil did not significantly reduce the cytokine expression in either the polyp or non-polyp tissue. In support of this, the slightly higher density of CD11b cells associated with polyps did not change in response to sildenafil treatment (Fig. 3D–E). These results support the idea that mucosal inflammation is not a central driver of intestinal carcinogenesis in the ApcMin/+ mouse, and that the suppressive effect of cGMP is not due to an effect on polyp inflammation.
Figure 3. Sildenafil treatment does not reduce inflammation within polyps of ApcMin/+ mice.
A, B, C, Real-time qPCR analysis of inflammatory cytokine gene expression in mucosa from C57BL/6 mice and mucosa and polyps from untreated or sildenafil-treated ApcMin/+ mice (n= 6). D, E, Representative images and quantification of Cd11b+ cells (red) in intestines of C57BL/6 mice and untreated or sildenafil-treated ApcMin/+ mice. Tissues were counterstained with DAPI, n= 3 mice, 3 polyps per mouse. Scale bars represent 50 µm.
The expression of cGMP-signaling components in the ApcMin/+ intestinal polyps
While both cGMP-elevating drugs examined in the present study reduced polyp multiplicity in the ApcMin/+ intestine, neither drug affected polyp size, proliferation, or apoptosis. Similar observations were reported recently using the AOM/DSS inflammatory carcinogenesis model, where a dramatic loss of cGMP-generating machinery in the polyps partially explained the phenomenon (23). To determine whether this occurred in the intestinal polyps in ApcMin/+ mice, the expression of cGMP-signaling components was examined using RT-qPCR. These studies revealed that the mRNA levels of uroguanylin and GCC were unchanged between non-polyp epithelium and the polyp tissue (Fig. 4A). Surprisingly, guanylin mRNA levels were significantly higher in the polyps compared to the surrounding tissue (p=0.007), but the physiological significance is not clear as the relative transcript levels were much lower than uroguanylin (data not shown). The expression of the effector protein kinases PKG1α, β did not differ significantly between polyp and non-polyp tissue in the ApcMin/+ mouse intestine, but the relative mRNA level of the PKG2 isoform was elevated in the polyps (p=0.02, Fig. 4B). Expression-analysis of phosphodiesterases with activity toward cGMP (PDE5, 9, and 10a) revealed little change in PDE5 or PDE9, but significantly higher levels of the dual-specificity PDE10a were observed in the polyps compared to non-polyp tissue (p=0.001, Fig. 4C). However, the increased expression of PDE10a in the ApcMin/+ polyps was independent of sildenafil treatment.
Figure 4. Expression of cGMP signaling components in intestinal polyps from ApcMin/+ mice.
A, The expression of cGMP generators was measured by RT-qPCR in non-neoplastic tissue and untreated and treated polyps from ApcMin/+ mice (Control, Ctrl; Sildenafil, Sild). B, The expression of cGMP effectors were measured by real-time qPCR in non-polyp tissue and polyps from untreated and treated ApcMin/+ mice. C, The expression of phosphodiesterases with activity toward cGMP were measured by RT-qPCR in non-polyp tissue and untreated and treated polyps from ApcMin/+ mice. n= 6 mice; Error bars show SEM, and the p-values are from a One-way ANOVA and Tukey’s post hoc analysis.
cGMP elevating agents regulate homeostasis in the preneoplastic tissue of ApcMin/+ mice
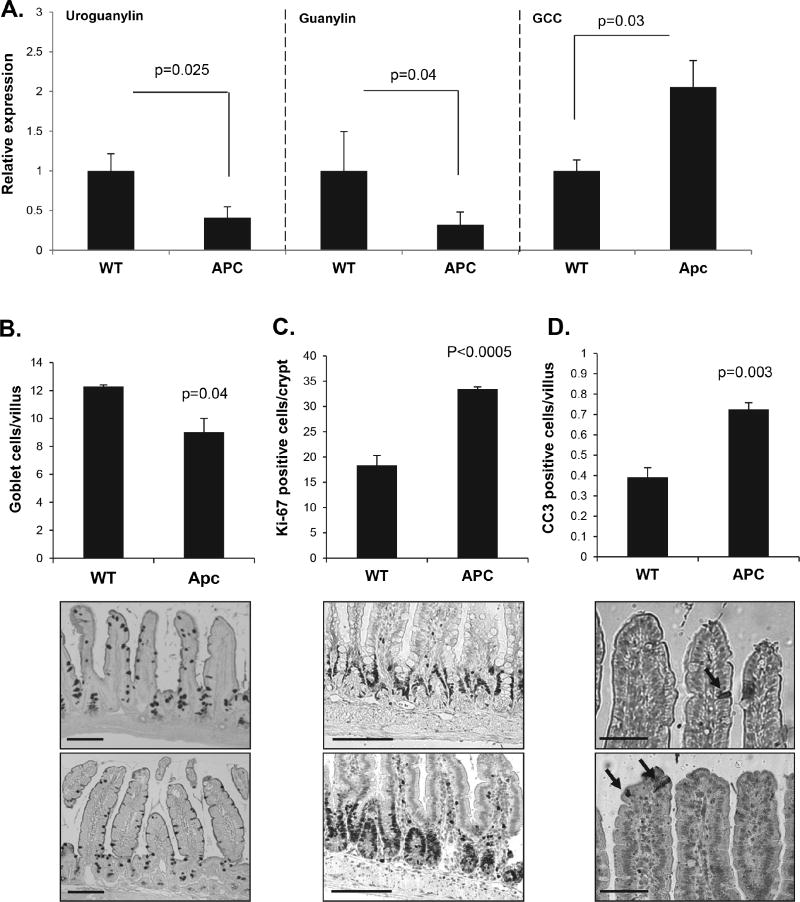
The suppression of tumorigenesis by both sildenafil and linaclotide in the absence of any effect on initiated polyps suggested that the principal effect of cGMP is on the preneoplastic epithelium. To explore this further, RT-qPCR analysis of cGMP signaling component expression in the non-polyp epithelium of the ApcMin/+ intestine was compared to wild type animals. These studies revealed significantly less uroguanylin and guanylin (p=0.025 and 0.04 respectively), but elevated levels of GCC in the ApcMin/+ intestine (p=0.03, Fig. 5A). To determine whether the altered expression of these components affected epithelial homeostasis, histological analysis of proliferation, apoptosis, and differentiation was examined in the non-polyp epithelium. These results showed that the intestine of ApcMin/+ mice exhibited significantly higher crypt proliferation as evidenced by Ki67 staining (Fig. 5A) and total PCNA levels (Fig. S1). In addition, ApcMin/+ mice had fewer goblet cells and more apoptosis at the villus tip compared to wild type animals (p=0.003, Fig. 5B–D).
Figure 5. Non-polyp epithelium from ApcMin/+ mice appears deficient in cGMP signaling.
A, RT-qPCR analysis of uroguanylin, guanylin, and GCC gene expression in C57BL/6 mucosal tissue and non-polyp mucosal tissue from ApcMin/+ mice. B, C, D, Quantification and representative images of goblet cells (AB/PAS), proliferation (Ki-67), and apoptosis (CC3) in stained sections of intestine from C57/BL6 mice and ApcMin/+ mice. A, n=6; B-D, n=3. Error bars show SEM, and p-values are from a two-tailed Student’s t-test. Scale bars represent 50 µm B, C and 25 µm D.
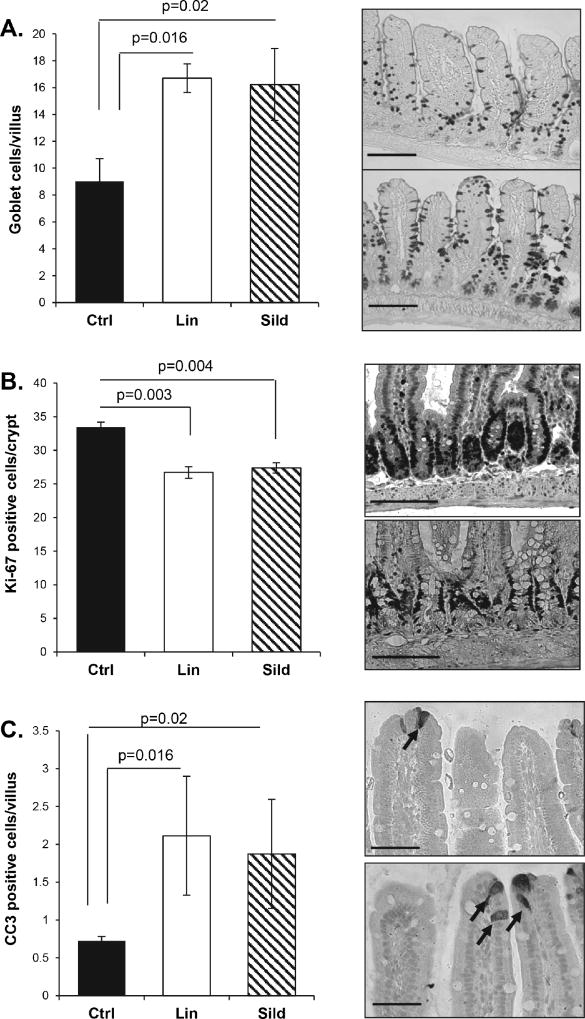
It has been reported that increasing cGMP in the intestine with PDE5 inhibitors can suppress proliferation and apoptosis in the mouse colon, but this has not been examined previously in the small intestine (22,24). To better understand the effect of cGMP elevating agents in the ApcMin/+ mice, the effects of linaclotide and sildenafil on homeostasis in the non-neoplastic intestinal epithelium was measured. Similar to the effect of these agents on the colon, both drugs increased goblet cell density and suppressed proliferation (Fig. 6A, B). In contrast to previously reported effects on the colon however, both linaclotide and sildenafil treatment increased apoptosis at the tip of the villi. These drugs had a similar effect in the small intestine of wild type mice as in the ApcMin/+, except that the effect on proliferation is much weaker and did not attain significance (Fig. S2).
Figure 6. Linaclotide and sildenafil treatment suppress proliferation in the non-polyp epithelium of ApcMin/+ mice.
Non-polyp tissue from ApcMin/+ mice treated with sildenafil or linaclotide for ten weeks were collected and prepared for histological analysis. A, B, C, Tissue was stained for goblet cell density (AB/PAS), proliferation (Ki-67), and apoptosis (CC3). Representative pictures of the staining are shown at the right of the graph. Arrows on Panel C indicate CC3 positive cells. Error bars show SEM, and the p-values are from a two-tailed Student’s t-test. Scale bars represent 50 µm A, B and 25 µm C.
Discussion
Recent reports show that the GCC agonist plecanatide can reduce DSS-induced tumorigenesis in the colon of ApcMin/+ mice (21), and that sildenafil can inhibit tumorigenesis in colon of AOM/DSS treated mice (23,25). However, these two classes of drugs have not been compared in a single model, and the tumor suppressive mechanism remains poorly understood. Results shown here demonstrate that the GCC agonist linaclotide and the PDE5 inhibitor sildenafil were equally able to suppress polyp multiplicity in the intestine of ApcMin/+ mice. Moreover, the magnitude of the tumor suppressive effect in the intestine was the same as reported for sildenafil in the colon of AOM/DSS-treated mice. Despite differences in tumor initiation and location, these observations underscore a common cGMP-dependent mechanism. PDE5 inhibitors have been shown to suppress DSS-induced inflammation, suggesting that this could play an important role in the tumor prevention effect of sildenafil (22). However, the AOM/DSS study concluded that the tumor suppressive effect of sildenafil was mostly during the mutagenesis/initiation phase, and based on the timing of drug administration it was independent of inflammation (23). The present study supports the idea that these drugs suppress initiation as it affected the smaller polyps but not the larger ones that presumably were initiated in the 4 weeks prior to drug administration. A separate study that used a very high dose of sildenafil in the AOM/DSS model suggested that the tumor suppressive effect is due to blockade of myeloid cell function (25). However, the results shown here do not support this idea because endogenous inflammation is not a driver of tumorigenesis in the small intestine of ApcMin/+ mice, and in contrast to the colonic polyps in AOM/DSS mice, inflammation in the intestinal polyps was not affected by sildenafil.
Sildenafil only slightly reduced proliferation in the colonic polyps of AOM/DSS-treated mice, but notably increased mucus differentiation (23). This contrasts with the intestinal polyps from ApcMin/+ mice, which were largely devoid of goblet cells and this was not altered by treatment with either drug (data not shown). The observation that neither linaclotide nor sildenafil affected proliferation or apoptosis of established polyps is ostensibly at odds with numerous reports describing antitumor effects of cGMP (27–29). The previous study of sildenafil in AOM/DSS treated mice, partly attributed the relatively benign effect of the drug on established polyps to reduced guanylin expression (23). Guanylin levels are also reduced in human colorectal tumors relative to non-tumor tissue (18,19,30). As GCC ligands have been shown to have cytostatic effects in colon cancer cell lines (5,16,29), “hormone replacement therapy” has been suggested as an approach to use exogenous ligands to treat colon tumors (31–33). The results shown here do not support this idea, as linaclotide did not affect mean size or proliferation of polyps. Moreover, the mRNA levels of uroguanylin and guanylin were not reduced in the intestinal polyps from ApcMin/+ mice. These observations indicate that the loss of GCC ligands is not the central reason for a lack of effect of cGMP-elevating drugs on initiated polyps. In support of several previous studies, it was shown here that the PDE10a increased dramatically in the intestinal polyps (23,34,35). It is possible that this dual-specificity PDE impedes the activation of cGMP signaling in tumors. Since inhibition of PDE10a inhibits proliferation of colon cancer cell lines (35), whether such inhibitors might be used as a treatment strategy for colon cancer will be an important area for future investigation.
The lack of effect of either sildenafil or linaclotide on established polyps strongly suggested that the reduced polyp formation resulting from treatment with these agents was due to an effect on the preneoplastic tissue. A growing body of evidence demonstrates that cGMP signaling controls intestinal homeostasis, and that increasing cGMP can suppress proliferation while increasing goblet cell density (13,14,24,36). It was shown here that treatment with either linaclotide or sildenafil caused an increase in epithelial apoptosis in the intestine, which contrasts with previous reports of reduced apoptosis in the colon epithelium (22,24). However, because the apoptosis is restricted to the luminal border, and because these agents prevent polyp formation in both colon and intestine, it is unlikely that the apoptosis contributes to the mechanism of tumor suppression.
A novel observation in the present study was the reduced expression of GCC ligands in the mucosa of ApcMin/+ mice relative to wild-type animals, and increased proliferation that is consistent with reduced cGMP signaling. While further study is needed to determine the significance of this to human FAP patients, both linaclotide and sildenafil were found to reduce proliferation in the intestine of ApcMin/+ mice. The carcinogenic mechanisms underlying the AOM/DSS and ApcMin/+ colon cancer models are different. In the AOM/DSS model carcinogenesis is due to mutations caused by azoxymethane, whereas in the ApcMin/+ model it is due to loss of heterozygosity (LOH) at the Apc gene locus (37,38). Assuming an equal rate of DNA-repair, the rate of LOH should be proportional to the size of the proliferative compartment. The suppression of proliferation in response to cGMP elevating agents would affect both the LOH in ApcMin/+ mice, and mutagenic efficacy in the AOM/DSS model, and is therefore likely to be part of the tumor preventative mechanism. The mechanism by which cGMP can reduce the proliferative compartment of both the intestine and colon of mice is presently unknown. However, this novel interpretation predicts that increasing epithelial cGMP is also likely to reduce tumorigenesis in sporadic and Lynch syndrome associated lesions that are also a function of proliferation.
Taken together, the results shown here demonstrate that PDE5 inhibitors and GCC agonists can equally suppress intestinal tumorigenesis in mice. The equal efficacy of the two drugs to suppress polyp multiplicity underscores the common mechanism involving elevation cGMP in the preneoplastic epithelium resulting in a reduced proliferative compartment. A recent report demonstrated that linaclotide administration can reduce proliferation in the colon epithelium of human patients (39). The present preclinical study therefore underscores the potential for targeting cGMP signaling for chemoprevention of colorectal cancer in human patients at increased risk.
Supplementary Material
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics 2017. CA: A Cancer Journal for Clinicians. 2017;67(3):177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16(3):173–86. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gwyn K, Sinicrope FA. Chemoprevention of colorectal cancer. Am J Gastroenterol. 2002;97(1):13–21. doi: 10.1111/j.1572-0241.2002.05435.x. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/s0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, et al. Exisulind induction of apoptosis involves guanosine 3',5'-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60(13):3338–42. [PubMed] [Google Scholar]
- 6.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, et al. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res (Phila) 2012;5(6):822–33. doi: 10.1158/1940-6207.capr-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang WC, Everley LC, Pfeiffer GR, 2nd, Cooper HS, Barusevicius A, Clapper ML. Sulindac sulfone is most effective in modulating beta-catenin-mediated transcription in cells with mutant APC. Ann N Y Acad Sci. 2005;1059:41–55. doi: 10.1196/annals.1339.020. [DOI] [PubMed] [Google Scholar]
- 8.Richter M, Weiss M, Weinberger I, Furstenberger G, Marian B. Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis. 2001;22(1):17–25. doi: 10.1093/carcin/22.1.17. [DOI] [PubMed] [Google Scholar]
- 9.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–6. doi: 10.1056/nejm199305063281805. [DOI] [PubMed] [Google Scholar]
- 10.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006;55(3):367–73. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis SH, Busch JL, Corbin JD. cGMP-Dependent Protein Kinases and cGMP Phosphodiesterases in Nitric Oxide and cGMP Action. Pharmacological Reviews. 2010;62(3):525–63. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaandrager AB, Bot AG, Ruth P, Pfeifer A, Hofmann F, De Jonge HR. Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon. Gastroenterology. 2000;118(1):108–14. doi: 10.1016/s0016-5085(00)70419-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Kwon IK, Thangaraju M, Singh N, Liu K, Jay P, et al. Type 2 cGMP-dependent protein kinase regulates proliferation and differentiation in the colonic mucosa. Am J Physiol Gastrointest Liver Physiol. 2012;303(2):G209–19. doi: 10.1152/ajpgi.00500.2011. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007;171(6):1847–58. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol. 2002;161(6):2169–78. doi: 10.1016/S0002-9440(10)64494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133(2):599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138(1):241–54. doi: 10.1053/j.gastro.2009.08.064. doi S0016-5085(09)01558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, et al. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun. 2000;273(1):225–30. doi: 10.1006/bbrc.2000.2917. [DOI] [PubMed] [Google Scholar]
- 19.Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, et al. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2328–37. doi: 10.1158/1055-9965.epi-14-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60(18):5151–7. [PubMed] [Google Scholar]
- 21.Chang WL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, et al. Plecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc+/Min-FCCC mice. World J Gastrointest Pharmacol Ther. 2017;8(1):47–59. doi: 10.4292/wjgpt.v8.i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Kwon IK, Singh N, Islam B, Liu K, Sridhar S, et al. Type 2 cGMP-dependent protein kinase regulates homeostasis by blocking c-Jun N-terMinal kinase in the colon epithelium. Cell Death Differ. 2014;21(3):427–37. doi: 10.1038/cdd.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam BN, Sharman SK, Hou Y, Bridges AE, Singh N, Kim S, et al. Sildenafil Suppresses Inflammation-Driven Colorectal Cancer in Mice. Cancer Prev Res (Phila) 2017;10(7):377–88. doi: 10.1158/1940-6207.capr-17-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharman SK, Islam BN, Hou Y, Usry M, Bridges A, Singh N, et al. Sildenafil normalizes bowel transit in preclinical models of constipation. PLoS One. 2017;12(4):e0176673. doi: 10.1371/journal.pone.0176673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, et al. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res. 2017;7(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 26.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. The Journal of Experimental Medicine. 2006;203(12):2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem. 2014;289(1):581–93. doi: 10.1074/jbc.M113.511311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deguchi A, Thompson WJ, Weinstein IB. Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res. 2004;64(11):3966–73. doi: 10.1158/0008-5472.CAN-03-3740. [DOI] [PubMed] [Google Scholar]
- 29.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98(14):7846–51. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36(2):170–9. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Lin JE, Li P, Pitari GM, Schulz S, Waldman SA. Guanylyl cyclase C in colorectal cancer: susceptibility gene and potential therapeutic target. Future Oncol. 2009;5(4):509–22. doi: 10.2217/fon.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Lin JE, Schulz S, Pitari GM, Waldman SA. Can colorectal cancer be prevented or treated by oral hormone replacement therapy? Curr Mol Pharmacol. 2009;2(3):285–92. doi: 10.2174/1874467210902030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Lin JE, Snook AE, Gibbons AV, Zuzga DS, Schulz S, et al. Colorectal cancer is a paracrine deficiency syndrome amenable to oral hormone replacement therapy. Clin Transl Sci. 2008;1(2):163–7. doi: 10.1111/j.1752-8062.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Lee K, Xi Y, Zhu B, Gary BD, Ramirez-Alcantara V, et al. Phosphodiesterase 10A: a novel target for selective inhibition of colon tumor cell growth and beta-catenin-dependent TCF transcriptional activity. Oncogene. 2015;34(12):1499–509. doi: 10.1038/onc.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, Lindsey AS, Li N, Gary B, Andrews J, Keeton AB, et al. beta-catenin nuclear translocation in colorectal cancer cells is suppressed by PDE10A inhibition, cGMP elevation, and activation of PKG. Oncotarget. 2016;7(5):5353–65. doi: 10.18632/oncotarget.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012;7(2):e31686. doi: 10.1371/journal.pone.0031686. PONE-D-11-23995 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertis MD, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. Journal of Carcinogenesis. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54(22):5947–52. [PubMed] [Google Scholar]
- 39.Weinberg DS, Lin JE, Foster NR, Della'Zanna G, Umar A, Seisler D, et al. Bioactivity of Oral Linaclotide in Human Colorectum for Cancer Chemoprevention. Cancer Prev Res (Phila) 2017;10(6):345–54. doi: 10.1158/1940-6207.capr-16-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.