Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is neurotoxic in animals but few studies have investigated its effects on the human brain. Related dioxin-like compounds have been linked to poorer cognitive and motor function in older adults, with effects more pronounced in women, perhaps due to the loss of neuro-protective estrogen in menopause. On 10 July 1976, a chemical explosion in Seveso, Italy, resulted in one of the highest known residential exposures to TCDD. In 1996, we initiated the Seveso Women’s Health Study, a retrospective cohort study of the health of the women who were newborn to 40 years old in 1976. Here, we investigate whether TCDD exposure is associated with physical functioning and working memory more than 20 years later. Individual TCDD concentration (ppt) was measured in archived serum collected soon after the explosion. In 1996 and 2008, we measured physical functioning (n=154) and working memory (n=459), respectively. We examined associations between serum TCDD and motor and cognitive outcomes with multivariate linear regression and semi-parametric estimators. A 10-fold increase in serum TCDD was not associated with walking speed (adjusted β=0.0006 ft/sec, 95% Confidence Interval (CI): −0.13, 0.13), upper body mobility (adjusted β=−0.06, 95% CI: −0.36, 0.23), or manual dexterity (adjusted β=0.34, 95% CI: −0.65, 1.33). We observed an inverted U-shaped association in grip strength, with poorer strength in the lowest and highest TCDD exposure levels. There was no association between TCDD and the Wechsler digit and spatial span tests. Neither menopause status at assessment nor developmental timing of exposure modified associations between TCDD and working memory. Our findings, in one of the only studies of TCDD’s effects on neuropsychological and physical functioning in women, do not indicate an adverse effect on these domains, with the exception of a U-shaped relationship with grip strength. Given the limited assessment and relative youth of the women at this follow-up, future work examining additional neuropsychological outcomes is warranted.
Keywords: dioxin, neurocognition, endocrine disruptors, women, Seveso
INTRODUCTION
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a global environmental pollutant released into the environment through industrial sources of combustion. Due to its exceptional toxicological potency and chemical stability, TCDD ranks among the 2001 Stockholm Convention’s “dirty dozen” of ubiquitous persistent organic pollutants (POPs) (Lallas 2001). TCDD exerts its biological toxicity primarily through its binding affinity for the aryl hydrocarbon receptor (AhR), a nuclear receptor and transcription factor that regulates myriad biological processes related to development, cell growth, apoptosis, and immune function (Denison et al. 2011). TCDD is a member of a wider class of halogenated aromatic compounds such as polychlorinated dibenzo-para-dioxins (PCDDs), dibenzofurans (PCDFs), and certain polychlorinated biphenyls (PCBs) that share this mechanism of action via the AhR. Dioxins bioaccumulate in adipose tissue (Kahn et al. 1988) and have a long half-life of 4 to 11 years in the human body (Milbrath et al. 2009; Warner et al. 2014). While levels of dioxins found in humans have decreased substantially over the last few decades, aging populations with high fish and meat consumption are particularly vulnerable to accruing higher, potentially toxic body burdens over the life course (LaKind et al. 2009).
In vitro studies demonstrate that TCDD-induced activation of the AhR, through altering endocrine function and expression of genes related to apoptosis and oxidative stress, promotes premature cell senescence in rat and human neurons and animal studies have reported impairments in memory, spatial and visual learning, and fear response with developmental exposure to TCDD and dioxin-like compounds (Curran et al. 2011; Haijima et al. 2010; Hojo et al. 2008; Schantz and Bowman 1989; Schantz and Widholm 2001; Seegal 2000; Wan et al. 2014). However, few studies have investigated the neurotoxic effects of TCDD in humans. Studies of U.S. veterans exposed to TCDD through the spraying of Agent Orange during the Vietnam War have found that men with the highest dioxin exposures performed poorly on tests of motor coordination and verbal memory compared to their unexposed peers (Barrett et al. 2001; Wolfe et al. 1992). Neuropathic signs, writer’s dystonia, and tremor have also been documented in small studies of individuals exposed to TCDD occupationally and during the Seveso accident (Barbieri et al. 1988; Klawans 1987; Singer et al. 1982; Urban et al. 2007). Several studies in general populations with exposures closer to background levels have found associations between dioxin-like PCB body burdens and impairments in motor function, memory, learning, and executive function (Fitzgerald et al. 2008; Haase et al. 2009; Peper et al. 1993; Schantz et al. 2001).
Susceptibility to the neurotoxic effects of dioxin may vary by sex and age. For example, an inverse association was observed between blood PCB concentrations and performance on tests of attention, visual memory, and learning ability among older Taiwanese women but not among men who were exposed as adults in 1979 to high levels of PCBs and dioxin-like compounds in contaminated cooking oil (the Yucheng cohort) (Lin et al. 2008). Similarly, a study of older adults in NHANES, where exposures were closer to background, found adverse associations between dioxin-like PCB serum concentrations and poorer cognitive scores, with the association most pronounced among women aged 70+ years (Bouchard et al. 2014). An excess of Parkinson’s disease, dementia, and amyotrophic lateral sclerosis was also observed among women occupationally exposed to PCBs (Steenland et al. 2006). Given the wide cross-talk of the AhR with several hormonal pathways, the mechanism underlying these interactions between age, sex, and exposure to dioxin-like compounds may be exacerbated by the loss of estrogen’s neuroprotective effects during and after menopause (Koyama et al. 2016). Estrogen-related loss of brain dopamine could also contribute to lowered physical functioning and reductions in muscle mass and strength following menopause (Sowers et al. 2007).
In the present study, we investigated the neurotoxic effects of dioxin in the Seveso Women’s Health Study (SWHS), a historical cohort study of women residing around Seveso, Italy at the time of an industrial accident on July 10, 1976 that resulted in one of the highest levels of residential TCDD contamination known (Mocarelli et al. 1988). We hypothesized that higher 1976 serum concentrations of dioxin would be inversely associated with physical and cognitive functioning and that adverse associations would be most pronounced in postmenopausal women. In addition to susceptibility factors at the time of assessment, we also considered differences in susceptibility among those exposed at younger ages, while the brain, particularly areas related to working memory such as the prefrontal cortex, are still developing (White and Swartzwelder 2005). The study of neurodevelopmental effects of dioxin have largely focused on the perinatal period but the continued susceptibility of the brain to environmental toxicants during its rapid growth, neuronal pruning, and maturation during childhood and young adulthood (up to about 25 years of age) is not well understood (Golub 2000; Weiss 2013).
METHODS
Study population
Recruitment of the SWHS cohort has been described previously (Eskenazi et al. 2000). Briefly, this historical cohort study recruited eligible women who were newborn to 40 years of age on July 10, 1976, resided at that time in the highest contaminated areas (Zones A and B), and had adequate stored serum for analysis of TCDD collected soon after the explosion. Enrollment took place from March 1996 to July 1998, and 981 women (80% of those eligible) participated. The oldest women (31–40 years in 1976) who were interviewed after September 1997 (n=173 of 229) were invited to participate in an assessment of physical functioning added as part of the study visit. Of those invited, 19 women refused to participate in any of the tests leaving 154 women (89% of eligible) who completed the physical function tests.
Between April 2008 and December 2009, we conducted a follow-up of the SWHS cohort: 833 (85%) of the original 981 women could be contacted and participated (16 were deceased and 36 could not be located). Data collection was already underway when findings of lowered working memory and other neuropsychological measures in the Yucheng cohort were published (Lin et al. 2008). This motivated development of an ad-hoc assessment of neuropsychological outcomes in the SWHS. Starting in December 2008, partway through the 2008–2009 follow-up of the cohort, remaining participants (n=459) were invited to complete an assessment of working memory as part of the study visit. We excluded two with Turner’s syndrome leaving 457 participants.
Procedure
The study was approved by the Institutional Review Boards of the participating institutions and written informed consent was obtained from all women prior to participation. Details of the study procedure for the 1996 and 2008 studies are described elsewhere (Eskenazi et al. 2000; Warner et al. 2011). In both 1996 and 2008, information on covariates such as demographic and lifestyle factors and medical history were obtained from a questionnaire administered in private by a trained nurse-interviewer and followed by a brief medical exam which included anthropometric and blood pressure measurements. Interviewers were blinded to participants’ serum TCDD levels and zones of residence.
Laboratory Analyses
Archived serum samples collected in 1976 were stored at −20°C until shipped to the Centers for Disease Control and Prevention (CDC) for analysis in 1998. TCDD was measured in archived sera by high-resolution gas chromatography/high-resolution mass spectrometry methods (Patterson et al. 1987). Prior to statistical analysis, serum TCDD levels were adjusted for blood lipid concentrations by dividing TCDD on a whole-weight basis by total serum lipid content, estimated from measurements of triglycerides and total cholesterol (Akins et al. 1989). Serum TCDD levels were reported in picograms per gram lipid or parts per trillion (ppt). The median serum sample weight for these samples was 0.65 g, and the median lipid-adjusted limit of detection was 18.8 ppt. Samples below the limit of detection (LOD) (9.4% in full cohort) were assigned a value equal to one-half of the LOD (Hornung and Reed 1990). Details of the serum sample selection and TCDD concentrations measured in 1976 serum are presented elsewhere (Eskenazi et al. 2000; Eskenazi et al. 2004b).
Physical function assessment in 1996
The physical function assessment administered in 1996 included four validated physical tasks chosen for their ease of implementation, reliability, and frequent use in studies of community-dwelling older adults (Tager et al. 1998): 1) a 10-foot walking test of functional mobility, 2) a coin-flipping test of manual dexterity, 3) a grip strength test, and 4) a reach down test of lower body mobility (Muriel Lezak 2012). Together, these tests represented a diverse cross-section of physical performance. For the 10-foot walking test, participants were asked to walk back and forth on a 10-foot long course for two minutes at their regular speed as if walking down the street to go to the store. We used the number of lengths walked in this time to calculate the average walking speed (ft/s). For the coin flip test, participants were timed on how quickly they could turn five 50 lire coins from the heads to tails position on a table without dropping them. For the grip strength test, participants, while in the standing position, were asked to squeeze a dynamometer three times in each hand. We analyzed grip strength in two ways: the average of the three measures and the highest of the three measures for the dominant and non-dominant hands. Lastly, in the reach down test, participants were timed (in seconds) on how quickly they could, from a standing position, reach down to pick a pen off the floor and return to standing.
Working memory assessment in 2008
The neurocognitive assessment in 2008 included the Wechsler Adult Intelligence Scale (WAIS) digit span and spatial span tests (Muriel Lezak 2012). The digit span test targets verbal working memory and engages executive function skills of attention, associability by linking items so as to better recall them, and mathematical ability; the backward task requires more complex storage and retrieval. The forward and backward subtests begin with the administrator saying aloud 2 digits (i.e. 1–7) and progresses until criterion up to 8 digits. The participant is then asked to retrieve and verbalize the span back to the administrator in either the forward or backward order and must successfully repeat two lists at each sequence length before another digit is added. The digit span (forward, backward) is the length of the longest sequence recalled correctly (maximum of 8 forward and 7 backward). In a normative sample of Italian adults (aged 30–70 years) the mean (±SD) of the forward and backward digit spans was 6.0 (±1.0) and 4.7 (±1.1), respectively (Monaco et al. 2013).
The spatial span test, an adaptation of the Corsi block-tapping test (Muriel Lezak 2012), is considered a visual-spatial analog of the digit span. The task assesses an individual’s ability to remember a 3-dimensional sequence of tapping on a grid of white squares by the administrator immediately after their presentation. The spatial span (forward, backward) is the length of the longest sequence correctly recalled with a maximum of 8. The normative mean (±SD) forward and backward spatial spans in Italian adults is 5.5 (±1.0) and 4.9 (±1.0), respectively (Monaco et al. 2013).
Each of the four subtests was repeated twice, and each subtest raw score was calculated as the sum of the two trials. The maximum raw score was 14 for the backward digit span subtest and 16 for the other three subtests (forward digit span, forward and backward spatial span).
Statistical analysis
Because the serum TCDD distribution was approximately log-normal, TCDD levels were log10-transformed. Serum TCDD was analyzed as both a continuous exposure (log10TCDD) and categorized to four levels. In the categorical analysis, TCDD levels ≤20ppt, which were comparable to background serum levels of unexposed Italian women in 1976, served as the reference group (Eskenazi et al. 2004a; Papke et al. 1994); the remaining three exposure categories were defined by exposure tertiles calculated across the full cohort, producing groups of ≤ 20, 20.1–47.0, 47.1–135.0, and > 135 ppt.
Potential confounding variables were chosen a priori based on the literature of human neurotoxicity of PCBs/dioxins and earlier work in the SWHS. We considered: educational attainment, smoking, alcohol consumption, age at interview, age at explosion, menarche status at explosion, menopause status (pre-/post-) at interview (>12 months without a menstrual cycle or surgical menopause), body mass index category [BMI; kilograms per meter squared categorized as underweight/normal (< 25 kg/m2), overweight BMI ≥ 25 kg/m2 and < 30 kg/m2) and obese (BMI ≥ 30 kg/m2)], and marital status. We used a directed acyclic graph (DAG) of the assumed underlying causal relationships among variables to inform covariate selection into the initial adjusted model and then, following a change-in-estimate approach, further pared down the adjustment set to contain covariates changing the association between TCDD and the outcome by more than 10% (Evans et al. 2012).
The physical function and working memory outcomes were initially all considered as continuous variables. We examined the functional form of the relationship between each of the outcomes and TCDD using locally weighted scatterplot smoothing (lowess) and restricted cubic splines, and compared regression models with and without a squared term on TCDD with a likelihood ratio test (Desquilbet and Mariotti 2010). If a linear model appeared adequate, we used multivariate linear regression to assess the dose-response relationship between dioxin and the physical and cognitive function tests, conditional on confounders. In sensitivity analyses, we modeled TCDD categorically and obtained marginal estimates of effect using semi-parametric estimation [targeted maximum likelihood estimation (tmle) implementing Superlearner] that allowed us to make fewer assumptions about the functional form, thus mitigating any bias in the effect measure introduced by mis-specified parametric modeling (van der Laan and Rubin 2006). For all regression models, variances were estimated using a robust sandwich estimator (Williams 2000).
For the working memory analysis, we considered effect modification by developmental status at exposure including menarche status in 1976 (premenarche versus postmenarche) and before/after peak brain development (< age 25 versus ≥ age 25) (Pujol et al. 1993; Sowell et al. 2001), and menopause status at assessment (premenopause vs. postmenopause). (All women assessed on physical functioning were post-menarche at the time of the explosion and post-menopause at assessment.) Effect modification was modeled by creating a cross-product term between log10TCDD and the effect modifier of interest. Interactions were considered significant if the p-value for the cross-product term was < 0.2.
In sensitivity analysis, to account for potential selection bias due to subsampling of women within each wave and for loss-to-follow-up across the 1996 and 2008 waves, we re-estimated the parameters with inverse-probability weights of censoring (Cole and Hernan 2008).
RESULTS
Participant characteristics
Descriptions of the 1996 physical function and 2008 working memory study samples in comparison to the full SWHS cohort are provided in Table 1. The 1996 physical function sample was older than the full SWHS cohort but did not differ with respect to other sociodemographic characteristics; the 2008 working memory sample was of similar age to the full SWHS cohort and was also similar in sociodemographic characteristics (see Table 1). In the 1996 assessment of physical functioning, the average age at interview was 57.3 (SD± 2.9) years and all women were postmenopausal. Almost all were married (97%) and the household primary wage earner had completed the required education (96%), 22% reported ever smoking, and 49% were regular alcohol drinkers. In the 2008 working memory sample, 53% of the women were postmenopausal and averaged 52.3 (SD± 11.3) years at the time of the memory assessment. The majority were married (91%) and reported the primary wage earner had completed the required education (65%), and 38% and 37% had a history of smoking and regular alcohol consumption, respectively. In addition, 30% were pre-menarche at the time of the explosion.
Table 1.
Select characteristics of participants in complete cohort and in physical function and working memory assessments, SWHS, Italy, 1996–2009 [n(%)]
| Characteristic | SWHS Full Cohort* |
1996 Physical Function Subgroup |
2008 Working Memory Subgroup |
|---|---|---|---|
| Total | n=981 | n=154 | n=459 |
| Characteristics at explosion | |||
| Age at explosion, years | |||
| 0–10 | 232 (23.7) | 0 | 111 (24.2) |
| 11–20 | 279 (28.4) | 0 | 144 (31.4) |
| 21–30 | 241 (24.6) | 1 (0.7) | 105 (22.9) |
| 31–40 | 229 (23.2) | 153 (99.3) | 99 (21.6) |
| Menarche status at explosion | |||
| Premenarche | 284 (28.9) | 0 | 139 (30.2) |
| Postmenarche | 69 (71.1) | 154 (100) | 320 (69.8) |
| Exposure before age 25 | |||
| <25 years | 613 (62.5) | 0 | 301 (65.6) |
| ≥25 years | 368 (37.5) | 154 (100) | 158 (34.4) |
| Smoking status | |||
| Never | 827 (84.3) | 126 (80.8) | 387 (84.3) |
| Ever | 154 (15.7) | 28 (18.2) | 72 (15.7) |
| Alcohol status | |||
| Never | 772 (78.8) | 85 (55.2) | 367 (80.0) |
| Ever | 209 (21.3) | 69 (44.8) | 92 (20.0) |
| Serum TCDD, ppt (median (IQR)) | 55.9 (28, 157) | 45.2 (28,100) | 60.1 (29, 150) |
| Characteristics at follow-up | |||
| Age at interview, years (Mean±SD) | NA | 57.3 ± 2.9 | 52.3 ± 11.3 |
| Menopause status | |||
| Premenopausal | 484 (49.3) | 0 | 216 (47.3) |
| Postmenopausal | 496 (50.6) | 154 (100) | 241 (52.7) |
| Primary wage earner's education | |||
| ≤ Required | 627 (63.9) | 148 (96.1) | 297 (64.7) |
| ≥High school | 354 (36.1) | 6 (3.9) | 162 (35.3) |
| Marital Status | |||
| Never | 76 (7.8) | 4 (2.6) | 43 (9.4) |
| Ever | 905 (92.3) | 150 (97.4) | 416 (90.6) |
| Smoking status | |||
| Never | 619 (63.1) | 120 (77.9) | 286 (62.3) |
| Former | 194 (19.8) | 11 (7.1) | 105 (22.9) |
| Current | 168 (17.1) | 23 (14.9) | 68 (14.8) |
| Alcohol status | |||
| Never | 618 (63) | 78 (50.6) | 288 (62.7) |
| Former | 44 (4.5) | 14 (9.1) | 16 (3.5) |
| Current | 319 (32.5) | 76 (49.4) | 155 (33.8) |
| BMI Category | |||
| Underweight | 26 (2.6) | 1 (0.7) | 8 (1.7) |
| Normal | 437 (44.6) | 71 (46.1) | 200 (43.6) |
| Overweight | 302 (30.8) | 57 (37.0) | 142 (30.9) |
| Obese | 216 (22.0) | 25 (16.2) | 109 (23.8) |
Last follow-up information on full cohort obtained from 1996 data for women who did not participate in 2008 follow-up.
The median 1976 TCDD serum concentration was 45.2 ppt [interquartile range (IQR) = 28 – 100] for women who completed the physical function assessment and 60.1 ppt (IQR = 29 – 150) for women who completed the working memory assessment compared to a median of 55.9 ppt (IQR = 28 – 157) for the full SWHS cohort. Among women who were pre- and post-menopausal at the time of the working memory assessment, the median levels were 74.3 ppt (IQR = 33 – 207) and 46.3 ppt (IQR = 25 – 103), respectively. Women who were pre-menarche at the time of the explosion had higher serum TCDD levels (median = 141.2 ppt; IQR = 48 – than those who were postmenarche (median= 45.6 ppt; IQR = 25 – 93). These associations between exposure levels and age have been documented previously (Eskenazi et al. 2004a).
The distribution of the neurophysiological function scores in 1996 and 2008 are presented in Table 2. Physical function and working memory scores were normally distributed with the exception of the reach down test where the majority (94%) of participants completed the task within the narrow range of 1–3 seconds while the rest of the distribution was positively skewed toward longer test times.
Table 2.
Summary of measures (mean ± SD) of physical functioning and working memory tests, SevesoWomen's Health Study, Italy 1996–2009
| Measurement | n | mean ± SD | median | min | max |
|---|---|---|---|---|---|
| Coin Flipping (sec) | 153 | 7.88 ± 2.43 | 7 | 3 | 19 |
| Walking Speed (ft/sec) | 149 | 2.12 ± 0.36 | 2.08 | 1.25 | 2.22 |
| Reach Down Test (sec) | 152 | 2.19 ± 1.50 | 2 | 1 | 16 |
| Grip Strength | |||||
| Dominant (N) | 98 | 23.73 ± 4.63 | 23.67 | 11 | 34.67 |
| Non-Dominant (N) | 98 | 22.52 ± 4.87 | 22.67 | 6 | 34.33 |
| Digit Span | |||||
| Forward | 459 | 8.03 ± 1.96 | 8 | 3 | 14 |
| Backward | 459 | 4.53 ± 1.98 | 4 | 0 | 12 |
| Spatial Span | |||||
| Forward | 459 | 6.94 ± 1.73 | 7 | 2 | 13 |
| Backward | 459 | 5.88 ± 2.03 | 6 | 0 | 11 |
TCDD and physical functioning
The linear models for the physical function outcomes are presented in Table 3. We observed no association between a 10-fold increase in 1976 TCDD serum concentrations and average walking speed (adjusted β = 0.0006; 95% Confidence Interval (CI): −0.13, 0.13), manual dexterity (coin flip)(adjusted β = 0.32, 95% CI: −0.65, 1.33), and lower body flexibility (reach down test)(adjusted β = −0.04; 95% CI: −0.36, 0.23) in the postmenopausal women twenty years after the accident, after adjusting for age at assessment, primary wage earner's highest education level (i.e. spouse for majority of participants), and BMI in 1996
Table 3.
Multivariable linear regression analyses for the relationship of serum TCDD (log10) with measures of physical functioning, Seveso Women’s Health Study, Italy, 1996–97.
| log10TCDD (ppt) | log10TCDD2 (ppt) | ||
|---|---|---|---|
| Outcome | n | Adj.βa (95% CI) | Adj.βa (95% CI) |
| Time to flip 5 coins (sec) | 153 | 0.34 (−0.65, 1.33) | - |
| Walking speed (ft/sec) | 148 | 0.0006 (−0.13, 0.13) | - |
| Reach down test time (sec) | 152 | −0.06 (−0.36, 0.23) | - |
| Average grip strength dominant hand (kg) | 98 | 8.50 (0.78, 16.22)* | −2.06 (−3.69, −0.43)* |
| Average grip strength non-dominant hand (kg) | 98 | 12.83 (5.61, 20.05)* | −3.15 (−4.66, −1.64)* |
| Highest grip strength dominant hand (kg) | 98 | 9.48 (1.59, 17.37)* | −2.26 (−3.90, −0.63)* |
| Highest grip strength non-dominant hand (kg) | 98 | 12.59 (5.39, 19.80)* | −3.11 (−4.61, −1.60)* |
Adjusted for age at interview, primary wage earner education in 1996, BMI in 1996
p<0.05
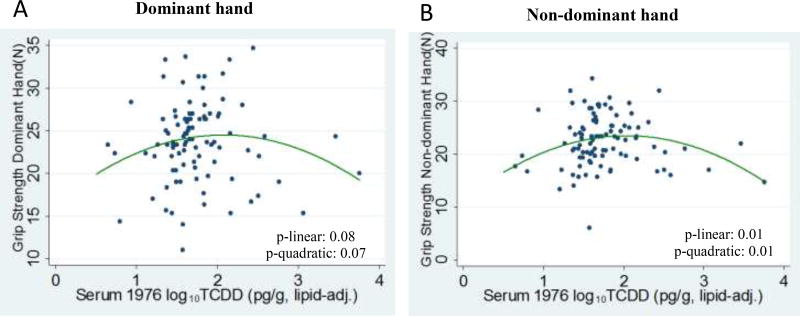
The relationship between log10TCDD and dominant and non-dominant hand grip strengths showed evidence of non-linearity. In separate quadratic models for average grip strength in dominant and non-dominant hands, the first and second order log10TCDD terms were statistically significant. In these two models, the first order log10TCDD terms were positive and the quadratic terms were negative, suggesting an inverted U-shape curve at which grip strength improved at low levels of TCDD but decreased with higher exposure levels (see Figure 1). This pattern was also supported by models using a restricted cubic spline function with 4 knots. Models examining the highest grip strength yielded similar findings (Table 3). Fifty-five women were unable to perform the grip strength test due to self report of arthritis (n=47), tendonitis (n=6), or recent hand/arm surgery (n=2); though the proportion with arthritis/hand pain was higher than the typical prevalence of osteoarthritis in Italian women at this age (Corti and Rigon 2003), these women did not differ significantly from those who did perform the test with respect to age or TCDD levels. Modeling all of the physical functioning outcomes as a function of categorized TCDD levels based on the distribution of exposure in the full cohort (Supplementary Table 1) provided similar inference as the continuous models.
Figure 1.
Plots of grip strength measurements for a 10-fold increase in 1976 levels of serum TCDD (ppt) in A) dominant hand and B) non-dominant hand. Green line shows fit of predicted values from quadratic model adjusted for age at assessment, BMI, and primary wage earner’s education.
In sensitivity analyses, when we adjusted for loss-to-follow up with inverse-probability weights results were similar (data not shown).
TCDD and working memory
Adjusting for age at assessment and primary wage earner’s highest education level, a 10-fold increase in 1976 serum TCDD levels was not associated with verbal or spatial working memory measured about thirty years after the accident (Table 4) and there was no evidence of non-linearity in the associations. We found some evidence of effect modification by menarche status in 1976 (Table 4). A 10-fold increase in serum TCDD was associated with a better forward digit span score (adjusted β = 0.42; 95% CI: 0.03, 0.81) among women who were post-menarche in 1976, but not among those who were premenarche in 1976 (adjusted β = −0.05; 95% CI: −0.43, 0.32) (p-interaction = 0.06). We found no evidence of effect modification of the association between continuous TCDD and memory scores by exposure before or after peak brain development (age 25) (Supplementary Table 2) or by menopause status at study visit (Supplementary Table 3).
Table 4.
Multivariable linear regression analyses for the relationship of serum TCDD (log10) with measures of working memory span, stratified by menarche status at explosion, Seveso Women’s Health Study, Italy, 2008–2009.
| All women (n=457) |
Pre-menarche in 1976 (n=139) |
Post-menarche in 1976 (n=318) |
||
|---|---|---|---|---|
| Outcome | Adj.βa (95% CI) | Adj.βa (95% CI) | Adj.βa (95% CI) | Pint |
| Digit Span | ||||
| Forward | 0.18 (−0.09, 0.45) | −0.05 (−0.43, 0.32) | 0.42 (0.03, 0.81)* | 0.06 |
| Backward | 0.06 (−0.22, 0.34) | −0.1 (−0.51, 0.32) | 0.17 (−0.23, 0.57) | 0.52 |
| Spatial Span | ||||
| Forward | 0.05 (−0.21, 0.31) | 0.17 (−0.28, 0.61) | 0.04 (−0.32, 0.39) | 0.47 |
| Backward | −0.03 (−0.30, 0.24) | −0.17 (−0.66, 0.32) | 0.06 (−0.31, 0.43) | 0.54 |
Models adjusted for age at interview, primary wage earner education in 2008
p<0.05
In sensitivity analyses, we examined the consistency of the working memory association when the assumptions of parametric models were relaxed with use of targeted maximum likelihood estimation (tmle) using SuperLearner (Supplementary Table 4). These models produced similar findings to the linear regression models using categorical exposure, suggesting no association between TCDD exposure and working memory (Supplementary Table 5). Further, results were robust in models including inverse-probability of censoring weights to account for selection bias in subsampling and loss-to-follow up in the cohort (data not shown). We also did not observe evidence of effect measure modification by exposure before menarche or during peak brain development at the explosion, when TCDD was categorized (data not shown).
DISCUSSION
In our study, we examined physical and cognitive function in women several decades after exposure to dioxin released in an industrial explosion in Seveso, Italy. We found no significant associations between their 1976 serum TCDD levels and several measures of motor function and working memory (digit span or spatial span) approximately twenty and thirty years after exposure, respectively. Average and highest grip strengths in the dominant and non-dominant hands were the only endpoints with a suggestive non-monotonic relationship characterized by diminished strength at the lowest and highest levels of dioxin exposure. At present, we are unaware of a biological explanation for the shape of this relationship and caution it may be a spurious result from a small sample. This finding warrants confirmation although few other populations will have exposure as high as the Seveso cohort.
In 1976 at the time of the explosion, the women in the physical function study were in their 30s – an age window not typically considered a critical period of brain development (Knudsen 2004; Schug et al. 2011). Further, the women were assessed for motor ability in their 50s and 60s when such functions are beginning to decline; though our findings suggest that TCDD may not accelerate this course in the short term, an investigation at later life stages, when physical function is in steeper decline, may better reveal dioxin’s potential to alter the aging process. Further, we did not assess the physical function of the younger women in the cohort (less than 30 years at the time of the explosion); as they age, these outcomes may warrant another look. Our findings are consistent with a cross-sectional study of older Michigan residents with diets high in PCB-contaminated fish in which no association was found between PCB exposure and hand steadiness nor visual-motor coordination in models adjusted for age and gender (Schantz et al. 1999). However, it is important to note that the physical function tests we examined are reflective of general physical performance and are not typically as sensitive as the tests of finer neuromotor changes in the Michigan study.
Our findings on working memory are not consistent with those reported by Lin et al. in the Yucheng cohort, which was exposed primarily to a mixture of PCBs and furans. They reported a dose-response relationship between exposure and poorer performance on learning and memory tests, including the digit span test, in 313 exposed women 25 years after the accidental rice oil poisoning (Lin et al. 2008). This discrepancy could be attributable to the older age of their sample (mean = 69.5 (±5.9) years versus 52.3 (±11.3) years in our sample), differences in the mix of compounds the two populations were exposed to, or perhaps differences in the populations’ genetic susceptibilities. Though our study examined cognitive susceptibility around the time of menopause, several studies of PCB/dioxins in older adults have reported cognitive reductions in women over the age of 70, suggesting that the reserve capacity defending the brain from neurotoxins may diminish with aging (Bouchard et al. 2014; Grandjean 1991; Haase et al. 2009). We note that the oldest subject in our analysis was 73 years old with fewer than 10% of the sample aged 70 years or more. Thus, any age-related susceptibility to dioxin neurotoxicity may not manifest nor be readily measureable until the cohort shifts toward elderly (as we also previously noted with the physical function tests).
Another potential explanation for the differences between our findings and those in the Yucheng cohort is the comprehensiveness of the latter’s neuropsychological assessment. While they found significant poorer performance on tests of forward and backward digit span in women with increased exposure to PCB/PCDFs, they also reported dose-response inverse relationships with additional measures of attention, verbal memory, psychomotor function, visual scanning, and learning ability that we were not able to assess in the SWHS (Lin et al. 2008). We only examined working memory and physical functioning in the SWHS, and thus the effects of TCDD exposure on additional neurophysiological domains cannot be gleaned from this study and remain unknown. Future work should consider a more rigorous and broad examination of neuropsychological function and include, for example, a refined battery of neuromotor tests and the digit symbol coding test which was sensitive to dioxin-like chemicals in two different studies (Bouchard et al. 2014; Lin et al. 2008).
With measurements of working memory in 2008, we were able to examine dioxin’s neurotoxic potential across two developmental windows in the female life course – menarche and menopause. Gonadal hormones are integral to brain development, transitioning from organizational effects on brain differentiation in early life to activational effects of neural plasticity and behavioral functions throughout adulthood (Weiss 2007). Thus, we expected initial TCDD exposure and subsequent body burdens in relation to these periods of neuroendocrine regulation could have important and disparate effects on the aging brains of women who were different ages, ranging from 0–40 years, at the time of the accident. However, in our analysis, we found that dioxin exposure at younger ages, either during the window of peak brain development before age 25 or before menarche in adolescence, was not associated with cognitive performance thirty years later.
We previously reported a non-monotonic relationship between TCDD and risk of earlier menopause in the SWHS, consistent with the hypothesized effects of endocrine-disrupting chemicals (Eskenazi et al. 2005). We expected that the hormonal declines of menopause could also modify a woman’s later life susceptibility to dioxin neurotoxicity since many women experience temporary neurological impairment, particularly related to memory, during perimenopause though the longevity of this effect is unclear (Dluzen 2000; Espeland et al. 2004). The inhibition of central dopamine resulting from the menopause-related decline of estrogen in older women as well as age-related loss of dopaminergic neurons, may be exacerbated by the neurotoxic activity of dioxin-like compounds on the dopamine system to hasten cognitive decline among highly exposed older women (Akahoshi et al. 2009; Seegal 2000; Weiss 2007). Toxicologic studies in vitro have suggested that TCDD, in addition to disrupting regulation of the neuroendocrine system, can alter neuronal biochemistry, inhibiting calcium uptake and neurotransmitter synthesis and signaling (Hong et al. 1998; Jiang et al. 2014; Kim et al. 2007; Lee et al. 2007; Li et al. 2013). However, the physiologic implications of these molecular changes have not been closely studied in adult animals or humans. In this study, we did not see evidence of modified sensitivity to TCDD neurotoxicity among menopausal women, the majority of whom were adults at the time of explosion.
TCDD has been shown to cross the placenta and is hypothesized to interfere with fetal development in both humans and animals (Chao et al. 2007; Nau et al. 1986). Animal studies and limited epidemiological evidence suggest those exposed in utero may be even more susceptible to the effects of TCDD (Arima et al. 2009; Bruner-Tran and Osteen 2011; Ding et al. 2011; Gray et al. 1995; Gray and Ostby 1995; Ikeda et al. 2005; Jin et al. 2008; Kakeyama et al. 2008; Manikkam et al. 2012). TCDD’s neurotoxicity in the human fetus, however, is less clear and the sex-specific effects of TCDD on human neural development remain to be characterized (White and Birnbaum 2009). In future analyses, we will examine the relationship of in utero TCDD exposure on the children born to the women in the SWHS.
The present study has several strengths. First, the SWHS cohort is one of the only studies of sufficient size and wide exposure variability to TCDD with background levels of exposure to other dioxin-like compounds (Warner et al. 2005) to examine the effects of dioxin on women’s health. Further, the study utilizes direct measurements of serum TCDD close to the time of the accident and a prospective study design.
In addition to the limited scope of the study’s neuropsychological assessment and the possibility that the window of the present study is too premature to observe the age-related neurotoxicity of TCDD, the small sample size of the physical function analysis raises concerns about power and selection bias. Further, a third of the women who completed the physical function assessment did not participate in the grip strength test due to hand pain or surgery. Since these women did not differ significantly in TCDD levels and age from those who completed the test, selection bias is unlikely to explain the grip strength finding. Nevertheless, the proportion of women refusing the test due to arthritis/hand pain is in excess of the expected prevalence in older Italian women (18%), further qualifying these findings. Our data in the working memory analysis lack a large cell size of younger people with lower TCDD levels (and older individuals with high exposure), leaving open the possibility of residual confounding by age. While we were only able to collect outcome data on a subsample of eligible women in both 1996 and 2008, sensitivity analyses exploring the possibility of selection bias with inverse probability of treatment weighting did not appreciably change the results. Nevertheless, the background exposures of 1976 are substantially higher than background levels today (around 2ppt), which makes our lowest exposed individuals fairly exposed by today’s standards, and thereby limits our ability to generalize the relationship to these lower levels.
CONCLUSIONS
In summary, we did not see evidence of an adverse relationship between TCDD exposure and long-term effects on working memory in a cohort of women exposed postnatally to high levels of dioxin released during the Seveso accident. TCDD exposure does not appear to be associated with physical functioning in the oldest women in the cohort, possibly with the exception of grip strength where we observed an inverted U-shaped association. However, the cohort was still relatively young at the age of assessment and continued follow-up of their neurophysiological health into old age, with a more comprehensive neurocognitive assessment, is warranted. Research on the interplay of past and current environmental exposures on the aging brain is understudied and of greater importance given the increasing longevity of populations in industrialized countries.
Supplementary Material
Highlights.
Little research exists on the neurotoxic effects of dioxins in older women.
TCDD was associated with grip strength but not other measures of physical function.
TCDD exposure was not adversely associated with working memory.
Menopause, age at exposure, and age at menarche did not modify the association.
TCDD susceptibility may increase as the women age, warranting further research.
Acknowledgments
We acknowledge the significant contributions in sample analyses made by D. Patterson, L. Needham, and W. Turner to the Seveso Women’s Health Study. We gratefully acknowledge A. Parigi for coordinating data collection at Hospital of Desio and A. Wesselink for assistance in data management. We also dedicate this work to W.A. Satariano, a leader in the field of aging and health and a beloved colleague and friend. He passed away in May 2017 and is greatly missed.
This study was supported by grants F06 TW02075-01 from the National Institutes of Health, R01 ES07171 and 2P30-ESO01896-17 from the National Institute of Environmental Health Sciences, R82471 from the U.S. Environmental Protection Agency, and 2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or potential competing financial interests.
References
- Akahoshi E, Yoshimura S, Uruno S, Ishihara-Sugano M. Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: A neurotoxicology study. Environ Health. 2009;8:24. doi: 10.1186/1476-069X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic 'summation' method. Clin Chim Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Arima A, Kato H, Ooshima Y, Tateishi T, Inoue A, Muneoka A, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) induces a reduction in epididymal and ejaculated sperm number in rhesus monkeys. Reprod Toxicol. 2009;28:495–502. doi: 10.1016/j.reprotox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Barbieri S, Pirovano C, Scarlato G, Tarchini P, Zappa A, Maranzana M. Long-term effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the peripheral nervous system. Clinical and neurophysiological controlled study on subjects with chloracne from the seveso area. Neuroepidemiology. 1988;7:29–37. doi: 10.1159/000110133. [DOI] [PubMed] [Google Scholar]
- Barrett DH, Morris RD, Akhtar FZ, Michalek JE. Serum dioxin and cognitive functioning among veterans of operation ranch hand. Neurotoxicology. 2001;22:491–502. doi: 10.1016/s0161-813x(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Oulhote Y, Sagiv SK, Saint-Amour D, Weuve J. Polychlorinated biphenyl exposures and cognition in older u.S. Adults: Nhanes (1999–2002) Environ Health Perspect. 2014;122:73–78. doi: 10.1289/ehp.1306532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Osteen KG. Developmental exposure to tcdd reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lin LY, Lee WJ, Päpke O. Placental transfer of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in taiwanese mothers in relation to menstrual cycle characteristics. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 2007;45:259–265. doi: 10.1016/j.fct.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti MC, Rigon C. Epidemiology of osteoarthritis: Prevalence, risk factors and functional impact. Aging Clin Exp Res. 2003;15:359–363. doi: 10.1007/BF03327356. [DOI] [PubMed] [Google Scholar]
- Curran CP, Vorhees CV, Williams MT, Genter MB, Miller ML, Nebert DW. In utero and lactational exposure to a complex mixture of polychlorinated biphenyls: Toxicity in pups dependent on the cyp1a2 and ahr genotypes. Toxicol Sci. 2011;119:189–208. doi: 10.1093/toxsci/kfq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological Sciences. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod Toxicol. 2011;31:351–358. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol. 2000;29:387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, et al. Seveso women's health study: A study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, et al. Relationship of serum tcdd concentrations and age at exposure of female residents of seveso, italy. Environ Health Perspect. 2004a;112:22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Samuels S, et al. Relationship of serum tcdd concentrations and age at exposure of female residents of seveso, italy. Environmental Health Perspectives. 2004b;112:22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, et al. Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005;113:858–862. doi: 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's health initiative memory study. JAMA : the journal of the American Medical Association. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Evans D, Chaix B, Lobbedez T, Verger C, Flahault A. Combining directed acyclic graphs and the change-in-estimate procedure as a novel approach to adjustment-variable selection in epidemiology. BMC Med Res Methodol. 2012;12:156. doi: 10.1186/1471-2288-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, et al. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper hudson river communities. Environ Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS. Adolescent health and the environment. Environ Health Perspect. 2000;108:355–362. doi: 10.1289/ehp.00108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P. Effects on reserve capacity: Significance for exposure limits. Sci Total Environ. 1991;101:25–32. doi: 10.1016/0048-9697(91)90099-z. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to tcdd during development permanently alters reproductive function in male long evans rats and hamsters: Reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol Appl Pharmacol. 1995;131:108–118. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) alters reproductive morphology and function in female rat offspring. Toxicology and applied pharmacology. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- Haase RF, McCaffrey RJ, Santiago-Rivera AL, Morse GS, Tarbell A. Evidence of an age-related threshold effect of polychlorinated biphenyls (pcbs) on neuropsychological functioning in a native american population. Environ Res. 2009;109:73–85. doi: 10.1016/j.envres.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijima A, Endo T, Zhang Y, Miyazaki W, Kakeyama M, Tohyama C. In utero and lactational exposure to low doses of chlorinated and brominated dioxins induces deficits in the fear memory of male mice. Neurotoxicology. 2010;31:385–390. doi: 10.1016/j.neuro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hojo R, Kakeyama M, Kurokawa Y, Aoki Y, Yonemoto J, Tohyama C. Learning behavior in rat offspring after in utero and lactational exposure to either tcdd or pcb126. Environmental Health and Preventive Medicine. 2008;13:169–180. doi: 10.1007/s12199-008-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Grover CA, Safe SH, Tiffany-Castiglioni E, Frye GD. Halogenated aromatic hydrocarbons suppress ca1 field excitatory postsynaptic potentials in rat hippocampal slices. Toxicol Appl Pharmacol. 1998;148:7–13. doi: 10.1006/taap.1997.8317. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- Ikeda M, Tamura M, Yamashita J, Suzuki C, Tomita T. Repeated in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure affects male gonads in offspring, leading to sex ratio changes in f2 progeny. Toxicol Appl Pharmacol. 2005;206:351–355. doi: 10.1016/j.taap.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Jiang J, Duan Z, Nie X, Xi H, Li A, Guo A, et al. Activation of neuronal nitric oxide synthase (nnos) signaling pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd)-induced neurotoxicity. Environ Toxicol Pharmacol. 2014;38:119–130. doi: 10.1016/j.etap.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Jin MH, Ko HK, Hong CH, Han SW. In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin affects the development of reproductive system in mouse. Yonsei Med J. 2008;49:843–850. doi: 10.3349/ymj.2008.49.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn PC, Gochfeld M, Nygren M, Hansson M, Rappe C, Velez H, et al. Dioxins and dibenzofurans in blood and adipose tissue of agent orange-exposed vietnam veterans and matched controls. JAMA. 1988;259:1661–1667. [PubMed] [Google Scholar]
- Kakeyama M, Sone H, Tohyama C. Perinatal exposure of female rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin induces central precocious puberty in the offspring. J Endocrinol. 2008;197:351–358. doi: 10.1677/JOE-08-0062. [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee HG, Choi EJ, Park KY, Yang JH. Tcdd alters pkc signaling pathways in developing neuronal cells in culture. Chemosphere. 2007;67:S421–427. doi: 10.1016/j.chemosphere.2006.05.138. [DOI] [PubMed] [Google Scholar]
- Klawans HL. Dystonia and tremor following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mov Disord. 1987;2:255–261. doi: 10.1002/mds.870020403. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Koyama AK, Tworoger SS, Eliassen AH, Okereke OI, Weisskopf MG, Rosner B, et al. Endogenous sex hormones and cognitive function in older women. Alzheimers Dement. 2016;12:758–765. doi: 10.1016/j.jalz.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Hays SM, Aylward LL, Naiman DQ. Perspective on serum dioxin levels in the united states: An evaluation of the nhanes data. J Expo Sci Environ Epidemiol. 2009;19:435–441. doi: 10.1038/jes.2008.63. [DOI] [PubMed] [Google Scholar]
- Lallas PL. The stockholm convention on persistent organic pollutants. American Journal of International Law. 2001;95:692–708. [Google Scholar]
- Lee HG, Kim SY, Choi EJ, Park KY, Yang JH. Translocation of pkc-betaii is mediated via rack-1 in the neuronal cells following dioxin exposure. Neurotoxicology. 2007;28:408–414. doi: 10.1016/j.neuro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen G, Zhao J, Nie X, Wan C, Liu J, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) induces microglial nitric oxide production and subsequent rat primary cortical neuron apoptosis through p38/jnk mapk pathway. Toxicology. 2013;312:132–141. doi: 10.1016/j.tox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Lin KC, Guo NW, Tsai PC, Yang CY, Guo YL. Neurocognitive changes among elderly exposed to pcbs/pcdfs in taiwan. Environ Health Perspect. 2008;116:184–189. doi: 10.1289/ehp.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrath M, Wenger Y, Chang C, Emond C, Garabrant D, Gillespie B, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast feeding. Environmental Health Perspectives. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P, Pocchiari F, Nelson N. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans--seveso, italy. MMWR Morb Mortal Wkly Rep. 1988;37:733–736. [PubMed] [Google Scholar]
- Monaco M, Costa A, Caltagirone C, Carlesimo GA. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an italian adult population. Neurol Sci. 2013;34:749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- Muriel Lezak DH, Erin Bigler, Daniel Tranel. Neuropsychological assessment. Fifth. New York, New York: Oxford University Press; 2012. [Google Scholar]
- Nau H, Bass R, Neubert D. Transfer of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) via placenta and milk, and postnatal toxicity in the mouse. Arch Toxicol. 1986;59:36–40. doi: 10.1007/BF00263955. [DOI] [PubMed] [Google Scholar]
- Papke O, Ball M, Lis A. Pcdd/pcdf in humans, a 1993-update of background data. Chemosphere. 1994;29:2355–2360. doi: 10.1016/0045-6535(94)90404-9. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Hampton L, Lapeza CR, Belser WT, Green V, Alexander L, et al. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Analytical Chemistry. 1987;59:2000–2005. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- Peper M, Klett M, Frentzel-Beyme R, Heller WD. Neuropsychological effects of chronic exposure to environmental dioxins and furans. Environ Res. 1993;60:124–135. doi: 10.1006/enrs.1993.1021. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Martivilalta JL, Capdevila A. When does human brain-development end - evidence of corpus-callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) Neurotoxicol Teratol. 1989;11:13–19. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gardiner JC, Gasior DM, Sweeney AM, Humphrey HE, McCaffrey RJ. Motor function in aging great lakes fisheaters. Environ Res. 1999;80:S46–S56. doi: 10.1006/enrs.1998.3904. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gasior DM, Polverejan E, McCaffrey RJ, Sweeney AM, Humphrey HE, et al. Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of great lakes fish. Environ Health Perspect. 2001;109:605–611. doi: 10.1289/ehp.01109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF. The neurotoxicological consequences of developmental exposure to pcbs. Toxicol Sci. 2000;57:1–3. doi: 10.1093/toxsci/57.1.1. [DOI] [PubMed] [Google Scholar]
- Singer R, Moses M, Valciukas J, Lilis R, Selikoff IJ. Nerve conduction velocity studies of workers employed in the manufacture of phenoxy herbicides. Environ Res. 1982;29:297–311. doi: 10.1016/0013-9351(82)90032-9. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Tomey K, Jannausch M, Eyvazzadeh A, Nan B, Randolph J., Jr Physical functioning and menopause states. Obstet Gynecol. 2007;110:1290–1296. doi: 10.1097/01.AOG.0000290693.78106.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT, 2nd, Prince MM, Nilsen NB, Whelan EA, et al. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- Tager IB, Swanson A, Satariano WA. Reliability of physical performance and self-reported functional measures in an older population. J Gerontol A Biol Sci Med Sci. 1998;53:M295–300. doi: 10.1093/gerona/53a.4.m295. [DOI] [PubMed] [Google Scholar]
- Urban P, Pelclova D, Lukas E, Kupka K, Preiss J, Fenclova Z, et al. Neurological and neurophysiological examinations on workers with chronic poisoning by 2,3,7,8-tcdd: Follow-up 35 years after exposure. Eur J Neurol. 2007;14:213–218. doi: 10.1111/j.1468-1331.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- van der Laan M, Rubin D. Targeted maximum likelihood learning. The International Journal of Biostatistics. 2006;2 doi: 10.2202/1557-4679.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, et al. 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (tcdd) induces premature senescence in human and rodent neuronal cells via ros-dependent mechanisms. PLoS One. 2014;9:e89811. doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Eskenazi B, Patterson DG, Clark G, Turner WE, Bonsignore L, et al. Dioxin-like teq of women from the seveso, italy area by id-hrgc/hrms and calux. J Expo Anal Environ Epidemiol. 2005;15:310–318. doi: 10.1038/sj.jea.7500407. [DOI] [PubMed] [Google Scholar]
- Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the seveso women's health study. Environ Health Perspect. 2011;119:1700–1705. doi: 10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Mocarelli P, Brambilla P, Wesselink A, Patterson DG, Turner WE, et al. Serum tcdd and teq concentrations among seveso women, 20 years after the explosion. Journal of Exposure Science & Environmental Epidemiology. 2014;24:588–594. doi: 10.1038/jes.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Can endocrine disruptors influence neuroplasticity in the aging brain? Neurotoxicology. 2007;28:938–950. doi: 10.1016/j.neuro.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Aging and vulnerability to environmental chemicals age-related disorders and their origins in environmental exposures introduction. Issues Toxicol. 2013;16:1–4. [Google Scholar]
- White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev Alcohol. 2005;17:161–176. doi: 10.1007/0-306-48626-1_8. [DOI] [PubMed] [Google Scholar]
- White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. Journal of Environmental Science and Health - Part C Environmental Carcinogenesis and Ecotoxicology Reviews. 2009;27:197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Wolfe WH, Michalek JE, Miner JC, Roegner RH, Grubbs WD, Lustik MB, et al. The air-force health study - an epidemiologic investigation of health-effects in air-force personnel following exposure to herbicides, serum dioxin analysis of 1987 examination results. Chemosphere. 1992;25:213–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.