Abstract
Addition of activated carbon to contaminated sediment is an established means of remediation but its applicability to sediments high in organic carbon is presently unknown. We evaluated the effects of adding either granular activated carbon (GAC) or pelletized fine-grained activated carbon (PfAC, containing ~ 50% AC) to contaminated sediments from Lake Apopka featuring a very high total organic carbon content (~ 39% w/w dry). Sediments showing background levels of legacy pesticides were spiked with a mixture of 5 chemicals (p,p’-DDE, dieldrin, triclosan, triclocarban, and fipronil) to a nominal concentration of 2 μg/g sediment for each chemical. Following incubation of spiked sediments with the addition of activated carbon for 30 days, we assessed the success on limiting bioaccumulation using Lumbriculus variegatus (blackworm). In contaminant-spiked sediments amended with PfAC, blackworm body burdens of triclosan, triclocarban, and fipronil decreased by >50% and those of p,p’-DDE and dieldrin decreased by <30%. GAC addition to spiked sediments was less impactful, and yielded notable benefits in worm body burden reduction only for fipronil (40%). Fipronil achieved high treatment efficiency within the 30 day amendment with both GAC and PfAC. This is the first study to examine AC treatment in artificially contaminated sediments intrinsically very rich in organic matter content. PfAC exhibited superior performance over GAC for mitigating the uptake of certain organochlorines by aquatic organisms. These results indicate that further studies focusing on additional types of sediments and a broader spectrum of hydrophobic pollutants are warranted.
Keywords: organochlorines, high organic carbon content, activated carbon, Lake Apopka
Introduction
Organochlorine chemicals (OCs) are of global concern due to their negative effects on wildlife and humans. OCs can include legacy pesticides such as p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) and dieldrin or contaminants of emerging concern such as the antimicrobials triclosan (TCS) and triclocarban (TCC), and the widely-used insecticide fipronil. Many organochlorine pesticides (OCPs) have been banned since the late 1970s due to their persistence, bioaccumulation, and toxicity. Although many uses of TCS and TCC in personal care products have been banned recently in the US, legacy contamination and ongoing use of these high production volume chemicals in commerce are still of potential concern. Recent data suggest adverse outcomes for TCS and TCC exposures, including endocrine disruption (Lee et al. 2014; Pollock and Tang, 2014), reduced sperm quality in men (Zhu et al. 2016), and decreased gestational age at birth (Geer et al. 2017). Fipronil, on the other hand, is a topical insecticide added to pet products to control fleas, ticks, and chewing lice infestations. Fipronil can undergo abiotic and biotic processes to yield several degradation products in sediments and wastewater (Gunasekara and Troung, 2007; Sadaria et al. 2017).
The north shore area of Lake Apopka, FL was contaminated with OCPs resulting from agricultural activities and chemical plant discharges in the 1970s. The area was a former muck farm (drained swamp land) with very high total organic carbon content (TOC, up to 39% w/w dry) in sediments due to the accumulation of aquatic-plant materials (Dang et al. 2016). There are several reports in the literature of reproductive impairment and immune dysfunction of local wildlife including alligator, fish and migratory birds on the site (Guillette et al. 1994; Gallagher et al. 2001; Martyniuk et al. 2016). The muck farms have been recently treated by mixing the top 15 – 20 cm of contaminated sediments with the lower 1.2 m of less contaminated sediments (SJRWMD, 2011), but contaminants are still present at high concentrations in organisms (Dang et al. 2016; Martyniuk et al. 2016), and animals, some of which serve as food for human consumption (e.g., fish and ducks) (Tucker and Dudley, 2011). It is therefore necessary to find an alternative approach that can effectively remediate the area.
Activated carbon (AC) amendment of surface water sediments is an established means of reducing the bioavailability of legacy pollutants including dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyls (PCBs) demonstrated both in the laboratory and at the field-scale (Tomaszewski et al. 2007, 2008; McLeod et al. 2008; Sharma et al. 2009). The AC is a porous and manufactured material created from coal or wood, featuring a high surface area and high sorption capacity (Ghosh et al. 2011). Activated carbon can be mixed physically with contaminated sediments or applied directly to the sediment surfaces and further contact sediment porewater via bioturbation. However, no study has focused yet on the benefits of adding AC to sediments containing very high levels of TOC, such as the sediments of the north shore of Lake Apopka. Literature also is lacking on the application of AC to reduce the bioavailability of TCC, TCS, and fipronil in sediments, despite knowledge of the bioaccumulation potential of these chemicals in organisms of ecological importance (Dang et al. 2016; Karlsson et al. 2016; Higgins et al. 2009). In this study, we assessed the efficacy of AC amendment for limiting bioaccumulation of organochlorines in sediments spiked with p,p’-DDE, dieldrin, TCC, TCS, and fipronil. The spiked sediments were used in this study due to lack of sediments historically contaminated with a mixture of the five chemicals. We focused on these five chemicals, as their octanol-water partitioning coefficient (log KOW) values are 6.5 (Sangster, 1994), 5.4 (De Bruijn et al. 1989), 4.9 (Halden and Paull, 2005), 4.8 (Halden and Paull, 2005), and 4.0 (BCPC, 1997), for p,p’-DDE, dieldrin, TCS, TCC, and fipronil, respectively (Table 1), resulting in different affinities for sediment and in bioaccumulation potentials. Two different types of AC were tested including granular AC (GAC) and pelletized fine-grained AC (PfAC). PfAC are pressed pellets consisting of approximately 50 ± 5% AC by dry weight with the remainder of the weight made up by clay, to make them sink faster to the bottom (Patmont et al. 2015). When added into water, the AC pellets quickly breakdown into fine particles, releasing the AC amendment materials and becoming integrated into the bioactive sediment zone via bioturbation (Menzie et al. 2016). To assess the success on limiting bioaccumulation, the freshwater oligochaete Lumbriculus variegatus was exposed to AC- amended, contaminant-spiked sediments. L. variegatus has been extensively used for bioaccumulation studies because worms can burrow into the sediment and tolerate highly contaminated areas (USEPA, 2000). Dang et al. (2016) also reported that OCPs remain in sediments of the north shore of Lake Apopka and are bioavailable to L. variegatus which could be a potential source of OCPs for fish in the lake.
Table 1.
Physical and chemical properties of 5 investigated chemicals
| Chemicals | Molecular formula | log KOW | log KOC | solubility (mg/L) |
|---|---|---|---|---|
| p,p’-DDE | C14H8Cl4 | 6.5 | 4.94a | 0.04 |
| dieldrin | C12H8Cl6O | 5.4 | 4.41b | 0.19 |
| TCS | C12H7Cl3O2 | 4.9 | 4.26c | 10 |
| TCC | C13H9Cl3N2O | 4.8 | 3.82c | < 0.03 |
| fipronil | C12H4Cl2F6N4OS | 4.0 | 2.91d | 1.90 |
Solubility values were obtained from TOXNET database
We aimed to compare the benefits of PfAC addition versus GAC addition, by concentrating on contaminants, both new and historically present in sediments, featuring challenging remediation conditions caused by a high content of organic carbon.
Materials and methods
Standards and solvents
Neat standards of p,p’-DDE (CAS# 72-55-9, 99% pure) and dieldrin (CAS# 60-57-1, 98.7% pure) were purchased from Aldrich (Milwaukee, WI). Neat standards of triclosan (TCS, CAS# 3380-34-5, 96% pure) and triclocarban (TCC, CAS# 101-20-2, 98% pure) were purchased from TCI America (Montgomeryville, PA), while fipronil (CAS# 120068-37-3, 98% pure) was obtained from Enzo Life Sciences (Farmingdale, NY). Isotope internal standards (13C12-p,p’- DDE, 13C12-dieldrin, 13C12-TCS, and 13C6-TCC) were purchased from Cambridge Isotope Laboratories (Tewksbury, MA), whereas 13C2,15N2-fipronil internal standard was purchased from Toronto Research Chemicals (TRC, Canada). Organic solvents (HPLC-grade hexane, acetone, and acetonitrile) were purchased from Fisher Scientific (Fair Lawn, NJ).
Sediment preparation
Sediments (top 40 cm) were collected from the least contaminated areas in the north shore area of Lake Apopka, FL. The TOC in sediments was measured at ~ 39% dry weight and the sediments still contained measurable concentration of weathered p,p’-DDE at 0.9 ± 0.1 (μg/g dw, mean ± SD), but concentrations for dieldrin, TCC, TCS, and fipronil were lower than the limit of quantitation (LOQ; 20 – 50 ng/g dry wt) (Dang et al. 2016). Sediment preparation and spiking were accomplished as described elsewhere (Dang et al. 2016). In brief, a mixture of 5 chemicals (2 mg of each chemical) dissolved into 10 ml of methanol was added dropwise into a mixing slurry of water and sediments (2:1 v/w) to obtain a nominal concentration of 2 μg chemical/g dry sediment. The slurry was then agitated in a cement mixer for a week to establish a steady state level. The spiked sediments were washed 3 times with 500 ml clean water to remove unbound chemicals and then divided into 2 portions: the first portion was not amended with AC and served as un-amended, artificially contaminated sediments while the second portion was amended with AC.
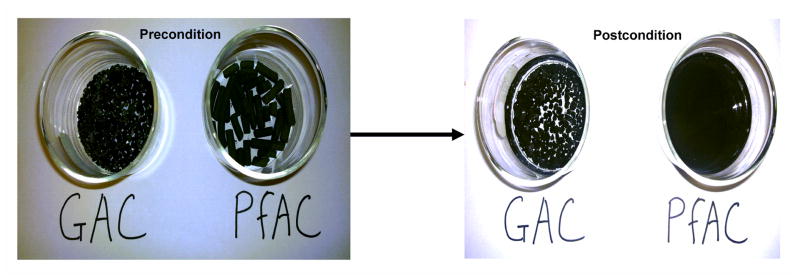
GAC (bituminous coal) was obtained from a tropical fish store (Aquatropics, Gainesville, FL) while PfAC was provided by Dr. Ghosh, University of Maryland-Baltimore County, MD. PfAC is also commercially available from Sediment Solutions, LLC (Ellicott City, MD). Their physical characteristics are presented in Table 2. The GAC remained a hard crumble throughout the experiment, whereas PfAC (tubular pellets that were approximately 1 cm in length and 3 mm in diameter) were completely broken down into fine-grained AC within 1 hr after wetting (Fig. 1). GAC and PfAC were soaked in clean water for 24 h and the supernatants were decanted to remove impurities prior to amendment.
Table 2.
Physical properties of GAC and PfAC used in the study
| Carbon type | Manufacturer | C (%) | Surface area (m2/g) | AC particle size (μm) | Bulk density (g/cm3) |
|---|---|---|---|---|---|
| Bituminous coal based AC (GAC) | Fritz Aquatics | 90 ± 2.5 | 1000 | 2380 – 4000 | 0.5 |
| Coal/coconut based virgin, fine-grained AC (PfAC) | Sediment Solut. | 50 ± 5 | 1050 | 90% < 300 | 0.7 |
Figure 1.
Pre- and post-condition of GAC and PfAC in water for 1 hr.
Artificially contaminated sediment amendments were conducted in eighteen 600 ml glass beakers (6 replicates per treatment) which contained ~ 100 g wet weight (ww) of amended or un-amended, artificially contaminated sediments and 300 ml clean water. Prepared GAC and PfAC were added into the spiked sediments at a dose of 5% dry weight which were equivalent to 0.2-fold and 0.1-fold of the sediment native TOC, respectively. Spiked sediments with AC addition were mechanically mixed for 2 h and further incubated for 30 days to reach a steady state level prior to use in exposure experiments. Clean water was added twice a week to compensate for any loss of water during the incubation.
Bioaccumulation study in spiked sediments lacking AC addition and with AC amendments
Lumbriculus variegatus (California blackworms) were purchased from Aquatic Foods (Fresno, CA) and cultured at the Center for Environmental and Human Toxicology (CEHT), University of Florida, FL. Worms were maintained unfed in an aerated, continuous-flow tank containing a 1.5 cm thick layer of quartz sand. A subsample of L. variegatus was collected for background levels of 5 chemicals.
Bioaccumulation tests were accomplished in 600 ml glass beakers containing un-amended and AC-amended, spiked sediments prepared previously. To each beaker, ~ 0.5 g of worms was added and no food was provided during the 7 days of the exposures. All bioaccumulation experiments were conducted at a constant temperature (25 ± 2 °C) with a 16:8 light:dark photoperiod, and with an aerated system. Worms were separated from sediments by sieving (500 μm mesh size) and rinsed 3 times with clean water, and allowed to depurate in water for 24 hr. About 40 ml of bulk sediments were collected at the end of the exposures. After sediment particles settled for 2 hr, overlying water was decanted and sediments were centrifuged at 2,300 × g for 10 min to collect porewater. About 5 g ww of sediments from each beaker containing spiked sediments lacking AC addition were also collected for measuring chemical concentrations. Samples were kept at −20 °C prior to analysis.
Sample extraction
Samples were thawed at room temperature and extractions were performed following the method of Dang et al. (2016). Approximately 0.5 g of dry sediment or entire sample of worms (~ 0.4 g ww of worm) were first homogenized using a mortar and pestle, spiked with 10 ng of internal standards and solvent extracted with 7 ml hexane:acetone (2:5 v/v) followed by 30 min sonication. The mixture was centrifuged for 10 min at 450 × g and supernatants were collected, concentrated to dryness, and subsequently re-dissolved into 1 ml acetonitrile.
Collected porewater samples (~ 5 – 7 ml) were diluted to a total volume of approximately 20 ml, spiked with 10 ng of internal standards, and then liquid-liquid extracted with 2 ml of hexane followed by 2 ml of dichloromethane. The organic extracts were combined, passed through a drying column containing 5 g of anhydrous Na2SO4, and concentrated to dryness. The extracts were re-dissolved into 1 ml acetonitrile prior to analysis.
Analytical methodology
Analysis of p,p’-DDE and dieldrin was conducted on an Agilent 7000 gas chromatography-tandem mass spectrometry (GC-MS/MS) (Santa Clara, CA) operating in the electron impact mode (70 eV). Analysis of TCS, TCC, and fipronil was accomplished on a liquid chromatography- tandem mass spectrometry (LC-MS/MS) using the QTRAP 6500 (Applied Biosystems, Framingham, MA) coupled to a Shimadzu Prominence UHPLC-30AD (Shimadzu Scientific Instruments, Inc., Columbia, MD), controlled by Analyst 1.6 software. Instrumental conditions are provided in Supporting Information. Multiple reaction monitoring (MRM) was used for quantitative analysis on both GC-MS/MS and LC-MS/MS. Transition ions of the target analytes and internal standards are summarized in Table S1.
Data analysis
Concentrations of chemicals measured in worms, spiked sediments, and porewater were expressed as the mean of 6 replicates. Limit of quantitation (LOQ) in samples analyzed by GC-MS/MS ranged from 10 – 50 ng for p,p’-DDE and dieldrin, while LOQ for those analyzed by LC-MS/MS ranged from 1 – 20 ng for TCC, TCS, and fipronil. Concentrations of chemicals were normalized to internal standards spiked into the samples prior to extractions. Difference in concentrations among treatments was compared by one-way analysis of variance (ANOVA) using Graphpad Prism 5.0. Tukey post-hoc t test was used to determine the significance (p<0.05).
Results
Body burdens of L. variegatus in spiked sediments with and without addition of AC
Concentrations of p,p’-DDE, dieldrin, TCC, TCS, and fipronil in spiked sediments lacking AC addition after 7 days of the exposure were 3.4 ± 0.6, 1.8 ± 0.5, 1.4 ± 0.2, 1.1 ± 0.4, and 0.8 ± 0.1 μg/g dry sediment, respectively. Concentrations of p,p’-DDE included both weathered and freshly added chemical. Measured concentrations of dieldrin and TCC in spiked sediments were close to a nominal concentration of 2 μg/g dry sediment, but measured concentrations of TCS and fipronil were much lower (~ 50%) than the nominal concentration. Huang et al. (2014) also reported a rapid decrease in concentrations of TCS in spiked water-sediment systems as compared to a relatively stable concentration of TCS in spiked water and suggested that the loss of TCS in the sediments was due to biological processes. Low concentrations of fipronil in spiked sediments may be attributable to transformative loss of fipronil. Results from prior work with spiked sediments from the north shore of Lake Apopka revealed the formation of fipronil sulfide and fipronil sulfone in spiked sediments, suggesting biodegradative loss mechanisms for fipronil in these sediments (Dang et al. 2016). However, biodegradation of TCS and fipronil in spiked sediments was not examined in the current study.
Sediments newly spiked with a mixture of five contaminants were amended with either GAC or PfAC at 0.2-fold and 0.1-fold of the sediment native TOC, respectively and then used for bioaccumulation studies with L. variegatus. Prior to these exposure studies, we confirmed experimentally that worms used in the study had no detectable levels of p,p’-DDE, dieldrin, TCC, TCS, and fipronil, and that the contaminant concentrations used in the experiments did not cause acute toxicity to L. variegatus. Body burdens of 5 chemicals in worms incubated in the spiked sediments with and without AC amendments are presented in Fig. 2.
Figure 2.
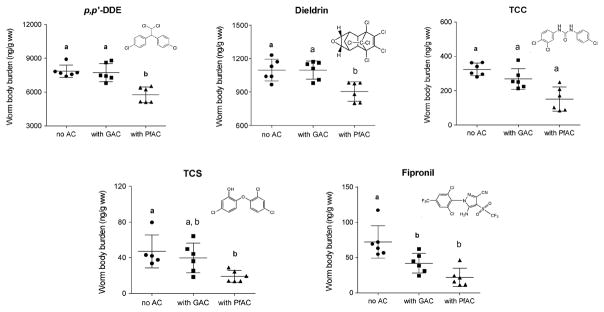
Worm body burdens (ng/g ww) of chemicals in spiked sediments lacking AC addition and with AC amendments. Horizontal bars represent concentration mean and standard deviations. Separate letters indicate significantly different body burdens of chemicals (p<0.05). N=6 replicates for each chemical except for TCS, which had n=5 replicates, in spiked sediments without AC addition.
Chemical concentrations varied among worm replicates per treatment with a considerably high variation observed for TCS and fipronil in spiked sediments lacking AC addition (Fig. 2). In L. variegatus, p,p’-DDE caused the highest body burden, followed by dieldrin, while the lowest body burdens were observed for TCS and fipronil. Our results are in agreement with a previous study that reported biota-sediment accumulation factor (BSAF) values in a rank order [fipronil (1.1) < TCS (1.4) < dieldrin (21.8) < p,p’-DDE (49.8)] corresponding to the increasing degree of hydrophobicity of these contaminants (Dang et al. 2016). Whereas TCC and TCS have similar KOW values and similar measured concentrations in spiked sediments, worm body burdens of TCC were about 6-fold higher than body burdens of TCS in both spiked sediments lacking AC addition and in spiked sediments with AC amendments. Possible explanations are involved with the ionization potential of TCS and/or its relatively greater susceptibility to biotransformation. In a study of earthworms in biosolids-amended soils, Higgins et al. (2011) also observed variable bioaccumulation of TCS and no clear relationship between TCC exposure levels and bioaccumulation in worm tissue. The authors attributed this difference to the biotransformation of TCS to by-products (e.g., methyl TCS) once taken up by the worms (Higgins et al. 2011). In addition, the pH of the spiked sediments in the current study was ~ 6.5 suggesting that a fraction of TCS was present in the ionic form, reducing its hydrophobicity and resulting in a much lower body burden relative to TCC concentrations in L. variegatus.
In the GAC amendment experiments, concentrations in L. variegatus of p,p’-DDE and dieldrin at 7740 ± 800 and 1100 ± 81 ng/g ww, respectively, were similar to worm body burdens in experiments lacking GAC amendment (7850 ± 540 and 1100 ± 100 ng/g ww for p,p’-DDE and dieldrin, respectively) (Fig. 2). For TCC and TCS, chemical concentrations in worms also were not significantly different between un-amended and GAC amended exposure experiments (p<0.05) (TCC: 324 ± 36 and 268 ± 70 ng/g ww, respectively; TCS: 50 ± 19 and 44 ± 15 ng/g ww, respectively). An exception was that the worm body burden for fipronil in the spiked sediments with GAC addition decreased significantly (~ 40%) compared to concentrations of fipronil in worms incubated in spiked sediments lacking GAC addition, implying that application of GAC may effectively reduce the body burden of fipronil in this circumstance. GAC appeared not to be effective for limiting bioaccumulation of p,p’-DDE, dieldrin, TCC, and TCS in L. variegatus. This could be the fact that p,p’-DDE, dieldrin, TCC, and TCS are more hydrophobic than fipronil which loosely binds to sediment particles and further is effectively sequestered by GAC.
In PfAC amendment experiments, concentrations of chemicals in L. variegatus were much lower than concentrations of chemicals in worms exposed to spiked sediments lacking PfAC addition. For example, worm body burdens of p,p’-DDE and dieldrin in the PfAC treatment were measured at 5758 ± 700 and 905 ± 89 ng/g ww, respectively, showing a decrease by 20 – 30% (p<0.05) in comparison to worm body burdens determined for spiked sediments lacking AC addition. For TCC and TCS, concentration decreases of ~ 60% were observed in the presence of PfAC relative to spiked sediments lacking AC amendment. Worm body burden of TCC was also lower in spiked sediments with PfAC amendment than body burdens of TCC in spiked sediments with GAC amendment, but concentrations of TCS in worms were not statistically different when comparing the PfAC and GAC amendment sediments (p<0.05) (Fig. 2). Concentration of fipronil in worms again decreased (~ 70%) and the decrease was not statistically different between GAC and PfAC amendment experiments (p<0.05).
Sediment porewater concentrations in the presence and absence of AC amendments
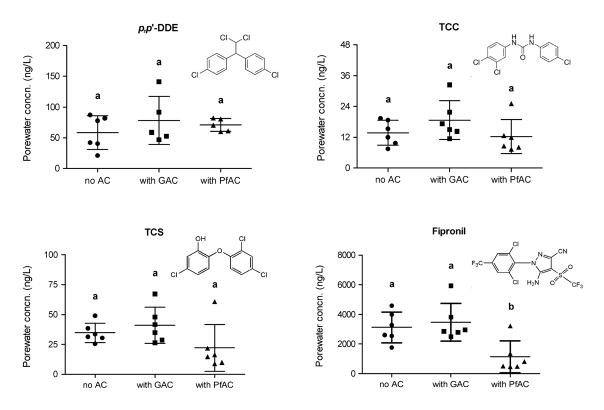
Porewater was collected from spiked sediments in the presence of AC addition and from spiked sediments lacking AC addition and also subjected for chemical analysis. Porewater was experimentally defined as sediment-derived water subjected to centrifugation at 2,300 × g for 10 min to remove sediment particles but not colloids. The measured concentrations of 5 chemicals in each replicate are presented in Fig. 3. Porewater concentrations of dieldrin were below the method detection limit in both sediments having received AC amendments and in spiked sediments lacking AC addition. Concentrations of p,p’-DDE, TCC, and TCS in porewater were not statistically different among treatments. Addition of PfAC to spiked sediments resulted in statistically lower porewater concentrations of fipronil (~ 50 – 60% reduction) relative to sediments having received either no PfAC or GAC (p<0.05). Concentration patterns of chemicals in sediment porewater were not similar to the pattern of chemicals in worms, suggesting that worm body burdens of chemicals likely included both uptake of truly dissolved chemicals in the porewater and ingestion of contaminated sediments.
Figure 3.
Porewater concentrations (ng/L) of chemicals in spiked sediments lacking AC addition and with AC amendments. Concentrations of dieldrin were below LOQ in sediment porewater. Horizontal bars represent concentration mean and standard deviations. Separate letters indicate significantly different concentrations (p<0.05). N=6 replicates for each chemical except for p,p’-DDE, which had n=5 replicates, in GAC and PfAC amendment experiments.
Discussion
Studies on the application of AC to remediate contaminated sites are often focused on the sediments containing low TOC contents (< 10% w/w), but no study evaluates AC amendment for sediments featuring very high TOC. High TOC in sediments tend to bind strongly to OCPs and therefore result in lower concentrations in porewater than expected and less bioavailable to organisms. However, we previously reported that organisms quickly incur their body burdens of chemicals after 7 days of exposure using spiked sediments from the north shore of Lake Apopka containing a TOC of 39% (Dang et al. 2016). Application of AC might thus be an effective means for limiting bioaccumulation of toxicants from sediments rich in organic matter. In this study, we monitored body burdens of both new and historical contaminants in L. variegatus in artificially spiked sediments lacking AC addition and with AC amendments.
Results provide strong evidence that use of conventional GAC was effective only for fipronil (log KOW ~ 4), but PfAC can significantly limit bioavailability and thereby reduce the body burdens in L. variegatus of a spectrum of contaminants of concern. For example, the PfAC amendment reduced worm body burdens by 50 – 60% for TCC and TCS, and 20 – 30% for p,p’-DDE and dieldrin, but no reduction was observed for these contaminants in spiked sediments receiving GAC. It should also be noted that PfAC contains approximately 50% AC and thus the actual amount of AC introduced into the spiked sediments were 2 fold less than the amount of AC added into spiked sediments in the GAC experiments. The difference in bioaccumulation reduction was likely due to the difference in particle sizes between PfAC (< 300 μm) and GAC (2380 – 4000 μm) and greater particle surface area for PfAC (Table 2). Our results are consistent with previous data indicating that smaller AC particle size increases treatment effectiveness in reducing the bioaccumulation of hydrophobic organic contaminants including polychlorinated biphenyls (PCBs) and OCPs (Zimmerman et al. 2005; Thompson et al. 2016). In addition, PfAC appears to completely disintegrate into fine particles after 1 hr incubation in water (Fig. 1) and perhaps, it is fully exposed to the sediment porewater via mechanical mixing with spiked sediments, making it more available for sorption of chemicals from the dissolved phase than GAC, which is still present in larger particle sizes and has less contact with the porewater. Choi et al. (2014) attributed this effect to the faster mass transfer within the AC particle and shorter diffusion distance between AC and sediment particles for smaller AC particles.
Although PfAC amendment appeared to be more effective than GAC amendment in the current study, only a small percent reduction (< 30%) in worm body burdens of two OCPs (p,p’-DDE and dieldrin) was observed in PfAC amendment experiments (Fig. 2) and porewater concentrations of dieldrin and p,p’-DDE were not significantly different between PfAC and no AC amendment (Fig. 3). Previous studies showed that bioaccumulation of PCBs, which have similar degrees of hydrophobicity or log KOW values, in worms reduced up to 99% after contaminated sediments were amended with AC (particle size < 300 μm) (Beckingham and Ghosh, 2011; Sun and Ghosh, 2007) (Table 3). Beckingham and Ghosh (2011) also reported a large reduction (95 – 99%) in aqueous concentrations of PCBs 1 year after AC amendment as compared to background levels. Contact time could play a significant role in treatment efficiency for highly lipophilic contaminants. The effectiveness of AC applications often increases over time assuming that there is not a significant flux of pollutants from the sediment porewater to the water column (Patmont et al. 2015). In addition, Werner et al. (2006) proposed a mass transfer model of PCBs in AC amended sediments and suggested that the higher chlorinated PCBs (high log KOW values) are slow to transfer from sediments to AC. In this study, spiked sediments with AC addition were only incubated for 30 days prior to the bioaccumulation study. Many studies indicate that full treatment effectiveness of AC amendments is achieved years after application (Thompson et al. 2016; Beckingham and Ghosh, 2011; Sun et al. 2009).
Table 3.
Carbon dosage, particle size, sediment TOC, contact time, and worm body burden reduction of chemicals
| Carbon amendment(% w/w) | Particle size(μm) | Sediment TOC (%) | Contact time | Body burden reduction(%) | |
|---|---|---|---|---|---|
| Current study | 2.5 | < 300 | 39 | 30 days | 20 – 30% for p,p’-DDE and Dieldrin 60 – 70% for TCC, TCS, and fipronil |
| Beckingham and Ghosh (2011) | 3.8 | 75 – 300 | 5.8 ± 0.7 | 3 years | 69–99% for PCBs |
| Sun and Ghosh(2007) | 2.6 | 45 – 180 | 5.2 ± 0.2 | 2 min | 92% for PCBs |
Application of AC to reduce the bioaccumulation of OCPs including DDT (precursor of p,p’-DDE) (Tomaszeeski et al. 2007; Thompson et al. 2016; Denyes et al. 2016) and legacy PCBs (Sun and Ghosh, 2007; Beckingham and Ghosh, 2011) has been reported, but no study addresses the bioaccumulation of TCC, TCS, and fipronil in AC amended sediments. Concentrations of TCC and TCS in L. variegatus from sediment exposures with PfAC amendment in the current study were reduced by 50 – 60% (Fig. 2). Beckingham and Ghosh (2011) indicated the percent reduction in bioaccumulation of PCBs with log KOW ~ 5.0, similar to TCC (log KOW of 4.8) and TCS (log KOW of 4.9), in L. variegatus after AC amendment ranging from 69 – 99% (Table 3). This percent reduction is higher than the percent reduction in bioaccumulation of TCC and TCS in the current study. However, sediment exposures were conducted 1 – 3 year after AC amendment in the previous study as compared to 1 month incubation of the spiked sediments with AC in the current study. Fipronil appeared to achieve relatively high treatment efficiency in both GAC and PfAC experiments, possibly requiring much less time (< year or months) than required for legacy contaminants. Further research is necessary to test the role of amendment dosage and incubation time of AC in high TOC sediments for these three chemicals.
Supplementary Material
Highlights.
GAC and PfAC were added into spiked sediments featuring a very high total organic carbon content.
Limiting bioaccumulation of organochlorines in AC-amended, spiked sediments was assessed using Lumbriculus variegatus.
GAC only reduced body burden of fipronil in worms.
PfAC achieved high treatment efficiency for chemicals with low log KOW values.
Acknowledgments
This research was supported by funding from the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (SRP) (Award Number R01-ES020899). This project was made possible by the National Institutes of Health Shared Instrumentation Grant (Grant Number 1S10OD018141). We thank Dr. Upal Ghosh for kindly providing pelletized activated carbon materials to support this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agyin-Birikorang S, Miller M, O’Connor GA. Retention-release characteristics of triclocarban and triclosan in biosolids, soils, and biosolids-amended soils. Environ Toxicol Chem. 2010;29(9):1925–1933. doi: 10.1002/etc.251. [DOI] [PubMed] [Google Scholar]
- Beckingham B, Ghosh U. Field-scale reduction of PCB bioavailability with activated carbon amendment to river sediments. Environ Sci Technol. 2011;45(24):10567–10574. doi: 10.1021/es202218p. [DOI] [PubMed] [Google Scholar]
- Choi Y, Cho Y, Werner D, Luthy RG. In situ sequestration of hydrophobic organic contaminants in sediments under stagnant contact with activated carbon. 2 Mass transfer modeling. Environ Sci Technol. 2014;48(3):1843–1850. doi: 10.1021/es404209v. [DOI] [PubMed] [Google Scholar]
- Dang VD, Kroll KJ, Supowit SD, Halden RU, Denslow ND. Bioaccumulation of legacy and emerging organochlorine contaminants in Lumbriculus variegatus. Arch Environ Contam Toxicol. 2016;71(1):60–69. doi: 10.1007/s00244-016-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruijn J, Busser F, Seinen W, Hermens J. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environ Toxicol Chem. 1989;8(6):499–512. [Google Scholar]
- Denyes MJ, Rutter A, Zeeb BA. Bioavailability assessments following biochar and activated carbon amendment in DDT-contaminated soil. Chemosphere. 2016;144:1428–1434. doi: 10.1016/j.chemosphere.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Gross TS, Sheehy KM. Decreased glutathione S-transferase expression and activity and altered sex steroids in Lake Apopka brown bullheads (Ameiurus nebulosus) Aquat Toxicol. 2001;55(3):223–237. doi: 10.1016/s0166-445x(01)00158-8. [DOI] [PubMed] [Google Scholar]
- Geer LA, Pycke BF, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater Part A. 2017;323:177–183. doi: 10.1016/j.jhazmat.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh U, Luthy RG, Cornelissen G, Werner D, Menzie CA. In-situ sorbent amendments: a new direction in contaminated sediment management. Environ Sci Technol. 2011;45(4):1163–1168. doi: 10.1021/es102694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekara AS, Troung T. Environmental fate of fipronil. Environmental Monitoring Branch; California Environmental Protection Agency; Sacramento, CA: 2007. http://cdpr.ca.gov/docs/emon/pubs/fatememo/fipronilrev.pdf. [Google Scholar]
- Halden RU, Paull DH. Co-occurrence of triclocarban and triclosan in U.S water resources. Environ Sci Technol. 2005;39(6):1420–1426. doi: 10.1021/es049071e. [DOI] [PubMed] [Google Scholar]
- Higgins CP, Paesani ZJ, Chalew TEA, Halden RU. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ Toxicol Chem. 2009;28(12):2580–2586. doi: 10.1897/09-013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CP, Paesani ZJ, Abbott Chalew TE, Halden RU, Hundal LS. Persistence of triclocarban and triclosan in soils after land application of biosolids and bioaccumulation in Eisenia foetida. Environ Toxicol Chem. 2011;30(3):556–563. doi: 10.1002/etc.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wu C, Xiong X, Zhang K, Liu J. Partitioning and degradation of triclosan and formation of methyl-triclosan in water-sediment systems. Water Air Soil Pollut. 2014;225:2099–2109. [Google Scholar]
- Karlsson MV, Marshall S, Gouin T, Boxall AB. Routes of uptake of diclofenac, fluoxetine, and triclosan into sediment-dwelling organisms. Environ Toxicol Chem. 2016;35(4):836–842. doi: 10.1002/etc.3020. [DOI] [PubMed] [Google Scholar]
- Koch R. Molecular connectivity index for assessing ecotoxicological behavior of organic compounds. Toxicol Environ Chem. 1983;6(2):87–96. [Google Scholar]
- Lee H, Hwang K, Nam K, Kim H, Choi K. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol. 2014;27(5):834–842. doi: 10.1021/tx5000156. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Doperalski NJ, Prucha MS, Zhang J, Kroll KJ, Conrow R, Barber DS, Denslow ND. High contaminant loads in Lake Apopka's riparian wetland disrupt gene networks involved in reproduction and immune function in largemouth bass. Comp Biochem Physiol Part D: Genomics and Proteomics. 2016;19:140–150. doi: 10.1016/j.cbd.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Menzie C, Amos B, Driscoll SK, Ghosh U, Gilmour C. Evaluating the efficacy of a low-impact delivery system for in situ treatment of sediments contaminated with methylmercury and other hydrophobic chemicals (ER-0835) Fact Sheet 2016 [Google Scholar]
- McLeod PB, Luoma SN, Luthy RG. Biodynamic modeling of PCB uptake by Macoma balthica and Corbicula fluminea from sediment amended with activated carbon. Environ Sci Technol. 2008;42(2):484–490. doi: 10.1021/es070139a. [DOI] [PubMed] [Google Scholar]
- Patmont CR, Ghosh U, LaRosa P, Menzie CA, Luthy RG, Greenberg MS, Cornelissen G, Eek E, Collins J, Hull J. In situ sediment treatment using activated carbon: A demonstrated sediment cleanup technology. Integr Environ Assess Manag. 2015;11(2):195–207. doi: 10.1002/ieam.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T, Tang B. Triclosan exacerbates the presence of 14 C-bisphenol A in tissues of female and male mice. Toxicol Appl Pharmacol. 2014;278(2):116–123. doi: 10.1016/j.taap.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Sadaria AM, Sutton R, Moran KD, Teerlink J, Brown JV, Halden RU. Passage of fiproles and imidacloprid from urban pest control uses through wastewater treatment plants in northern California, USA. Environ Toxicol Chem. 2017;36(6):1473–1482. doi: 10.1002/etc.3673. [DOI] [PubMed] [Google Scholar]
- Sangster J. LogKOW - A databank of evaluated octanol-water partition coefficient (log P) on microcomputer diskette. Sangster Research Laboratory; Montreal, Quebec, Canada: 1994. [Google Scholar]
- Sharma B, Gardner KH, Melton J, Hawkins A, Tracey G. Evaluation of activated carbon as a reactive cap sorbent for sequestration of polychlorinated biphenyls in the presence of humic acid. Environ Eng Sci. 2009;26(9):1371–1379. [Google Scholar]
- Sharom MS, Miles JRW, Harris CR, McEwen FL. Behaviour of 12 insecticides in soil and aqueous suspensions of soil and sediment. Water Res. 1980;14(8):1095–1100. [Google Scholar]
- St. Johns River Water Management District (SJRWMD) Human health risk assessment update, Lake Apopka north shore restoration area (NSRA) Lake and Orange Counties; FL: 2011. AMEC Project#: 6063110187. [Google Scholar]
- Sun X, Ghosh U. PCB bioavailability control in Lumbriculus variegatus through different modes of activated carbon addition to sediments. Environ Sci Technol. 2007;41(13):4774–4780. doi: 10.1021/es062934e. [DOI] [PubMed] [Google Scholar]
- Sun X, Werner D, Ghosh U. Modeling PCB mass transfer and bioaccumulation in a freshwater oligochaete before and after amendment of sediment with activated carbon. Environ Sci Technol. 2009;43(4):1115–1121. doi: 10.1021/es801901q. [DOI] [PubMed] [Google Scholar]
- The British Crop Protection Council (BCPC) The pesticide manual: A world compendium. 11 1997. [Google Scholar]
- Tomlin CDS, Tomaszewski JE, Werner D, Luthy RG. Activated carbon amendment as a treatment for residual DDT in sediment from a superfund site in San Francisco Bay, Richmond, California, USA. Environ Toxicol Chem. 2007;26(10):2143–2150. doi: 10.1897/07-179R.1. [DOI] [PubMed] [Google Scholar]
- Tomaszewski JE, McLeod PB, Luthy RG. Measuring and modeling reduction of DDT availability to the water column and mussels following activated carbon amendment of contaminated sediment. Water Res. 2008;42(16):4348–4356. doi: 10.1016/j.watres.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Tucker WA, Dudley JL. Human health risk assessment update. Lake Apopka North Shore restoration area (NSRA), Lake and Orange Counties, FL AMEC E&I, Inc; Newerry, FL: 2011. [Google Scholar]
- Thompson JM, Hsieh C, Hoelen TP, Weston DP, Luthy RG. Measuring and modeling organochlorine pesticide response to activated carbon amendment in tidal sediment mesocosms. Environ Sci Technol. 2016;50(9):4769–4777. doi: 10.1021/acs.est.5b05669. [DOI] [PubMed] [Google Scholar]
- USEPA. Methods for measuring the toxicity and bioaccumulation of sediment- associated contaminants with freshwater invertebrates. 2. Office of Research and development, Mid-Continent Ecological Division, and Office of Water, Office of Science and Technology. USEPA; Duluth: 2000. EPA 600/R–9/064. [Google Scholar]
- Werner D, Ghosh U, Luthy RG. Modeling polychlorinated biphenyls mass transfer after amendment of contaminated sediment with activated carbon. Environ Sci Technol. 2006;40(13):4211–4218. doi: 10.1021/es052215k. [DOI] [PubMed] [Google Scholar]
- Ying GG, Kookana RS. Sorption of fipronil and its metabolites on soils from South Australia. J Environ Sci Health. 2001;B36(5):545–558. doi: 10.1081/PFC-100106184. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhang H, Tong C, Xie C, Fan G, Zhao S, Yu X, Tian Y, Zhang J. Environmental exposure to triclosan and semen quality. Int J Environ Res Public Health. 2016;13(2):224–236. doi: 10.3390/ijerph13020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JR, Werner D, Ghosh U, Millward RN, Bridges TS, Luthy RG. Effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environ Toxicol Chem. 2005;24(7):1594–1601. doi: 10.1897/04-368r.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.