Abstract
Host immunity influences the impact of radiotherapy (RT) in cancer but mechanistic connections remain obscure. In this study, we investigated the relationship of indoleamine 2,3-dioxygenase (IDO) systemic activity on clinical outcomes in RT-treated non-small cell lung cancer (NSCLC). IDO-mediated production of kynurenine and the kynurenine:tryptophan ratio in patient blood serum were determined for stage III NSCLC patients at times before, during and after RT administration and then correlated to overall survival (OS), progression-free survival and disease progression rate in patients. We found the impact of RT on these serum IDO markers to be heterogeneous in patients. On average, kynurenine:tryptophan ratios were reduced during RT but restored after RT. Notably, both baseline levels of kynurenine:tryptophan and changes in the levels of kynurenine after RT were significantly associated with OS. When combined, favorable change and favorable baseline corresponded with very long-term OS (median OS was not reached after 57 months median follow-up). Favorable change combined with unfavorable baseline still corresponded with a lack of distant metastases. Our results suggest that RT alters IDO-mediated immune status in NSCLC patients and that changes in this serum biomarker may be useful to predict outcomes and perhaps personalize RT dosage to improve survival.
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80-85% of lung cancer, which is the leading cause of cancer death in the United States (1). Approximately 64% of patients with NSCLC require radiotherapy (RT) at least once during their course of disease (2). Recently, it has been reported that radiation induced tumor killing can activate the immune system by generating tumor specific antigens and converting the tumor into an individualized in situ vaccine (3). Animal studies have demonstrated that a combination of hypo-fractionated RT with immunotherapy generated synergetic effects on local tumor control and even abscopal effects for tumor killing outside the treatment fields (3-5). The RT abscopal effect has been reported in a few clinical cases, including one melanoma case in the New England Journal of Medicine (4).
Indoleamine 2, 3-dioxygenase (IDO) is an intercellular enzyme that converts the essential amino acid tryptophan into kynurenine through the IDO/kynurenine pathway (6). IDO depletes tryptophan in the tumor microenvironment and activates immune checkpoint via amino acid sensor general control nonderepressible 2 (GCN2) (7,8). Kynurenine, the direct metabolite of this pathway, and its further downstream metabolites, promotes potent immune suppression by enhancing Foxp3-regulatory T cell functions and attenuating responses of effector T cells and natural killer cells (9,10). Previous studies have validated IDO as a potent immune checkpoint in cancers and other chronic inflammatory diseases (11-13). Because tryptophan and kynurenine concentrations can be measured from patients' serum, IDO activity can be monitored by computing kynurenine to tryptophan (K:T) ratio. Clinical studies have reported that IDO activity correlated with the number of tumor infiltrating lymphocytes in esophageal and colorectal cancers (14,15), and elevated IDO activity correlated with poor clinical outcomes in several types of cancers (16-19), including lung cancer (20-22). These clinical data further support that IDO is an immune checkpoint that mediates anti-tumor immune activity. However, it is not clear how this IDO-mediated immune activity changes during or after RT in cancer patients, and whether these changes have any impact on tumor control or survival.
In this study, we hypothesized that RT can alter the IDO-mediated anti-tumor immune activity which will impact on tumor progression or survival in patients with NSCLC. To test this hypothesis, we assessed IDO-mediated immune activity by quantifying the key molecules associated with the IDO checkpoint, including kynurenine and the K:T ratio (a commonly used surrogate for IDO activity), at pre-RT, 2-week (during-RT), 4-week (during-RT) and post-RT (3 months after RT completion). We then performed survival analysis to correlate these parameters and their changes with progression free survival (PFS), overall survival (OS) and distant tumor progression.
Materials and Methods
Patients and Treatment
Patients with stage III inoperable/unresectable NSCLC enrolled institutional review board (IRB) approved prospective protocols were eligible. The protocols were conducted according to requirements of Belmont Report and U.S. Common Rules. All subjects signed informed written consent before enrollment. All patients received conventionally fractionated daily RT, which was given using 3D conformal radiotherapy (3DCRT) as previously described (23,24). Details of these prospective trials were summarized in supplement Table S1. Equivalent dose at 2 Gy fraction (EQD2) were computed for those received other than 2 Gy daily doses, using alpha/beta of 10.
Patients Follow-up and Samples/Clinical Data Collection
Patients were examined weekly during the course of RT, followed up approximately every 3 months during the first year, every 6 months during the second year and then annually thereafter. Clinical and follow-up data, including age, gender, smoking history, histology, clinical stage, tumor volume, Karnofsky performance score (KPS), EQD2 and chemotherapy, were recorded prospectively up to 2 years after completion of RT. Red top blood collection tubes without anticoagulants were used for serum. Blood samples of patients were collected at up to four time points of pre-RT, 2- and 4-week during-RT, and post-RT (generally first follow-up at 3 months after RT completion).
Measurements of Serum Tryptophan and Kynurenine
Serum tryptophan and kynurenine concentrations were measured using a high performance liquid chromatography system (Shimadzu LC20) as described before with minor modification (25,26). 25μl of serum samples were diluted with equal volumes of 30mM NaAc pH4.0 and deproteinated with perchloric acid. Kynurenine was detected on an UV channel at 360nm and tryptophan was detected on a fluorescence channel at 285nm excitation and 365nm emission. Samples were analyzed using Lab SolutionTM software (Shimadzu). Samples were quantified with external standards and at least one quality control samples were randomly inserted into every plate for reference. For quality control purposes, double-blinding duplicate/triplicate testing verified the assay reproducibility to be over 95%.
Statistical Analysis
OS and PFS were the primary endpoints. OS was computed from RT start to the date of death of any cause. PFS was defined from start of RT to the date of any progression or death. Patients were censored at last follow-up if progression or death had not occurred. Kaplan-Meier log-rank test was applied to compare the survival difference between groups. Clinical variables with log-rank P<0.05 under univariate analysis were selected as co-variants for adjustment in multivariate analysis. Multivariate Cox proportional hazards model was used to estimate hazard ratios (HR) with 95% confidence interval (95% CI). The IDO-checkpoint associated molecules from the 4 time points were compared using repeated analysis of variance (ANOVA) (equivalent to paired t test for 2 groups). Considering 2 markers × 2 time points in survival analysis, Bonferroni correction for multiple testing was performed for statistical significance at a P<0.0125 (0.05/4) level. All statistical analyses were performed using IBM® SPSS Statistics 22.0 (IBM, Inc.). Scatter plot figures of IDO dynamic changes were generated using GraphPad Prism® (version 5.01).
Results
Study Population and Overall Clinical Outcomes
Of 182 patients enrolled, 110 patients staged III with quality samples available for IDO testing formed the primary study population. There were 103 Caucasian (94%) and 29 (26%) females. The median age was 66 years. All patients (100%) received definitive dose (≥ 60 Gy) of 3DCRT and 103 (94%) of whom underwent concurrent platinum-based chemotherapy. Table 1 details the patient demographic and clinical features. The median follow-up time was 56 months (95% CI, 49-63 months). The median OS and PFS were 23 (95% CI, 17-29) and 11 (95% CI, 7-15) months, respectively. Univariate analysis showed that age (P=0.016), gender (P=0.040), tumor volume (P=0.010), KPS (P<0.001) and EQD2 (P=0.014) were significant for OS. Younger, female, smaller tumor volume, higher KPS and higher EQD2 had better OS. However, only tumor volume (P=0.041), KPS (P=0.010) and EQD2 (P=0.004) were significant for PFS (Table 1). These clinical factors were thus selected as clinical co-variants for further multivariate analysis of IDO checkpoint associated parameters.
Table 1. Comparison of Overall Survival and Progression Free Survival Based on Clinical Characteristics of Patients.
| Clinical factors | Patients (n) | Overall Survival | Progression Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Death n (%) | MST (months) | P* | HR (95% CI) | Progression n (%) | MPT (months) | P* | HR (95% CI)* | ||
| Age (years) | |||||||||
| ≤ 66 | 59 | 38 (64) | 31 | 0.016 | 1.00 (reference) | 44 (75) | 16 | 0.235 | 1.00 (reference) |
| > 66 | 51 | 41 (80) | 14 | 1.03 (1.01-1.05) | 42 (82) | 8 | 1.01 (0.99-1.04) | ||
| Gender | |||||||||
| Male | 81 | 63 (78) | 22 | 0.040 | 1.00 (reference) | 66 (81) | 12 | 0.583 | 1.00 (reference) |
| Female | 29 | 16 (55) | 41 | 0.56 (0.32-0.97) | 20 (69) | 10 | 0.87 (0.53-1.44) | ||
| Smoking | |||||||||
| No | 7 | 4 (57) | 43 | 0.402 | 1.00 (reference) | 5 (71) | 19 | 0.599 | 1.00 (reference) |
| Yes | 103 | 75 (73) | 23 | 1.54 (0.56-4.21) | 81 (79) | 11 | 1.27 (0.52-3.15) | ||
| Histology | |||||||||
| Adenocarcinoma | 33 | 18 (54) | 31 | 0.061 | 1.00 (reference) | 23 (70) | 11 | 0.918 | 1.00 (reference) |
| Squamous cell | 38 | 28 (74) | 16 | 1.83 (1.01-3.31) | 29 (76) | 10 | 1.12 (0.65-1.93) | ||
| NOS | 39 | 33 (85) | 19 | 1.93 (1.08-3.42) | 34 (87) | 14 | 1.09 (0.64-1.86) | ||
| Tumor volume (10 cc) | |||||||||
| ≤ 16 | 51 | 35 (69) | 25 | 0.010 | 1.00 (reference) | 38 (74) | 14 | 0.041 | 1.00 (reference) |
| > 16 | 51 | 39 (76) | 22 | 1.02 (1.00-1.03) | 41 (80) | 9 | 1.01 (1.00-1.02) | ||
| KPS | |||||||||
| ≤ 80 | 43 | 36 (84) | 13 | < 0.001 | 1.00 (reference) | 38 (88) | 9 | 0.010 | 1.00 (reference) |
| > 80 | 67 | 43 (64) | 32 | 0.95 (0.93-0.98) | 48 (72) | 14 | 0.96 (0.94-0.99) | ||
| EQD2 (Gy) | |||||||||
| ≤ 70 | 63 | 50 (79) | 18 | 0.014 | 1.00 (reference) | 53 (84) | 8 | 0.004 | 1.00 (reference) |
| > 70 | 47 | 29 (62) | 32 | 0.97 (0.95-0.99) | 33 (70) | 19 | 0.97 (0.95-0.99) | ||
| Chemotherapy | |||||||||
| No | 7 | 5 (71) | 7 | 0.243 | 1.00 (reference) | 5 (71) | 5 | 0.476 | 1.00 (reference) |
| Yes | 103 | 74 (72) | 23 | 0.58 (0.23-1.44) | 81 (79) | 12 | 0.72 (0.29-1.78) | ||
Abbreviations: NOS, non-otherwise specified; MST, median OS; MPT, median PFS; KPS, Karnofsky Performance Score; EQD2, the 2 Gy-per-fraction equivalent dose; HR, hazard ratio; 95% CI, 95% confidence interval.
By univariate analysis. Age, tumor volume, KPS and EQD2 were analyzed as continuous variables.
Dynamics of Kynurenine and K:T Ratio at Different Time Points
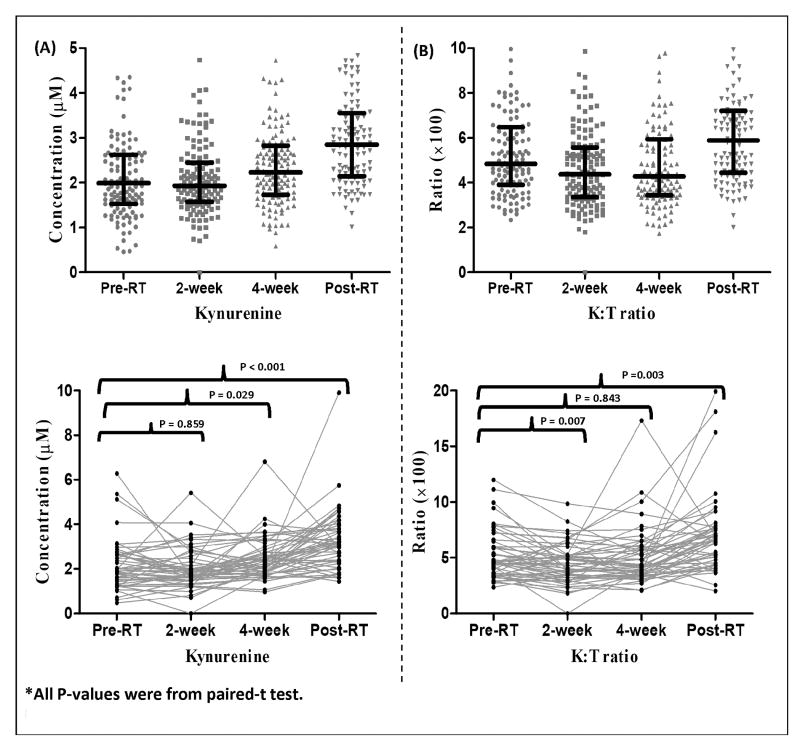
Serum kynurenine and K:T ratio changed heterogeneously through 4 time points from pre-RT, to 2- and 4-week during-RT, and post-RT (Figure 1A-B). Mean kynurenine concentrations did not change significantly at 2-week of RT, but increased at 4-week during-RT, and increased further post-RT. Kynurenine concentrations post-RT were significantly higher than that of other time points. The mean K:T ratio decreased from pre to 2-week and to 4-week during-RT, and increased sharply post-RT. The K:T ratio at the post-RT was significantly higher than that of pre- and during-RT time points. K:T ratio at 2-week was significantly lower than that of pre-RT under the paired t-test analysis (P=0.007).
Figure 1.
Dynamic changes of IDO-associated molecular activity during and post radiotherapy. The top panel shows individual and mean activity levels, and the bottom panel shows spaghetti plots for kynurenine (A) and K:T ratio (B) at four time points. P-values shown were from paired t-tests. Error bars at figures show 95% confidence interval (CI).
Baseline IDO Biomarkers and Outcome
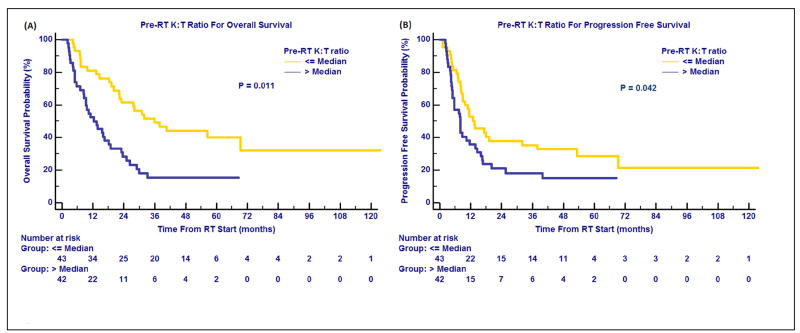
Kynurenine at pre-RT was not significantly correlated with OS (HR=1.03, 95%CI=0.73-1.45; P=0.887) or PFS (HR=0.99, 95%CI=0.71-1.37; P=0.927) (Table 2). K:T ratio at pre-RT correlated significantly with OS (HR, 2.36; 95% CI, 1.21-4.59; P=0.011) and PFS (HR, 1.77; 95% CI, 1.02-3.05; P=0.042) (Figure 2A and 2B, Table 2) after adjusting for the clinical significant factors. However, only the P-value for OS remained significant after Bonferroni's correction. High K:T ratio correlated significantly with poorer survival.
Table 2. Association Between IDO Activity and Changes with Overall Survival and Progression Free Survival of NSCLC.
| Time points | IDO activities | Patients (n) | Overall Survival | Progression Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Death n (%) | MST (months) | P* | HR (95% CI)* | Progression n (%) | MPT (months) | P* | HR (95% CI)* | |||
|
Pre-RT (n = 85) |
Kynurenine | |||||||||
| ≤ median | 43 | 30 (70) | 22 | 0.887 | 1.00 (reference) | 33 (77) | 9 | 0.927 | 1.00 (reference) | |
| > median | 42 | 30 (71) | 18 | 1.03 (0.73-1.45) | 32 (76) | 12 | 0.99 (0.71-1.37) | |||
| K:T ratio | ||||||||||
| ≤ median | 43 | 25 (58) | 36 | 0.011 | 1.00 (reference) | 30 (70) | 14 | 0.042 | 1.00 (reference) | |
| > median | 42 | 35 (83) | 12 | 2.36 (1.21-4.59) | 35 (83) | 8 | 1.77 (1.02-3.05) | |||
|
Post-RT (n = 76) |
Kynurenine | |||||||||
| ≤ median | 38 | 22 (58) | 36 | 0.384 | 1.00 (reference) | 26 (68) | 15 | 0.235 | 1.00 (reference) | |
| > median | 38 | 27 (71) | 25 | 1.07 (0.92-1.24) | 29 (76) | 16 | 1.08 (0.95-1.23) | |||
| K:T ratio | ||||||||||
| ≤ median | 38 | 21 (55) | 41 | 0.277 | 1.00 (reference) | 26 (68) | 25 | 0.739 | 1.00 (reference) | |
| > median | 38 | 28 (74) | 24 | 1.44 (0.75-2.78) | 29 (76) | 14 | 1.10 (0.62-1.98) | |||
|
Post/Pre (n = 57) |
Kynurenine | |||||||||
| ≤ median | 28 | 13 (46) | 41 | 0.006 | 1.00 (reference) | 16 (57) | 15 | 0.025 | 1.00 (reference) | |
| > median | 29 | 22 (76) | 28 | 1.35 (1.09-1.68) | 23 (79) | 14 | 1.31 (1.04-1.66) | |||
| K:T ratio | ||||||||||
| ≤ median | 29 | 18 (62) | 32 | 0.278 | 1.00 (reference) | 19 (65) | 17 | 0.268 | 1.00 (reference) | |
| > median | 28 | 17 (61) | 28 | 1.62 (0.68-3.87) | 20 (71) | 11 | 1.57 (0.71-3.46) | |||
Abbreviations: MST, median OS; MPT, median PFS; HR, hazard ratio; 95% CI, 95% confidence interval.
From multivariate Cox proportional hazards regression models by adjusting for tumor volume, KPS, EQD2. IDO parameters were analyzed as continuous variables. P<0.0125 was considered statistical significance.
Figure 2.
Baseline IDO-mediated immune activity and treatment outcomes. Pre-RT K:T ratio for overall survival (A); Pre-RT K:T ratio for progression free survival (B).
Post-RT IDO Biomarkers and Outcome
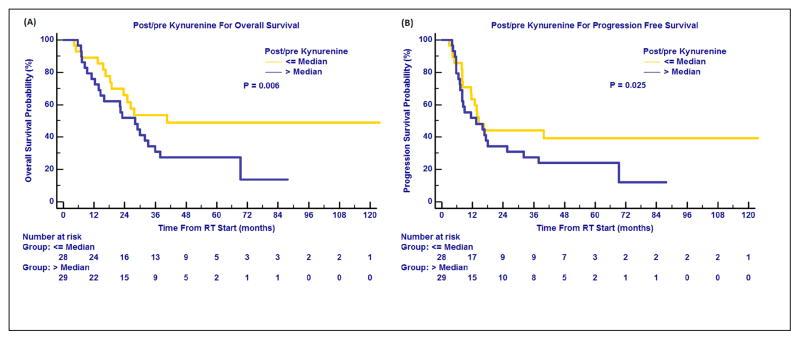
None of the post-RT levels in kynurenine or K:T ratio was significant for OS or PFS. Among various RT-induced changes of these IDO checkpoint molecules, only change in kynurenine was significant. Using median as the cut-off, greater levels of post/pre kynurenine correlated significantly with worse OS (HR, 1.35, 95% CI, 1.09-1.68, P=0.006) and poorer PFS (HR, 1.31, 95% CI, 1.04-1.66, P=0.025). (Figure 3A and 3B, Table 2). However, only the P-value for OS remained significant after Bonferroni's correction.
Figure 3.
Changes of IDO-mediated immune activity and treatment outcomes. Changes of kynurenine after radiotherapy (Post/pre kynurenine) associated with overall survival (A) and progression free survival (B).
Combined Effects of Baseline and Change of IDO Biomarker on OS, PFS and Metastasis
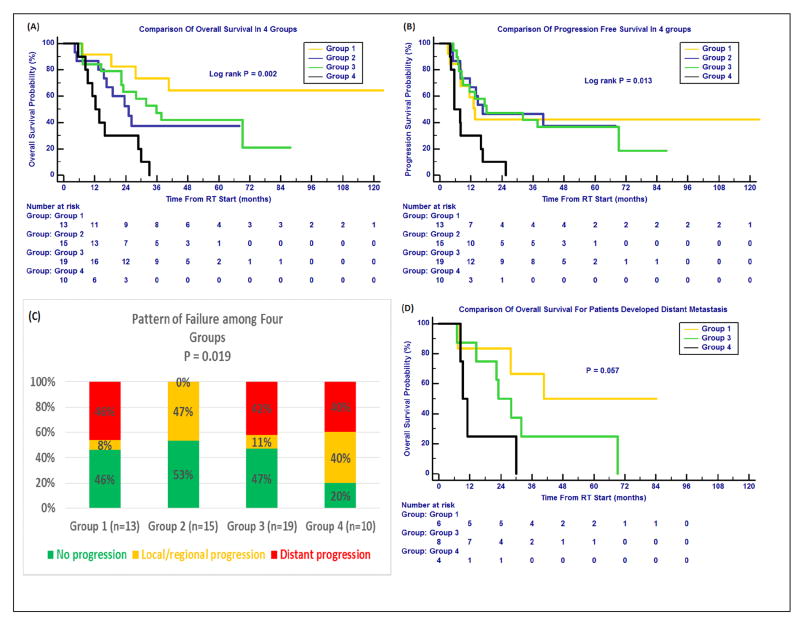
To study the combined effects of baseline and change of IDO activities, patients were stratified into the following 4 groups according to the medians of pre-RT K:T ratio and post/pre kynurenine: 1) low pre-RT K:T ratio and low post/pre kynurenine (favorable baseline and favorable change); 2) high pre-RT K:T ratio and low post/pre kynurenine (unfavorable baseline and favorable change); 3) low pre-RT K:T ratio and high post/pre kynurenine (favorable baseline and unfavorable change); 4) high pre-RT K:T ratio and high post/pre kynurenine (unfavorable baseline and unfavorable change). There were significant differences in OS (P=0.002) (Figure 4A) and PFS (P=0.013) (Figure 4B) for these 4 groups. Patients in Group 1 achieved extremely long-term OS (the median OS has not been reached after up to 57 months of median follow-up), but a relatively low PFS of 14 (10-17) months. Patients in Group 2 had median OS and PFS of 25 (14-36) and 17 (0-45) months, respectively. The median OS and PFS (and their 95% CIs) were 36 (22-50) and 18 (0-45) months for Group 3, and 12 (8-17) and 6 (2-9) months for Group 4, respectively.
Figure 4.
Combined effects of baseline IDO biomarkers and post-RT change with survival and tumor progression. Patients were stratified into 4 groups according to the medians of pre-RT K:T ratio (baseline immune activity) and post/pre kynurenine (change of immune activity). Group 1: favorable baseline and favorable change; Group 2: unfavorable baseline and favorable change; Group 3: favorable baseline and unfavorable change; Group 4: unfavorable baseline and change. (A) Comparison of overall survival (OS) in 4 groups, Group 1 showing extremely long-term OS. (B) Comparison of progression free survival (PFS) in 4 groups.
(C) Comparison of tumor local/regional and distant progressions in 4 groups. The favorable baseline (Groups 1 and 3) corresponded to less local/regional progression, while Group 2 had 0% distant progression.
(D) Comparison of OS for patients developed distant metastasis for Groups 1, 3 and 4.
Pattern of failure analysis was performed for these 4 groups to study how baseline and change of the IDO differently impact on OS and PFS. Distant and local/regional failure were 6/13 (46%) and 1/13 (8%), 0/15 (0%) and 7/15 (47%), 8/19 (42%) and 2/19 (11%), 4/10 (40%) and 4/10 (40%) for Groups 1, 2, 3 and 4, respectively (P=0.019) (Figure 4C). Furthermore, those patients who developed distant progression in Group 1 had a median OS of 41 months, which was longer than that in group 3 and group 4 (23 and 9 months) (P=0.057) (Figure 4D).
Discussion
This study demonstrated that 1) RT caused significant reductions in IDO activity (represented by K:T ratio) during-RT but IDO increased significantly post-RT (after more radiation); 2) IDO at baseline and post-RT correlated significantly with OS and PFS; 3) RT-induced change in kynurenine post-RT was significant for either OS or distant progression in an unusual manner (median OS did not reach, corresponded to 88 months mean OS with favorable baseline, and 0% distant progression under unfavorable baseline). These results support our hypotheses that RT can alter the IDO-mediated anti-tumor immune activity.
This study demonstrates that IDO activity at baseline is an important prognostic marker for patients with NSCLC treated with fractionated RT. This is in agreement with previous studies that baseline IDO immune status plays a prognostic role after surgery or chemotherapy (16,17,20-22). Our data demonstrated low activity of IDO, i.e. low level of kynurenine or low K:T ratio (favorable baseline), was significantly associated with better survival. The prognostic significance of baseline IDO biomarkers in our patients treated with RT may serve as a strong validation of above studies and clinically confirms the immune suppressive role of IDO in NSCLC (27). Although studies with larger sample sizes are needed for RT, this has clinical significance as knowing the prognosis of an individual patient may help triage the care plan of that patient.
More importantly, this study is the first to demonstrate that fractionated RT in NSCLC induces significant change of IDO-mediated immune activity level, and such change correlates with OS, PFS and unusual treatment outcomes in combination with baseline IDO activity. Studies in animal models have shown that hypo-fractionated RT can generate synergetic immune responses (3,5), and there are speculations and single-case reports about activation of anti-tumor immunity by hypo-fractionated RT (3,4). However, there are no reports in human studies showing that RT can generate anti-tumor immunity in a significant number of patients. Recent results in KYNOTE-001 phase 1 trial suggested that previous treatment with radiotherapy in patients with advanced NSCLC results in longer PFS and OS with pembrolizumab treatment than that seen in patients who did not have previous radiotherapy (28). This findings supported that radiotherapy affects host immunity in NSCLC patients. Our study showed that patients with favorable RT-induced change in combination with favorable baselines (Group 1) corresponded to extremely long-term OS (>60% OS up to 57 months median follow-up), suggesting RT-induced immune activity change may generate memory T-cells for long-term tumor control. In addition, patients with favorable change and unfavorable baseline (Group 2) had 0% distant progression, suggesting that this RT-induced anti-tumor immunity may control occult tumor cells outside the RT fields, a phenomenon similar to abscopal effects. However, it is unclear why patients in Group 1 did not have similar “abscopal” effect as in Group 2. The underlying biologic mechanism for this phenomenon will require further research. We speculate that relatively small amount of anti-tumor immune cells were activated in Group 1 patients due to their favorable baseline status. They were not sufficient to control the occult diseases outside the RT fields in a short time, and local salvage treatment was required and able to control the distant metastasis developed from these occult diseases. Our data showed that the metastasis patients still had 41 months median OS, supporting our speculation.
Most interestingly, our data showed that RT can lead favorable or unfavorable changes of IDO immune activity varied with time point (dose level) or patient level. It is unclear what patient-specific factors influence these heterogeneous RT responses. We hypothesize that both underdose and overdose of RT will lead to unfavorable changes of immune activity. Underdose will not be sufficient to generate the favorable change, while overdose will damage the immune system and revert into the unfavorable status. Therefore, there is an optimal RT dose for maximal immune activation. Because patients and tumors have heterogeneous radiosensitivities, the optimal RT dose could be different for different patients. We found that median K:T ratio and median kynurenine concentration reduced in the initial phase of RT (at 2-week, lower doses). This statistically significant K:T ratio reduction suggests that IDO activity may be suppressed by anti-tumor immunity in some patients as early as 2-week after starting RT. Though median kynurenine levels tended to increase, median K:T ratio remained at low levels in the middle phase of RT (at 4-week), while both increased at later stages (post-RT status, high doses). Therefore, changes of these IDO-associated molecules during-RT may serve as potential biomarkers to determine the optimal individualized RT dose for each patient by determining when to stop excessive RT. However, a slight-increase of IDO or kynurenine concentration during RT may not be an indication of unfavorable change, because immune response to radiation damage of other normal tissues may also partially contribute to the increase. Further study is required to understand better the underlying mechanisms.
Additionally, the findings from this study may be relevant to blood biomarker to guide multimodality clinical trials. Anti-IDO agents are available commercially and clinical trials using various IDO inhibitors are in progress (29). Animal studies have revealed synergies between RT and anti-IDO agents (30). A pilot study using an IDO inhibitor after chemotherapy in stage III-IV NSCLC patients has generated promising results (31). A key question about combining RT with IDO inhibitor therapy and other immunotherapy drugs is the optimal RT dose and optimal time points to give IDO inhibitors. RT doses that activate anti-tumor immunity but do not cause excessive immune depletion are desirable.
This study has limitations. One of them is serum sample availability. The numbers of available samples at different time points were diverse, which may impact study results, especially for inter-group comparisons. Also, the study was not powered for a subgroup analysis on chemotherapy-IDO activity association. Previous studies have shown synergy between chemotherapy and IDO inhibitors in promoting clinical responses. It is possible that potential synergistic effects of chemotherapy may have partially biased our results. Rather than a sole effect of RT, the observed phenomenon could be the combined effects of chemotherapy and radiotherapy.
Supplementary Material
Acknowledgments
We are grateful to Theodore S Lawrence MD, PhD for his valuable review comments of the Manuscript.
Grant Support: This work was supported in part by NIH grants R01CA142840 (PI: Kong) and a start-up award (PI: Kong) from Indiana University School of Medicine.
Abbreviations list
- IDO
Indoleamine 2, 3-dioxygenase
- NSCLC
Non-small cell lung cancer
- RT
Radiotherapy
- OS
Overall survival
- PFS
progression-free survival
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66(4):271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49(4):973–85. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 3.Formenti SC. Silvia Formenti on the promise of combining radiotherapy and immunotherapy to treat cancer. Oncology (Williston Park) 2016;30(4):289, 92. [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72(21):5435–40. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 7.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer CC, Wang Y, Hough KP, Sawant A, Grant SC, Thannickal VJ, et al. Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget. 2016 doi: 10.18632/oncotarget.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends in immunology. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines (Basel) 2015;3(3):703–29. doi: 10.3390/vaccines3030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Liu L, Liu K, Bizargity P, Hancock WW, Visner GA. Reduced cytotoxic function of effector CD8+ T cells is responsible for indoleamine 2,3-dioxygenase-dependent immune suppression. J Immunol. 2009;183(2):1022–31. doi: 10.4049/jimmunol.0900408. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Liu WL, Zhang L, Wang JY, Kuang MH, Liu P, et al. Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinoma. Clinical & developmental immunology. 2011;2011:384726. doi: 10.1155/2011/384726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12(4):1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 16.Ino K. Indoleamine 2,3-dioxygenase and immune tolerance in ovarian cancer. Curr Opin Obstet Gynecol. 2011;23(1):13–8. doi: 10.1097/GCO.0b013e3283409c79. [DOI] [PubMed] [Google Scholar]
- 17.Pelak MJ, Snietura M, Lange D, Nikiel B, Pecka KM. The prognostic significance of indoleamine-2,3-dioxygenase and the receptors for transforming growth factor beta and interferon gamma in metastatic lymph nodes in malignant melanoma. Polish journal of pathology : official journal of the Polish Society of Pathologists. 2015;66(4):376–82. doi: 10.5114/pjp.2015.57249. [DOI] [PubMed] [Google Scholar]
- 18.Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93(12):1894–8. doi: 10.3324/haematol.13113. [DOI] [PubMed] [Google Scholar]
- 19.Cavia-Saiz M, Muniz P, De Santiago R, Herreros-Villanueva M, Garcia-Giron C, Lopez AS, et al. Changes in the levels of thioredoxin and indoleamine-2,3-dioxygenase activity in plasma of patients with colorectal cancer treated with chemotherapy. Biochem Cell Biol. 2012;90(2):173–8. doi: 10.1139/o11-077. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Kachala SS, Kadota K, Shen R, Mo Q, Beer DG, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17(16):5247–56. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 21.Creelan BC, Antonia S, Bepler G, Garrett TJ, Simon GR, Soliman HH. Indoleamine 2,3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer. Oncoimmunology. 2013;2(3):e23428. doi: 10.4161/onci.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67(3):361–5. doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Chapet O, Kong FM, Quint LE, Chang AC, Ten Haken RK, Eisbruch A, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63(1):170–8. doi: 10.1016/j.ijrobp.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 24.Kong FM, Hayman JA, Griffith KA, Kalemkerian GP, Arenberg D, Lyons S, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65(4):1075–86. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Lemos HP, Li L, Li M, Chandler PR, Baban B, et al. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J Immunol. 2012;188(10):4913–20. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laich A, Neurauter G, Widner B, Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clinical chemistry. 2002;48(3):579–81. [PubMed] [Google Scholar]
- 27.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16(4):354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3(10):e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca SB, Bolduc AK, et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer. 2014;2:21. doi: 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20(1):221–32. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.